Abstract
Recently, it has been shown that multiple mammalian cell types express daily rhythms in vitro. Although the suprachiasmatic nucleus (SCN) of the hypothalamus is known to regulate a wide range of circadian behaviors, the role for intrinsic rhythmicity in other tissues is unknown. We tested whether the main olfactory bulb (OB) of mice mediates daily changes in olfaction. We found circadian rhythms in cedar oil-induced c-Fos, a protein marker of cellular excitation, in the mitral and granular layers of the OB and in the piriform cortex (PC). These oscillations persisted in constant darkness with a fourfold change in amplitude and a peak ∼4 h after the onset of daily locomotor activity. Electrolytic lesions of the SCN abolished circadian locomotor rhythms, but not odor-induced c-Fos rhythms in the OB or PC. Furthermore, removal of the OB abolished spontaneous circadian cycling of c-Fos in the PC, shortened the free-running period of locomotor rhythms, and accelerated re-entrainment after a 6 h advance and slowed re-entrainment after a 6 h delay in the light schedule. OB ablation or odorant altered the amplitude of c-Fos rhythms in the SCN and ablation of one OB abolished c-Fos rhythms in the ipsilateral PC, but not in the contralateral OB and PC. We conclude that the OB comprises a master circadian pacemaker, which enhances olfactory responsivity each night, drives rhythms in the PC, and interacts with the SCN to coordinate other daily behaviors.
Keywords: olfaction, circadian rhythms, c-Fos, oscillator, suprachiasmatic nucleus, piriform cortex
Introduction
The suprachiasmatic nucleus (SCN) of the hypothalamus is considered the master circadian clock, which controls rhythmic expression of many physiological variables in mammals (Moore and Card, 1985; Reppert and Weaver, 2001). The recent discoveries of extra-SCN tissues and cell types that express daily rhythms in gene expression in vitro have motivated analyses of coordination among multiple circadian oscillators in vivo and their relevance to timing-related disorders, including jet lag (Balsalobre et al., 1998, 2000a,b; Yamazaki et al., 2000; Stokkan et al., 2001; Schibler and Sassone-Corsi, 2002; Tosini and Fukuhara, 2003; Panda and Hogenesch, 2004; Guo et al., 2005). Among mammalian neural tissues, the SCN, retina, and main olfactory bulb (OB) have been shown to generate near 24 h rhythms in hormone secretion or firing rate, which can be synchronized to 24 h cycles in their environment (Tosini and Menaker, 1996; Abe et al., 2002; Granados-Fuentes et al., 2004a,b; Abraham et al., 2005). Importantly, the roles for clocks outside the SCN are unknown.
The OB is the first relay of the mammalian olfactory system and is critical to detection and discrimination of odorants using precisely wired neural circuits. Mitral-layer neurons of the OB send olfactory information to higher brain centers, including the piriform cortex (PC), anterior olfactory nucleus (AON), amygdala, and hypothalamus (Komiyama and Luo, 2006; Mori et al., 2006). Using the protein of the immediate early gene, c-Fos, as an indicator of cellular responses, Amir et al. (1999a) reported day–night differences in odor-induced responses in multiple brain areas associated with olfaction. Furthermore, indirect projections from the OB to the SCN have been reported (Krout et al., 2002), and olfactory stimulation enhances light-induced phase shifts of locomotor activity and c-Fos expression in the SCN (Amir et al., 1999b). Combined, the anatomical evidence suggests that the OB could gate daily olfactory processing and interact with the canonical SCN-driven circadian system.
The field of circadian biology has relied heavily on daily running-wheel activity in rodents as an indicator of the state of intrinsic circadian timekeeping. Although this behavior is easy to measure and highly repeatable from day to day, it may not reflect rhythmicity in all circadian clocks. We sought to measure an alternative output that might reflect the action of a pacemaker in the olfactory system. We measured running-wheel activity patterns and spontaneous and odor-evoked c-Fos expression at different times of day in the olfactory system of intact, SCN-lesioned (SCNX), and bilateral or unilateral bulbectomized (OBX) mice. The results indicate that the OB clock regulates olfactory responsivity within the OB and PC as a function of time of day.
Materials and Methods
Animals and locomotor activity recordings.
Male C57BL/6 mice (2 months of age) were purchased from Charles River (Boston, MA) and housed individually for 1 week in a 12 h light/dark (LD) cycle (lights on at 7:00 A.M.). For locomotor activity assays, cages were then placed in light-tight, ventilated chambers illuminated by fluorescent bulbs (3.9 × 1017 to 6.9 × 1018 photons/s/m2 at the bottom of the cages). We recorded running-wheel revolutions in 1 min bins using Clocklab (Actimetrics, Evanston, IL) as described previously (Aton et al., 2004). All procedures were approved by the Animal Care and Use Committee at Washington University and conformed to National Institutes of Health guidelines.
Surgical procedures.
For SCN lesions, mice were anesthetized with ketamine (75 mg/kg) and medetomidine (0.5 mg/kg, i.p.) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Bilateral lesions were made by passing an anodal current (40 s of 1.25 mA DC) through a tungsten electrode (563410; A-M Systems, Carlsborg, WA) placed 0.6 mm posterior to, ±0.1 mm lateral to, and 5.7 mm below bregma (Paxinos, 2003). Control animals received sham SCN lesions in which no current was passed through the electrode.
For bulbectomies, animals were anesthetized with ketamine (75 mg/kg) and medetomidine (0.5 mg/kg, i.p.), and bilateral or unilateral holes 3 mm wide were drilled above the OB and the bulbs suctioned. Control animals were treated identically, but no suction was applied.
Operated animals received atipamezole (0.1 mg/kg) to reverse the anesthetic and, after 1 h, were returned to their home cages. After behavioral and histological examination, we excluded mice with incomplete SCN lesions (n = 3) or incomplete bulbectomies (n = 1).
c-Fos expression in the OB and PC.
To test for circadian changes in OB and PC excitability, we measured odor-induced c-Fos expression in mice maintained in a 12 h LD cycle (lights on at 7:00 A.M.) for 5 d and then in constant darkness (DD) for 10 d. We defined the time of daily activity onset in DD as circadian time 12 (CT12) and other times in circadian hours (24 divided by the free-running period of the locomotor activity) from CT12.
Under infrared illumination (number 11 filter; Kodak, Rochester, NY) and wearing night-vision goggles (model E1700-4C; Excalibur Electronics, Fogelsville, PA) and disposable gloves, D.G.-F. exposed mice to cedar oil (S79956; Fisher Scientific, Pittsburgh, PA) diluted 1:1000 in mineral oil (M-8410; Sigma, St. Louis, MO) for 5 min at CT0, 4, 8, 12, 16, or 20 (n = 4 mice per CT). We exposed mice to odorant in a room adjacent to the room where locomotor activities were monitored to avoid stimulation of neighboring mice. The tip of a cotton swab, soaked with 100 μl of the odorant, was placed 2 cm below the lid of the home cage. Mice typically investigated the cotton swab for nearly the full 5 min of exposure so that the latency to sniff and duration of active investigation did not vary with circadian time (data not shown).
To test whether the canonical master circadian pacemaker plays a role in c-Fos expression in the OB and PC, a second group of mice was exposed to odorant after receiving bilateral electrolytic lesions to their SCN. All lesions were done at CT4, and locomotor activity was recorded for eight additional days in DD. We stimulated mice with cedar oil at projected CT0, 4, 8, 12, 16, or 20 (n = 4 per CT). Projected CT12 was extrapolated from the onsets of free-running locomotor activity on the 12–15 d before SCN ablation and defined as the time of projected activity onset on the eighth day after SCN ablation (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Other circadian times were defined in circadian hours from projected CT12. Some sections were also stained for Nissl to identify the layer boundaries and cellular identities in the OB. Granular cells were characterized by their smaller size and darker nuclei.
Animals in each of these two groups were anesthetized (avertin, 0.75 mg/g) 50 min after odor presentation, perfused intracardially with 0.9% saline followed with 4% paraformaldehyde (441244)-lysine (L5626)-periodate (PLP) (S-1878; all from Sigma; pH 7.2) fixative, and their brains were dissected. Each isolated brain was stored in PLP for 1 h at 4°C and then transferred successively to 10%, 20% (for 1 d each), and 30% sucrose phosphate-buffered (PB) solutions for 3 d until brains sank. Intact and SCNX animals were divided randomly into two groups that were fixed on two separate days. Serial coronal sections (40 μm thick) were obtained with a cryostat (CM1850; Leica, Maryland Heights, MO) and stored in 0.1 m PBS, pH 7.2, at 4°C for 24 h. Sections were processed in two groups with two mice from each CT for c-Fos (s.c.-52 rabbit polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA) immunoreactivity according to the avidin-biotin method (ABC kit, pk-6101; Vector Laboratories, Burlingame, CA). Specificity controls for this antibody in mouse brain have been published previously (Van Der et al., 2000; Schwartz et al., 2004). Sections were mounted on precleaned slides (12-550-14; Fisher Scientific) and coverslipped with Permount (SP15-500; Fisher Scientific).
Digitized images (Retiga 1350EX; QImaging, Burnaby, British Columbia, Canada) using Northern Eclipse software (Empix, North Tonawanda, NY) were taken using standardized illumination for all sections. We assigned sections randomly to two individuals, blind to experimental conditions. The numbers of c-Fos-expressing cells in a volume of 1 × 107 μm3 were counted in 100 nonadjacent fields (50 × 50 × 40 μm) of two sections containing the medial OB and two sections containing the PC and SCN. Some sections were counted by both observers; counts never differed by >5%.
Regulation of c-Fos expression in the PC by the OB.
To test the hypothesis that a circadian clock in the OB modulates excitability in the olfactory cortex, three groups of mice were maintained in LD for 5 d and received sham, bilateral (OBX), or unilateral bulbectomies (UOBXs). Sham and OBX mice were then kept for 5 d in LD followed by 10 d in DD and killed at CT0, 4, 8, 12, 16, or 20 (n = 4 per CT). UOBX animals (n = 6 per CT) were stimulated with cedar oil (diluted 1:1000) at CT8 or CT16 and killed 50 min later. The fixed brains of all mice were processed for c-Fos immunoreactivity as described above.
Modulation of SCN controlled circadian rhythms by the OB.
To investigate whether the OB affects rhythms controlled by the SCN, we studied whether bulbectomies or olfactory stimulation changed c-Fos expression in the SCN. From the sham, odor-exposed, and OBX groups (n = 24 mice in each group; six CTs), we counted c-Fos-immunopositive cells in the core and shell of the SCN. Two observers, blind to experimental conditions, combined to count immunopositive cells in 93 nonadjacent fields (40 × 40 × 40 μm) from two medial SCN sections of each mouse.
In addition, we analyzed running-wheel activity of sham (n = 10) and OBX (n = 24) mice from 10 d in a 12 h LD cycle (lights on at 7:00 A.M.), from 11 d after a 6 h delay in the light cycle (lights on at 1:00 P.M.), from 11 d after advancing the light schedule (lights on at 7:00 A.M.), and finally from 6 d in DD. Onsets and offsets of daily activity were determined using a 6 h on/6 h off template with Clocklab software. We measured the number of days to re-entrain after each shift in the light schedule, the average onset time of daily activity (termed the phase angle of entrainment) during the last 3 d in each light cycle, and the free-running period during the 6 d in DD.
Results
Odor-evoked c-Fos is circadian in the OB and PC
To test whether the circadian patterns of gene expression and firing rate in the cultured OB (Abe et al., 2002; Granados-Fuentes et al., 2004b) relate to olfactory function in vivo, we counted c-Fos-immunoreactive (c-Fos-IR) cells in the olfactory system of mice after stimulation with cedar oil at different times of the day. In constant darkness, cedar oil induced similar daily rhythms in the number of c-Fos-IR cells of the OB and PC (Fig. 1) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Approximately four times as many cells were labeled during the early subjective night compared with the subjective day in all three areas. Labeling was found throughout the OB and PC, regardless of the CT, and in some cells of the glomerular layer. Nissl-stained sections revealed that c-Fos-IR cells were predominantly granular cells in both the mitral and granular layers.
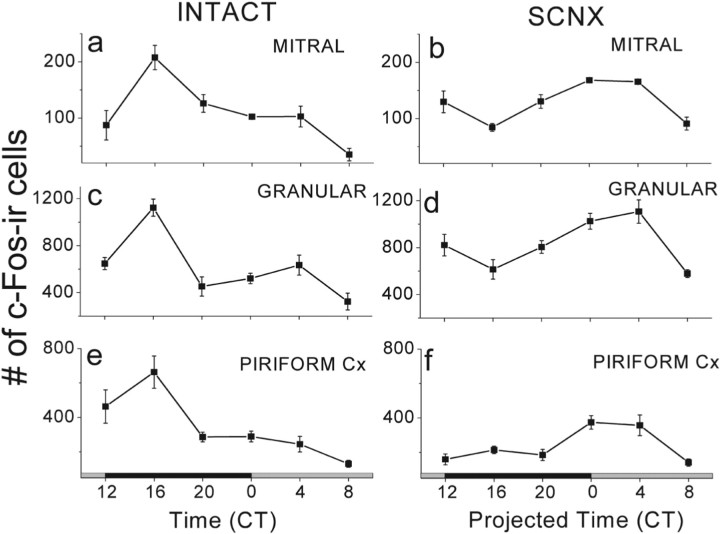
Figure 1.
Odor-evoked c-Fos rhythms persisted in the OB and PC of SCNX mice. Intact mice showed high-amplitude circadian rhythms in running-wheel activity and in cedar oil-induced c-Fos expression in their OB and PC (n = 4 per CT). a, c, e, The numbers of c-Fos-positive cells in the mitral and granular layers of the OB (a, c) and in the PC (e) peaked during the subjective night, approximately CT16, and declined approximately fourfold to a minimum during the subjective day, approximately CT8 (F(2,23) = 10.3, p < 0.0001 for mitral; F(2,23) = 15.5, p < 0.0001 for granular; F(2,23) = 9.6, p < 0.0001 for PC counts; one-way ANOVA and Tukey's test). SCNX mice were arrhythmic in running-wheel activity but showed a circadian rhythm in odor-induced c-Fos expression in their OB and PC (n = 4 per CT). b, d, f, The numbers of c-Fos-positive cells in the mitral (b) and granular (d) layers of the OB and in the PC (f) peaked during the projected subjective day (CT0–4) and declined approximately twofold to a minimum during the subjective night (F(2,23) = 7, p = 0.001 for mitral; F(2,23) = 8.05, p < 0.0001 for granular; F(2,23) = 7.6, p = 0.001 for PC counts; one-way ANOVA and Tukey's test).
Odor-evoked c-Fos rhythms do not require the SCN
To assess the role of the canonical circadian pacemaker in the SCN on rhythms in the olfactory system, we measured c-Fos induction by cedar oil in SCN-lesioned mice. Because destruction of the SCN abolished locomotor rhythms during the 8 d before olfactory stimulation, we exposed mice to odorant for 5 min at a projected circadian time determined by extrapolating the daily onsets of locomotor activity before lesioning the SCN. We found that the odor-evoked rhythms persisted in the mitral and granular layers of the OB and in the PC of SCNX mice (Fig. 1) (supplemental Fig 1, available at www.jneurosci.org as supplemental material). Critically, odor-induced rhythms peaked ∼8 h before the time of projected locomotor activity onset and 12 h out of phase with the c-Fos rhythm seen in SCN-intact mice. Peak-to-trough rhythm amplitude was unaffected by SCN ablation in the mitral (207.5 ± 21.5 c-Fos-IR cells in SCN-intact mice vs 163.3 ± 3.8 in SCNX; mean ± SEM; p = 0.09, Student's t test) or granular layers of the OB (1123 ± 76.2 intact vs 1024.8 ± 67.8 SCNX; p = 0.4, Student's t test) but reduced in the PC (663 ± 93.7 intact vs 374.3 ± 39.5 SCNX; p = 0.03, Student's t test). Thus, odor induced a greater response in the OB and PC during times when mice would typically be active, but this rhythm did not require the SCN or daily rhythms in sleep-wake.
Spontaneous and odor-induced circadian rhythms in c-Fos in the PC require the OB
Because the PC receives the vast majority of afferent fibers from the OB (Brunjes et al., 2005), we predicted that the OB clock could regulate circadian rhythms in the PC. We found circadian rhythms peaking at approximately CT0 in the number of c-Fos-IR cells of the OB and PC from mice that were not exposed to cedar oil (Fig. 2). This “spontaneous” circadian rhythm provided the opportunity to test whether rhythms in the PC are driven by the OB. We found that removal of the bilateral OB resulted in arrhythmic numbers of c-Fos-IR cells in the PC across the circadian cycle (Fig. 3). Because levels were intermediate compared to intact, nonstimulated animals, we conclude that the OB normally augments spontaneous c-Fos expression in the PC around subjective dawn.
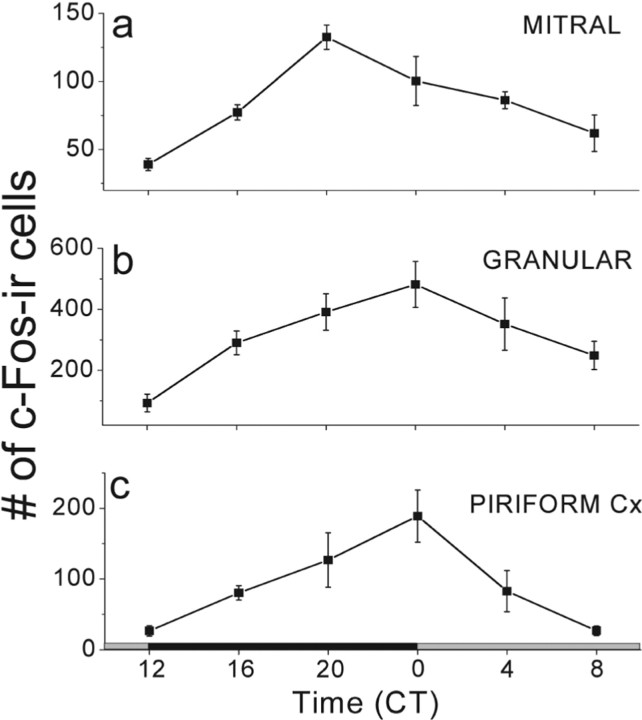
Figure 2.
Spontaneous c-Fos rhythms in the OB and PC. Behaviorally rhythmic mice (n = 4 per CT time), which were not exposed to cedar oil, showed a peak in c-Fos expression around subjective dawn (CT20–0) in the mitral and granular layer of the OB (a, b) and the PC (c). The number of c-Fos-positive cells declined nearly fivefold to a minimum during the late subjective day (F(2,23) = 9.2, p < 0.0001 for mitral; F(2,23) = 5.1, p = 0.004 for granular; F(2,23) = 6, p = 0.002 for PC counts; one-way ANOVA and Tukey's test).
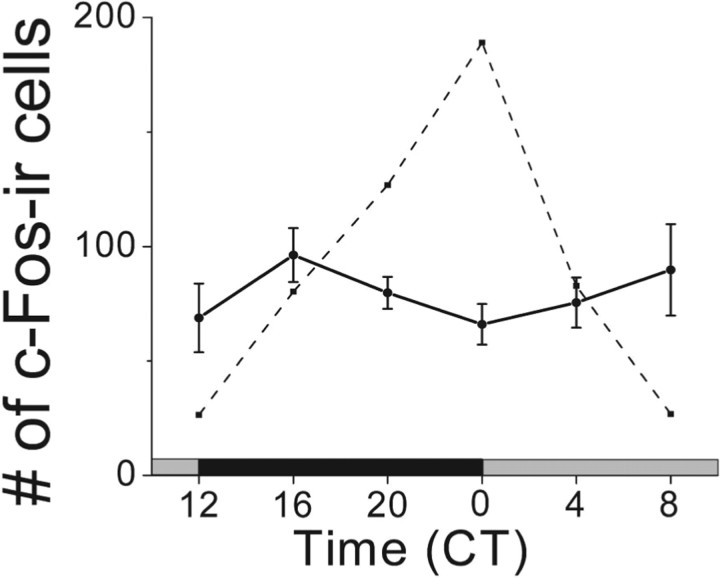
Figure 3.
OBX abolished the PC rhythm in spontaneous c-Fos expression. Bulbectomized mice showed free-running locomotor rhythms but lost rhythmicity in the PC (continuous line) (F(2,23) = 0.9; p = 0.5; one-way ANOVA). The number of c-Fos-IR cells was constant and intermediate compared with intact mice (dashed line; replotted from Fig. 2c).
Because OB afferents exclusively project ipsilaterally to the PC (Brodal, 1981; Brunjes et al., 2005), we predicted that OB regulation of PC rhythms would be strongly lateralized. We performed unilateral bulbectomies and found that cedar oil-induced higher c-Fos at CT16 than CT8 in the remaining OB and in the ipsilateral PC but no day–night difference in the contralateral PC (Fig. 4). These data indicate that the OB normally augments odor-evoked responses in the ipsilateral PC during the subjective night.
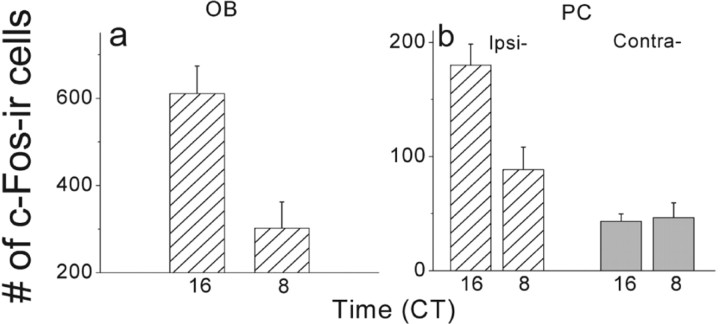
Figure 4.
PC rhythms require the ipsilateral OB. Removal of one OB did not abolish locomotor rhythms or cedar oil-induced c-Fos expression in the remaining OB (cumulative counts from mitral and granular layers) (a) or the ipsilateral PC (Ipsi-; b, dashed bars) with higher expression at approximately CT16 and lower at approximately CT8 (t = 3.9, p = 0.003 for OB; t = 3.3, p = 0.007 for PC; Student's t test). In contrast, the number of c-Fos-positive cells in the contralateral PC (Contra-; b, gray bars) was low during both the subjective day and night (t = −0.2, p = 0.8; Student's t test; n = 5 per CT). Thus, UOBX specifically eliminated the odor-evoked rhythm in the ipsilateral PC.
The OB modulates circadian rhythms regulated by the SCN
Some anatomical and behavioral studies have suggested an OB-to-SCN signaling pathway but have not revealed functional evidence for this interaction (Goel and Lee, 1997; Goel et al., 1998; Possidente et al., 1990; Governale and Lee, 2001; Krout et al., 2002). We explored the potential role of the OB in SCN-controlled locomotor activity rhythms and in c-Fos expression rhythms in the SCN.
We tested whether removal of the OB affects SCN-driven rhythms in wheel running. OBX significantly reduced the number of days needed to re-entrain to a 6 h advance in the LD cycle and significantly lengthened the time needed to re-entrain to a 6 h delay (Table 1). Consistent with these changes in re-entrainment rates, OBX shortened the free-running period of locomotor rhythms but did not change the phase angle of entrainment to a 12 h LD cycle. These results indicate that, in mice, the OB can modulate circadian behaviors that require the SCN.
Table 1.
OBX modifies motor activity rhythms
| Phase angle of entrainment (h) (lights on) |
Rate of re-entrainment (d) |
Free-running period (h) | ||||
|---|---|---|---|---|---|---|
| 7:00 A.M. | 1:00 P.M. | 7:00 A.M. | 6 h delay | 6 h advance | ||
| OBX (n = 24) | 6.9 ± 0.08 | 1.1 ± 0.02 | 7.01 ± 0.05 | 0.6 ± 0.2 | 4.3 ± 0.6 | 23.9 ± 0.04 |
| Sham (n = 10) | 7.0 ± 0.03 | 1.1 ± 0.04 | 6.9 ± 0.09 | 1.7 ± 0.3 | 2.4 ± 0.3 | 23.2 ± 0.02 |
| Student's t test | p = 0.6 | p = 0.9 | p = 0.09 | p = 0.004 | p = 0.04 | p = 0.0005 |
OBX and sham animals did not differ in their phase angles of entrainment but differed in the number of days needed to re-entrain to advances or delays in the LD cycle and their free-running periods. The delay from the daily onset of running-wheel activity to the onset of light (phase angle of entrainment), the number of days required to synchronize locomotor activity after a shift in the light schedule (rate of re-entrainment), and the free-running period under DD are expressed as mean ± SEM.
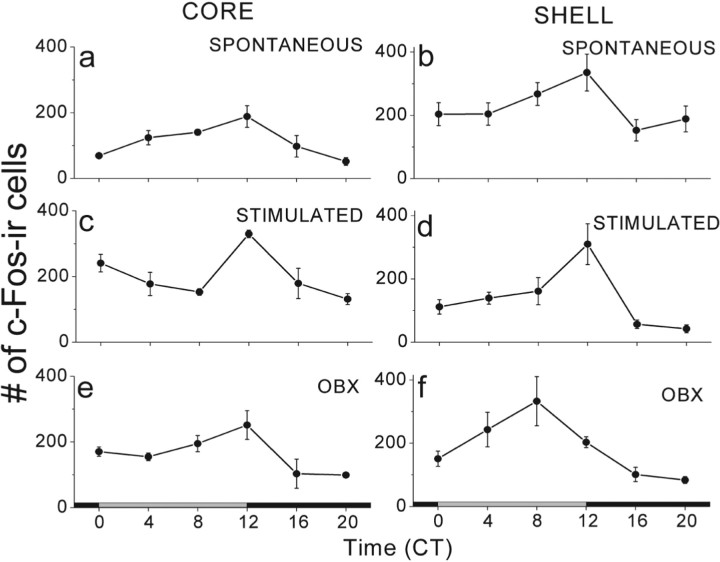
We examined the effects of odor presentation on c-Fos expression in the SCN. Previous reports highlighted spontaneous daily rhythms peaking near CT12 in c-Fos expression in the ventrolateral (“core”) and, with lower amplitude, in the dorsomedial (“shell”) of the rat SCN (Sumova et al., 2000). We found significant circadian rhythms in the numbers of c-Fos-IR cells in the core and shell of the SCN of mice that had or had not been exposed to cedar oil (Fig. 5). The rhythms of cedar oil-stimulated and control animals peaked at approximately CT12 in the SCN core and shell and had a significantly higher peak in the core of odor-exposed mice. OBX increased the rhythm amplitude in the core at four of six circadian points but had no significant effect in the SCN shell. These results show that stimulation or removal of the OB induces changes in SCN c-Fos patterns.
Figure 5.
Olfactory stimulation or OBX modify c-Fos rhythms in the SCN. A spontaneous rhythm in c-Fos expression in the core and shell regions of the SCN (a, b) increased in amplitude after odor-exposure or OBX (c, e) in the core SCN at CT12 (F(2,9) = 4.5; p = 0.04; one-way ANOVA) but not in the shell (d, f; F(2,9) = 2.4; p = 0.1; one-way ANOVA). Peak phase was similar across treatments in the SCN core (a, c, e) and appeared to peak earlier in the SCN shell of OBX mice (f vs b and d).
Discussion
A fundamental question in circadian biology is whether and how daily changes in gene activity relate to physiology and behavior. We previously reported that circadian oscillations in Per1 activity in the OB are entrainable and temperature compensated in vitro and persist in animals whose SCN were arrhythmic or had been lesioned (Granados-Fuentes et al., 2004a,b). The present data implicate a circadian clock in the OB in the daily regulation of olfactory responsivity.
Circadian regulation of olfaction
Circadian regulation of olfactory responsivity may be highly conserved. We found that olfactory stimulation induced circadian changes in c-Fos expression in the OB and PC of mice peaking at approximately CT16 (Fig. 6). In rats, cedar oil-induced c-Fos was also higher at CT15 than CT6 in the OB, PC, AON, infralimbic, orbital, perirhinal-entorhinal cortices, and basolateral and central nuclei of the amygdala (Amir et al., 1999a; Funk and Amir, 2000). Flies show circadian rhythms in odor-evoked antennal field potentials and odor-cued avoidance or attraction behaviors (Krishnan et al., 1999; Zhou et al., 2005). Moths show larger responses to pheromone during the subjective night (Silvegren et al., 2005), and salamanders reduce their foraging when exposed to predator extracts more at approximately CT14 (Maerz et al., 2001). In humans, odor-evoked event-related potentials are largest at ∼4:00 P.M. (Nordin et al., 2003). Indeed, it may turn out that circadian modulation of sensory processing is ubiquitous, because rhythms in acuity or sensitivity have been reported in vision, audition, touch, and electroreception (Lotze et al., 1999; Kavakli and Sancar, 2002; Kimchi and Terkel, 2002; Zupanc, 2002).
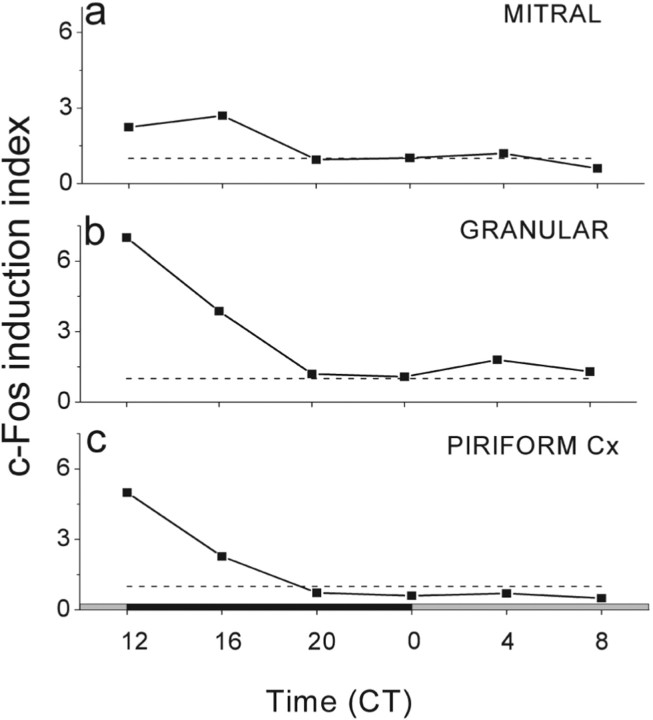
Figure 6.
The c-Fos induction index of odor-stimulated mice indicates peak responsivity during the subjective night. The index was calculated as the number of c-Fos-IR cells within the specified brain region of odor-exposed mice divided by the number of immunopositive cells in the same region of mice not exposed to the odorant. From CT12 to CT20, the ratios of induced to spontaneously expressing c-Fos cells in the OB (a, b) and PC (c) were >1 (dashed line), indicating that circadian modulation activates responsiveness during the subjective night.
It is interesting that olfactory responsivity oscillates in phase with wakefulness in mice, rats, humans, and moths but peaks at night in the diurnal fruit fly. It may be that daily modulation of olfactory sensitivity is species specific or depends on which process is measured. In the mouse OB and PC, the antiphase rhythms of spontaneous and odor-evoked c-Fos likely reflect different underlying neural processes that could improve odor detection, perhaps at the expense of discrimination, during the night. Plotting the ratio of odor-induced to spontaneous c-Fos, we see a peak in the signal-to-noise around dusk in the OB and PC (Fig. 6). This is reminiscent of the circadian enhancement of visual responsiveness and suppression of spontaneous photopigment isomerizations in the eye the horseshoe crab, Limulus polyphemus. A circadian clock in the Limulus brain augments visual sensitivity in the lateral eyes at night by increasing the likelihood of photon capture (Barlow, 1983; Chamberlain and Barlow, 1987). At the same time, the clock reduces noise in the eye by protonating rhodopsin, increasing its energy of activation (Kaplan et al., 1990; Barlow et al., 1993). The increased sensitivity at night comes at the cost of reduced spatial acuity because each photoreceptor views a larger and overlapping field with its neighbors (Barlow et al., 1980). The benefit is that horseshoe crabs can use vision to find mates equally well day and night (Powers et al., 1991). It will be important to learn the sites of action for the nocturnal increase in odor-evoked c-Fos and decrease in spontaneous c-Fos, the number of circadian clocks involved, and how this relates to olfactory-guided behaviors.
A separable circadian timing system
There is growing evidence for SCN-independent timekeeping in mammals. Here, we present evidence that the olfactory system performs similarly to the SCN-based canonical circadian system: A master pacemaker drives rhythms in spontaneous and odor-evoked activity in the OB and in its primary synaptic targets within the PC. Although we cannot rule out a contribution from the olfactory epithelium or a rhythm imposed by breathing, the clock within the OB is likely a master circadian pacemaker in the olfactory system, because it is circadian in the absence of the SCN in vitro and in vivo and is required for rhythms in the PC. The PC, which shows no intrinsic oscillations in clock genes in vitro (Abe et al., 2002) and loses rhythms after bulbectomy, likely depends on rhythmic input from the OB to oscillate in vivo. We do not know whether the PC is the only brain area controlled by the OB clock; other olfactory- and nonolfactory-related processing are likely regulated by this clock, because its projections include areas within the amygdala and entorhinal cortex (McDonald, 1998; Mouly and Di Scala, 2006), some of which have been shown to express day-night differences in c-Fos (Amir et al., 1999a). This parallels the role of the SCN in driving rhythms in, for example, the PVN to regulate daily oscillations in plasma glucose and melatonin (Perreau-Lenz et al., 2003; Kalsbeek et al., 2004). Similarly, antennal neurons, but not the central clock neurons that drive locomotor rhythms, are necessary for daily oscillations in olfactory function in flies. Targeted rescue of clock gene expression in antennal neuron oscillators in clock-mutant flies shows that these neurons are also sufficient for olfaction rhythms (Tanoue et al., 2004). Thus, there is growing evidence for circadian pathways in vertebrates and invertebrates that parallel the circuits controlling locomotion.
There is evidence for other SCN-independent pacemakers in mammals. For example, a food entrainable (FEO) produces anticipatory behaviors when food availability is restricted daily, even when the SCN or the OB are ablated (Davidson et al., 2001; Stephan, 2002; Herzog and Muglia, 2006). Recent evidence has suggested the FEO may be within the dorsal medial hypothalamus (Gooley et al., 2006; Mieda et al., 2006; but see Landry et al., 2006). A clock within the retina releases melatonin and dopamine on a daily basis (Tosini and Menaker, 1996; Doyle et al., 2002), and other oscillators exist, which are revealed during methamphetamine treatment or during forced desynchrony in rats and humans (Folkard et al., 1984; Hiroshige et al., 1991; Strijkstra et al., 1999). Understanding how these multiple circadian oscillators interact to coordinate daily behavior will likely provide insights into timing-related disorders like jet lag.
Interaction between oscillators
Although capable of self-sustained circadian cycling, the OB likely interacts with the SCN and its pathways. For example, projections from the OB to the SCN suggest indirect synaptic communication (Krout et al., 2002), and removal of the bulbs lengthens the free-running periods of hamsters and mice (Possidente et al., 1990; Pieper and Lobocki, 1991) and slows photic re-entrainment in male Octogon degus (Goel and Lee, 1997; Lee and Labyak, 1997; Goel et al., 1998; Governale and Lee, 2001). Consistent with these results, we found that OBX lengthened the period of mice locomotor rhythms in DD, altered resynchronization rates in response to advances or delays in the light cycle, and enhanced c-Fos rhythms in the SCN core. In vivo, the SCN is not necessary to maintain OB oscillations but for entrainment of the OB (Granados-Fuentes et al., 2004b). This may explain the change in phase of c-Fos rhythms in the OB of SCNX mice compared with SCN intact mice. After SCN ablation, the entraining signal from the SCN is lost so the OB likely free-runs. The intrinsic period of the in vitro rat OB is ∼1 h shorter than that of the in vitro SCN or in vivo locomotor rhythms (Abe et al., 2002; Granados-Fuentes et al., 2004b) so that, 8 d after SCN ablation, the phase of the OB in vivo might be ∼8-12 h advanced relative to the projected onset of locomotor activity, consistent with the results in Figure 1.
The effects of OBX on SCN-controlled rhythms may be caused by direct or indirect loss of OB input to the SCN. OBX has been shown to increase cAMP levels in the SCN and not in the hippocampus (Vagell et al., 1991), and odorant exposure enhanced c-Fos expression within the SCN core, not shell, suggesting some level of specificity. However, OBX induces a depression-like state and changes neurotransmitter expression in many brain areas (Song and Leonard, 2005). This may relate to the hypothesis that some psychiatric disorders arise from internal desynchronization between circadian oscillators (Kripke et al., 1978; Lumia et al., 1992).
We conclude that the OB contains a clock that controls circadian olfactory responsivity in mice and interacts with as a separable, but integrated, part of the circadian system. Our results highlight the need to study alternate circadian outputs to elucidate roles for extra-SCN pacemakers and the potential for odorants to alter, directly or indirectly, SCN and locomotor rhythms.
Footnotes
This work was supported by National Institutes of Health Grant MH63104. We thank Don Wilson, Alexis Webb, Luciano Marpegan, and Christian Beaulé for comments and suggestions on this manuscript and figures.
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham U, Prior JL, Granados-Fuentes D, Piwnica-Worms DR, Herzog ED. Independent circadian oscillations of Period1 in specific brain areas in vivo and in vitro. J Neurosci. 2005;25:8620–8626. doi: 10.1523/JNEUROSCI.2225-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J. In rats, odor-induced Fos in the olfactory pathways depends on the phase of the circadian clock. Neurosci Lett. 1999a;272:175–178. doi: 10.1016/s0304-3940(99)00609-6. [DOI] [PubMed] [Google Scholar]
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J. Olfactory stimulation enhances light-induced phase shifts in free-running activity rhythms and Fos expression in the suprachiasmatic nucleus. Neuroscience. 1999b;92:1165–1170. doi: 10.1016/s0306-4522(99)00222-5. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Block GD, Tei H, Yamazaki S, Herzog ED. Plasticity of circadian behavior and the suprachiasmatic nucleus following exposure to non-24-hour light cycles. J Biol Rhythms. 2004;19:198–207. doi: 10.1177/0748730404264156. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000a;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol. 2000b;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- Barlow RB. Circadian rhythms in the Limulus visual system. J Neurosci. 1983;3:856–870. doi: 10.1523/JNEUROSCI.03-04-00856.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB, Chamberlain SC, Levinson JZ. Limulus brain modulates the structure and function of the lateral eyes. Science. 1980;210:1037–1039. doi: 10.1126/science.7434015. [DOI] [PubMed] [Google Scholar]
- Barlow RB, Birge RR, Kaplan E, Tallent JR. On the molecular origin of photoreceptor noise. Nature. 1993;366:64–66. doi: 10.1038/366064a0. [DOI] [PubMed] [Google Scholar]
- Brodal A. Neurological anatomy. New York: Oxford UP; 1981. The olfactory pathways; pp. 640–697. [Google Scholar]
- Brunjes PC, Illig KR, Meyer EA. A field guide to the anterior olfactory nucleus (cortex) Brain Res Brain Res Rev. 2005;50:305–335. doi: 10.1016/j.brainresrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Chamberlain SC, Barlow RB. Control of structural rhythms in the lateral eye of Limulus: interactions of natural lighting and circadian efferent activity. J Neurosci. 1987;7:2135–2144. doi: 10.1523/JNEUROSCI.07-07-02135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Aragona BJ, Werner RM, Schroeder E, Smith JC, Stephan FK. Food-anticipatory activity persists after olfactory bulb ablation in the rat. Physiol Behav. 2001;72:231–235. doi: 10.1016/s0031-9384(00)00417-0. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Folkard S, Minors DS, Waterhouse JM. Is there more than one circadian clock in humans? Evidence from fractional desynchronization studies. J Physiol (Lond) 1984;357:341–356. doi: 10.1113/jphysiol.1984.sp015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Amir S. Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 2000;866:262–267. doi: 10.1016/s0006-8993(00)02249-6. [DOI] [PubMed] [Google Scholar]
- Goel N, Lee TM. Olfactory bulbectomy impedes social but not photic reentrainment of circadian rhythms in female Octodon degus. J Biol Rhythms. 1997;12:362–370. doi: 10.1177/074873049701200408. [DOI] [PubMed] [Google Scholar]
- Goel N, Lee TM, Pieper DR. Removal of the olfactory bulbs delays photic reentrainment of circadian activity rhythms and modifies the reproductive axis in male Octodon degus. Brain Res. 1998;792:229–236. doi: 10.1016/s0006-8993(98)00134-6. [DOI] [PubMed] [Google Scholar]
- Gooley J, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006 doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Governale MM, Lee TM. Olfactory cues accelerate reentrainment following phase shifts and entrain free-running rhythms in female Octodon degus (Rodentia) J Biol Rhythms. 2001;16:489–501. doi: 10.1177/074873001129002169. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. 2004a;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Saxena MT, Prolo LM, Aton SJ, Herzog ED. Olfactory bulb neurons express functional, entrainable circadian rhythms. Eur J Neurosci. 2004b;19:898–906. doi: 10.1111/j.0953-816x.2004.03117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Muglia LJ. You are when you eat. Nat Neurosci. 2006;9:300–302. doi: 10.1038/nn0306-300. [DOI] [PubMed] [Google Scholar]
- Hiroshige T, Honma K-I, Honma S. SCN-independent circadian oscillators in the rat. Brain Res Bull. 1991;27:441–445. doi: 10.1016/0361-9230(91)90139-b. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Barlow RB, Renninger GH, Purpura K. Circadian rhythms in Limulus photoreceptors. II. Quantum bumps. J Gen Physiol. 1990;96:665–685. doi: 10.1085/jgp.96.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavakli IH, Sancar A. Circadian photoreception in humans and mice. Mol Intervent. 2002;2:484–492. doi: 10.1124/mi.2.8.484. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Terkel J. Seeing and not seeing. Curr Opin Neurobiol. 2002;12:728–734. doi: 10.1016/s0959-4388(02)00381-1. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Luo L. Development of wiring specificity in the olfactory system. Curr Opin Neurobiol. 2006;16:67–73. doi: 10.1016/j.conb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Mullaney DJ, Atkinson M, Wolf S. Circadian rhythm disorders in manic-depressives. Biol Psychiatry. 1978;13:335–351. [PubMed] [Google Scholar]
- Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience. 2002;110:73–92. doi: 10.1016/s0306-4522(01)00551-6. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistance of a behavioral food anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Lee TM, Labyak SE. Free-running rhythms and light- and dark-pulse phase response curves for diurnal Octodon degus (Rodentia) Am J Physiol. 1997;273:t-86. doi: 10.1152/ajpregu.1997.273.1.R278. [DOI] [PubMed] [Google Scholar]
- Lotze M, Wittmann M, von Steinbuchel N, Poppel E, Roenneberg T. Daily rhythm of temporal resolution in the auditory system. Cortex. 1999;35:89–100. doi: 10.1016/s0010-9452(08)70787-1. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B. Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res. 1992;587:181–185. doi: 10.1016/0006-8993(92)90995-l. [DOI] [PubMed] [Google Scholar]
- Maerz JC, Panebianco NL, Madison DM. Effects of predator chemical cues and behavioral biorhythms on foraging activity of terrestrial salamanders. J Chem Ecol. 2001;27:1333–1344. doi: 10.1023/a:1010309108210. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci USA. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Card JP. Visual pathways and the entrainment of circadian rhythms. [Rev] Ann NY Acad Sci. 1985;453:123–133. doi: 10.1111/j.1749-6632.1985.tb11805.x. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Di Scala G. Entorhinal cortex stimulation modulates amygdala and piriform cortex responses to olfactory bulb inputs in the rat. Neuroscience. 2006;137:1131–1141. doi: 10.1016/j.neuroscience.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Nordin S, Lotsch J, Murphy C, Hummel T, Kobal G. Circadian rhythm and desensitization in chemosensory event-related potentials in response to odorous and painful stimuli. Psychophysiology. 2003;40:612–619. doi: 10.1111/1469-8986.00062. [DOI] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB. It's all in the timing: many clocks, many outputs. J Biol Rhythms. 2004;19:374–387. doi: 10.1177/0748730404269008. [DOI] [PubMed] [Google Scholar]
- Paxinos G. New York: Academic; 2003. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, Van D V, Van Heijningen C, Simonneaux V, Pevet P, Buijs RM. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur J Neurosci. 2003;17:221–228. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- Pieper DR, Lobocki CA. Olfactory bulbectomy lengthens circadian period of locomotor activity in golden hamsters. Am J Physiol. 1991;261:R973–R978. doi: 10.1152/ajpregu.1991.261.4.R973. [DOI] [PubMed] [Google Scholar]
- Possidente B, Lumia AR, McGinnis MY, Teicher MH, deLemos E, Sterner L, Deros L. Olfactory bulb control of circadian activity rhythm in mice. Brain Res. 1990;513:325–328. doi: 10.1016/0006-8993(90)90475-q. [DOI] [PubMed] [Google Scholar]
- Powers MK, Barlow RB, Kass L. Visual performance of horseshoe crabs day and night. Vis Neurosci. 1991;7:179–189. doi: 10.1017/s0952523800004016. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Nunez AA, Smale L. Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience. 2004;127:13–23. doi: 10.1016/j.neuroscience.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Silvegren G, Lofstedt C, Qi RW. Circadian mating activity and effect of pheromone pre-exposure on pheromone response rhythms in the moth Spodoptera littoralis. J Insect Physiol. 2005;51:277–286. doi: 10.1016/j.jinsphys.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Strijkstra AM, Meerlo P, Beersma DG. Forced desynchrony of circadian rhythms of body temperature and activity in rats. Chronobiol Int. 1999;16:431–440. doi: 10.3109/07420529908998718. [DOI] [PubMed] [Google Scholar]
- Sumova A, Travnickova Z, Illnerova H. Spontaneous c-Fos rhythm in the rat suprachiasmatic nucleus: location and effect of photoperiod. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2262–R2269. doi: 10.1152/ajpregu.2000.279.6.R2262. [DOI] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Tosini G, Fukuhara C. Photic and circadian regulation of retinal melatonin in mammals. J Neuroendocrinol. 2003;15:364–369. doi: 10.1046/j.1365-2826.2003.00973.x. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Vagell ME, McGinnis MY, Possidente BP, Narasimhan VN, Lumia AR. Olfactory bulbectomy increases basal suprachiasmatic cyclic AMP levels in male rats. Brain Res Bull. 1991;27:839–842. doi: 10.1016/0361-9230(91)90219-a. [DOI] [PubMed] [Google Scholar]
- Van Der GE, Vandenbussche E, Orban GA, Vandesande F, Arckens L. A new cat Fos antibody to localize the immediate early gene c-fos in mammalian visual cortex after sensory stimulation. J Histochem Cytochem. 2000;48:671–684. doi: 10.1177/002215540004800511. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Zhou X, Yuan C, Guo A. Drosophila olfactory response rhythms require clock genes but not pigment dispersing factor or lateral neurons. J Biol Rhythms. 2005;20:237–244. doi: 10.1177/0748730405274451. [DOI] [PubMed] [Google Scholar]
- Zupanc GK. From oscillators to modulators: behavioral and neural control of modulations of the electric organ discharge in the gymnotiform fish, Apteronotus leptorhynchus. J Physiol (Paris) 2002;96:459–472. doi: 10.1016/S0928-4257(03)00002-0. [DOI] [PubMed] [Google Scholar]