Abstract
The endoplasmic reticulum (ER) Ca2+ store plays a key role in integration and conveyance of Ca2+ signals in highly polarized neurons. The interconnected ER network in neurons generates Ca2+ signals in local domains, but the regional interaction is unclear. Here, we show that continuous or repetitive applications of caffeine produced robust Ca2+ release from the ER Ca2+ store in dendritic areas without severe store depletion, but that similar stimuli applied to soma caused rapid store depletion in acutely isolated midbrain dopamine neurons. Partial emptying of the ER Ca2+ store within a dendrite caused a similar level of store depletion in unstimulated dendrites, as well as in soma. Photobleaching and local stimulation experiments revealed that Ca2+ and the dye trapped within the ER diffused rapidly from the soma to dendrites up to 90 μm, which we could resolve, suggesting that the ER network acts as a functional tunnel for rapid Ca2+ transport. These data imply that the ER in soma acts as a Ca2+ reservoir supplying Ca2+ to the dendritic store, and that the dendritic store, hence, is able to respond to Ca2+-mobilizing input signals endurably.
Keywords: Ca2+ signal, endoplasmic reticulum, intracellular Ca2+ store, neuron, dopaminergic neuron, Ca2+ concentration in the endoplasmic reticulum, photobleaching
Introduction
The endoplasmic reticulum (ER) is the largest intracellular organelle and constitutes an interconnected network that extends from the soma to axons, dendrites, and even dendritic spines (Verkhratsky and Petersen, 1998; Berridge et al., 2000). The ER is a site of protein synthesis and maturation that can be modulated by Ca2+ levels and fluctuations in the lumen of the ER (Michalak et al., 2002). The ER also acts as a key player for determining complex patterns of local or global Ca2+ signals as a Ca2+ store or as a sink (Friel and Tsien, 1992; Pozzan et al., 1994; Berridge, 1998; Verkhratsky, 2005). In addition, the ER can also generate Ca2+ sparks or oscillations without external stimulation (Llano et al., 2000; Emptage et al., 2001) and can also regulate neuronal excitability (Stutzmann et al., 2003). Disturbed ER Ca2+ signals appear to cause several types of neurodegenerative diseases (Verkhratsky, 2005). Therefore, precise understanding of the structural and functional organization of the ER in neurons is very important. In general, most neurons are highly polarized and receive numerous subsets of synaptic inputs in local domains so that Ca2+ signals occur in local domains (Finch and Augustine, 1998; Augustine et al., 2003; Spitzer et al., 2004; Goldberg and Yuste, 2005). Therefore, despite its wide connection and distribution, a small part of the ER appears to participate in the modulation of Ca2+ signals as a local domain according to stimulus intensities and localities. Consequently, it has been demonstrated that the ER present in distinct parts of a neuron apparently behaves as an independent Ca2+ store (Blaustein and Golovina, 2001). Many pharmacological studies have postulated that the ER is composed of separate Ca2+ stores that are individually loaded and unloaded (Golovina and Blaustein, 1997; Montero et al., 1997; Blaustein and Golovina 2001). However, this model cannot explain how the ER, as a multifunctional but signal-integrating organelle, is integrated into a coherent system, because the ER not only governs Ca2+ signals but also controls generalized cell functions such as protein processing, regulation of metabolism, and gene expression (Patil and Walter, 2001; Verkhratsky and Petersen, 2002). Recently, it has been proposed that the ER is a interconnected single Ca2+ store in pancreatic acinar cells, functioning as a whole system by allowing Ca2+ movement and equilibration through the connected lumen (Park et al., 2000, 2001; Petersen et al., 2001). By analogy, the ER in neurons may be composed of a single homogenous Ca2+ pool, and its functions in different regions may be harmonized or communicated by luminal connectivity. However, no experimental evidence exists to demonstrate the regional interactions of the ER between different regions of a neuron.
Therefore, we set out to determine whether the ER is a functionally connected Ca2+ store or whether it is composed of separate subunits with the regional interaction of ER Ca2+ signals between soma and dendrites in acutely isolated dopamine neurons. We show here that the ER is a luminally interconnected organelle through which Ca2+ can diffuse rapidly from soma to dendrites.
Materials and Methods
Preparation and identification of dopamine neurons.
Single dopamine neurons were acutely isolated from Sprague Dawley rats at postnatal day 9–14. Brains were cut into midbrain blocks containing the substantia nigra pars compacta (SNc), and coronal slices of 300–400 μm thickness were obtained using a vibratome (TPI, St. Louis, MO). The substantia nigra pars compacta of the slices, demarcated by their dark color, were dissected out with a scalpel and digested with fully oxygenated HEPES-buffered saline containing papain (4–10 U/ml; Worthington, Freehold, NJ) for 20–60 min at 34–37°C. Gentle agitation with varying sizes of Pasteur pipettes produced single neurons of various shapes. Among these neurons, large multipolar neurons containing three to six large neurites were immunostained with tyrosine hydroxylase antibody. Thus, we used only these typical neurons in this experiment. Detailed procedures and staining results have been described previously (Choi et al., 2003).
Solutions and chemicals.
The normal bath solution contained the following (in mm): 140 NaCl, 5 KCl, 10 HEPES, 10 d-glucose, 1 CaCl2, and 1 MgCl2. Osmolarity and pH were adjusted to ∼300 mOsm and 7.4 with sucrose and NaOH, respectively. The Ca2+-free solution was made by replacement of 1 mm CaCl2 with 0.2 mm EGTA. The bath volume was ∼0.7 ml, and solution exchanges were made within 8 s. (S)-3,5-Dihydroxyphenylglycine (DHPG) (group 1 metabotropic glutamate receptor agonist) and tetrodotoxin (Na+ channel blocker) were obtained from Tocris Cookson (Ballwin, MO). All other materials were purchased from Sigma (St. Louis, MO).
Measurement of cytosolic Ca2+ concentrations.
Isolated SNc neurons were incubated with 2–5 μm fura-2 AM at room temperature (20–24°C) for 20–35 min. The cells were then washed twice with normal physiological salt solution. All cells were used within 3 h of isolation. Single-cell fluorescence intensities were measured using an Olympus Optical (Tokyo, Japan) IX70 inverted microscope (40× objective or 60× water immersion objective) attached to a frame-transfer and back-illuminated charge-coupled device camera (Quantix; Photometrics, Tucson, AZ) and Metafluor software (Molecular Devices, Sunnyvale, CA). We used 340/380 nm dual excitations with a 400 nm dichroic mirror, and emitted light was collected using a long-pass filter of 450 nm. The details have been described previously (Choi et al., 2003). To visualize the localization of Ca2+ signals in local stimulation experiments, 340 nm excitation images were divided by 380 nm excitation images, and then the basal ratio images were subtracted from the post-caffeine ratio images, which showed maximum cytosolic Ca2+ concentration ([Ca2+]c) rises (ΔR).
Ratios (340/380 nm) of cell fluorescence intensities were calibrated using maximum (R max) and minimum ratio values (R min) obtained by exposing cells to 15 μm ionomycin and 10 mm Ca2+ or 10 mm EGTA, by assuming a dissociation constant (K d) of 150 nm for Ca2+–fura-2 at room temperature, according to the following well known formula (Grynkiewicz et al., 1985):
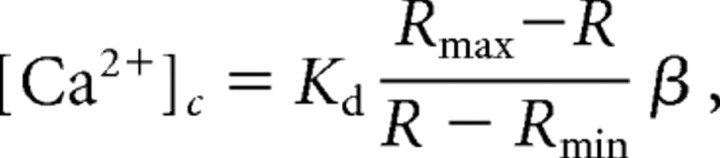 |
where β is a specific constant.
Micropressurized injection.
A Narishige (Tokyo, Japan) microinjection system (IM 300 Microinjector) was used to rapidly apply caffeine to cells. The internal diameters of the micropressure glass pipettes were <0.5 μm, and their resistance ranged between 10 and 20 MΩ. Glass pipettes contained 50 mm caffeine. To avoid backflow reapplication of agonists to cells, we injected agonists parallel to perfusion solution flow. The injection pressure was maintained between 40 and 50 psi, and single pulse durations were 150–200 ms (or 200 s for extended stimulation).
Visualization of the endoplasmic reticulum.
We used ER-Tracker to observe the endoplasmic reticulum. SNc neurons were loaded with ER-Tracker Blue-White DPX (Invitrogen, Carlsbad, CA). A stock solution of ER-Tracker was prepared at 1 mm in DMSO. After cell preparation, the cells were incubated in 100 nm ER-Tracker for 20–30 min. Specimens were excited at 364 nm (UV laser), and images of ER-Tracker fluorescence were obtained using a Zeiss (Oberkochen, Germany) 510 confocal laser scanning microscope.
Photobleaching experiments.
Isolated SNc neurons were incubated with 2–5 μm MagFluo 4 AM for 20–35 min at 37°C. We used the UV laser at 80 mW power to induce fast bleaching of Magfluo-4 under a Zeiss 510 confocal microscope together with the 488 nm laser line at 30 mW. Bleaching was iterated at 5× with the dwell time per pixel of 2.56–10.2 μs.
Statistics.
Paired and unpaired Student's t tests were used, and p values of <0.05 were regarded as significant difference.
Results
Caffeine empties the ER Ca2+ store
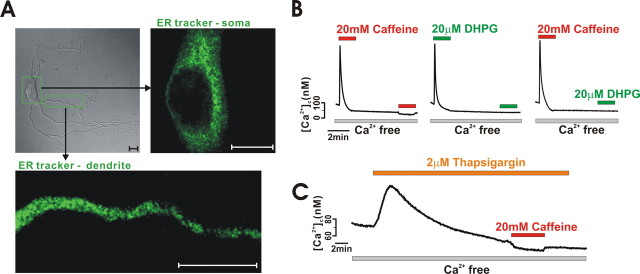
Acutely isolated midbrain dopamine neurons are immunopositive to tyrosine hydroxylase antibody and are characterized by a large cell body with multiple dendrites (Choi et al., 2003; Kim et al., 2004). Because dopamine neurons show a strong ER Ca2+ release property and possess several large dendrites (Fiorillo and Williams, 1998; Morikawa et al., 2000; Choi et al., 2003), they are a good model for examining the functional organization of the ER Ca2+ store in central neurons. Because the ER is a major Ca2+ store, we examined the distribution of the ER in acutely isolated dopamine neurons by staining the ER with ER-Tracker. As shown in Figure 1 A, the ER was observed everywhere in soma and dendrites, except within a nucleus, which is similar to the ubiquitous distribution of the ER shown in several types of other neurons (Verkhrasky and Petersen, 1998), thereby contributing to generation of Ca2+ signals in the whole area of a polarized neuron. To examine Ca2+ release from intracellular Ca2+ stores, we stimulated cells with caffeine in a Ca2+-free bath medium and measured cytosolic Ca2+ levels using fura-2. Although caffeine is known to both open ryanodine receptors and to inhibit IP3 receptors (Cancela et al., 2000), it has been widely used to release Ca2+ from the ER in neurons (Verkhratsky, 2005). As shown in Figure 1 B, application of 20 mm caffeine produced a transient [Ca2+]c rise in the Ca2+-free bath solution; there was an initial rise in [Ca2+]c and a subsequent decay to the basal level within 1.2 ± 0.2 min (n = 15). This suggests that caffeine rapidly depletes the ER Ca2+ store in the Ca2+-free medium. The metabotropic glutamate receptor agonist DHPG (20 μm) also produced a similar response in Ca2+-free medium, and the increased [Ca2+]c also returned to the basal level in a similar amount of time (1.3 ± 0.2 min; n = 15). After depletion of the intracellular Ca2+ store during administration of a high concentration of caffeine, the application of DHPG failed to cause additional release of Ca2+ (Fig. 1 B, right), which suggests that caffeine shares a Ca2+ pool with DHPG in dopamine neurons. Therefore, caffeine appears to not only deplete the ryanodine-mediated Ca2+ pool, but it also depletes IP3-mediated Ca2+ pools. Therefore, dopamine neurons seem to have a single Ca2+ pool on which IP3 and ryanodine receptors reside. Thapsigargin, a sarcoplasmic/endoplasmic reticulum calcium ATPase pump inhibitor, also raised the [Ca2+]c level transiently in Ca2+-free bath medium, perhaps through unidentified leak channels (Lomax et al., 2002). After depleting the thapsigargin-sensitive store, caffeine failed to increase [Ca2+]c (Fig. 1 C). All of these findings suggest that caffeine releases Ca2+ from the ER store in dopamine neurons.
Figure 1.
Distribution of the ER and Ca2+ release in midbrain dopamine neurons. Neuronal ER can release Ca2+ in response to caffeine. A, Transmitted image of an acutely isolated dopamine neuron and fluorescence image of the ER (ER-Tracker), obtained using a confocal microscope. Scale bars, 10 μm. B, Caffeine or DHPG releases Ca2+ from the ER. DHPG failed to release Ca2+ from the ER after pretreatment with caffeine (n = 9). C, Thapsigargin prevents caffeine-induced Ca2+ release (n = 17). Fluorescence intensities were measured from soma.
Single or low-frequency Ca2+ release from the local dendritic ER does not significantly deplete the ER Ca2+ store
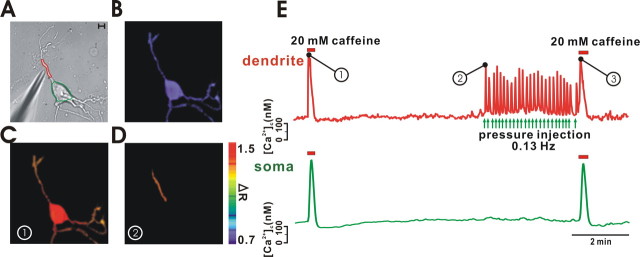
To examine and compare the Ca2+ signals of the ER in different areas of a neuron, we attempted to elicit ER Ca2+ release locally in midbrain dopamine neurons using a micropressurized micropipette containing caffeine. To prevent backflow or diffusion of caffeine during experiments, the micropipette was positioned in parallel with the flow direction in the bath. Although metabotropic glutamate receptor agonists, such as DHPG, are capable of releasing Ca2+ from the ER, they also modulate Ca2+-permeable ion channels and change membrane potential (Guatteo et al., 1999; Prisco et al., 2002; Choi et al., 2003), which leads to difficulties in the interpretation of [Ca2+]c signals. Thus, caffeine seems to be a better tool to trigger Ca2+ release from the ER. Therefore, to evoke Ca2+ release from the ER in a localized area of a dendrite, we applied caffeine to a dendrite by using a micropressurized pipette containing 50 mm caffeine. As shown in Figure 2 A, the dopamine neuron that we used had multiple dendrites, and the caffeine-containing micropipette, which is shown in the left bottom corner, pointed at one of the dendrites. The fura-2 fluorescence ratio image (340/380 nm excitation) obtained is shown in Figure 2 B, and, in Figure 2 A, the green (soma) and red (dendritic region) circles from which fluorescence intensities were measured are shown. To trigger the release of Ca2+ from the ER in the entire neuron, we first applied 20 mm caffeine to the bath. As shown in Figure 2 C, this caused a rise in [Ca2+]c throughout the whole neuron. The subtracted image (ΔR) shows a global [Ca2+]c rise in the soma and dendrites, and [Ca2+]c changes in the soma and dendrite, which are marked in green and red circles in Figure 2 A, are presented in Figure 2 E in the same colors. In this case, the shapes of [Ca2+]c changes in the soma and dendrite were very similar (the first [Ca2+]c rise, marked by the number 1). After that, we allowed the ER to refill with Ca2+ for several minutes, and then, using a micropipette containing 50 mm caffeine, we repeatedly stimulated a dendritic area in a pulsatile manner. As shown in Figure 2 D, the [Ca2+]c rose only in the dendrite close to the micropressurized pipette, and there was no rise in [Ca2+]c elsewhere in unstimulated areas such as soma and remote dendrites. This was also presented in Figure 2 E, in which the unstimulated soma showed no [Ca2+]c change during the local dendritic caffeine stimulations (number 2). In this case, caffeine pulses were applied at ∼0.13 Hz, and the peak values in each Ca2+ spike were very similar. This finding indicates that, despite repeated stimulation (sequential Ca2+ release from the ER), the local dendritic ER store was able to release Ca2+ persistently without severe store depletion. To estimate the remaining Ca2+ levels of the ER at the end of the repetitive local stimulation, 20 mm caffeine was immediately applied to the bath again. This evoked a global Ca2+ release from the ER (the last [Ca2+]c change in Fig. 2 E, which is marked number 3). As expected, the amplitudes of the Ca2+ spikes (number 3) measured in the soma and a dendrite were similar to those of the Ca2+ spikes (number 1) that were first evoked before the local repetitive stimulations (number 2), suggesting that local repetitive stimulation to a limited area of a dendrite at this slow frequency did not significantly deplete the Ca2+ store in dendrites or in soma.
Figure 2.
Pulsatile low-frequency Ca2+ release from the local dendritic ER does not deplete the Ca2+ store in soma. A dopamine neuron was stimulated globally or locally by bath application of 20 mm caffeine or by micropressurized application of 50 mm caffeine, respectively. A, A transmitted image shows an isolated neuron and a micropressurized pipette containing 50 mm caffeine. The two different measured areas are circled in different colors, and Ca2+ signals are presented in the same color in E. Scale bar, 10 μm. B, A fluorescence ratio (F 340/F 380) image of fura-2. C, A subtracted ratio image (ΔR), which was obtained by subtraction of a ratio (F 340/F 380) image before and after global stimulation of a neuron with caffeine, shows a [Ca2+]c rise throughout the soma and dendrites. D, A subtracted ratio image (ΔR) of a neuron before and after local stimulation of caffeine to a dendrite shows local increase in [Ca2+]c only in the stimulated region of a dendrite (red circle in A). E, Global–local–global stimulations with caffeine elicited [Ca2+]c changes in a dendrite (red circle marked in A) and/or in the soma (green circle marked in A), in a stimulation-dependent manner. Local Ca2+ releases within a dendrite by local applications of caffeine (number 2) did not affect the second global Ca2+ release (number 3) when compared with the first global Ca2+ release (number 1), suggesting that the store was not depleted significantly in this condition.
Different depletion rates of the ER Ca2+ store between soma and dendrites in response to continuous or high-frequency stimulation
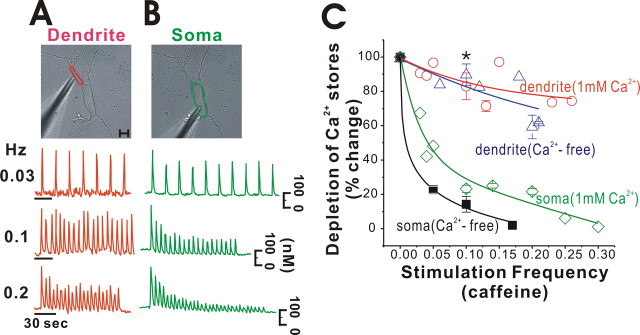
To examine how differently the ER in dendrites and soma releases Ca2+ according to the stimulation frequency, we stimulated neurons at different frequencies with a micropipette containing 50 mm caffeine. As shown in Figure 3 A, when we stimulated a dendrite (marked in red in the left) with caffeine pulses at 0.03 Hz, repetitive Ca2+ spikes were generated in dendrites, and the amplitudes in each Ca2+ spike were relatively similar between Ca2+ spikes, which indicates that the dendritic ER was able to release Ca2+ persistently at this condition. However, when we raised caffeine stimulation frequencies from 0.03 Hz, we observed a sequential reduction in amplitudes of Ca2+ spikes from 0.2 Hz (Fig. 3 A, bottom). To compare this with the ER Ca2+ release behavior in soma, we performed the same experiment in soma. Pulsatile application of 50 mm caffeine to soma at 0.03 Hz generated similar amplitudes of Ca2+ spikes (Fig. 3 B, top), indicating that the soma ER was able to release Ca2+ repeatedly in this condition. However, stimulation at 0.1 Hz led to a clear sequential reduction of amplitudes in Ca2+ spikes at levels much greater than in the dendrites (Fig. 3 B, middle). At 0.2 Hz caffeine stimulation, a more dramatic reduction of amplitudes in Ca2+ spikes was observed (Fig. 3 B, bottom). In Figure 3 C, we plotted the relative changes of the reduction in amplitude in the dendrites (red circles) and soma (green diamonds) versus stimulation frequencies, in which each point was obtained from one to five measurements from several neurons. In contrast to the dendritic areas, the sequential reductions in peak [Ca2+]c values with repetitive stimulations were greater in soma at the same stimulation frequencies. This phenomenon was also observed in the Ca2+-free medium (Fig. 3 C, blue triangles in dendritic areas and black rectangles in soma areas), in which the Ca2+ influx was limited. Therefore, it is unlikely that the different Ca2+ release behaviors in soma and dendrites are attributable to differences in Ca2+ refilling rates of the ER between soma and dendrites, because a similar result was obtained in Ca2+-free medium. This sequential reduction behavior in peak [Ca2+]c values with repetitive caffeine stimulations may indicate that the ER Ca2+ store was being depleted gradually. Otherwise, it may be attributable to the desensitization of the Ca2+ release process by repetitive caffeine applications (Verkhratsky, 2005); the behavior may also result from a combination of these processes. Because the sequential reductions in amplitudes of Ca2+ spikes in soma were greater than those in dendrites, the ER in the soma may have a more sensitive desensitization process to caffeine or may be depleted more quickly than in dendrites, or a combination of the two.
Figure 3.
Different depletion rates of the ER Ca2+ store in soma and dendrites caused by repetitive local applications of caffeine. A, B, When a cell was stimulated by the repetitive application of 50 mm caffeine, the [Ca2+]c responses differed in the soma and dendrites in a frequency-dependent manner. Ca2+ release in dendrites was much more persistent than that in soma. Scale bar, 10 μm. C, In several cells, relative changes in [Ca2+]c versus stimulation frequencies are presented in soma (green diamonds, from 15 neurons) and dendrites (red circles, from 14 neurons) in the bath solution containing 1 mm Ca2+ and soma (black rectangles, from 8 neurons) and dendrites (blue triangles, from 20 neurons) in Ca2+-free bath medium. At 0.1 Hz stimulation, the relative depletion of Ca2+ store at dendrites and soma in 1 mm Ca2+ or Ca2+-free solutions were 82.95 ± 7.82% (n = 3), 23.24 ± 2.09% (n = 3), 89.67 ± 6.34% (n = 3), and 14.33 ± 4.09% (n = 3), respectively. *Statistical differences (p < 0.05, Student's t test); p = 0.02 and 0.01 between soma and dendrites in 1 mm Ca2+ or in Ca2+-free solutions, and p = 0.31 and 0.18 between soma with 1 mm Ca2+ and without Ca2+ and between dendrites with 1 mm Ca2+ and without Ca2+.
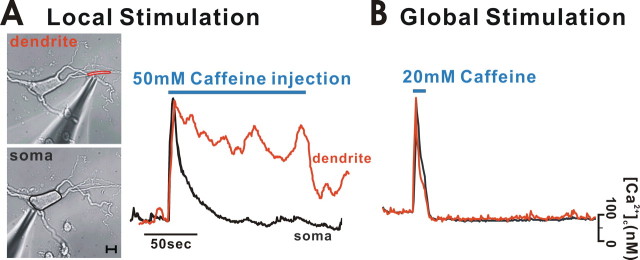
Therefore, we investigated further to distinguish between these possible explanations. First, we continuously applied caffeine locally into only a dendrite or soma, using the same micropressurized pipette. As shown in Figure 4 A, when caffeine was applied continuously to only one dendrite with a micropipette containing 50 mm caffeine, the [Ca2+]c in the stimulated dendritic area rose immediately and was then sustained without a severe decay (a red curve). However, when we stimulated soma with the same pipette, although [Ca2+]c increased similarly, the level was not maintained, instead rapidly decaying to the basal level. This is similar to the results obtained from the repetitive caffeine stimulations (Fig. 3). Then, in the same neuron, we applied 20 mm caffeine to the bath and stimulated the soma and dendrites at the same time. In this case, the reductions of peak [Ca2+]c values in soma and dendrites were remarkably similar, and there was no difference in the decay processes of soma and dendrites (Fig. 4 B). If caffeine-induced desensitization processes are different in soma and dendrites, the decay processes would be different in soma and dendrites under this condition. This was not the case. Therefore, this finding strongly indicates that the decline in peak amplitudes with repetitive local stimulations is attributable to the different depletion rates of the ER Ca2+ store in soma and dendrites, rather than being attributable to the different desensitization processes to caffeine. This also likely suggests that the dendritic Ca2+ store is more resistant to depletion when repetitively stimulated than the soma store.
Figure 4.
Different depletion rates of the ER Ca2+ store in soma and dendrites by continuous application of caffeine. A, When cells were stimulated by the continuous injection of 50 mm caffeine, [Ca2+]c responses differed in the soma (black line; [Ca2+]c decay at 50 s after stimulation, 96.2 ± 0.7%; n = 9) and dendrites (red line; [Ca2+]c decay at 50 s after stimulation, 36.7 ± 2.8%; n = 9). The Ca2+ release in dendrites was much more robust than that in the soma. Scale bar, 10 μm. B, When cells were stimulated by bath application of 20 mm caffeine, the relative changes in [Ca2+]c in the soma (black line) and dendrites (red line) were similar. This experiment was performed 12.5 min after the experiment in A. The elevated [Ca2+]c returned to the basal level at 22.3 ± 1.7 min in soma and 22.1 ± 1.3 min in dendrites (n = 9), respectively.
Luminal connection of the ER Ca2+ store in soma and dendrites
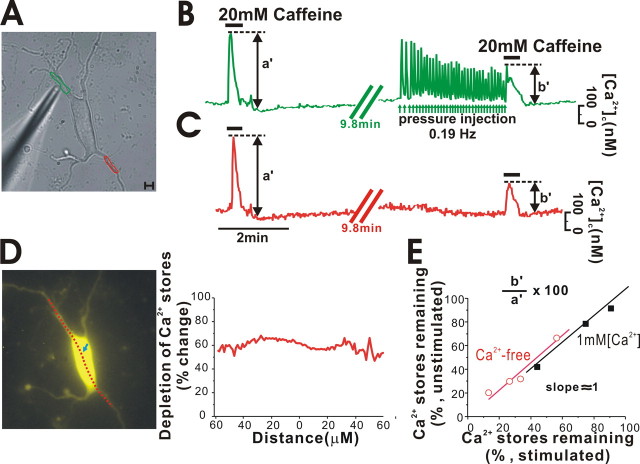
The observed differences between Ca2+ store depletion rates in response to the local repetitive stimulations in soma and dendrites may be simply explained by either the existence of distinct Ca2+ stores in soma and dendrites or the tunneling model. In the former case, when repetitively stimulated, the dendritic store would be more resistant to depletion than the soma store. Nevertheless, this model could not explain why global stimulations with caffeine depleted the ER Ca2+ stores at similar rates in soma and dendrites (Fig. 4 B), because this differed greatly from the cases in local stimulations (Fig. 4 A). Therefore, the tunneling model is more likely to explain the findings. If the large soma Ca2+ store acts as Ca2+ reservoir and continuously supplies Ca2+ to the remote dendritic store, the above phenomenon can be explained. To test this hypothesis, we performed the following experiment. First, 20 mm caffeine was applied to the bath and [Ca2+]c was measured in two different dendritic areas, as shown in Figure 5 A (green and red circles). The peak amplitudes in [Ca2+]c changes in the two areas are marked as a′ in Figure 5, B and C, in which the peak values were similar. Some time after allowing the ER to refill with Ca2+, we repetitively applied 50 mm caffeine locally onto one dendrite (Fig. 5 A, green circle) and depleted the local store sequentially, by observing the gradual reduction of amplitudes in Ca2+ spikes (Fig. 5 B, arrows). After this treatment, to estimate the ER Ca2+ level remaining, we applied 20 mm caffeine to the bath and again evoked a Ca2+ release from the whole ER. The peak values in [Ca2+]c changes in this second global stimulation are marked as b′ (Fig. 5 B,C). In this case, the relative ratio (b′/a′) seems to reflect the extent of Ca2+ depletion in the ER store inversely. Interestingly, despite partial depletion of the ER Ca2+ store in the left top dendrite, as shown in Figure 5 A, the ER Ca2+ store in the right bottom dendrite, which was not stimulated, was also found to be depleted. Moreover, b′/a′ ratios between stimulated and unstimulated regions were very similar, indicating that depletion of the Ca2+ store in one dendritic region caused store depletion in remote unstimulated dendritic regions to a similar extent. When we measured [Ca2+]c changes along the line marked in Figure 5 D before and after the local stimulations, we found that relative depletion levels (b′/a′) along the line were similar up to >90 μm from the soma, despite the fact that peripheral regions became noisy as a result of the low fluorescence intensity in our experimental condition. When we examined the ER depletion levels in three neurons in stimulated and unstimulated dendrites, although depletion varied according to stimulation intensity and duration, depletion levels of the ER Ca2+ store in the unstimulated area were very similar to those in the stimulated area (Fig. 5 E, black rectangles). In Ca2+-free medium, we obtained the same result from four cells (red open circles), which indicates that the above results are not attributable to the different kinetics of Ca2+ influx or refilling rates in different regions of a neuron. These results strongly support that the aforementioned ER tunneling model explains the observed differences between Ca2+ store depletion rates in dopamine neurons.
Figure 5.
Luminal connectivity of the ER Ca2+ store between soma and dendrites. A neuron was first stimulated globally, then locally, and finally globally by the bath application of 20 mm caffeine or by pressurized application of 50 mm caffeine with a micropipette. [Ca2+]c changes (graphs B and C) were measured in two different regions, marked by green and red circles in A. After the partial depletion of a local Ca2+ store in the left top dendrite by local application of caffeine (B, arrows), Ca2+ stores were found to be depleted to a similar level in the bottom right dendrite (C). Amplitudes of b′ were similar between B and C. D, After local depletion of the Ca2+ store in the green circled area (A), fluorescence intensities along the line at the time of the first (a′) and last (b′) global stimulations were compared. The relative store depletion along the line was similar. E, The relative emptying of ER Ca2+ stores (b′/a′ × 100) in locally stimulated (x-axis) and unstimulated (y-axis) regions of dendrites in the same neuron were plotted under the Ca2+-free condition (red circles; n = 4) and normal condition (black squares; 1 mm Ca2+; n = 3) in separate neurons. Scale bar, 10 μm.
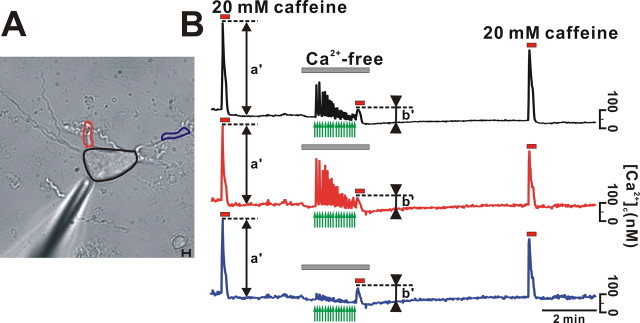
Next, to further confirm that the tunneling model is working in neurons, we attempted to deplete the Ca2+ store of soma by directly applying caffeine to soma. As shown in Figure 6 A, we positioned a micropipette containing 50 mm caffeine near the soma and measured [Ca2+]c changes in the three different areas. We initially applied 20 mm caffeine to the bath and observed [Ca2+]c rises in the three marked areas (Fig. 6 B, a′). We then replaced the bath medium with Ca2+-free solution and stimulated soma with repetitive pulses of caffeine, which rapidly depleted the ER Ca2+ store in the soma and nearby dendrites. Store depletion was reflected by a rapid reduction of the [Ca2+]c peaks. The nearby dendritic region (red circle and graph) and the soma (black circle and graph) (Fig. 6 B) showed large [Ca2+]c changes, but the remote dendritic region, marked as a blue circle, shows only a small response (blue graph). Nevertheless, when we applied 20 mm caffeine to the bath again, the relative amplitudes of the [Ca2+]c changes (marked b′) in the three measured regions were very similar, despite the fact that the stores measured in the different areas released Ca2+ differently. These results indicate that the depletion of the ER Ca2+ store in soma depletes the Ca2+ store in remote unstimulated dendritic areas to a similar level. Interestingly, when we reapplied 20 mm caffeine to the bath again some time after allowing the ER to refill with Ca2+, relative [Ca2+]c rises were similar in the three areas, indicating that the refilling rates were similar in all regions of a neuron. These findings again support the ER tunneling model, in which the ER is connected and Ca2+ equilibration between the ER in different regions occurs through the lumen. The above experiment was performed on four cells, and their relative extents of the ER Ca2+ depletions in soma or dendritic areas were exactly the same, similarly as shown in Figure 5 E.
Figure 6.
Luminal connectivity of the ER Ca2+ store between soma and dendrites. Some time after the global bath application of 20 mm caffeine, which elicited global rises in [Ca2+]c (marked as a′), a cell was stimulated by rapid and local application of 50 mm caffeine (arrows) in Ca2+-free bath solution. At the end of repetitive local stimulation, 20 mm caffeine was applied to the bath and [Ca2+]c peaks were measured (marked as b′). Changes in [Ca2+]c in three different regions (A), which were measuring simultaneously, were plotted in B. Although the Ca2+ releases induced by local stimulation in three measured areas differed, the relative depletions of the local Ca2+ stores (b′/a′) in the three regions were similar. Scale bar, 10 μm.
Photobleaching experiments reveal that the ER between soma and dendrites is connected luminally
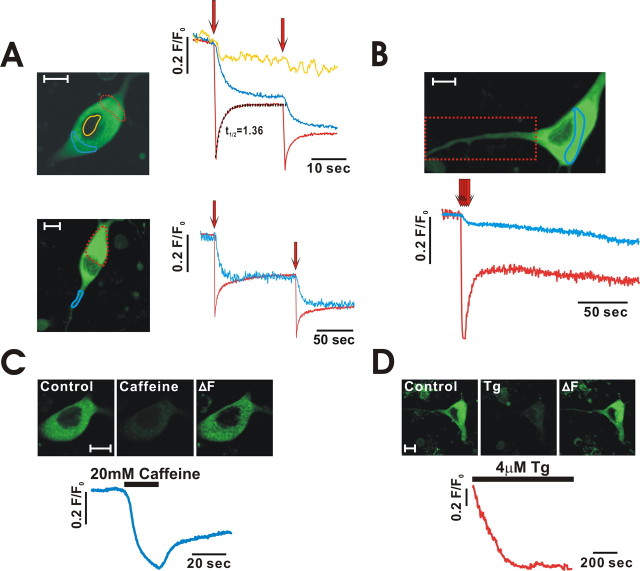
To directly examine whether or not the ER is a luminally connected organelle, we loaded dopamine neurons with MagFluo-4, a low-affinity Ca2+ dye that is used to measure Ca2+ concentrations in the lumen of the ER (Park et al., 2000). As shown in Figure 7 A, after loading neurons with MagFluo-4, fluorescence was very low in a nucleus, which indicates that the dye was specific for Ca2+-containing organelles such as the ER, mitochondria, and granules (Park et al., 2002). Under these conditions, we bleached one part of the soma (Fig. 7 A, red dotted circle) and measured fluorescence intensities in both bleached (red dotted circle) and unbleached (blue circle) areas across a nucleus (yellow circle). As shown in the top right of Figure 7 A, fluorescence intensity in the bleached area was rapidly decreased by photobleaching and was subsequently recovered (half-recovery time of 1.6 ± 0.1 s; n = 14), whereas the remote unbleached area (blue circle) showed reciprocal reductions in fluorescence. The same fluorescence change was also observed in the proximal dendritic area (Fig. 7 A, bottom). This finding suggests that the dye trapped in the lumen of the ER can quickly move across a nucleus to the remote ER in the opposite soma as well as in the proximal dendrite. Conversely, when we bleached one dendritic region for a very short period of time (<800 ms), intensity changes in the soma were negligible (data not shown). However, the repetitive bleaching of dendrites (Fig. 7 B, red dotted rectangle) reduced fluorescence intensity in the soma (green circle). This finding could be explained by the assumption that the total volume of the ER in the bleached area is much smaller than that of the soma. Next, to verify whether the Magfluo-4 fluorescence comes from the functionally active ER Ca2+ store, we stimulated neurons with caffeine or thapsigargin and then observed decreases in MagFluo-4 fluorescence intensity throughout the neuron, except within the nucleus (Fig. 7 C,D).
Figure 7.
Photobleaching experiments revealed that the ER in the soma is luminally connected with that in dendrites. Neurons were loaded with 5 μm MagFluo-4, which is often used for ER Ca2+ measurement, and local areas were photobleached. A, The bleaching of dye in one part of a soma (red dotted line) revealed a rapid recovery in fluorescence intensity after the initial sharp decrease, whereas the intensity measured in remote areas of soma (top) and dendrites (bottom) concomitantly decreased. B, Continuous bleaching of a wide dendritic area (red dotted rectangle) led to a decrease in the fluorescence of soma (green). C, D, Fluorescence intensity in MagFluo-4-loaded cells was decreased by treatment with caffeine (44.4 ± 1.3%; n = 9) or thapsigargin (47.4 ± 4.9%; n = 7), which suggests that most fluorescence originated from the ER. Scale bars, 10 μm.
Speed of Ca2+ diffusion through the ER lumen in dendrites
To examine the extent to which the ER in soma is luminally connected to the distal dendrites, we enhanced the detector sensitivity so that fluorescence in the soma was partly saturated, which then made it possible to detect MagFluo-4 fluorescence in more distal dendrites. In this condition, when we bleached only the soma, we were able to detect decreases in fluorescence intensity through the unbleached dendrite (Fig. 8 A). In Figure 8 A, the dotted rectangle indicates bleached area, and fluorescence changes were measured in the circles drawn in different colors along the dendrite. Interestingly, fluorescence decreases through the dendrite after soma bleaching were delayed as the distance of the measured sites from the bleached soma increased (Fig. 8 A, right). These data more clearly show that the dye trapped within the ER in the soma could luminally diffuse to the ER in distal dendrites, up to nearly 90 μm, which was a limit of our resolution. When we plotted the half-fluorescence decreasing time versus the distance from a soma that was photobleached (Fig. 8 C, red rectangles), we found that the diffusion speed of MagFluo-4 was 3.78 ± 0.30 μm/s (n = 29 from 7 cells).
Figure 8.
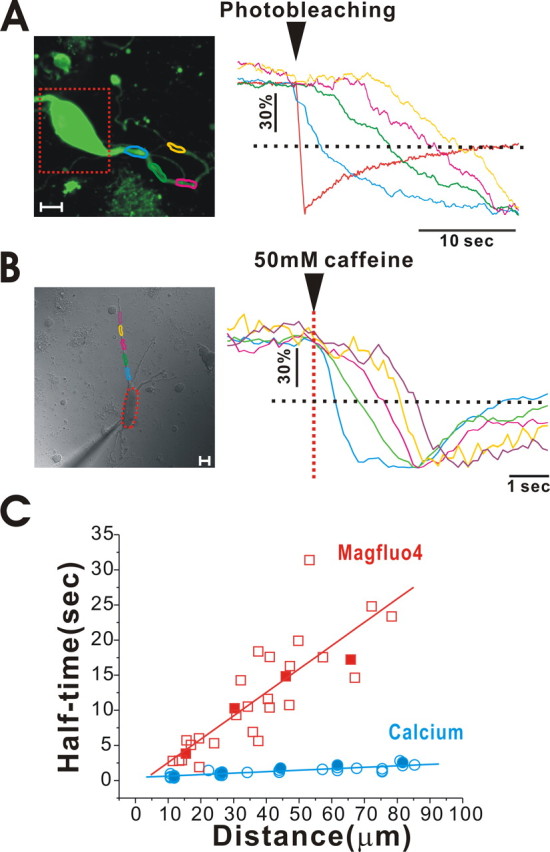
Measurement of Ca2+ and MagFluo-4 diffusion speed through the ER lumen in a dendrite. An acutely isolated dopamine neuron was loaded with MagFluo-4, and its fluorescence was measured using a confocal microscope. A, A soma (dotted red rectangle) was photobleached by 351, 364, and 488 nm combined laser lines, and the fluorescence intensity through a dendrite was measured in the areas marked by differently colored circles. The intensity was normalized and plotted in the graph on the right. The time at which fluorescence decreased through the dendrite depended on the distances from the soma. The distances were measured from the margin of the bleached areas, which contains most of soma. Time lags were more clearly detected at half-fluorescence decreasing time (dotted line). Scale bar, 10 μm. B, Caffeine was locally applied to soma with a micropressurized pipette, and fluorescence intensity through a dendrite was measured in the areas marked by differently colored circles. The intensity was normalized and plotted in the graph on the right. The time at which fluorescence decreased through the dendrite depended on the distances from the soma. In this neuron, soma fluorescence signal was saturated. Scale bar, 10 μm. C, The half-fluorescence decreasing times were plotted versus the distances from the soma. These data were obtained from 29 points from six neurons for MagFluo-4 and 30 points from 7 neurons for Ca2+. Data points from A and B were specifically marked with filled symbols. The diffusion speeds of Ca2+ and MagFluo-4 through the dendritic ER lumen were 31.57 ± 1.94 μm/s (n = 30 from 6 neurons) and 3.78 ± 0.30 μm/s (n = 29 from 7 neurons), respectively. They were calculated from the graphs shown in data in C.
Finally, to measure the diffusion speed of Ca2+ through the dendritic ER lumen, we directly measured MagFluo-4 fluorescence changes after locally applying caffeine to soma; this process was able to generate a Ca2+ gradient within the ER between soma and dendrites. To detect changes in fluorescence in dendrites, we raised the detector sensitivity and visualized fluorescence in distal dendrites at distances nearly 90 μm from the soma. In this condition, caffeine application caused a serial reduction in fluorescence intensity along the dendrite, as marked in different colors (Fig. 8 B). The drops in fluorescence (decreases in ER Ca2+ level) were first observed in the proximal dendrite and were later observed in the distal dendrite. It indicates that sudden reduction of Ca2+ concentration within the soma ER by caffeine causes Ca2+ diffusion from dendrites to soma, thereby leading to an earlier dropping in luminal Ca2+ concentration in the ER in a proximal dendrite rather than in a distal dendrite. When we plotted the half-fluorescence decreasing time versus the distance through the dendrite from the soma (Fig. 8 C, blue circles), we found that the diffusion speed of Ca2+ through the dendritic ER was ∼31.57 ± 1.94 μm/s (n = 30 from 6 cells). The diffusion speed of Ca2+ was ∼10 times faster than that of MagFluo-4.
Discussion
In this study, we show that the ER in dopamine neurons exists as an interconnected Ca2+ pool that allows rapid Ca2+ movement between the soma and dendrites through the lumen of the ER. Because the soma contains a very large Ca2+ pool and each dendrite possesses a relatively small amount of the ER, this transit Ca2+ allows dendrites to persistently respond to continuous Ca2+-mobilizing input signals without suffering from severe depletion of local store. Ca2+ store in dendritic areas can be rapidly refreshed by a continuous supply of Ca2+ from a large soma Ca2+ reservoir. Accordingly, because of the functional luminal connectivity of the ER, which we prove here, we could speculate that local ER Ca2+ signals occurring in distal dendrites may be transferred to the ER in remote unstimulated dendrites, as well as to the main ER in soma at a relatively rapid speed. In our experimental condition, the diffusion speed of Ca2+ through the dendritic ER was 31.57 ± 1.94 μm/s (n = 30) (Fig. 8). However, in this study we are not able to rule out the possibility that mobile Ca2+ dye would affect the real speed of Ca2+ movement through the ER lumen. Nevertheless, if free Ca2+ diffuse at the above speed, it would be worth noting that, although the ER is luminally connected, the local ER Ca2+ signals generated by synaptic inputs take time to transfer information to a distant part of the ER, several seconds to travel over 100 μm. Considering the diffusion speed of Ca2+ that we measured at normal central neurons (Fig. 8), single or weak synaptic activities may not propagate to a distant area and may dissipate, thereby generating signals limited within local domains. Nevertheless, it is likely that strong and long-lasting changes in Ca2+ signals in local ER domains would affect Ca2+ levels in the remote ER. Therefore, the ER Ca2+ signals in different areas would be harmonized or communicated. We also found that dendritic ER Ca2+ signals are robust and endurably respond to successive input signals without severe reduction or desensitization in dopamine neurons (Figs. 2 –4). This could be explained by the ER tunneling model (Petersen et al., 2001). Because the ER Ca2+ store in the soma is large and is connected to the dendritic ER (Figs. 1, 7, 8), it appears to act as Ca2+ reservoir and serves to keep dendrites active by luminally supplying Ca2+ (Figs. 3, 9).
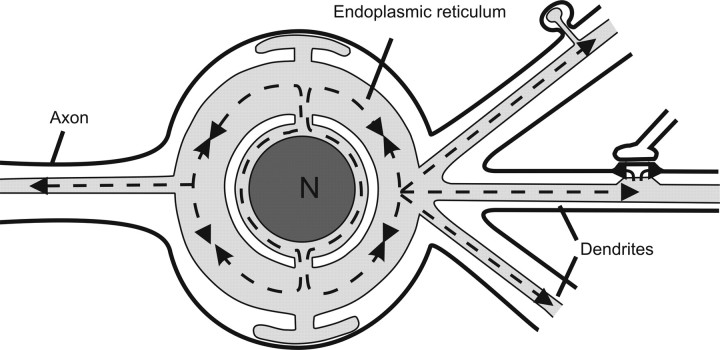
Figure 9.
A model of the ER Ca2+ pool in a central neuron. The ER in soma and dendrites are luminally connected, which allows rapid Ca2+ movement and equilibration. Thus, if a local Ca2+ store in a dendritic area is depleted, it can be quickly replenished by a supply of Ca2+ from the large Ca2+ reservoir in the soma. Conversely, intensive and massive release of Ca2+ in the local dendritic store could cause Ca2+ depletion in the ER of the soma. Thus, ER Ca2+ signals in different cellular regions would communicate. The subsurface ER cisterns in the soma would be connected to the Ca2+ reservoir of the soma and appear to actively reabsorb Ca2+ in subplasmalemmal spaces (adapted from Berridge, 1998).
The ER is an organelle that is responsible for protein synthesis and storage, and fluctuations in luminal Ca2+ concentrations modulate protein processing (Wileman et al., 1991; Gallin and Greenberg, 1995; Finkbeiner and Greenberg, 1997; Patil and Walter, 2001; Michalak et al., 2002; Burgoyne et al., 2004). Therefore, Ca2+ signals, which are often generated at remote dendritic areas, should be transferred to the soma, which contains the main body of the ER and nucleus. Neurons may integrate multiple local dendritic events via luminally connected ER, because profound changes in luminal Ca2+ concentration in multiple dendrites are likely to affect Ca2+ concentration in the central part of the ER (Figs. 5, 7). The luminally connected ER model (Fig. 9) could, therefore, explain how the ER integrates local signals and how the ER in different compartments is harmonized or communicates. In hippocampal neurons, it has been reported that synaptic activities elevate the ER Ca2+ levels in dendrites for several minutes (Pozzo-Miller et al., 2000; Pivovarova et al., 2002). If we consider the diffusion speed of Ca2+, we estimate that successive burst of presynaptic action potentials occurring within 1 s would affect postsynaptic ER Ca2+ levels at nearby synapses at distances up to ∼32 μm. Nevertheless, if the local ER Ca2+ levels change for several minutes attributable to synaptic activity, the ER in the soma would be affected, because luminal Ca2+ would diffuse and eventually equilibrate between the dendrites and the soma. Thus, the memory of ER Ca2+ signals would be both time dependent and space limited.
Morphologically, it is clear that the ER is a continuous and interconnected network of tubules and cisterns in neurons. Direct visualization of neuronal ER by electron microscopy (Spacek and Harris, 1997) and by fluorescent staining with lipophilic carbocyanine dyes (Terasaki et al., 1994) supports the membrane connectivity of the ER throughout the whole of a neuron. However, many pharmacological experiments in various types of neurons and glial cells have divided the ER into two distinct models, i.e., a single functional ER Ca2+ pool or separate ER Ca2+ pools (Verkhratsky, 2005). The experimental strategy has mostly been based on pharmacological experiments. Because some chemicals or drugs could empty certain Ca2+ store compartments in different ways, it has been concluded that there are spatially distinct separate compartments in the ER in some cell types (Verkhratsky, 2005). However, these experiments were not free of contamination of non-ER Ca2+ stores, such as the Golgi apparatus, nuclear envelope, granules, endosomes, and mitochondria (Pozzan et al., 1994; Michelangeli et al., 2005). Considering these aspects, it is noteworthy to mention that synaptic activity in hippocampal slices induced a rise in Ca2+ in some fractions of the ER and caused no change in other portions of the ER (Pozzo-Miller et al., 2000; Pivovarova et al., 2002). Thus, the possibility that some of Ca2+-binding proteins would hamper Ca2+ diffusion and act as a barrier within the connected ER in dendrites has been suggested (Berridge et al., 2000; Carafoli et al., 2001; Collin et al., 2005; Konur and Ghosh, 2005). We showed here that Ca2+ can move freely through the lumen of the ER at distances of up to 90 μm. However, we did not rule out the possibility that the distal dendrites >90 μm from the soma may express a lot of fixed Ca2+-binding proteins at local ER, thereby acting as a local diffusion barrier for Ca2+. In truth, we do not know the actual interactions between far more distant dendrites because we did not confirm the functional connection at distances of >90 μm because of the technical limits of our experimental conditions. Nevertheless, our results provide direct evidence to show the functional connectivity of the ER in different neuronal regions. To our knowledge, this is the first study to demonstrate emptying of local ER stores and comparison of ER responses elicited in different regions of a single neuron. More importantly, we directly measured the diffusion speed of Ca2+ through the dendritic ER for the first time. Thus, we believe that our study provides the strongest evidence available to demonstrate that the ER is a functionally connected organelle that acts as a functional tunnel for Ca2+ in central neurons.
The single ER Ca2+ pool model and its functional implications have been proposed in polarized pancreatic acinar cells (Park et al., 2000). Because pancreatic acinar cells should secrete zymogen granules through the apical luminal membrane by Ca2+ signals, hormone- or neurotransmitter-driven Ca2+ spikes usually occur at the apical pole. Additionally, pancreatic acinar cells contain a very large amount of the ER in the basal portion of a cell, whereas zymogen granules containing many digestive enzymes are tightly packed within the apical part of a cell. Thus, because of the limited space in the apical area, only a small amount of ER exists in the apical pole (Petersen, 1999). Nevertheless, Ca2+ oscillations could occur continuously in the apical pole, even in the Ca2+-free bath solution (Park et al., 2000). Thus, it has been questioned how pancreatic acinar cells continuously and sufficiently raise [Ca2+]c at the apical pole with only such a small amount of ER (Petersen et al., 1999). This mystery can be explained by the connected ER tunnel model, in which Ca2+ in the ER at the apical area of a cell can be continuously supplied by a large ER Ca2+ reservoir in the basal part of the cell attributable to luminal Ca2+ movement (Park et al., 2000; Petersen et al., 2001). Similarly, in this study, we demonstrate for the first time that this is the case in central neurons. In midbrain dopamine neurons, the ER is abundant in soma and in dendrites (Figs. 1, 9). Although the total amount of ER in dendrites may exceed the total amount of ER in soma in the midbrain dopamine neuron in vivo, the relative amount of ER in a small area of dendrites that receive synchronized synaptic inputs would be smaller than that in the soma. Thus, decreases in Ca2+ concentration in dendrites in response to synaptic activities could be refreshed by a Ca2+ supply from the soma ER store and nearby ER. Thus, the ER in dendrites would quickly become ready to respond to the next synaptic input. Conversely, large and intensive afferent inputs into local dendrites would lead to a severe reduction in the ER Ca2+ level and, hence, cause Ca2+ changes in the soma ER via ER Ca2+ movement through functional tunnels. Thus, a neuron may recognize what is happening in remote regions of the neuron, such as the dendrites and axons. This model may help us to understand and investigate many other intracellular signaling complexes in highly polarized neurons.
Footnotes
This work was supported by Neurobiology Research Program from the Korea Ministry of Science and Technology Grant M1-0108-00-0027 and Basic Research Program of the Korea Science and Engineering Foundation Grant R01-2006-000-10478-0.
References
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, O'Callaghan DW, Hasdemir B, Haynes LP, Tepikin A. Neuronal Ca2+-sensor proteins: multitalented regulations of neuronal function. Trends Neurosci. 2004;27:203–209. doi: 10.1016/j.tins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Cancela JM, Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. Two different but converging messenger pathways to intracellular Ca2+ release: the roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose and inositol trisphosphate. EMBO J. 2000;19:2549–2557. doi: 10.1093/emboj/19.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. doi: 10.1080/20014091074183. [DOI] [PubMed] [Google Scholar]
- Choi YM, Kim SH, Uhm DY, Park MK. Glutamate-mediated [Ca2+]c dynamics in spontaneously firing dopamine neurons of the rat substantia nigra pars compacta. J Cell Sci. 2003;116:2665–2675. doi: 10.1242/jcs.00481. [DOI] [PubMed] [Google Scholar]
- Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Cur Opin Neurobiol. 2005;15:275–281. doi: 10.1016/j.conb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Greenberg ME. Spatial features of calcium-regulated gene expression. BioEssays. 1997;19:657–660. doi: 10.1002/bies.950190803. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurons modulates effects of Ca2+ entry on [Ca2+]i . J Physiol (Lond) 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin WJ, Greenberg ME. Calcium regulation of gene expression in neurons: the mode of entry matters. Curr Opin Neurobiol. 1995;5:367–374. doi: 10.1016/0959-4388(95)80050-6. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R. Space matters: local and global dendritic Ca2+ compartmentalization in cortical interneurons. Trends Neurosci. 2005;28:158–167. doi: 10.1016/j.tins.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Blaustein MP. Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science. 1997;275:1643–1648. doi: 10.1126/science.275.5306.1643. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guatteo E, Mercuri NB, Bernardi G, Knopfel T. Group I metabotropic glutamate receptors mediate an inward current in rat substantia nigra dopamine neurons that is independent from calcium mobilization. J Neurophysiol. 1999;82:1974–1981. doi: 10.1152/jn.1999.82.4.1974. [DOI] [PubMed] [Google Scholar]
- Kim SH, Choi YM, Chung S, Uhm DY, Park MK. Two different Ca2+-dependent inhibitory mechanisms of spontaneous firing by glutamate in dopamine neurons. J Neurochem. 2004;91:983–995. doi: 10.1111/j.1471-4159.2004.02783.x. [DOI] [PubMed] [Google Scholar]
- Konur S, Ghosh A. Calcium signaling and the control of dendritic development. Neuron. 2005;46:401–405. doi: 10.1016/j.neuron.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Llano I, González J, Caputo C, Lai AF, Blayney LM, Tan YP, Marty A. Ryanodine-sensitive Ca2+ stores underlie large-amplitude miniature IPSCs and spontaneous presynaptic Ca2+ transients at Purkinje cell synapses. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lomax RB, Camello C, Van Coppenolle F, Petersen OH, Tepikin AV. Basal and physiological Ca2+ leak from the endoplasmic reticulum of pancreatic acinar cells. Second messenger-activated channels and translocons. J Biol Chem. 2002;277:26479–26485. doi: 10.1074/jbc.M201845200. [DOI] [PubMed] [Google Scholar]
- Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Curr Opin Cell Biol. 2005;17:135–140. doi: 10.1016/j.ceb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Montero M, Alvarez J, Scheenen WJJ, Rizzuto R, Meldolesi J, Pozzan T. Ca2+ homeostasis in the endoplasmic reticulum: coexistence of high and low [Ca2+] subcompartments in intact HeLa cells. J Cell Biol. 1997;139:601–611. doi: 10.1083/jcb.139.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Imani F, Khodakhah K, Williams JT. Inositol 1,4,5-triphosphate-evoked responses in midbrain dopamine neurons. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-20-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Tepikin AV, Petersen OH. What can we learn about cell signalling by combining optical imaging and patch clamp techniques? Pflügers Arch. 2002;444:305–316. doi: 10.1007/s00424-002-0832-y. [DOI] [PubMed] [Google Scholar]
- Patil C, Walter P. Intracellular signalling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13:349–356. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- Petersen OH. Waves of excitement: calcium signals inside cells. Biologist. 1999;46:227–230. [Google Scholar]
- Petersen OH, Burdakov D, Tepikin AV. Polarity in intracellular calcium signaling. BioEssays. 1999;21:851–860. doi: 10.1002/(SICI)1521-1878(199910)21:10<851::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Tepikin A, Park MK. The endoplasmic reticulum: one continuous or several separate Ca2+ stores? Trends Neurosci. 2001;24:271–276. doi: 10.1016/s0166-2236(00)01787-2. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Pozzo-Miller LD, Hongpaisan J, Andrews SB. Correlated calcium uptake and release by mitochondria and endoplasmic reticulum of CA3 hippocampal dendrites after afferent synaptic stimulation. J Neurosci. 2002;22:10653–10661. doi: 10.1523/JNEUROSCI.22-24-10653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium store. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Connor JA, Andrews SB. Microheterogeneity of calcium signalling in dendrites. J Physiol (Lond) 2000;525:53–61. doi: 10.1111/j.1469-7793.2000.t01-1-00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco S, Natoli S, Bernardi G, Mercuri NB. Group I metabotropic glutamate receptors activate burst firing in rat midbrain dopaminergic neurons. Neuropharmacology. 2002;42:289–296. doi: 10.1016/s0028-3908(01)00192-7. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–421. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, LaFerla FM, Parker I. Ca2+ signaling in mouse cortical neurons studied by two-photon imaging and photoreleased inositol triphosphate. J Neurosci. 2003;23:758–765. doi: 10.1523/JNEUROSCI.23-03-00758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Slater NT, Fein A, Schmidek A, Reese TS. Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc Natl Acad Sci USA. 1994;91:7510–7514. doi: 10.1073/pnas.91.16.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Petersen OH. Neuronal calcium stores. Cell Calcium. 1998;24:333–343. doi: 10.1016/s0143-4160(98)90057-4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Petersen OH. The endoplasmic reticulum as an integrating signalling organelle: from neuronal signalling to neuronal death. Eur J Pharmacol. 2002;447:141–154. doi: 10.1016/s0014-2999(02)01838-1. [DOI] [PubMed] [Google Scholar]
- Wileman T, Kane LP, Carson GR, Terhorst C. Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J Biol Chem. 1991;266:4500–4507. [PubMed] [Google Scholar]