Abstract
Introduction:
The feasibility and accuracy of text messaging to monitor events after influenza vaccination throughout pregnancy and the neonatal period has not been studied, but may be important for seasonal and pandemic influenza vaccines and future maternal vaccines.
Methods:
This prospective observational study was conducted during 2013–2014 and analyzed in 2015–2016. Enrolled pregnant women receiving inactivated influenza vaccination at a gestational age <20 weeks were sent text messages intermittently through participant-reported pregnancy end to request fever, health events, and neonatal outcomes. Text message response rates, Day 0–2 fever (≥ 100.4°F), health events, and birth/neonatal outcomes were assessed.
Results:
Most (80.2%, n=166) eligible women enrolled. Median gestational age was 8.9 (SD=3.9) weeks at vaccination. Response rates remained high (80.0%–95.2%). Only one Day 0–2 fever was reported. Women reported via text both pregnancy-and non-pregnancy–specific health events, not all associated with medical visits. Most pregnancy-specific events in the electronic medical record (EMR) were reported via text message. Of all enrollees, 84.9% completed the study (131 reported live birth, ten reported pregnancy loss). Two losses reported via text were not medically attended; there was one additional EMR-identified loss. Gestational age and weight at birth were similar between text message–reported and EMR-abstracted data and 95% CIs were overlapping for proportions of prematurity, low birth weight, small for gestational age, and major birth defects, as identified by text message–reported versus EMR-abstracted plus text message–reported versus EMR-abstracted data only.
Conclusions:
This study demonstrated the feasibility of text messaging for influenza vaccine safety surveillance sustained throughout pregnancy. In these women receiving inactivated influenza vaccination during pregnancy, post-vaccination fever was infrequent and a typical pattern of maternal and neonatal health outcomes was observed.
INTRODUCTION
Rapid monitoring of vaccine safety is an important component of national and international pandemic influenza plans, and pregnant women are a target population for vaccination, owing to increased risk of influenza morbidity and mortality.1–8 Such monitoring may also be important for other vaccines for pregnant women, particularly when medical records are not readily available.9,10
Text messaging can be used to rapidly collect individualized data on both medically attended and non-attended events in large populations over a long period of time.11 Although the use of text messaging for vaccine adverse event reporting in children has been successful,12,13 and limited studies have assessed its use in pregnant women,14–16 the feasibility of its use to monitor vaccine safety in women enrolled early in pregnancy and monitored throughout pregnancy and the neonatal period has yet to be reported. Such a system would ideally capture immediate post-vaccination events, such as fever, short-term adverse events in the first 42 days post-vaccination, longer-term pregnancy-related conditions, and pregnancy and neonatal outcomes.
The goal of this prospective study was to assess the:
feasibility of recruiting pregnant women <20 weeks gestational age (GA) to participate in a text messaging—based vaccine adverse event monitoring program after receipt of inactivated influenza vaccine (IIV); and
utility of text messaging to monitor health events, including fever frequency on vaccination and next 2 days (Days 0–2), health events during pregnancy, pregnancy outcomes, and neonatal outcomes.
METHODS
This prospective observational study was conducted during the 2013–2014 influenza season at a family planning clinic and three obstetrics and gynecology practices. All four sites are affiliated with New York-Presbyterian Hospital/Columbia University Medical Center (NYP/CUMC) in New York City. One practice serves a primarily publicly insured and Latino population, and three a commercially insured, primarily non-minority, English-speaking one. Patients from both sites deliver at one of two NYP sites. All vaccination decisions were made by patients and healthcare providers as part of usual practice; it was not routine practice to provide antipyretics at vaccination. CUMC’s IRB approved the study and the Centers for Disease Control and Prevention relied on the CUMC IRB. Analyses were conducted in 2015–2016.
Study Population
Women were eligible to enroll if they (1) were pregnant with a GA <20 weeks by last menstrual period and/or ultrasound; (2) were aged ≥18 years; (3) elected to receive IIV (trivalent) at time of enrollment; (4) had a cell phone with text messaging capabilities; (5) were English or Spanish speaking; and (6) were willing to report via text message through pregnancy end. Exclusion criteria included (1) decision to not continue pregnancy at time of enrollment; (2) temperature ≥ 100.4°F at vaccination; (3) anti-pyretic administration within 6 hours pre-vaccination or stated intent to use prophylactically; and (4) inability to read text messages. Receipt of additional vaccines at enrollment or any time during pregnancy was not an exclusion criterion.
At the time of vaccination, eligible women were approached. After consent, they completed an intake form, reviewed text message procedures, and enrolled by sending a text message to the text messaging platform. They also received a digital thermometer and a paper diary in a pre-addressed, pre-stamped envelope to return after the 2-day fever observation period; these procedures were not part of usual practice.
Participants were instructed to take their oral temperature at least once daily, or more often if they felt febrile, from Day 0 (day of vaccination) and continuing over the next 2 days (Days 1–2), and were sent messages nightly to report their highest temperature, antipyretic use, and care sought. Text messages were next sent on Days 7, 14, 28, and 42 to capture short-term adverse events temporally associated with vaccination. From Day 70 onward, text messages were sent once a month for 4 months, then biweekly for 4 weeks, and then weekly. All text messages first assessed whether the participant was still pregnant and, if affirmative, continued into an interactive message cascade assessing any health problems. This included both close-ended queries assessing specifically for vaginal bleeding, contractions/cramping, and fever, and open-ended questions for other health events. If a participant reported she was no longer pregnant, a cascade assessed the pregnancy outcome (i.e., delivery, pregnancy loss, or termination). After reporting a delivery, GA, birth weight, and any neonatal health problems were assessed via open-ended questions. After reporting a pregnancy loss or termination, GA at time of the event was elicited. All cascades ended with a note informing women to contact their healthcare provider if they needed medical care. Messages were stopped when a pregnancy outcome was reported or 4 weeks after due date. Ten pregnancy-related health tips were sent between assessments through Day 175 to aid in study engagement. All messages were sent in English or Spanish based on patient preference.
Research staff reviewed incoming responses daily and initiated contact via phone with non-responders to collect missing data and answer technical questions. Using an electronic medical record (EMR) abstraction tool, vaccinations and all healthcare visits (prenatal, ambulatory, emergency department, and hospital) to NYP/CUMC throughout pregnancy were recorded. An exit survey was conducted.
Measures
Primary outcomes included the proportion of (1) eligible patients enrolled; (2) data reported on Days 0–2; and (3) data reported through the end of pregnancy. Secondary outcomes included (1) fever (temperature ≥ 100.4°F/38°C) on Days 0–2 post-vaccination; (2) pregnancy-specific events (e.g., gestational diabetes) reported; (3) pregnancy outcomes (delivery, pregnancy loss [spontaneous abortion {SAB} defined as loss at <20 weeks or stillbirth ≥ 20 weeks] or termination); and (4) proportion of preterm (< 37 weeks gestation), low birth weight (< 2,500 grams), and small for gestational age births and major birth defects.17–20
Statistical Analysis
The associations between baseline text message plan and frequency and response rates and study completion were assessed using chisquare tests. Reported health events were described. Pregnancy and neonatal outcomes using text message—reported only versus both sources (text plus EMR-abstracted) versus EMR-abstracted data only, including 95% CIs as applicable, were assessed. Pregnancy outcomes used the number of pregnant women as the denominator; neonatal outcomes used the number of live new-borns. All analyses were conducted in SPSS, version 23.
RESULTS
Nearly all approached women (97.1%) had a cell phone with text messaging. One hundred sixty-six women enrolled, 80.2% of those eligible (Appendix Figure 1, available online). Median GA at vaccination was 8.9 weeks (Table 1). Nearly all (90.4%) had unlimited text messaging plans and sent text messages daily (94.0%), but few (9.0%) had received text messages from their doctor or their doctor’s office. All received only IIV on enrollment, and 70.5% had documented receipt of tetanus—diphtheria—pertussis vaccine during the same pregnancy.
Table 1.
Characteristics of Study Population (N=166)
| Characteristics | Data |
|---|---|
| Age | |
| Median, years | 32 |
| 19–25 years | 38 (22.9) |
| 26–34 years | 75 (45.2) |
| 35–46 years | 53 (31.9) |
| GA (by last menstrual period at vaccination) | |
| Median, weeks | 8.9 |
| Range, weeks | 3.9–19.5 |
| 1–13 weeks (first trimester) | 141 (84.9) |
| 14– <20 weeks (second trimester) | 25 (15.1) |
| Primigravida | 69 (41.6) |
| Ethnicity, Latina | 96 (57.8) |
| Race (self-reported) | |
| White | 73 (44.0) |
| Black | 15 (9.0) |
| Asian | 8 (4.8) |
| Indigenous Latin American | 7 (4.2) |
| Multiracial | 21 (12.7) |
| Other | 42 (25.3) |
| Primary language | |
| Spanish | 55 (33.1) |
| English | 111 (66.9) |
| Insurance (at enrollment) | |
| Commerical | 85 (51.2) |
| Public | 36 (21.7) |
| Uninsured | 45 (27.1) |
| Education | |
| Less than HS | 11 (6.6) |
| HS/trade/vocational school | 38 (22.9) |
| Some college | 35 (21.1) |
| College graduate | 82 (49.4) |
| Type of text message plan | |
| Unlimited number of text messages per month | 150 (90.4) |
| Limited number of text messages per month/pay per text | 9 (5.4) |
| Don’t know | 7 (4.2) |
| How often send text messages | |
| Never | 1 (0.6) |
| Daily | 156 (94.0) |
| Weekly | 7 (4.2) |
| Monthly | 1 (0.6) |
| Less than once a month | 1 (0.6) |
| Ever received text messages from their doctor or doctor’s office | 15 (9.0) |
Note: Data are n (%) unless otherwise noted.
GA, gestational age; HS, high school.
Daily text message response rates were high on Days 0–2 (Figure 1A) with 84.9% providing data on all 3 days. There was no statistically significant difference in responding for all 3 days by text message plan (88.9% limited vs 84.7% unlimited plan, p=0.73) or frequency of baseline text message use (84.0% text daily vs 100.0% text less than daily, p=0.17). Only one participant reported a Day 0–2 fever (100.4°F on Day 1 via diary, 100.3°F via text message). Three quarters (77.7%) of participants returned the paper temperature diary. Most (n=141, 84.9%) continued to report through pregnancy end. Seventeen women stopped reporting (median stopped reporting 42 days; interquartile range, 21–161 days). Eight women never responded via text or phone. There was no difference in the 25 women who stopped or were non-responders versus those completing the study based on kind of text message plan (11.1% limited vs 16.0% unlimited plan, p=0.70) or baseline frequency of text message use (15.4% text daily vs 10.0% text less than daily, p=0.64). Two thirds of women received a phone call owing to not responding or questionable response. Of those, 84.8% remained active after the phone contact. Of the 95 remaining active, 51.6% only required one call.
Figure 1.
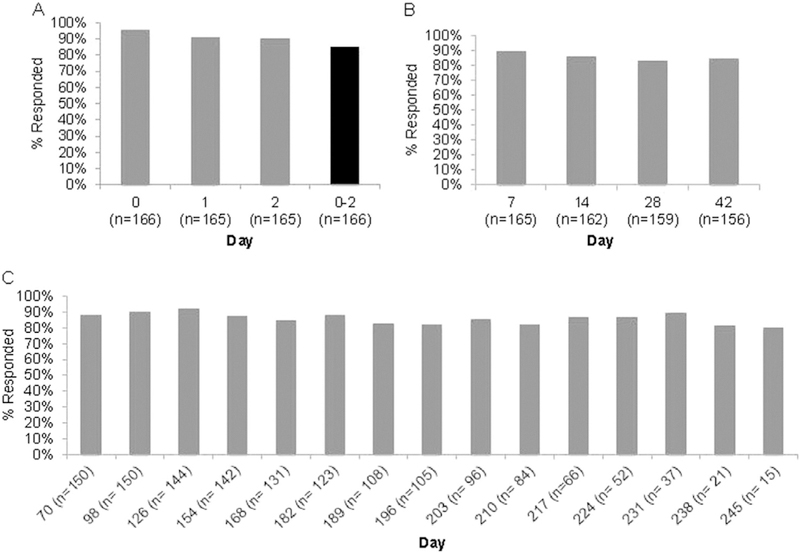
Text message response rates by day post-vaccination and response types. (A) Day 0–2 responses to text messages assessing fever; (B) Day 7–42 responses to text messages assessing short-term adverse events temporally associated with vaccination; (C) Day 70–245 responses to text messages assessing health events through the end of pregnancy, and pregnancy and neonatal outcomes.
Note: Response rates end on Day 245 due to having fewer than five participants from Day 252–259. Denominator includes all women who had not yet reported a pregnancy outcome who were still within 2 weeks of their estimated due date.
Response rates remained high throughout pregnancy (Figures 1B and 1C). In both the Day 7–42 and Day ≥ 70 periods, women reported via text both pregnancy-specific and non-pregnancy—specific health events. These included pre-specified responses for vaginal bleeding, contractions/cramping and fever, as well as in-depth free-text responses, not all of which resulted in medical visits (Table 2, Appendix Table 1, available online). On EMR review, there were pregnancy-related events that were not reported via text message. These included emergency department visits for vaginal bleeding (n=3), contractions (n=3), anemia/syncope (n=1), and oligohydramnios (n=1).
Table 2.
Pregnancy Events Post Influenza Vaccination Reported via Text Message and in the Electronic Medical Record
| Sourcea |
||
|---|---|---|
| Variable | Text report (with EMR confirmation) | No text report, documented in EMR |
| Days 0–7 | ||
| 2 vaginal bleedingb | 2 text (with 2 EMR) | |
| Days 8–14 | ||
| 2 vaginal bleedingb | 2 text (with 1 in EMR) | |
| 1 contractionsb | 1 text (with 1 in EMR) | |
| Days 15–28 | ||
| 4 vaginal bleedingb | 4 text (with 3 in EMR) | |
| 1 contractionsb | 1 text (with 1 in EMR) | |
| 2 gestational diabetesc | 0 text | 2 EMR |
| 3 anemiac | 0 text | 3 EMR |
| Days 29–42 | ||
| 5 vaginal bleedingb | 4 text (with 2 in EMR) | 1 additional in EMR |
| 2 contractionsb | 1 text (with 1 in EMR) | 1 additional in EMR |
| 1 anemiac | 0 text | 1 EMR |
| 1 gestational hypertensionc | 0 text | 1 EMR |
| 1 gestational thrombocytopeniac | 0 text | 1 EMR |
| Days 43–70 | ||
| 3 vaginal bleedingb | 2 text (with 0 in EMR) | 1 additional in EMR |
| 4 contractionsb | 2 text (with 0 in EMR) | 2 additional in EMR |
| 4 anemiac | 0 text | 4 EMR |
| 1 gestational diabetesc | 0 text | 1 EMR |
| 1 placenta previac | 0 text | 1 EMR |
| Days 71–249 | ||
| 5 vaginal bleedingb | 4 text (with 3 in EMR) | 1 additional in EMR |
| 31 contractionsb | 21 text (with 2 in EMR) | 10 additional in EMR |
| 11 gestational diabetesc | 2 text (with 2 in EMR) | 9 additional in EMR |
| 24 anemiac | 1 text (with 1 in EMR) | 23 additional in EMR |
| 3 gestational hypertensionc | 2 text (with 2 in EMR) | 1 additional in EMR |
| 4 oligohydramniosc | 2 text (with 2 in EMR)d | 2 additional in EMR |
| 3 gestational thrombocytopeniac | 0 text | 3 EMR alone |
| 1 mastitisc | 0 text | 1 EMR alone |
Note: Each event is unique within the time period indicated, but may occur for a given person in multiple time periods.
Text, reported by text message; EMR, documented in electronic medical record.
Events reported via a pre-specified text message response.
Events reported via free text.
Oligohydramnios: 1 leaking amniotic fluid, 1 low amniotic fluid.
EMR, electronic medical record.
Of the 141 participants completing the study, 131 reported a live birth and ten a pregnancy loss. Most (n=9) reporting a loss also reported the GA (8–23 weeks). Pregnancy loss was documented in the EMR for eight of the ten participants. One additional loss was noted in the EMR in a woman who last reported via text message on Day 14 post-vaccination and had a loss at Day 28 (15 5/7 weeks GA). The proportion of women with an SAB was similar when using text message reports alone versus text message plus EMR-abstracted data versus EMR-abstracted data alone (Table 3). There were three losses not classified as SABs: one stillbirth at 20 weeks, one elective termination at 22 weeks due to premature rupture of membranes and inevitable neonatal demise, and one pregnancy where it was unclear whether it was an SAB or elective termination prior to 18 weeks gestation. The first two were noted by text message and EMR abstraction; the third was noted by text message only.
Table 3.
Pregnancy Outcomes Using Text Message–Reported Data, EMR-Abstracted Data, and Both Sources
| Pregnancy and neonatal outcomes |
Text message–reported data only |
Text message–reported and EMR- abstracted data |
EMR-abstracted data only |
|---|---|---|---|
| Spontaneous abortion | 5.0 (2.4, 9.9) | 5.0 (2.5, 9.5) | 4.2 (1.9, 8.9) |
| Preterm birth | 6.3 (3.2, 11.9) | 6.6 (3.6, 11.8) | 7.4 (4.1, 13.1) |
| Low birth weight | 8.2 (4.6, 14.1) | 8.9 (5.4, 14.3) | 9.9 (6.0, 15.9) |
| Small-for-gestational age | 4.6 (2.1, 9.7) | 7.2 (4.1, 12.4) | 7.8 (4.4, 13.3) |
| Major birth defects | 1.5 (0.4, 5.3) | 2.5 (1.0, 6.3) | 2.8 (1.1, 7.0) |
Note: Values are % (95% CI). Spontaneous abortion is per pregnancy; preterm birth, birth weight, small for gestational age, and birth defects are per newborn.
EMR, electronic medical record.
There were 151 live-birth deliveries (131 reported via text message and an additional 20 from the EMR for women who stopped reporting or never reported). There were 145 singleton births, five sets of twins, and one set of triplets, for a total of 158 infants. Of the 131 deliveries reported via text message, EMR records were available for 115. Of those who reported birth via text, most (96.9%) reported a usable GA with a range of 30–42 weeks. When comparing GA reported via text message versus EMR-abstracted data, the text message–reported GA was within 7 days for 97.3% of records. Eight mothers reported a preterm birth via text message; all were confirmed by EMR review. Two additional preterm births were found in the EMR in women who stopped reporting via text message, for a total of ten. The proportion with preterm births was similar using the three data source categories (Table 3).
Birth weight reported via text message was within 8% of that recorded in the EMR for all infants and 5% for all but two infants. There were 11 low birth weight infants reported via text message, all of which were confirmed on EMR review. Three additional low birth weight infants were found in the EMR, two in a woman who stopped reporting and one in a woman with multiple births who reported for one infant only, for a total of 14 overall, with similar proportions from the three data source categories (Table 3). Although infant sex was not captured via text message, but would be needed to accurately assess small for gestational age using the Fenton growth chart,21 sex was abstracted for those with an EMR record and defaulted to male for the remaining for the most conservative estimate. There were six small for gestational age infants identified by text message alone, 11 infants each using text message plus EMR-abstracted data and EMR-abstracted data alone (Table 3).
Two major birth defects in two infants (hip dysplasia, situs in versus/hypospadias) were reported via text message, both EMR confirmed.18 Two other major birth defects were identified on EMR abstraction: penile webbing in two infants whose mothers had stopped reporting (one stopped 168 days before delivery and the other 36 days), resulting in similar proportions by data source (Table 3). Four minor defects were not reported by text message (three isolated penile torsions, one partial syndactyly of second and third toes). There were 15 other pregnancies with neonatal problems not considered to be birth defects. Thirteen of these women sent free text messages regarding neonatal health problems, eight of which were also identified in the EMR, including acid reflux, hypoglycemia (n=2), tachypnea, infection, jaundice (n=2), and prematurity (sent as free text in addition to providing GA). One woman identified jaundice by text message post-hospital discharge not documented in the birth hospital EMR. One woman indicated via text message that there was a problem but did not specify. Three women texted there was not a problem but one was identified in the EMR (blood ABO incompatibility in one infant, two infants with multiple issues in the neonatal intensive care unit). Two women did not send final pregnancy outcomes via text but a neonatal problem (hyperbilirubinemia, hypoglycemia) was identified in the EMR. When assessing EMR-confirmed outcomes for the 25 women who stopped reporting/never reported to the 141 who completed the study, pregnancy and neonatal outcomes were similar (Appendix Table 2, available online).
An exit survey was completed in 129 of 131 women reporting delivery. Nearly all (96.9%) were satisfied with the study and 94.6% would take part in a future text message study. Most (76.6%) preferred text message, whereas 4.7% preferred the paper diary. Most women (>95%) were satisfied with each of the different text message frequencies. Nine women reported not liking the ongoing questions that started with asking if “they were still pregnant.” Nearly all women (97.6%) believed that influenza vaccine is safe. Few (5.5%) had perceived side effects from influenza vaccine, including being tired (n=1), cold or “flu-like” symptoms (n=5), and fever (n=1). Finally, 14.2% reported that taking part in the study positively affected how they felt about vaccine safety, with the remaining reporting no or an unknown effect; there were no reports of a negative impact on attitudes.
DISCUSSION
This study demonstrates the feasibility of text messaging surveillance throughout pregnancy after influenza vaccination, including pregnancy and neonatal outcomes, across a bilingual population of different socioeconomic levels. Both medically attended and non-attended health events were identified, and both pregnancy losses and deliveries occurring outside the medical center were captured as well. Neonatal outcomes were identified with good correlation between text message—reported and EMR-recorded gestational age and weight. All major EMR-documented birth defects were identified by text message except penile webbing; however, minor birth defects, primarily penile torsion, were not identified. Similar to previous literature, post-vaccination fever was infrequent,22,23 and pregnancy-specific health events were few.22,24–26 Proportions of preterm births, low birth weight, and SGA births were consistent with those reported in national surveillance literature.27 Text message surveillance, with its ability to rapidly send thousands of messages from a centralized location, could enhance vaccine safety monitoring for pregnant women for clinical and public health use, including during an influenza pandemic or other outbreak, when such large-scale surveillance may be needed to detect potentially less common adverse events. The study also supports the potential of text messaging to collect product safety information in clinical research studies involving pregnant women.
The ability to capture non-medically attended events may be important because pregnant women and their providers may have concerns about vaccine safety and efficacy and the risks of influenza, and non-medically attended events can affect vaccination views.28 It may be helpful for reassurance from a vaccine safety surveillance perspective that all kinds of potential events are being captured. Interestingly, although there may have been a concern that involving women directly in reporting may make them more concerned about vaccine safety, the opposite was true. There was also an a priori concern of the study team about women being comfortable texting about sensitive topics. However, ten of 11 women with a pregnancy loss reported it via text message, as did all women with a child with a major birth defect, who were still reporting via text message at the time of the birth.
There were a number of lessons learned. Though it might be best to create a list of specific outcomes of importance with related text queries, it is valuable to maintain an open-ended question querying other events that pregnant women deem significant. Similarly, phone call follow-up may be needed for more precise information in those with a vaccine safety response of potential concern or for missing information; this could be more challenging in large-scale monitoring. For neonatal out-comes, the system needs to be able to capture multiple births. Additionally, depending on public health or research interest, more information may need to be captured including sex, GA in weeks and days, and neonatal intensive care unit versus well-baby/transitional nursery admission. A different message cascade might need to be developed for those infants admitted to the neonatal intensive care unit, for whom there may be multiple problems that may not be known right after birth or may be resolved by the time the mother replies to the text message in case of delay. In addition, although asking women if “they were still pregnant” was an important trigger question, it was not well received by a few women and likely should not be the opening message in a cascade. Also, as the messages were spaced so that women were not overwhelmed, the 1-month interval in the middle of the study may have been too long, leading to decreased event reporting. Finally, although the women were not asked to text about any urgent medical needs and all message cascades ended with a note to contact their healthcare provider if any medical care was needed, it is important that those instructions also be given upfront to women so that they do not mistakenly think they are texting their doctor’s office.
Limitations
The greatest strength of this study is that it is the first use of text message vaccine adverse event surveillance for pregnant women throughout pregnancy, not only in the immediate post-vaccination period as has been demonstrated.14–16,29 There are also a number of limitations. There could be under-reporting of pregnancy-specific outcomes, and there were 15% of women for whom information was inadequate. In addition, this study took place in one academic medical center system during one influenza season; however, the study population was relatively sociodemographically diverse. Finally, women had to be willing to enroll in a lengthy text message reporting study, but enrollment rates were high.
CONCLUSIONS
This study demonstrated the feasibility of text messaging for influenza vaccine safety surveillance throughout pregnancy. In addition, in these women receiving IIV during pregnancy, post-vaccination fever was infrequent and a typical pattern of maternal and neonatal health outcomes for pregnancies was observed.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Naomi Tepper, MD, MPH, from the Centers for Disease Control and Prevention (CDC) and Geeta K. Swamy, MD (Duke University), for their technical contributions to the study, New York-Presbyterian Hospital for its support of the EzVac Immunization Information System, and the New York-Presbyterian Hospital Ambulatory Care Network and Columbia Faculty Practice Organization, its staff, and patients. We would like to thank Caroline Torres, Michelle M. DiVito, RN, MSN, Claudia Roca, MPH, Luis Alba, Margarita Velasco, Yenny Villela, Luiza Kalemi, Minh-Chau Nguyen, and Cara R. Rabin from Columbia University for their help in this study.
This study was supported through the Clinical Immunization Safety Assessment Project Contract No 200–2012-53665–0003 from CDC. The findings and conclusions are those of the authors and do not necessarily represent the official position of CDC. This study was presented at the Annual Conference on Vaccine Research 2016 (Baltimore, MD). Trial Registration is ClinicalTrials.gov Identifier: NCT01974050.
Footnotes
M.S. Stockwell is a co-investigator but receives no financial support for an unrelated, investigator-initiated grant from the Pfizer Medical Education Group. The other authors have no conflicts of interests or financial disclosures.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2017.03.014.
REFERENCES
- 1.CDC. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep. 2013;62 (RR-07):1–43. [PubMed] [Google Scholar]
- 2.Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skowronski DM, De Serres G. Is routine influenza immunization warranted in early pregnancy? Vaccine. 2009;27(35):4754–4770. https://doi.org/10.1016Aj.vaccine.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14(1):95–100. 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094–1102. 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 6.Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev. 2006;28(1):47–53. 10.1093/epirev/mxj002. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374 (9688):451–458. 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 8.Phadke VK, Omer SB. Maternal vaccination for the prevention of influenza: current status and hopes for the future. Expert Rev Vaccines. 2016;15(10):1255–1280. 10.1080/14760584.2016.1175304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhi SA, Cutland CL, Jose L, et al. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in healthy women and their infants: a randomised phase 1b/2 trial. Lancet Infect Dis. 2016;16(8):923–934. 10.1016/S1473-3099(16)00152-3. [DOI] [PubMed] [Google Scholar]
- 10.Modjarrad K, Giersing B, Kaslow DC, et al. WHO consultation on Respiratory Syncytial Virus Vaccine Development Report from a World Health Organization Meeting held on 23–24 March 2015. Vaccine. 2016;34(2):190–197. https://doi.org/10.1016Aj.vaccine.2015.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nippita S, Oviedo JD, Velasco MG, et al. A randomized controlled trial of daily text messages versus monthly paper diaries to collect bleeding data after intrauterine device insertion. Contraception. 2015;92(6):578–584. 10.1016/j.contraception.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stockwell MS, Broder K, LaRussa P, et al. Risk of fever after pediatric trivalent inactivated influenza vaccine and 13-valent pneumococcal conjugate vaccine. JAMA Pediatr. 2014;168(3):211–219. 10.1001/jamapediatrics.2013.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockwell MS, Broder KR, Lewis P, et al. Assessing fever frequency after pediatric live attenuated versus inactivated influenza vaccination. J Pediatric Infect Dis Soc. In Press. Online June 14, 2016. 10.1093/jpids/piw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regan AK, Blyth CC, Mak DB, Richmond PC, Effler PV. Using SMS to monitor adverse events following trivalent influenza vaccination in pregnant women. Aust N Z J Obstet Gynaecol. 2014;54(6):522–528. 10.1111/ajo.12266. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie IS, MacDonald TM, Shakir S, et al. Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes. Br J Clin Pharmacol. 2012;73(5):801–811. 10.1111/j.1365-2125.2011.04142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan AK, Tracey LE, Blyth CC, Richmond PC, Effler PV. A prospective cohort study assessing the reactogenicity of pertussis and influenza vaccines administered during pregnancy. Vaccine. 2016;34(20): 2299–2304. https://doi.org/10.10167j.vaccine.2016.03.084. [DOI] [PubMed] [Google Scholar]
- 17.CDC. Preterm birth. www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm. Accessed August 14, 2016.
- 18.CDC. Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57(1):1–5. [PubMed] [Google Scholar]
- 19.CDC. Birthweight and gestational age. www.cdc.gov/nchs/fastats/birthweight.htm. Accessed August 14, 2016.
- 20.CDC. QuickStats: percentage of small-for-gestational-age births, by Race and Hispanic ethnicity—United States, 2005. www.cdc.gov/mmwr/preview/mmwrhtml/mm5750a5.htm. Accessed August 14, 2016.
- 21.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am J Obstet Gynecol. 2011;204 (2):146 e141-e147. 10.1016/j.ajog.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008;8(1):44–52. 10.1016/S1473-3099(07)70311-0. [DOI] [PubMed] [Google Scholar]
- 24.Moro PL, Tepper NK, Grohskopf LA, Vellozzi C, Broder K. Safety of seasonal influenza and influenza A (H1N1) 2009 monovalent vaccines in pregnancy. Expert Rev Vaccines. 2012;11(8):911–921. 10.1586/erv.12.72. [DOI] [PubMed] [Google Scholar]
- 25.Nordin JD, Kharbanda EO, Benitez GV, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol. 2013;121(3):519–525. 10.1097/AOG.0b013e3182831b83. [DOI] [PubMed] [Google Scholar]
- 26.Tamma PD, Ault KA, del Rio C, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009;201(6):547–552. 10.1016/j.ajog.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton BE, Martin JA, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2014. Natl Vital Stat Rep. 2015;64(12). www.cdc.gov/nchs/nvss/births.htm. Accessed May 5, 2016. [PubMed] [Google Scholar]
- 28.Kharbanda EO, Vargas CY, Castano PM, et al. Exploring pregnant women’s views on influenza vaccination and educational text messages. Prev Med. 2011;52(1):75–77. 10.1016/j.ypmed.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Regan AK, Blyth CC, Tracey L, et al. Comparison of text-messaging to voice telephone interviews for active surveillance of adverse events following immunisation. Vaccine. 2015;33(31):3689–3694. 10.1016/j.vaccine.2015.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


