To the Editor:
The largely stochastic process of T-cell antigen receptor (TCR) gene assembly inevitably produces self-reactive T cells. Although most self-reactive T cells are deleted in the thymus, rare thymocytes survive strong self-antigen engagement and differentiate into type A precursors of CD8αα+ intestinal intraepithelial lymphocytes (type A IELps) in the thymic cortex1 or CD4+ forkhead box P3–positive regulatory T (Treg) cells in the thymic medulla.2 Because TCR sequencing revealed amino acid motifs in complementarity-determining region 3 (CDR3) that are enriched in type A IELps or Treg cells,3,4 we reasoned that the expression of these self-reactive TCR motifs might serve as a biomarker to evaluate T-cell self-tolerance.
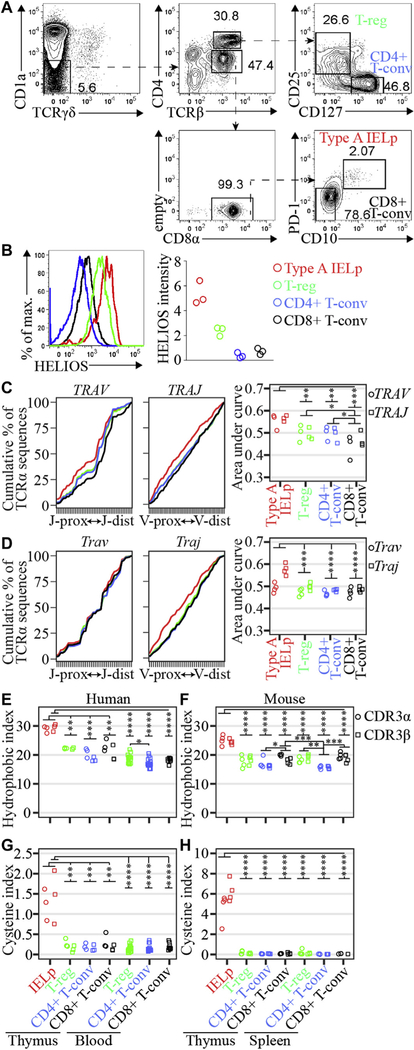
In human thymus samples we used programmed cell death protein 1 and CD10 to sort type A IELps5 and CD8+ conventional T (Tconv) cells, whereas CD127 and CD25 were used to distinguish Treg and CD4+ Tconv cells (Fig 1, A). Human type A IELps and Treg cells expressed more Helios than CD4+ and CD8+ Tconv cells (Fig 1, B), which is indicative of stronger TCR signaling. TCR-sequencing (for polymerase chain reaction primers, see Table E3 in this article’s Online Repository at www.jacionline.org) revealed that, compared with other lineages, type A IELps used more proximal TCRα variable (TRAV/Trav) and joining (TRAJ/Traj) gene segments in human subjects (Fig 1, C) and mice (Fig 1, D),4 indicating that type A IELps tend to receive a TCR signal early after initiating TCRα recombination.5
FIG 1.
Strong and early TCR activation in type A IELps. A, Phenotypes of sorted human thymocyte subsets. B, Electronically gated subsets (see color code at right) analyzed for Helios expression, with a summary of mean Helios labeling intensity normalized to CD4+CD8+ thymocytes in the same sample. C, Cumulative percentage of unique sequences that used each TRAV or TRAJ segment as a function of chromosomal location in the TRAV (left) or TRAJ (right) locus, with a summary of the area under the curve for each sample (right). D, Trav and Traj use in mouse T-cell subsets4 analyzed as in Fig 1, C. E-H, Percentage of unique sequences with a self-reactive CDR3 position 6 and 7 doublet (hydrophobic index; see Fig 1, E and F) or percentage of unique sequences with cysteine within 2 positions of the CDR3 apex (cysteine index; Fig 1, G and H; Fig 1, E and G, for human and Fig 1, F and H, for mouse T-cell subsets). Two-way ANOVA was used with repeated measures based on sample donor and TCR chain (α and β), whereas 1-way ANOVA was used to compare TCRβ repertoires. ns, P > .05. Mouse data in Fig 1, H, have been published4 and are included here to enable comparison with human data.
Compared with CD4+ Tconv cells, Treg cells are enriched in hydrophobic amino acid doublets at positions 6 and 7 of the CDR3 in the TCRβ chain (CDR3β), a biomarker of self-reactivity.3 The frequency of sequences carrying these position 6 and 7 doublets (hydrophobic index) was greater in the TCRα and/or TCRβ repertoires of type A IELps than in thymic and peripheral Treg cells and CD4+ and CD8+ Tconv cells in human subjects (Fig 1, E) and mice (Fig 1, F). These findings broaden the scope of the hydrophobic index to encompass both TCRα and TCRβ repertoires and reveal heightened self-reactivity in the T-cell population that undergoes strong TCR signaling in the thymic cortex.1,5
The frequency of TCRα and/or TCRβ sequences with cysteine within 2 positions of the CDR3 apex (cysteine index) was also greater in type A IELps than in other thymic and peripheral T-cell subsets in human subjects (Fig 1, G) and mice (Fig 1, H).4 This was not due to preferential use of TCR gene segments because none of the cysteine residues detected by using the cysteine index in this study were encoded by germline codons; rather, they were encoded by codons spanning the junctions of TCR gene segments. Unlike the hydrophobic index, the cysteine index was not greater in Treg cells than in CD4+ Tconv cells, suggesting that T cells with cysteine within 2 positions of the CDR3 apex typically acquire tolerance in the thymic cortex at a stage preceding the onset of Treg cell selection.
To investigate the effects of reduced thymopoiesis on T-cell self-reactivity indices, we examined patients with biallelic mutations in recombination-activating gene 1 (RAG1) or RAG2. In patients with RAG mutations, the hydrophobic index was not significantly altered in CDR3α, but it was increased in CDR3β (Fig 2, A, and see Table E2 in this article’s Online Repository at www.jacionline.org). Because the cysteine index was also not significantly altered in CDR3α but was increased in the CDR3β repertoire of Treg cells from patients with RAG mutations, subsequent analyses were confined to CDR3β (Fig 2, B). Significant correlations were detected when each index among unique sequences was plotted as a function of the same index among total sequences (Fig 2, C and D). Samples were on either side of the diagonal for the hydrophobic index (Fig 2, C), whereas those cysteine indices that deviated from the diagonal had a higher index among unique sequences than among total sequences (Fig 2, D), suggesting that clonal expansion is lower than average in cells carrying a cysteine near the CDR3β apex.
FIG 2.
Aberrant self-reactivity indices of T-cell populations selected in the presence of RAG mutations. A and B, Hydrophobic index (Fig 2, A) and cysteine index (Fig 2, B) in CDR3α and CDR3β repertoires of T-cell subsets (x-axis) from healthy control subjects (squares) and patients with RAG mutations (circles). Fifteen samples from patients with RAG mutations were excluded from Fig 2, B, because no sequences were detected with cysteine within 2 positions of the CDR3 apex, and hence the cysteine index was undefined (see Table E2 in this article’s Online Repository at www.jacionline.org). C and D, Hydrophobic index (Fig 2, C) and cysteine index (Fig 2, D) among unique sequences (y-axis) as a function of the same index among total sequences (x-axis). E and F, Hydrophobic index (Fig 2, E) and cysteine index (Fig 2, F) as a function of mean RAG recombination activity. G, Cysteine index (left) and hydrophobic index (right) in unique TCRβ sequences of CD4+ Tconv and Treg cells sorted from B6 control mice or mice with homozygous mutations in Rag2 (R229Q) or Rag1 (R972Q, F971L, or R972W). In Fig 2, A and B, 2-way ANOVA was used to compare healthy control subjects with patients with RAG mutations adjusted with the Sidak multiple comparisons test. **P < .01 and ***P < .001. In Fig 2, C-F, P and ρ values were determined by using the Spearman test for correlation. Horizontal lines in Fig 2, E and F, show the ranges observed in healthy control subjects.
For some mutations, the extent of recombination activity was determined by expressing the mutant RAG protein in Abelson virus–transformed Rag1−/− or Rag2−/− pro-B cells (see Table E1 in this article’s Online Repository at www.jacionline.org).6 RAG function was not correlated with the hydrophobic index (Fig 2, E) but was inversely correlated with the cysteine index (Fig 2, F). Thymic tissue in patients with RAG mutations lacks segregated K5+ and K8+ thymic epithelial cell populations,7 indicating immaturity of thymic stroma. The expression level and diversity of self-peptide/MHC complexes presented by such immature thymic tissue might be insufficient to induce tolerance in a normal proportion of nascent self-reactive αβ TCR+ thymocytes. Aberrant differentiation of these thymocytes would account for the high self-reactivity indices observed in peripheral TCR repertoires of most patients with RAG mutations.
To explore the self-reactivity of T cells differentiated in vitro, we analyzed TCRβ repertoires of CD3−CD4+CD8− immature single-positive and CD3+CD4+CD8+ double-positive cells cultured from induced pluripotent stem cells. The cysteine indices of immature single-positive and double-positive samples were low (see Fig E1 in this article’s Online Repository at www.jacionline.org), suggesting that deletion by the cysteine-dependent mechanism can occur in vitro.
We also sequenced the TCRb repertoires of Treg and CD4+ Tconv cells from mice with hypomorphic mutations in Rag2 (R229Q) or Rag1 (R972Q, F971L, and R972W).8 The 3 missense Rag1 mutations support different capacities to accomplish V(D)J recombination and T- and B-cell development (R972Q > F971L > R972W), and T-cell infiltrates were detected in target organs of the most severe R972W model (see Fig E2 in this article’s Online Repository at www.jacionline.org). The cysteine index was normal in all mouse strains examined (Fig 2, G), suggesting that thymocytes with cysteine in the CDR3 apex are rendered tolerant in the cortex of mice with hypomorphic Rag1 or Rag2 mutations. Unlike in human subjects, thymic architecture is partially intact in mice lacking RAG function completely, including segregated K8+K5− (cortical) and K8−K5+ (medullary) thymic epithelial cell populations.9 By contrast, hydrophobic indices were increased in the F971L and R972W mice, with a greater increase in the R972W strain (Fig 2, G). Together with small thymic medullary regions and reduced expression of autoimmune regulator,8 the simplest interpretation of these data is that defects in medullary tolerance underlie the increased hydrophobic indices in F971L and R972W mice.
Both cysteine and hydrophobic indices are highly reproducible in healthy control subjects, revealing a surprising predictability in normal T-cell selection. Hydrophobic doublets can promote self-reactivity by forming hydrophobic bonds that exclude water molecules from the TCR–peptide/MHC interface, whereas cysteine residues can form stronger disulfide bonds between the CDR3 apex and the peptide. The available data suggest that an increased cysteine index is a specific biomarker of defective cortical tolerance mechanisms. The hydrophobic index appears more sensitive for detection of a self-tolerance defect but is not specific for either cortical or medullary tolerance mechanisms. Thus the cysteine and hydrophobic indices provide complementary information in the diagnosis and classification of T-cell self-tolerance defects.
Supplementary Material
Acknowledgments
Supported by the Monash Biomedicine Discovery Institute, the National Health and Medical Research Council (grant 1107464), and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland.
Footnotes
Disclosure of potential conflict of interest: L. D. Notarangelo is supported by the National Institute of Allergy and Infectious Diseases; has board memberships with the Journal of Clinical Immunology, Clinical Immunology, and Frontiers in Immunology; and has received royalties from UpToDate. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Ruscher R, Kummer RL, Lee YJ, Jameson SC, Hogquist KA. CD8aa intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol 2017;18:771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med 2005;202:901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stadinski BD, Shekhar K, Gomez-Tourino I, Jung J, Sasaki K, Sewell AK, et al. Hydrophobic CDR3 residues promote the development of self-reactive T cells. Nat Immunol 2016;17:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirasinha RC, Singh M, Archer SK, Chan A, Harrison PF, Goodnow CC, et al. ab T-cell receptors with a central CDR3 cysteine are enriched in CD8aa intraepithelial lymphocytes and their thymic precursors. Immunol Cell Biol 2018;96:553–61. [DOI] [PubMed] [Google Scholar]
- 5.Verstichel G, Vermijlen D, Martens L, Goetgeluk G, Brouwer M, Thiault N, et al. The checkpoint for agonist selection precedes conventional selection in human thymus. Sci Immunol 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tirosh I, Yamazaki Y, Frugoni F, Ververs FA, Allenspach EJ, Zhang Y, et al. Recombination activity of human recombination-activating gene 2 (RAG2) mutations and correlation with clinical phenotype. J Allergy Clin Immunol 2019;143:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poliani PL, Facchetti F, Ravanini M, Gennery AR, Villa A, Roifman CM, et al. Early defects in human T-cell development severely affect distribution and maturation of thymic stromal cells: possible implications for the pathophysiology of Omenn syndrome. Blood 2009;114:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villa A, Notarangelo LD. RAG gene defects at the verge of immunodeficiency and immune dysregulation. Immunol Rev 2019;287:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc Natl Acad Sci U S A 1998;95:11822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.