Abstract
Bernstein (1967) hypothesized that preparation of the periphery was crucial for correct responses to motor output. To test this hypothesis in a behaving animal, we examined the roles of two identified motor neurons, B7 and B8, which contribute to feeding behavior in the marine mollusk Aplysia californica. Neuron B7 innervates a hinge muscle and has no overt behavioral effect during smaller-amplitude (type A) swallows, because the hinge muscle is too short to exert force. Neuron B8 activates a muscle (I4) that acts solely to grasp material during type A swallows. During larger-amplitude (type B) swallows, the behavioral actions of both motor neurons change, because the larger-amplitude anterior movement of the grasper sets up the periphery to respond differently to motor outputs. The larger anterior movement stretches the hinge muscle, so that activating neuron B7 mediates the initial retraction phase of swallowing. The changed position of the I4 muscle allows neuron B8 not only to induce grasping but also to pull material into the buccal cavity, contributing to retraction. Thus, larger-amplitude swallows are associated with the expression of two new degrees of freedom (use of the hinge to retract and use of the grasper to retract) that are essential for mediating type B swallows. These results provide a direct demonstration of Bernstein's hypothesis that properly positioning the periphery can be crucial for its ability to correctly respond to motor output and also demonstrate that biomechanical context can alter the functions of identified motor neurons.
Keywords: Aplysia, motor coordination, pattern generator, mechanical context, biomechanics, feeding
Introduction
Bernstein's analysis of the kinematics of human movement demonstrated that there was no simple correspondence between motor neuronal output and movement. Because of external forces (within the periphery and in the environment) and variations in initial conditions, the same neural output could generate different behavioral effects, so that the ultimate effect of neural outputs would be determined by the periphery and environment (Bernstein, 1967). At the same time, his analysis of human locomotion demonstrated that there were regular stable “biodynamic waves” of activation, some of which were attributable to leg mechanics. Thus, complexity did not preclude overall coordination. He hypothesized that an important aspect of coordination was “preparing the periphery” so that it would be maximally able to respond to the correct neural impulse at the right moment.
Previous work has provided indirect support for Bernstein's hypothesis by demonstrating the importance of limb configuration for behavioral output, implying that limb configuration must be properly prepared for appropriate responses to motor output. Lombard and Abbott (1907) emphasized that understanding neural output required understanding of the mechanical properties of a limb and showed in leopard frog and bullfrog that muscular action depended on limb configuration. More recent studies have confirmed and extended these results for the human shoulder (Buneo et al., 1997), the frog hindlimb during swimming (Kargo and Rome, 2002), and in simulations of leg muscles during bicycling and walking (Zajac et al., 2002, 2003). However, because of the difficulty of simultaneously characterizing neural control and peripheral biomechanics in vertebrates and humans, Bernstein's hypothesis that coordination involves preparing the periphery to be receptive to neural activity has not been directly tested in a behaving animal.
An ideal system to test this would be tractable to the analysis of motor neuronal activity, biomechanics, and behavior. This has been the rationale for studying the marine mollusk Aplysia californica. The feeding apparatus of the animal is a grasping device that the animal uses to manipulate and ingest food (Kupfermann, 1974). Activity of individual nerve cells that control the grasper during feeding has been monitored in intact animals (Warman and Chiel, 1995), movements of the feeding musculature have been visualized in intact animals (Neustadter et al., 2002), and semi-intact preparations have been used to explore the effects of motor neuronal activity on grasper biomechanics (Weiss et al., 1986; Cropper et al., 1990; Morton and Chiel 1993b; Evans et al., 1996; Orekhova et al., 2001).
We used this system to address Bernstein's prediction that preparation of the periphery is critical for the correct expression of neural control. In larger-amplitude swallows, prolonged activation of a protractor muscle allows two new degrees of freedom to be expressed that prepare the grasper to be appropriately receptive to motor neuronal output from the B7 and B8 motor neurons, altering the behavioral functions of these identified motor neurons. These results provide a first description of a neuromechanical mechanism for Bernstein's pioneering prediction.
Materials and Methods
Animals and feeding stimuli.
Aplysia californica (300–350 g) were obtained from Marinus Scientific (Garden Grove, CA) and maintained in an aerated aquarium containing artificial seawater (Instant Ocean; Aquarium Systems, Mentor, OH) cooled to 18 ± 1°C. Animals used for these experiments exhibited interbite intervals of 2–8 s. To study swallows in response to a uniform mechanical stimulus whose biomechanics were unchanged by feeding, we induced animals to swallow polyethylene tubes (outer diameter, 1.27 mm; inner diameter, 0.86 mm; PE90; BD Biosciences, Sparks, MD). The tube was marked every 1.67 mm (6 marks/cm) with a Redimark permanent dry marker (Dixon, Maitland, FL) to measure its inward movement (Morton and Chiel, 1993a).
Measurement of tube movements during swallowing.
During smaller-amplitude swallows (which we designated as type A swallows), a tube moved into the buccal cavity. During larger-amplitude swallows (which we designated as type B swallows), a tube moved inward while rotating ventrally within the midsagittal plane intersecting the animal's jaws and then moved further inward while rotating dorsally within the same plane. To accurately analyze the tube movements during some swallows in freely moving animals, a two-axis video system was used. Two digital video camcorders (ZR40 and ZR60; Canon, Tokyo, Japan) were mounted within a Styrofoam platform to be at right angles to one another, to be coplanar, and to have the same magnification. Recordings were synchronized with a light-emitting diode (LED) that flashed at the end of each trial. In other studies using a single camera, we were sometimes able to obtain very clear lateral views of the entire swallow, and these were also used for analysis. Based on these measurements, criteria were developed that allowed us to classify swallows as either type A or type B (see Results).
The line of the jaws was defined as the line joining the dorsal and ventral tips of the cartilage of the jaws, which can clearly be seen throughout a swallow (these external landmarks are indicated with small arrows in Fig. 2B3). The external angle that the tube made with the line of the jaws could be measured unequivocally in every animal. In studies of test objects held at known angles, we found that our reconstruction algorithm for this angle had less than a 5% error.
Figure 2.
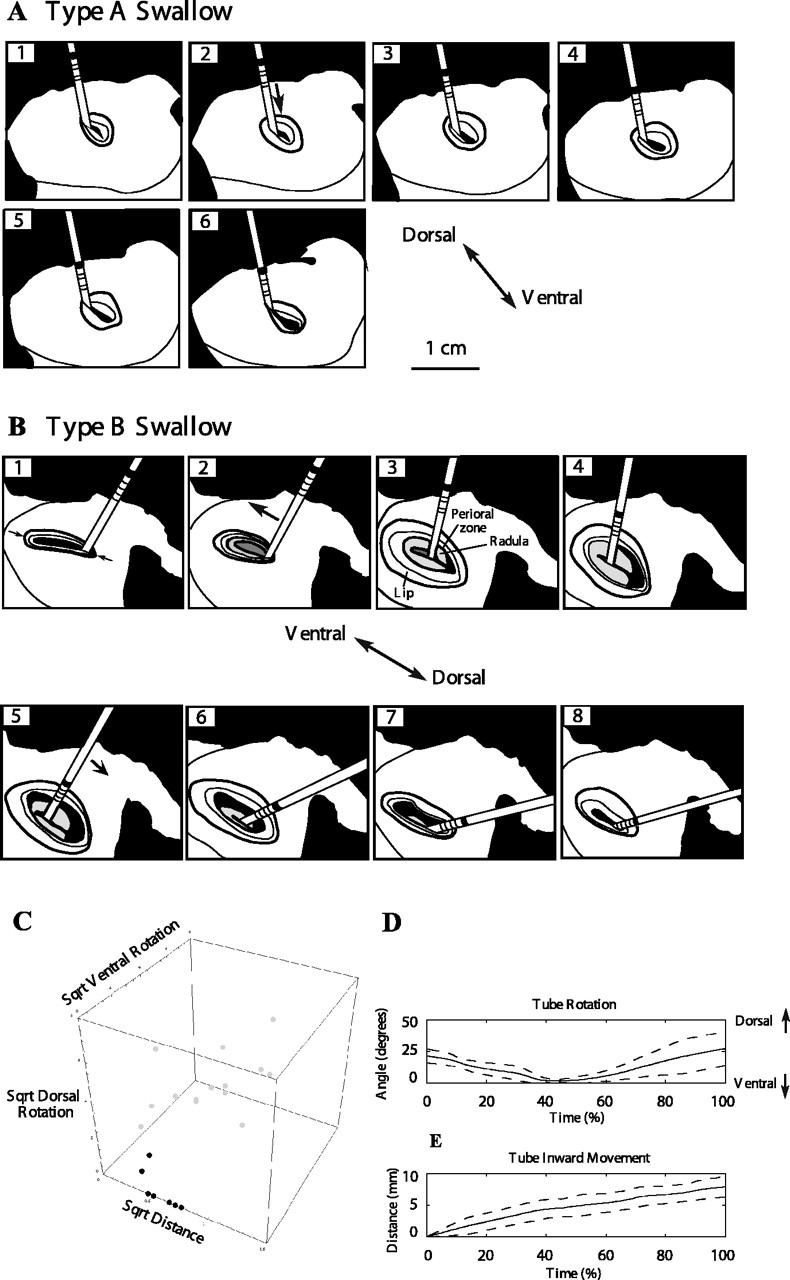
Type A versus type B swallows. Drawings are based on frames from videos of swallowing behavior. A, Type A swallow. The dark band on the tube moves toward the jaws in frames 1–5. Note that the tube translates inwards with no rotation. B, Type B swallow. The tube rotates ventrally (frames 2–4) and then rotates dorsally (frames 5–7) at the same time that the dark band on the tube moves toward the jaws (frames 2–8). During the type B swallow, the surface of the radula is visible (frames 2–7), suggesting that a stronger protraction occurs than during a type A swallow. The tube moves into the cleft between the radular halves during its initial ventral rotation (frames 2–4). The radular surface then rotates dorsally and is withdrawn behind the jaws as the tube rotates dorsally (frames 5–7). Anatomical features are indicated in frame 3 (the radular surface is labeled Radula). C, A plot of the total inward movement, dorsal rotation, and ventral rotation during seven type A (black dots) and 14 type B (gray dots) swallows. A square root transformation was applied to the data to normalize the distribution and increase the similarity of the variances of the two groups (distance was measured in centimeters, and dorsal and ventral rotations were measured in degrees). D, E, Averaged tube rotation (D) and inward movement (E) during type B swallows reconstructed from two-axis video measurements. Solid line represents the average of six swallows from four animals; dotted lines represent 1 SD from the average.
In some large-amplitude swallows, in which the surface of the radula was visible, it was also possible to observe the angle of the tube with respect to the surface of the radula (Fig. 2B, frames 2–4). Precisely quantifying the angle of the tube with the radular surface required the development of an in vitro preparation (see next section).
Animals were placed in a cylindrical glass chamber and induced to swallow a tube, which they did in <1 min. As we have shown in previous studies (Morton and Chiel, 1993a; Hurwitz et al., 1996), animals initially swallow the tube, and then, after a variable number of swallows during which both type A and type B swallows were observed, they reject the tube. Once the animal had rejected the tube, a trial would end. For each animal, each trial was repeated two to three times, and the number of swallows in a given trial ranged from two to eight swallows; intermediate and transitional swallows (i.e., swallows just before rejections) were not analyzed (Morton and Chiel, 1993a). During video recordings of behavior, to minimize bubbles and vibration, the chamber was not aerated. Between video recordings, the chamber was aerated for ∼5 min. Type A and type B swallows were observed with similar frequency in the presence or absence of aeration, and the time without aeration was no more than ∼5 min. Given the slow respiration rate of Aplysia and its ability to show little change in feeding behavior in tide pools for the first 0.5 h of exposure to lower oxygen conditions (Levy et al., 1997), it is unlikely that the brief periods without aeration had any effect on behavior. Digital video movies were analyzed on a Macintosh G4 computer (Apple Computers, Cupertino, CA). To reconstruct the movement of the tube, pairs of synchronized frames were captured and saved in PICT format (800 × 600 pixels) in iMovie 1.02 (Apple Computers). Image files were then opened in Adobe Photoshop 5.5 (Adobe Systems, San Jose, CA) for measurement. Typically, 20–40 pairs of frames were analyzed. The distance between a mark on the tube and the intersection of the tube and the jaws was measured, and the angle of the tube relative to the line of the jaws was calculated from the two orthogonal views. Other swallows were analyzed using a single camera, which was sufficient to determine whether the tube rotated or not, and thus to classify a swallow as type A or type B, to analyze the sequence of type A and type B swallows, and to determine the total inward tube movement during each swallow.
In vitro preparations for studying neural control of tube movements during swallowing.
To study ventral tube rotation, animals were anesthetized, and the radula/odontophore was dissected out of their buccal mass and anchored to the base of a Sylgard-coated dish using fine pins. The buccal ganglion was left attached to the radula/odontophore solely through the radular nerve (RN) (nomenclature of nerves based on Gardner, 1971a,b), and the cerebral ganglion was left attached to the buccal ganglion via the cerebral–buccal connectives. The buccal ganglion was pinned caudal side up and desheathed, and motor neurons of interest were impaled for recording and stimulation. A 4 cm polyethylene tube was placed on the radular surface. A mirror placed at 45° above the preparation made it possible to determine the angle of the tube relative to the radular surface to accurately place the tube.
To study dorsal tube rotations, animals were anesthetized, and the buccal mass was dissected out and anchored to the base of a Sylgard-coated dish using fine pins. The buccal ganglion was left attached to the radula/odontophore through buccal nerves 2 and 3 (BN2 and BN3), and the cerebral ganglion was left attached to the buccal ganglion via the cerebral–buccal connectives. The buccal ganglion was pinned caudal side up and desheathed, and motor neurons of interest were impaled for recording and stimulation. A 4 cm polyethylene tube was placed on the radular surface. Because dorsal rotations occur after the radula has closed on the tube and firmly grips the tube between its halves, in some experiments we kept the tube within the radular cleft and induced the halves of the radula to shut by gluing them with cyanoacrylate glue. Similar results were obtained with or without the use of glue.
Preparations were kept chilled on ice during both desheathing and the experiment, making it unnecessary to perfuse the preparation. A six digit LED timer (Veeder Root C342 Time Totalizer; Danaher Controls, Gurnee, IL) was filmed and activated with the same timing pulse that activated stimulation, synchronizing each video image. Thus, it was possible to calculate the delay from stimulation to onset of tube movement, as well as the magnitude of the tube movement.
Intracellular electrodes were made from single-barreled capillary glass (catalog #6150; A-M Systems, Everett, WA) pulled on a Flaming-Brown pipette puller (model P-80; Sutter Instruments, Novato, CA). Electrodes were backfilled with 3 m potassium acetate, and their resistances were 7–8 MΩ. The bridge was balanced for both stimulation and recording. Neurons were identified by their location in the ganglion, their projections on buccal nerves, and their motor effects (Gardner, 1971a,b; Morton and Chiel, 1993b; Church and Lloyd, 1994). Depolarizing or hyperpolarizing currents were passed into the soma of a cell using either direct currents (DCs) or individual pulses (1 ms duration) at fixed frequencies. Because neurons fired steadily at a nearly fixed frequency in response to DC depolarizing currents (variations of <5% in frequency), we report data that were obtained at a given frequency using either technique. Intracellular electrode signals were amplified using a DC-coupled amplifier (model 1600; A-M Systems) and filtered using a 1 kHz low-pass filter.
To induce feeding-like motor programs, carbachol (10−3 m) was applied to the cerebral ganglion (Susswein et al., 1996). To isolate the cerebral ganglion from the buccal ganglion during carbachol application, a small cylindrical well whose bottom was sealed with a Vaseline/mineral oil mixture was placed over the cerebral ganglion. Feeding-like motor programs were initiated within 5 min of carbachol application and lasted 20–30 min. To obtain extracellular nerve recordings, polyethylene tubing (1.27 mm outer diameter; PE90; Becton Dickinson) was heated and pulled to form extracellular electrodes. Nerves were suctioned into the tapered end of the electrode, whose other end was connected to an alternating current (AC)-coupled differential amplifier.
In vivo extracellular recordings.
In vivo extracellular electrodes were made as described previously (Morton and Chiel, 1993a). Briefly, hook electrodes were made from insulated stainless steel wire (25.4 μm diameter; California Fine Wire, Grover City, CA), whose ends were deinsulated. The end of a second electrode, wound around the first, was deinsulated and used as a reference electrode within the animal's hemocoel.
Animals used for in vivo recording were anesthetized by an injection of isotonic MgCl2 (∼25% of body weight) and kept on ice. A 1.5 cm incision was made from between the rhinophores toward the anterior tentacles. A cuff electrode was applied by slipping the hook of the electrode under the tissue (nerve or muscle), raising the electrode and nerve or muscle above the solution, applying a drop of cyanoacrylate glue (Duro, Quick Gel, product SGG-1; Loctite, Cleveland, OH), and using cold artificial seawater to cure the glue. A wire loop was used to create slack for the buccal mass to move as the animal fed. BN2 was identified by its characteristic trifurcation at the lateral groove and BN3 by its more distal location relative to BN2. To record from RN, a small incision was made in the left side of the intrinsic muscle 2 (I2), exposing the large branches of the RN. Recordings from the I2 muscle were made in the far lateral I2 muscle band, located between BN2 and BN3. Once the electrodes were in place, the skin incision was sutured (needle size, C1; silk size, 5-0; Ethicon, New Brunswick, NJ). Animals fed 24 h after the surgery, and their behavior did not appear to be altered by implantation of hook electrodes. To minimize disruption to feeding responses, we generally implanted electrodes on one nerve at a time or on the I2 muscle alone. We recorded individually from BN2 in six animals, from BN3 in eight animals, from I2 in seven animals, and from the RN in five animals. We were able to obtain simultaneous recordings from both BN2 and BN3 in two animals, RN and BN2 recordings in three animals, and I2 and BN2 recordings in two animals. Each animal performed at least five swallows. Electrode signals were amplified using an AC-coupled differential amplifier (model 1700; A-M Systems) and filtered with cutoffs of 1 kHz (low-pass filter) and 100 Hz (high-pass filter).
Analysis of electrophysiological recordings.
The in vivo electromyographic (EMG) recordings from the I2 muscle and the electroneurographic recordings from BN2, BN3, or RN were analyzed using a window discriminator algorithm that detected units of different sizes (Morton and Chiel, 1993a). Three different windows were used to detect the activity of the three largest units observed in the BN2 and BN3 recordings. A single window was used to detect units from the RN and I2 recordings. Thresholds for each window discriminator were selected individually for each animal using several representative swallow responses. The instantaneous frequency of the spikes (reciprocal of the interspike interval) from each window discriminator was computed. Instantaneous frequencies generated by this method usually fell within the range of 0–60 Hz, so an upper threshold of 60 Hz was used. The average instantaneous frequency profile was computed using a 300 ms data window that was moved through the data in 20 ms increments.
All values are reported as mean ± SD. Statistical significance for single comparisons was computed using Student's t test. Discriminant analysis was performed using SPSS (SPSS, Chicago, IL). Statistical significance for multiple dependent variables was computed using multivariate ANOVA (MANOVA) (NCSS Statistical Software, Kaysville, UT). Multiple comparisons were evaluated using the recently described false discovery rate procedure, which has been shown to be even more effective than the Bonferroni's criterion for avoiding both false positives and false negatives (Curran-Everett, 2000).
Results
Larger-amplitude swallows have different kinematics than smaller-amplitude swallows
Animals engage in swallowing once they have grasped food. The goal of an effective swallow is to reposition the grasper so that the animal can pull more food into the buccal cavity. The grasper first opens and moves toward the outside world (protracts), so that it is positioned further forward on food (Fig. 1A). The grasper then closes on food and strongly pulls the food inwards (retracts), toward the esophagus and digestive system. Finally, the grasper releases the food into the esophagus.
Figure 1.
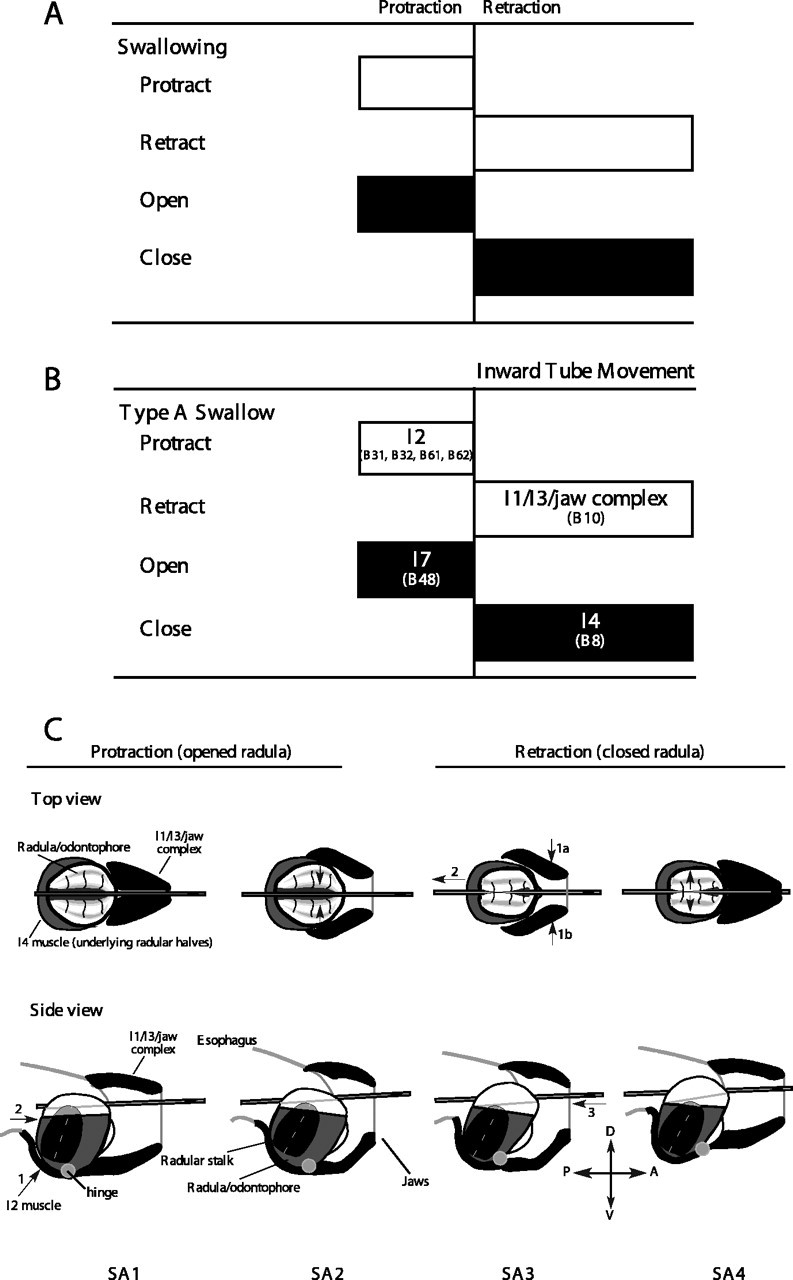
Schematic view of the process of swallowing. A, Arrangement of the four elementary functions (protraction, retraction, opening, and closing) during swallowing. The grasper protracts open on food in the buccal cavity so as to reposition itself further anteriorly. At the peak of protraction, the grasper closes and retracts, pulling food into the buccal cavity. B, Neural and muscular implementation of swallowing. Specific muscle groups implement each of the four functions. C, Schematic diagram of the process of swallowing. For details, see Results.
Studies of the neural and biomechanical mechanisms of swallowing have focused on smaller-amplitude swallows, which we will refer to as type A swallows throughout this paper. To ensure a uniform biomechanical input that is not changed by the swallowing process itself, the swallowing of polyethylene tubes has been studied (Kupfermann, 1974).
Previous studies of the functional anatomy and neural control of the feeding apparatus (referred to as the buccal mass) have clarified the control of the grasper and assigned the four functions of protraction, retraction, opening, and closing of the grasper to specific muscles, many of whose motor neurons have been identified (Fig. 1B). The grasper itself consists of a cartilaginous surface covered by fine teeth (referred to as the radula), which is controlled by underlying muscles (referred to as the odontophore) that open and close the grasper. The grasper rotates about a hinge muscle, which consists of the interdigitation of the muscle fibers of several major muscles of the grasper itself and the muscles surrounding the grasper (Sutton et al., 2004a). Protraction is mediated by the I2 muscle, whose motor neurons are B31, B32, B61, and B62 (Hurwitz et al., 1996). Retraction is mediated by the I1/I3/jaw complex, whose motor neurons include B10, B3, B6, and B9 (Morton and Chiel, 1993b; Church and Lloyd, 1994). Opening is mediated by the I7 muscles, which are controlled by motor neuron B48 [I7 is not shown in Fig. 1C because it is internal to the odontophore (see Evans et al., 1996)]. Closing is mediated primarily by the I4 muscles, which are controlled by the B8 motor neurons (Morton and Chiel, 1993b; Church and Lloyd, 1994). The I5 (or accessory radular closer muscle) may also assist closing and is controlled by the B15 and B16 motor neurons (Cohen et al., 1978; Cropper et al., 1990; Orekhova et al., 2001). In preliminary studies, we found that bilateral lesions of the I5 muscles did not affect tube swallows, and thus we did not study their role in swallowing.
Previous studies suggested that the following sequence of events mediates smaller-amplitude (type A) swallows (Fig. 1C, each step is labeled S for swallow, A for type A, and with a number for that specific step; thus, the first step in the sequence is SA1). (1) To position the grasper so that a tube can be pulled further into the buccal cavity, motor neurons for the I2 muscle protract the open radula/odontophore (i.e., the grasper) (Fig. 1C, SA1; direction of contraction of I2 shown by arrow 1 in side view, and direction of movement of radula/odontophore shown by arrow 2). (2) To grasp the tube, motor neurons B8 activate the I4 muscles, firmly closing the halves of the radula/odontophore around the tube (Fig. 1C, SA2; note in the top view that the grasper is much further forward relative to the mark on the tube after protraction; the direction of closure of the radular halves is shown by the arrows in the top view). (3) To pull the tube into the buccal cavity, motor neurons (e.g., B10) for the I1/I3/jaw complex are activated, inducing the radula/odontophore and the firmly held tube to move posteriorly into the buccal cavity (Fig. 1C, SA3; the direction of I1/I3 contraction is shown by arrows 1a and 1b, the direction of movement of the radula/odontophore is shown by arrow 2, and the inward movement of the tube is shown by arrow 3). (4) To release material into the esophagus, the B48 motor neuron activates I7, inducing the halves of the radula/odontophore to open (Fig. 1C, SA4; arrows in top view indicate direction of opening of radular halves; after radular halves open, the anatomy will be identical to SA1, but the mark on the tube will have moved into the buccal cavity).
We initially assumed that a larger-amplitude swallow was generated by positioning the grasper further forward on the tube (i.e., by activating the motor neurons for the protractor muscle I2 for a longer duration), followed by a phase in which the tube was pulled more strongly into the buccal cavity (i.e., by activating the motor neurons for the retractor muscles I1/I3 for a longer duration). Thus, we predicted that larger-amplitude swallows would be associated only with graded increases in the inward amplitude of tube movement.
Although larger protractions were associated with larger-amplitude swallows, we were surprised to find that swallows of larger amplitude were also associated with an initial ventral rotation of the tube, followed by a dorsal rotation of the tube [Fig. 2, compare A, which illustrates a smaller-amplitude swallow, with B, which illustrates a larger-amplitude swallow; 2C compares the inward distance, ventral angle, and dorsal angle of seven type A swallows (black dots) and 14 type B swallows (gray dots)]. The rotations occurred within the midsagittal plane through the jaws of the animal (Fig. 2, D and E show averaged time courses of rotation and inward movement for six larger-amplitude swallows from four animals). We refer to the larger-amplitude swallows associated with tube rotation as type B swallows.
In four animals, it was possible to obtain excellent lateral views during swallowing, and, in four other animals, it was possible to analyze both type A and type B swallows using two simultaneous orthogonal views. These data were analyzed using discriminant analysis to determine whether it was possible to distinguish the two types of swallows. We found that 100% of the swallows could be distinguished by the ventral and dorsal rotations (this is suggested by the nonoverlapping regions occupied by the black and gray dots in Fig. 2C; cross-validation by leaving out one or several observations at random confirmed that the two responses could be discriminated). Rotations >7° either ventrally or dorsally were never observed in type A swallows, and this was used as a criterion to classify all swallows.
Of the 67 swallows analyzed from 10 animals, 42% (28 of 67) were type A and 58% (39 of 67) were type B. Type B swallows were associated with a significantly larger inward movement of the tube than type A swallows (4.6 ± 1.4 mm during type A swallows vs 7.4 ± 1.4 mm during type B swallows; p < 0.001; n = 67). During some larger-amplitude type B swallows, the surface of the radula could be glimpsed at the peak of protraction (15%, 6 of 39; this is illustrated in Fig. 2B), which was never observed during the smaller-amplitude type A swallows.
The amplitude of a swallow did not appear to affect the amplitude of a succeeding swallow. We used 17 swallowing sequences from 10 animals to construct a 2 × 2 contingency table (i.e., type A followed by type A, type A followed by type B, type B followed by type A, or type B followed by type B swallows). If an animal generated a type A swallow (19 cases), it followed this with a type A swallow 12 times and a type B swallow seven times. If an animal generated a type B swallow (31 cases), it followed this with a type A swallow 11 times and a type B swallow 20 times. The χ2 statistic was not significant (3.63; p > 0.05), suggesting that each swallow could be analyzed as an independent response. These results are consistent with recent data suggesting that, during feeding in Aplysia, there is significant variability from response to response (Horn et al., 2004).
Larger-amplitude (type B) swallows are mediated by different neuromechanical mechanisms than smaller-amplitude (type A) swallows
The ventral and then dorsal rotations of the tube suggested that larger-amplitude type B swallows are generated using neural and biomechanical mechanisms that are distinct from those used to generate smaller-amplitude type A swallows (Fig. 3). (1) To position the grasper so that it is further forward than in a type A swallow, motor neurons for the I2 protractor muscle are activated for a longer duration than in type A swallows. The radula/odontophore protracts further forward and also rotates about the hinge muscle, causing the surface of the radula/odontophore to rotate ventrally relative to the tube, which has not yet been grasped (Fig. 3B, SB1; arrow 1 shows the direction of contraction of the I2 muscle, and arrow 2 shows the direction of movement of the entire radula/odontophore). (2) To grasp the tube and initiate its inward movement, the B8 motor neurons activate the I4 muscle. As the halves of the radula/odontophore close (Fig. 3, SB2; direction of closure of radular halves indicated by arrows in top view), they initially contact the tube at a posterior location, because the surface of the radula/odontophore is rotated ventrally (Fig. 3, SB2; arrow indicates initial contact point in bottom view; the initial position of the ends of the tube are labeled a1 and a2). Once the radular halves grasp the tube, it is both pulled inwards and rotated ventrally until it is firmly held. Thus, B8 now mediates both inward movement and ventral rotation of the tube (the direction of rotation of the tube is shown by arrows in SB3a; the final positions of the ends of the tube are labeled b1 and b2 after rotation). (3) To pull the tube further inward, the B7 motor neuron activates the hinge muscle. Because the hinge muscle is stretched by the stronger protraction (Fig. 3B; compare SB1, SB2, side views), the hinge muscle can act to initially retract the odontophore, inducing the tube to move inward and rotate dorsally (for the sake of clarity, the view of SB3a is repeated in the bottom half of images in Fig. 3B; arrow 1 shows the direction of the hinge muscle contraction, arrow 2 shows the movement of the odontophore, and arrow 3 shows the inward movement and dorsal rotation of the tube). The hinge muscle also moves the anterior of the odontophore within the lumen of the I1/I3/jaw complex so that the motor neurons (e.g., B10) of the I1/I3/jaw complex can move the entire radula/odontophore posteriorly, causing the tube to move still further inward and to rotate further dorsally (Fig. 3, SB3b; arrows 1a and 1b show the direction of contraction of the I1/I3 muscle, arrow 2 shows the direction of movement of the radula/odontophore, and arrow 3 shows direction of the tube movement). (4) To release material into the esophagus, the B48 motor neuron activates I7, inducing the halves of the radula/odontophore to open (SB4; arrows in top view indicate direction of opening of the radular halves; after the radular halves relax, the anatomy looks like SB1, but the tube is pulled much further into the buccal cavity).
Figure 3.
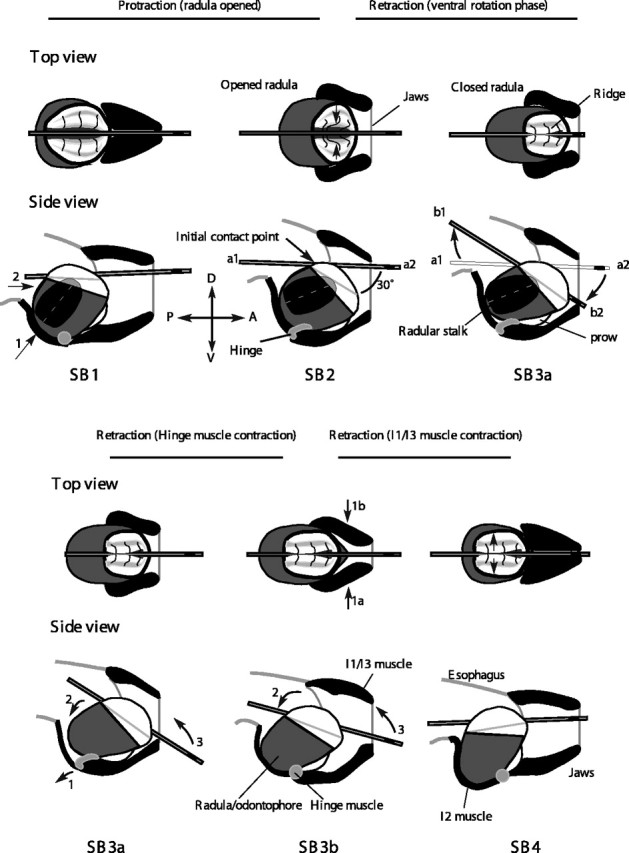
Schematic mechanisms of type B swallows. The retraction function is now shared by I4, the hinge muscle, and their motor neurons. Motor neurons B8 and B7 have new functions as a consequence of their new mechanical contexts. Schematic top and side views of movements of muscles of the buccal mass during a type B swallow. For additional details, see Results. A, Anterior; D, dorsal; P, posterior; V, ventral.
Thus, the initial larger duration protraction of the type B swallow (SB1) prepares the periphery so that the B8 motor neuron can induce the radula to initiate tube retraction (SB2), and the B7 motor neuron can initiate retraction of the radula/odontophore (SB3), behavioral functions that neither neuron has during type A swallows.
In the remainder of Results, we document each of these mechanisms as follows: (1) larger protractions of I2 induce a ventral rotation of the radular surface (Fig. 4); (2) when the radular surface is rotated ∼30° relative to the tube, activating B8 can induce the tube to move inward and rotate ventrally, and a single B8 can exert this effect during a feeding-like motor program (Fig. 5); (3) motor neuron B7, which is activated before and during retraction in feeding-like motor programs (Fig. 6), can induce inward movement and dorsal rotation of a tube once the hinge is stretched, and a single B7 can exert this effect during a feeding-like motor program (Fig. 7); and (4) motor neuron B10 can induce retraction alone when the grasper is in a type A position, and a single B10 can exert this effect during a feeding-like motor program (Fig. 8). B10 works in conjunction with B7 to induce retraction when the grasper is in a type B position (Fig. 9). Finally, we show (5) that the timing and duration of neural and muscular activity observed in intact, behaving animals can be predicted based on these in vitro results (Figs. 10–12).
Figure 4.
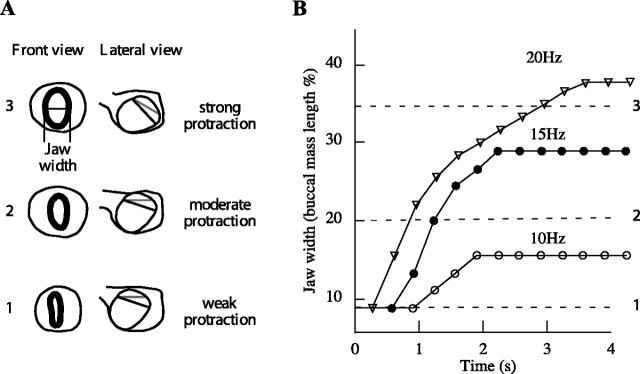
Long-duration stimulation of the I2 protractor muscle can induce protractions and ventral rotations of the surface of the radula similar in magnitude to those seen at the peak of a type B swallow. A, Increase in jaw width and position of surface of radula/odontophore as radula and odontophore are moved into increasingly protracted positions. The schematic diagrams are based on frames of video taken of isolated buccal masses. B, Activation frequency of I2 that is necessary to generate different amounts of radula/odontophore protraction monitored by measuring jaw width. The y-axis is width of the jaw divided by the length of the buccal mass to normalize measurements across different buccal masses. The I2 muscle must be stimulated at a frequency of 20 Hz for 2.8 s to induce a protraction similar to that observed at the peak of a type B swallow (top dashed line).
Figure 5.
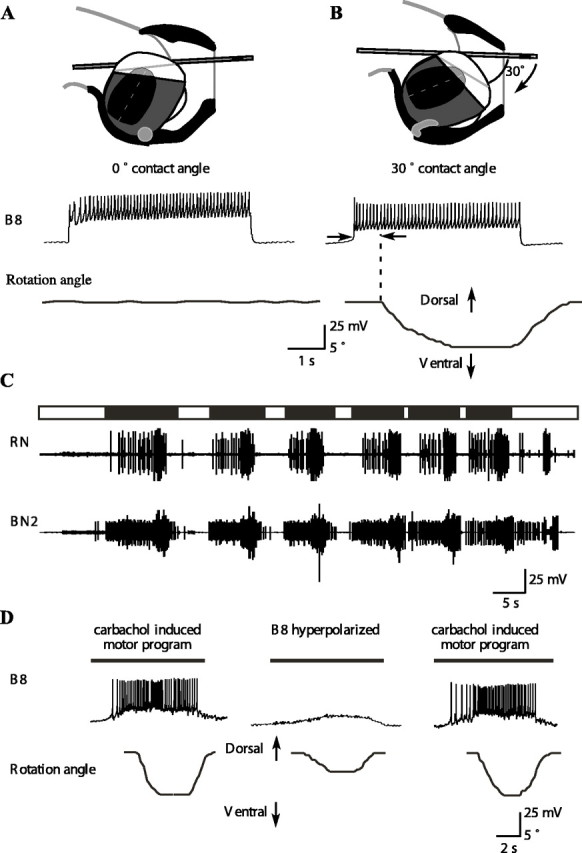
A B8 motor neuron can induce inward movements and ventral rotations of a tube if the tube is at a 30° angle to the surface of the radular cleft in isolation and during feeding-like motor programs. A, The tube was placed on the surface of the radular cleft at an angle of 0° (top schematic diagram). Stimulation of B8 had no effect on rotation or translation of the tube. B, The tube was placed on the radula at an angle of 30° relative to the radular cleft. Stimulation of B8 induced inward movement and ventral rotation of the tube (after a delay indicated by the arrows). Once the neuron was no longer stimulated, the muscle relaxed, and the tube returned to its original position. Data in A and B are from the same preparation. C, Ingestive-like motor program induced by carbachol. The retraction phase, corresponding to the activity on BN2, is indicated schematically above the traces using a black bar. The protraction phase, during which BN2 is inactive, is indicated using a white bar. Note that, during these patterns, large-unit RN activity (corresponding to B8 activity) occurs during retraction, i.e., closure and retraction occur simultaneously, an indicator of an ingestion-like pattern (Morton and Chiel, 1993a,b). D, Effects of hyperpolarizing a single B8 motor neuron during ingestion-like motor programs. Data are from the same preparation. Before and after hyperpolarization (left and right panels), B8 strongly depolarized at the same time that the tube moved inward and rotated ventrally. When B8 was hyperpolarized, the inward movement and ventral rotation were reduced. Dark bars above each trace are the estimated times of the retraction phase. Total inward movement is significantly reduced during hyperpolarization of a B8 motor neuron (n = 14 from 3 different animals; p < 0.001; the inward movement was reduced from 3.5 ± 0.7 to 2.4 ± 1.0 mm). Total angular rotation was also significantly reduced during hyperpolarization of a B8 motor neuron (n = 14 from 3 different animals; p < 0.001).
Figure 6.

Timing of activity of B7 and evidence that the activity of B7 corresponds to the third largest unit on BN3. A, B7 is activated before and during the retraction phase of a feeding-like motor program induced by carbachol. Extracellular recordings from RN, BN2, and BN3 were made simultaneously with an intracellular recording from B7. A schematic bar above the traces indicates the protraction phase (open bars) and the retraction phase (filled bars). Note that large-unit extracellular activity in RN and BN2 occurs at essentially the same time, which is characteristic of an ingestion pattern (Morton and Chiel, 1993a,b). Neuron B7 becomes active just before the onset of the retraction phase and remains active throughout the retraction phase (as judged by activity in BN2). Action potentials in B7 correspond to the third largest extracellular unit on BN3 (line labeled 3 pointing to the BN3 trace; second largest and largest units are indicated with lines labeled 2 and 1, respectively). Between the first and second ingestive-like patterns, B7 was injected with strong hyperpolarizing current (marked with a bar labeled B). This blocked action potentials in B7 and abolished the third largest extracellular units in BN3. The hyperpolarizing artifact has been suppressed so that the voltage trace from B7 is visible; note that B7 is not generating action potentials. B, Expanded timescale excerpt of the data marked with a bar and the “B” in A. Note the one-to-one correspondence between action potentials in B7 and extracellular units in BN3. During hyperpolarization of B7, the third largest extracellular units on BN3 are completely abolished and only return when B7 is released from hyperpolarization. The hyperpolarizing artifact has again been suppressed so that the voltage trace from B7 can be shown.
Figure 7.
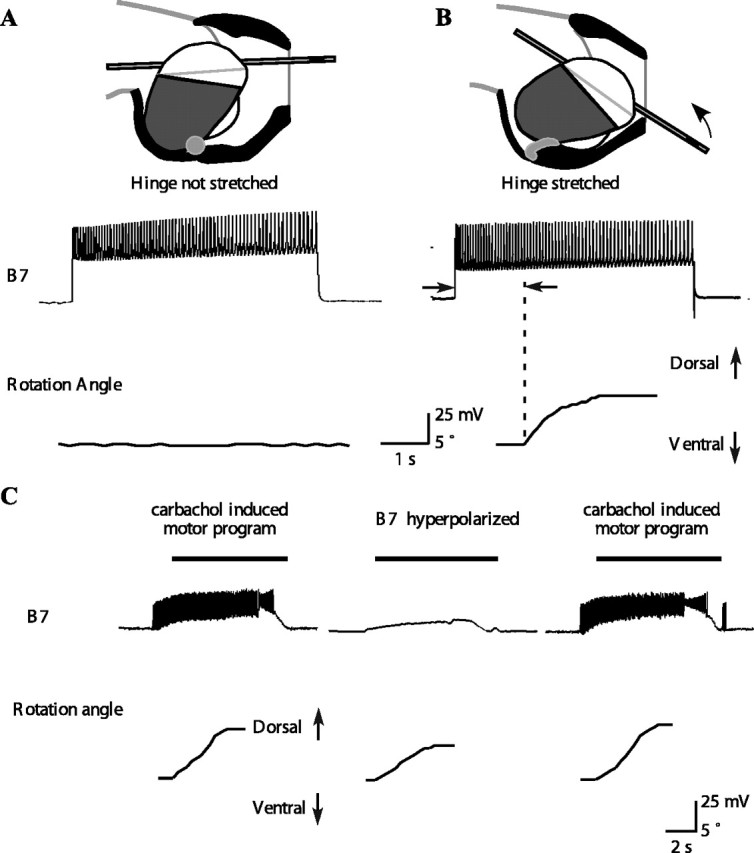
A B7 motor neuron can induce inward tube movement and a dorsal tube rotation if the hinge muscle is stretched either in isolation or during feeding motor programs. A, Top shows a schematic diagram of the peak protraction of a type A swallow. Based on previous in vitro studies (Sutton et al., 2004a), hinge stretch was estimated from the position of the tip of the odontophore. In the experiment shown in the bottom, the hinge was stretched to 0.19 buccal mass lengths. Activating B7 caused no rotation of the tube. In general, B7 had no effect when the hinge was stretched to a length near the minimum hinge stretch estimated from in vivo magnetic resonance images of swallows (Sutton et al., 2004a). The experiment was repeated four times at an average stretch of 0.21 ± 0.03 buccal mass lengths, and activating B7 had no effect in any of these experiments. B, Top shows a schematic diagram of the peak protraction of a type B swallow. The arrow shows the direction of tube inward movement and dorsal rotation. In the experiment shown in the bottom, the hinge muscle was stretched to 0.34 buccal mass lengths. Activating B7 caused a dorsal rotation of the hinge. The minimum stretch of the hinge at which B7 could begin to exert force was 0.35 ± 0.02 buccal mass lengths (n = 4). These values were similar to the maximum hinge stretch during swallowing estimated from in vivo magnetic resonance images (Sutton et al., 2004a). C, During carbachol-induced feeding motor programs, B7 strongly depolarizes and fires action potentials at the same time that the tube moves inward and rotates dorsally. Hyperpolarizing B7 (middle) significantly reduces the inward movement and dorsal rotation. Black bars above the B7 traces indicate the timing of the retraction phase. The magnitudes of the inward movement and rotation are restored when B7 is no longer hyperpolarized (n = 19 from 3 different animals; overall MANOVA highly significant, p < 0.05 for each individual comparison; the inward movement was reduced from 3.9 ± 0.9 to 2.1 ± 0.7 mm).
Figure 8.
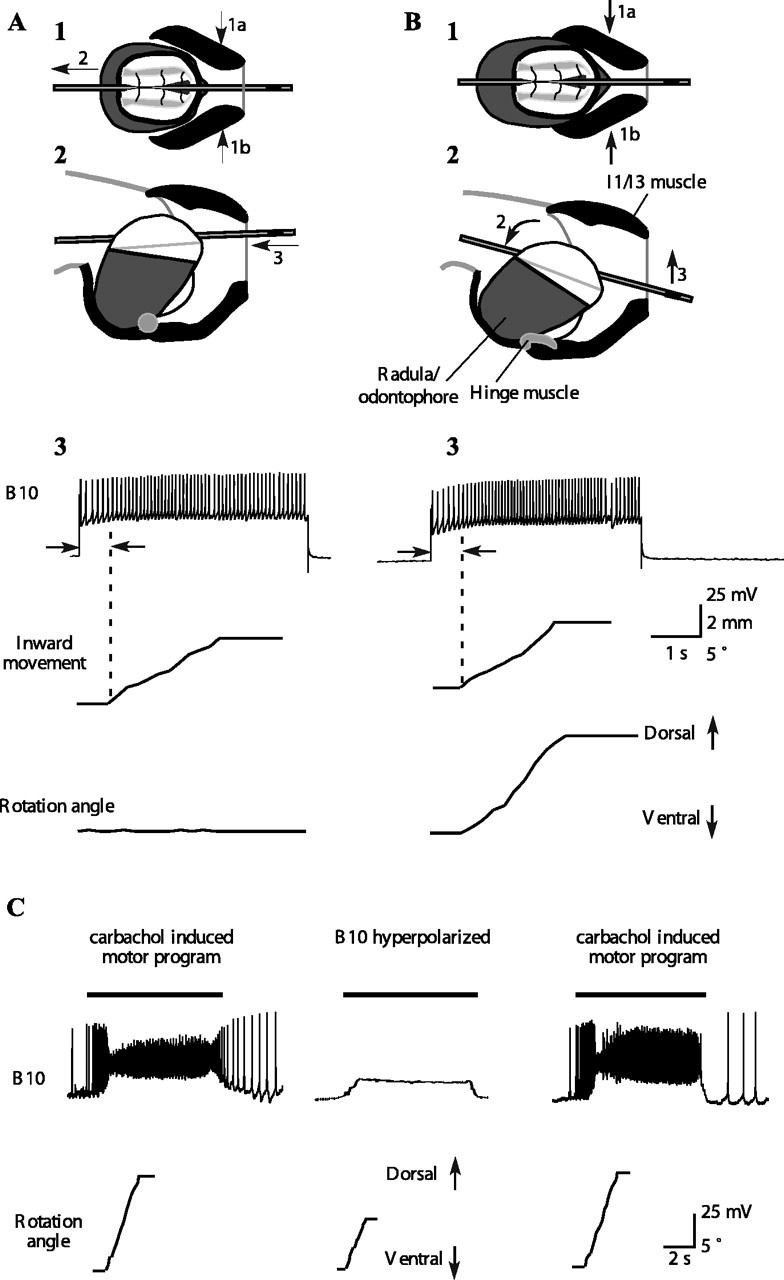
Activity in motor neuron B10 contributes to inward movement and dorsal rotation of a tube during ingestive-like feeding programs. A1, A2, Schematic top and side views of grasper and I1/I3/jaw complex in response to activating motor neuron B10 when the radula/odontophore is protracted to the peak position of a type A swallow. Direction of contraction of the I1/I3 muscle is shown by arrows 1a and 1b, and the resulting direction of the movement of the grasper is shown by arrow 2; in the side view (2), arrow 3 indicates the movement of the tube. A3, Activating B10 in a quiescent preparation protracted to the peak of a type A swallow induces a strong inward movement of a tube but no rotation. B1, B2, Schematic top and side views of grasper and I1/I3/jaw complex in response to activating motor neuron B10 when the radula/odontophore is protracted to the peak position of a type B swallow. Direction of contraction of the I1/I3 muscle is shown by arrows 1a and 1b, and the resulting direction of movement of the grasper is shown by arrow 2 in the side view; movement of the tube is indicated by arrow 3. B3, Activating B10 in a quiescent preparation protracted to the peak of a type B swallow induces a strong inward movement of the tube and a dorsal rotation. C, During carbachol-induced feeding motor program, B10 strongly depolarizes at the same time that the tube moves inward and rotates dorsally. Black bars above the B10 traces indicate the timing of the retraction phase. Hyperpolarizing B10 (middle) significantly reduces the inward movement and dorsal rotation. The magnitude of the inward movement and rotation is restored when B10 is no longer hyperpolarized (p < 0.05; n = 20 from 2 different animals; the inward movement of the tube was reduced from 4.4 ± 0.5 to 3.2 ± 0.7 mm).
Figure 9.
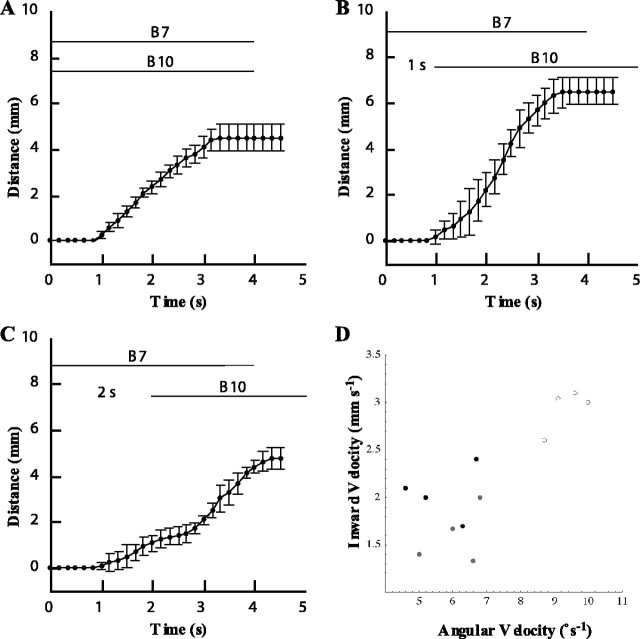
Timing of activation of motor neurons B7 and B10 affects magnitude and speed of inward movement and dorsal tube rotation. A, The B7 and B10 neurons were both activated simultaneously, and the magnitude of the inward movement and dorsal rotation was measured. B, The B7 neuron was activated 1 s before the B10 neuron. C, The B7 neuron was activated 2 s before the B10 neuron. The largest, fastest, and smoothest inward movement and dorsal rotation occurred when B7 was activated 1 s before B10 (n = 4). At a separation of 0 s between the onset of B7 and B10, the net inward movement was 4.8 ± 0.5 mm. At a separation of 1 s, the net inward movement was 6.5 ± 0.6 mm. At a separation of 2 s, the net inward movement was 4.5 ± 0.6 mm. Overall ANOVA was significant (p < .002), and the net inward movement with 1 s separation was significantly greater than either 0 s separation (p < 0.004) or 2 s separation (p < 0.003). The net inward movements at 0 s separation and 2 s separation were not significantly different from one another. D, A scatter plot of average angular velocity (in degrees per second) versus average inward velocity (in millimeters per second) for delay of 0 s from B7 to B10 activation (black dots), 1 s (open circles), and 2 s (gray dots). Note that the data from the 1 s delay are significantly separated from the other two datasets. Overall MANOVA is significant (p < 0.001), and post hoc differences between 1 s and either 0 or 2 s are significant (p < 0.001), but differences between 0 and 2 s are not.
Figure 10.
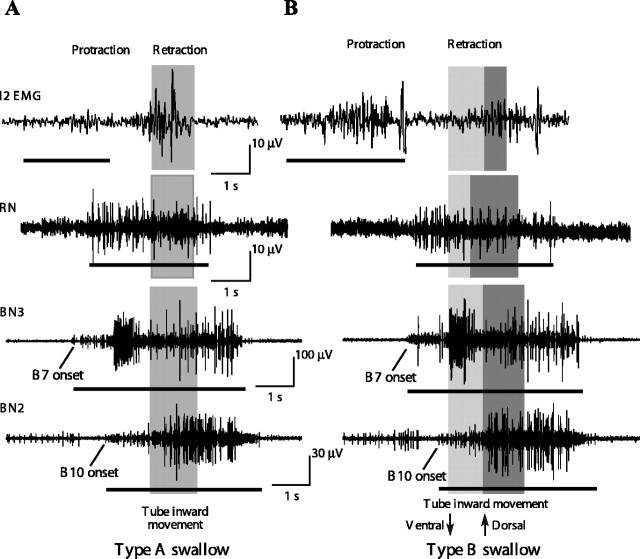
In vivo neural and muscular recordings from intact, behaving animals during type A and type B swallows. Bars underneath traces in both parts of the figure indicate large-unit activity identified based on a window discriminator algorithm (see Materials and Methods). A, Activity on muscles and nerves during a type A swallow. Traces are from three different animals and are aligned on the onset of the inward translation of a tube, which is indicated using the shaded gray rectangle. Note that the large burst on the I2 EMG that occurs during the inward translation of the tube (i.e., during retraction rather than protraction) is likely to reflect activity on the underlying I4 muscle because it is larger in amplitude, lower in frequency, facilitates, and is not abolished by lesions of the I2 nerve; this late activity is therefore not analyzed [Hurwitz et al. (1996), their Figs. 11B, 14]. B, Activity on muscles and nerves during a type B swallow. Traces from three different animals are aligned on the border between the ventral and dorsal rotation of the tube, which is indicated using a light or dark gray rectangle, respectively.
Figure 11.
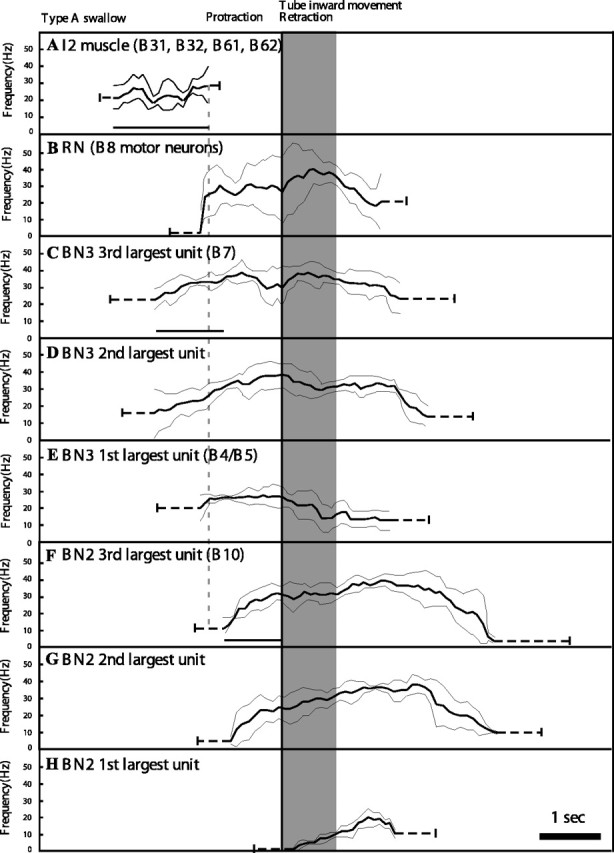
Statistical summary of nerve and muscle activity during type A swallows. Neural and muscular activity from recordings of single nerves or the I2 muscle in freely behaving animals (n = 5 for each trace) were synchronized with the inward translation of the tube, and their durations were normalized by the duration of the inward movement to create a composite summary. Three different frequency profiles were obtained from BN2 and BN3, respectively, corresponding to motor units of different sizes (see Materials and Methods). One frequency profile was generated for RN activity. Neural recordings from multiple type A swallowing responses were averaged (solid curves), and their SDs were calculated and plotted (thin curves). Before and after the solid central line in each panel, the mean ± SD of the onset and offset time are shown using dotted lines. Lines are drawn under the durations that are compared statistically in this and Figure 12: duration of I2 (A); time from onset of activity in B7 to onset of activity in B10 (C); and time from onset of B10 activity to onset of inward movement (F). Time from end of I2 activity to onset of activity in B10 is highlighted using a dotted line. Compare with Figure 12. For statistical comparisons, see Results.
Figure 12.
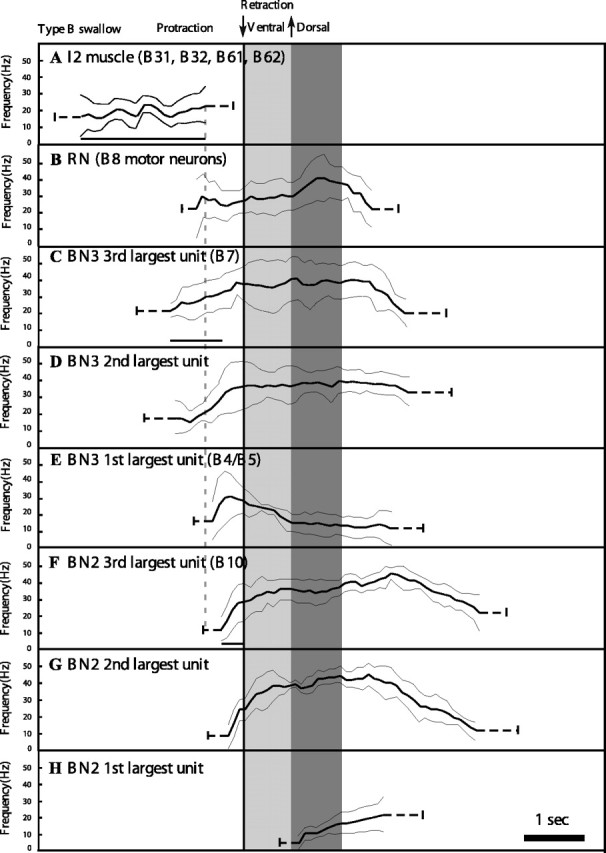
Statistical summary of nerve and muscle activity during type B swallows. Light gray bar indicates timing of tube ventral rotation; dark gray bar indicates timing of tube dorsal rotation. For details, see legend to Figure 11 and Materials and Methods.
Longer activation of I2 rotates the surface of the radula/odontophore ventrally (SA1 vs SB1)
We determined the effect of stimulating the I2 protractor muscle on the position of the surface of the radula/odontophore in an isolated buccal mass. To ensure maximum activation of the I2 muscle, we stimulated the I2 nerve, which contains the axons of all of the motor neurons of I2 (Hurwitz et al., 1996). To provide an objective measure of the degree of protraction of the anterior part of the radula/odontophore, we measured the jaw width, a standard measure of protraction in this system (Susswein et al., 1976). To deduce the position of the surface of the radula/odontophore, we also manually moved the odontophore anteriorly into three increasingly protracted positions and then measured the width of the jaws as well as the position of the radular surface (shown schematically in Fig. 4A). We observed that, in vivo, type B swallows correspond to a jaw width that is 35 ± 4.4% of the buccal mass length. The most protracted position of the odontophore in vitro expanded the jaws to 35% of the buccal mass length, and only at this position did type B movements occur. The I2 nerve had to be stimulated at a high frequency (20 Hz) for long durations (2.8 ± 0.2 s; n = 4) to achieve a jaw width and radular surface position equivalent to that seen at the peak of type B swallows (Fig. 4B). Stimulating I2 for 2.4 s was sufficient to reach this protracted position after a delay of 600 ms (n = 4), because I2 continues to contract after it ceases to receive stimulation (Yu et al., 1999).
B8 can induce inward movement and ventral tube rotation if there is an angle between the tube and the radular surface (SA2 vs SB2)
Once the surface of the radula rotates ventrally relative to the surface of the buccal mass, we predicted that the radula would be capable of pulling a tube inward as well as rotating the tube ventrally as the radular halves closed on the tube. Furthermore, because the motor neurons responsible for closing the halves of the radula are the B8 motor neurons, we predicted that activation of the B8 motor neurons when a tube was at the appropriate contact angle would allow the B8 motor neurons to induce the tube to move inwards and rotate ventrally. To test this hypothesis, we impaled B8 using an intracellular electrode and examined its effects on a tube placed on an isolated radula/odontophore (Fig. 5A,B). When the tube was placed at a contact angle of 0° (i.e., placed in the cleft between the halves of the radula), activating B8 induced neither inward movement nor rotation (Fig. 5 A). In contrast, when the tube was placed at a contact angle of 30° (the angle observed in intact, behaving animals at the end of a protraction in a type B swallow), activating B8 induced a strong inward movement and ventral rotation of the tube (Fig. 5B) (n = 4 different animals; inward movement was 3.5 ± 0.5 mm at 15 Hz; ventral rotation was 8.1 ± 1.4°). Thus, B8 can induce inward movement and ventral rotation as a function of its mechanical context.
What effect does a single B8 motor neuron have on tube movement during a feeding motor program? To test the hypothesis that changes in the activity of a single B8 motor neuron could significantly affect tube inward movement and ventral rotation, we induced ingestion-like motor programs in an isolated radula/odontophore by applying carbachol to the cerebral ganglion. To establish that the patterns were ingestive-like, we recorded activity on the RN. The large extracellular units on RN correspond to activity in the B8 motor neurons (Morton and Chiel, 1993b). We also recorded from BN2, whose large extracellular units innervate the I1/I3/jaw complex and indicate the onset of retraction (Morton and Chiel, 1993b). We studied those carbachol patterns in which activity in RN (closure) overlapped activity in BN2 (retraction), representing an ingestive pattern (Fig. 5C) (Morton and Chiel, 1993a,b). During the retraction phase of these motor programs, B8 was depolarized and fired, and the tube moved inward and rotated ventrally (Fig. 5D, left and right panels). We impaled the B8 neuron with an intracellular electrode and were able to hyperpolarize it so that it did not fire action potentials. The inward movement and rotation of the tube were significantly reduced when B8 was hyperpolarized (Fig. 5D, middle panel) (n = 14 from 3 different animals; during hyperpolarization of B8, inward movement was reduced from 3.5 ± 0.7 to 2.4 ± 1.0 mm, p < 0.001; ventral rotation was reduced from 8.2 ± 1.2 to 4.4 ± 1.3°, p < 0.001). Thus, activity of a single B8 motor neuron can move a tube inward and ventrally rotate it.
After the hinge is stretched, B7 can initiate retraction and dorsal rotation (SA3 vs SB3)
After a strong protraction, the hinge muscle (Fig. 3, SB2, bottom view) is stretched. Previous studies have shown that the B7 motor neuron innervates the hinge (Church and Lloyd, 1994). Does neuron B7 fire before the onset of retraction and during retraction, so that it could contribute to retraction? In an isolated buccal ganglion, we induced feeding-like motor programs (Fig. 6A) while recording intracellularly from B7 (Fig. 6A, bottom trace) and determined that B7 was active before the onset of retraction (as determined by onset of large unit activity in BN2) and continued to be active throughout the retraction phase (note filled bars at top of Fig. 6A). We also demonstrated that B7 corresponded to the third largest unit on BN3. When B7 was hyperpolarized intracellularly, the third largest unit on BN3 disappeared completely (Fig. 6A; an expanded timescale view is shown in B).
Does the ability by B7 to mediate retraction depend on the stretch of the hinge? If the radula/odontophore was moved to the peak protraction observed during type A swallows, activating B7 had no effect (Fig. 7A). In contrast, if the radula/odontophore was moved to the peak protraction observed during type B swallows, activating B7 induced an inward movement and dorsal rotation of the tube (Fig. 7B). Thus, mechanical context affects the ability of the B7 motor neuron to have an observable behavioral effect.
Can a single B7 neuron affect the inward movement and dorsal rotation of a tube? We measured the effects of hyperpolarizing a single B7 neuron on inward movement and dorsal rotation of the tube (Fig. 7C). During the retraction phase of the feeding-like motor programs, B7 received very strong excitation and fired strongly (Fig. 7C, left and right). When B7 was hyperpolarized (Fig. 7C, middle), the inward movement and dorsal rotation of the tube were significantly reduced (inward movement was reduced from 3.9 ± 0.9 to 2.1 ± 0.7 mm; p < 0.05; n = 19 from 3 different animals).
The B10 motor neuron for the I1/I3/jaw complex works with B7 to complete retraction and dorsal rotation during larger-amplitude swallows (SA3 vs SB3)
How does the role of the retractor muscles, the I1/I3/jaw complex, change during a type A as opposed to a type B swallow? We addressed this question in two stages. First, we examined the ability of an identified motor neuron for the I1/I3/jaw complex, motor neuron B10, to induce retraction from a protracted position similar to that observed in type A or type B swallows and also determined the contribution of a single B10 motor neuron to retraction during feeding-like motor programs. We then examined the interaction between B7 and B10 after a type B protraction.
Activating the B10 neuron once the radula/odontophore has been protracted to the position of a type A swallow induces a strong inward movement of a tube (Fig. 8A). After the radula/odontophore has been protracted to the position of a type B swallow, activating a B10 neuron induces both a strong inward movement of a tube and a dorsal rotation (Fig. 8B). Can a single B10 neuron affect tube retraction during a feeding-like motor program? Previous work established that B10 is the third largest extracellular unit on BN2 and that it is active throughout the retraction phase (Morton and Chiel, 1993b). We induced feeding-like motor programs and impaled a B10 neuron intracellularly. B10 fired intensely throughout the retraction phase of the feeding motor program (Fig. 8C, left and right). When B10 was hyperpolarized, it significantly reduced the inward movement and dorsal rotation of the tube (Fig. 8C, middle) (inward movement was reduced from 4.4 ± 0.5 to 3.2 ± 0.7 mm; p < 0.05; n = 20 from 2 different animals).
After strong protractions, similar to those observed at the peak of a type B swallow, we found that motor neurons B7 and B10 must be activated within a specific time window to generate maximal retractions. Activating B7 1 s before activating B10 produced the largest inward movement of the tube (Fig. 9B), which was significantly greater (p < 0.002) than that achieved if B7 was activated simultaneously with B10 (Fig. 9A) or if B7 was activated 2 s before B10 was activated (Fig. 9C). A summary scatter plot of angular velocity versus inward velocity demonstrates that the velocity of rotation and inward movement were greatest when B7 was activated 1 s before B10 [Fig. 9D; note that the points corresponding to the 1 s delay (open circles) are completely separated from those corresponding to no delay (black dots) or 2 s delay (gray dots)] [overall MANOVA on these data were highly significant (p < 0.001); post hoc comparisons showed that the 1 s delay data were significantly different from either the 0 or 2 s delay data (p < 0.001) but that the 0 and 2 s delay data were not significantly different from each other].
Observations of the isolated buccal mass showed that the initial contraction of the hinge muscle moved the anterior wedge-shaped portion of the radula/odontophore to the correct position for the I1/I3/jaw complex to push the radula/odontophore most strongly in a posterior direction (Fig. 3, bottom row, SB3a, SB3b). During a type A swallow, the I1/I3/jaw complex can act alone to induce a retraction (because the hinge muscle is not stretched at the peak protraction of a type A swallow, so motor neuron B7 can exert no force). In contrast, during a type B swallow, the hinge muscle pulled the radula/odontophore back into a position within the I1/I3 lumen at which the I1/I3/jaw complex could exert maximal retractive force if motor neuron B7 was activated ∼1 s before activation of the motor neurons of the I1/I3/jaw complex.
In vivo neuromuscular activity is consistent with in vitro studies
The in vitro data that we have presented supports the hypothesis that, if the periphery is correctly positioned, swallowing responses of different amplitudes can be generated in response to similar neural outputs, directly supporting Bernstein's hypothesis. The most rigorous test of the hypothesis would be to monitor neural activity and control the position of the periphery in an intact, behaving animal and demonstrate that, although the nervous system generated the identical neural output, changes in the position of the periphery alone were both necessary and sufficient to induce changes in an animal's behavior. Although it may be possible to do these experiments in the future (Chestek et al., 2004), the best test of the hypothesis currently available to us is to use the in vitro data to predict the changes in different identified motor units that should be observed during type A versus type B swallows. Then we can determine, based on in vivo recordings from nerves and muscles, whether these patterns of activity are actually observed. Of course, we cannot rule out the possibility that there are subtle changes in neural output that we have not been able to detect with our current, relatively crude techniques, but at least we can test the large changes in activity predicted by our in vitro studies.
Previous work has demonstrated that it is possible to track the activity of groups of identified motor neurons by recording from appropriate nerves and muscles in Aplysia. In particular, EMG activity recorded from the I2 muscle monitors activity in the motor neurons of I2 (B31, B32, B61, and B62) (Hurwitz et al., 1996), large-unit extracellular activity on RN is a monitor of action potentials in the B8 motor neurons (Morton and Chiel, 1993b), the third largest extracellular unit on BN2 corresponds, at least in part, to activity in the B10 motor neurons (Morton and Chiel, 1993b), and the largest extracellular unit on BN3 corresponds to activity in the multi-action B4/B5 neurons (Warman and Chiel, 1995). Data presented above suggest that the third largest unit on BN3 corresponds, at least in part, to activity in the B7 motor neurons (Fig. 6). Thus, by recording from the I2 muscle, RN, BN2, and BN3 and using a window discriminator algorithm (see Materials and Methods), it is possible to obtain evidence for the activity of individual identified motor neurons in intact, behaving animals.
The central hypothesis makes several testable predictions about how neural recordings should be altered from type A to type B swallows if the primary determinant of the larger-amplitude type B swallows is the new position of the periphery. (1) The duration of I2 activation should be longer in type B swallows to induce a larger protraction, which prepares the periphery so that the hinge and the grasper can initiate the larger-amplitude retraction (Figs. 3, 4). (2) In the new position, motor neurons that had a primary role in initiating retraction (i.e., the motor neurons for the I1/I3/jaw complex) no longer have this role. As a consequence, the onset of their timing relative to the onset of the behavior is no longer critical, and they do not have to become active early enough to induce the tube to move inwards. Instead, in the new position, it is activation of the grasper (i.e., the B8 motor neurons for the I4 muscle) and the hinge muscle (i.e., through activation of the B7 motor neuron) that initiate retraction (Figs. 5, 7). (3) In the new position, the hinge is stretched. Our in vitro studies (Fig. 9B) demonstrated that the largest, smoothest retraction sequence requires that activation of hinge motor neuron B7 should occur ∼1 s before activation of motor neuron (B10) for the I1/I3/jaw complex (B10 is the first motor neuron to become active for the I1/I3/jaw complex) (Church and Lloyd, 1994). If the new biomechanical relationship between the hinge muscle and the I1/I3/jaw complex observed in vitro is operative in vivo, then B7 should become active 1 s before B10 in vivo. Moreover, if essentially the same neural output is generated during type A behaviors, the temporal relationship between onset of activity in B7 and in B10 should also be observed, even if it is not behaviorally functional for a type A swallow. (4) If the position of the periphery allows the grasper and the hinge to mediate the initial part of retraction for the more intense swallowing behavior observed during type B swallows, then there should be no significant increase in the duration or frequency of activity of the main retractor motor neurons for the I1/I3/jaw complex, although the swallowing amplitude is larger.
We confirmed all four predictions by recordings in intact, behaving animals [Fig. 10 shows data from individual animals; Fig. 11 shows averaged data for type A swallows; and Fig. 12 shows averaged data for type B swallows (n = 5 different animals for each average trace)]. (1) The duration of I2 activity is significantly longer in type B than in type A swallows (1.6 ± 0.2 s for type A vs 2.4 ± 0.6 s for type B; n = 5; p < 0.01) (note that the bar underneath the traces labeled I2 EMG in Fig. 10, A and B, is longer for the type B swallow; also compare Figs. 11A, 12A). Thus, I2 activity is sufficient to set up the new position of the periphery in a type B, but not a type A, swallow. (2) Initiation of tube movement after activation of B10 requires ∼900–1000 ms, whether the radula is positioned in a type A or type B position (Fig. 8A3,B3). During type A swallows, for which our in vitro studies predict that the I1/I3/jaw complex serves as the sole retractor, the time of onset of activity in B10 occurs 1000 ± 180 ms before the initiation of tube movement (mean ± SD; n = 5) (see B10 onset in Fig. 10A and time of onset of B10 activity in Fig. 11F relative to the onset of tube movement). In contrast, during a type B swallow, our in vitro studies predict that the grasper and the hinge initiate retraction, so that B10 does not have to become active early for tube retraction to be initiated. Indeed, we found that the time of onset of activity in B10 during type B swallows was too short for B10 to play a significant role in initiating retraction movements (460 ± 98 ms; n = 5; p < 0.05 from the value for type A swallows) (see B10 onset in Fig. 10B and time of onset of B10 activity in Fig. 12F relative to the onset of tube movement). Of course, B10 is likely to contribute to the later retraction movements of the tube. (3) During type B swallows, activity in the third largest units on BN3 (i.e., motor neuron B7 activity) precedes activity in the third largest unit on BN2 (i.e., motor neuron B10 activity) by 0.9 ± 0.6 s (n = 5) [see line labeled B7 onset in Fig. 10B, trace labeled BN3, and compare with line labeled B10 on trace labeled BN2 (these data are from the same animal); also see Fig 12C,F], which is consistent with the predictions of the in vitro study (Fig. 9B). As predicted by the hypothesis that the neural output is very similar in both type A and type B swallows, a similar time difference is observed in type A swallows (1.0 ± 0.4 s; n = 5) (Figs. 10A, 11C,F). These results support the hypothesis that the differences in observed behavior are attributable to differences in positioning of the periphery, not to differences in the neural pattern. (4) The duration and frequencies of all units on BN2, which represent the main pool of motor neurons innervating the I1/I3/jaw complex, are not significantly different between type A and type B swallows (MANOVA comparing the duration of these units in type A and type B swallows was not significant). These results are consistent with our previous kinematic studies of swallowing in intact behaving animals (Neustadter et al., 2002). Although many features of swallowing were highly variable, the posterior translation and rotation of the odontophore at the peak of retraction showed relatively little variation, although the four swallows that were studied varied greatly in their overall amplitude [Neustadter et al. (2002), their Fig. 11].
These results suggest that the function of a larger-amplitude protraction is primarily to create a new mechanical configuration that allows animals to generate stronger swallows. By exposing two new degrees of freedom (the ability of the hinge to retract the odontophore and the ability of the radular halves to retract the tube in addition to grasping it), the stronger protraction allows an animal to generate a larger-amplitude swallow without a larger retraction. Thus, the larger I2 protraction prepares the periphery to be appropriately receptive to subsequent motor outputs. Furthermore, because activation of the hinge muscle has no overt behavioral effect if the animal does not generate the larger protraction, but occurs in both type A and type B swallows, it is an example of a motor output that is, at least initially, purely preparatory; its only function is to prepare the periphery for subsequent motor outputs, but not itself to effect any motor behavior.
Although the appropriate positioning of the periphery attributable to the longer activation of the I2 muscle may account for most of the differences between type A and type B swallows, we did find an additional difference in neural output. This difference is, however, also attributable to the biomechanical differences between the two types of swallows. Previous studies have shown that the I1/I3/jaw complex can induce strong retractions (Morton and Chiel, 1993a) and could therefore antagonize the strong protraction that initiates a type B swallow. Thus, we predicted that the onset of activity in the I1/I3/jaw complex motor neurons relative to the end of activity in the I2 protractor muscle would be delayed in type B swallows relative to type A swallows, so as not to antagonize the larger protraction that prepares the periphery for type B swallows.
As predicted, the delay was significantly greater in type B swallows compared with type A swallows (0.12 ± 0.08 s for type A swallows; 0.28 ± 0.1 s for type B swallows; n = 5; p < 0.02) (Figs. 11, 12, compare end of I2 activity in A with onset of activity in B10 in F; the delay is small but statistically significant). Previous studies have also shown that the identified multi-action neurons B4/B5 make widespread inhibitory connections to the motor neurons of the I1/I3/jaw complex (Gardner, 1993) and could therefore delay the onset of activity in the I1/I3/jaw complex during type B swallows. Indeed, we found that the peak frequency of B4/B5 (the largest extracellular unit on BN3) was higher in type B swallows than in type A swallows before the onset of activity in B10 (the third largest unit on BN2; 25.8 ± 3.8 Hz for type A swallows; 34 ± 7 Hz for type B swallows; n = 5; p < 0.05) (Figs. 11, 12, compare peak frequency of activity in B4/B5 in E before onset of activity in B10 in F).
Thus, our results suggest that that the change in the position of the periphery is primarily (but not solely) responsible for the change in intensity of swallowing in type B as opposed to type A swallows.
Discussion
Preparation of the periphery for motor coordination
The data presented in this paper support Bernstein's hypothesis that “The … highest stage of co-ordinational freedom corresponds to a degree of co-ordination at which the organism is not only unafraid of reactive phenomena in a system with many degrees of freedom, but is able to structure its movements so as to use entirely the reactive phenomena which arise… the mastery of co-ordination must consist in the ability to give the necessary impulse at the necessary moment… . Co-ordination… lies basically… in the accuracy of some sort of preparatory… effector impulses which organize and prepare the periphery for the reception of the right impulse at the right moment” (emphasis as in the original; Bernstein, 1967). To our knowledge, our results are the first direct evidence for Bernstein's hypothesis. Preparatory motor activity (e.g., longer protractor activity) exposes new degrees of freedom (use of the hinge for retraction and use of the grasper for retraction) and allows the system to respond properly to subsequent effector motor activity. Initial activation of the hinge is an example of a purely preparatory output, because it has no overt behavioral effect.
One might have expected that Aplysia's neural control would be significantly simpler than those of complex vertebrate locomotion systems, in which position, velocity, and acceleration play critical roles (Zajac, 1993). Previous studies of Aplysia's feeding system suggest that position and velocity alone are sufficient to describe system mechanics (Sutton et al., 2004b). Even in this simpler system, the periphery must be correctly positioned for motor output to be effective. It is highly likely, therefore, that similar phenomena will be observed in more complex systems. These results also suggest that prosthetic devices that used external actuators to “set up” an artificial limb to be properly receptive to neural outputs at the right time could help restore normal movements.
Dissociations between motor neuronal activity and behavior
The relationship between neural activity, EMG, and actual behavior is complex (Zajac, 1993). This paper provides examples of three dissociations between motor neuronal activity and behavior.
First, neural activity may not correspond to any behavioral output. Motor neuron B7 is intensely active before the onset of retraction in both type A and type B swallows (Figs. 11C, 12C), but, during type A swallows, the hinge muscle is not stretched sufficiently to exert force. More generally, if a muscle receives activation when it has little mechanical advantage or is at the extremes of its length/tension curve, it will generate little or no force (Zajac, 1989).
Second, neural activity of a muscle may correspond to more than one behavioral function. Thus, activity in the B8 motor neurons is generally interpreted as representing radular closure. During type B swallows, however, B8 motor neuronal activity also mediates tube retraction. More generally, if the function of a muscle changes with its mechanical context, behavioral interpretation of its activity must also change (Murray et al., 1995, 2000; Buneo et al., 1997; Kargo and Rome, 2002).
Third, neural activation of a muscle mediating a function may not change, but behavioral output may change as a result of recruitment of other muscles. Thus, activity of I1/I3/jaw complex motor neurons does not increase during type B swallows, but retraction amplitude does increase, because tube retraction is now mediated in part by the grasper and the hinge muscle. Activation of the I1/I3/jaw complex is no longer an accurate indicator of retraction amplitude. More generally, if other muscles act agonistically with a particular muscle in different biomechanical contexts, activity of all relevant muscles must be monitored to determine the overall behavioral output (Zajac et al., 2002, 2003).
Neuromechanical modules
Previous work has proposed the existence of movement control modules (Grillner, 1981, 1985; Loeb, 1985; Bizzi et al., 1991), for which there is evidence in both vertebrates (Tresch et al., 2002; d’Avella et al., 2003; Hart and Giszter, 2004; Stein and Daniels-McQueen, 2004) and invertebrates (Jing et al., 2004). Control modules incorporating both neural and biomechanical components may be referred to as “neuromechanical modules.” The position of the radula/odontophore at the end of the protraction phase of swallowing defines the borders of two neuromechanical modules, one to set up the initial position of the grasper and the second to actually ingest food. If an animal ingests seaweed whose resisting force pulls the radular surface forward, this alone might be sufficient to generate the second half of a type B swallow. Activity of B7 observed in type A swallows would stiffen the hinge muscle, allowing it to induce initial retraction; the ability of the radular halves to initiate retraction depends only on their angle relative to food; and the fixed timing of activity in B7 and B10, observed even in type A swallows, would allow the strongest retraction to occur once retraction was initiated by the hinge. Thus, despite the different previous history of the system, once put into the correct condition, it could execute the second neuromechanical module. A recent modeling study of locomotion in the stick insect demonstrated the role of appropriate local sensory feedback for maintaining coordination of normal walking (Ekeberg et al. 2004), suggesting how changes in leg position and feedback could be properly coordinated to maintain walking despite environmental perturbations (Bässler and Büschges, 1998).
Implications for pattern generation
Our data provide additional evidence that Aplysia's feeding behavior is not stereotyped but highly flexible. Studies of the feeding pattern generator have shown variability in vitro (Rosen et al., 1991; Church and Lloyd, 1994) and in vivo (Horn et al., 2004). Variability could be attributable to the changing biomechanics of food, e.g., changes in mechanical load (Hurwitz and Susswein, 1994), to intrinsic variability within the pattern generator, or to changing stimuli within the buccal cavity during ingestion. The two types of swallows we described appear in response to natural food: in studies of juvenile Aplysia swallowing small seaweed stipes, we observed both smooth inward movements and dorsal and ventral rotations during swallowing (Drushel et al., 1997). Because type A and type B swallows can be distinguished in vitro by the duration of I2 activity and the peak frequency of activity in B4/B5, studies of the sensory and interneuronal mechanisms underlying swallowing variability can now be done in reduced preparations induced to perform ingestive-like behaviors (Susswein et al., 1996).
Our results have implications for pattern generation in Aplysia and in other animals. At the neuronal level, the major difference between the two types of swallowing is protraction duration, which could be controlled by several mechanisms. The duration of the plateau in the B31/B32 neurons could be increased by excitation of the coupled B63 neuron (Dembrow et al., 2003). The protraction strength of muscle I2 could be enhanced by recruiting the motor neurons B61 and B62 (Hurwitz et al., 1996). Inhibition of the B64 neuron, which terminates the protraction phase, could also delay the onset of retraction and thereby prolong protraction (Jing et al., 2003).
Our data are relevant to understanding the structure of the feeding pattern generator in Aplysia. In Helisoma and Lymnaea, it is possible to define three phases for feeding motor patterns, corresponding to protraction, retraction, and hyper-retraction (Murphy, 2001; Elliott and Susswein, 2002). A study of 22 motor neurons in reduced preparations of Aplysia during ingestive-like and egestive-like motor patterns demonstrated that there was a clear boundary between protraction and retraction in the synaptic inputs to the motor neurons, but many subcomponents of the motor output could be distinguished [Church and Lloyd (1994), their Fig. 14]. It is clear that the timing of onset and offset of motor units on the buccal nerves in vivo falls into multiple phases, probably more than four (note the timing of onset and offset of motor units on I2, RN, BN2, and BN3 in Figs. 11, 12). This may reflect and contribute to the greater flexibility with which Aplysia can deploy its periphery during feeding.
The interneuronal mechanisms that generate different feeding motor patterns in Aplysia have been studied intensively (Jing and Weiss, 2001, 2002; Jing et al., 2004) using neural patterns identified through in vitro recordings of individual identified neurons or nerves. For example, to discriminate biting from swallowing, the authors use the duration of activity in the I2 motor neurons (Jing et al., 2004). However, the duration of I2 activity may be an indicator of a shift from type A to type B swallowing rather than from biting to swallowing. It will therefore be important to establish biomechanically based criteria for discriminating different in vitro feeding patterns.
More generally, pattern generators must be understood within a biomechanical context. During locust flight, cycle-to-cycle sensory feedback is critical for generating normal motor activity (Pearson et al., 1989). Rapid visco-elastic responses (preflexes) may regulate peripheral responses during rapid locomotion (Jindrich and Full, 2002). Our results suggest that the position of the periphery at each instant determines what neural control can do next. These results provide additional evidence that adaptive behavior emerges from the interaction of the nervous system, periphery, and the environment (Chiel and Beer, 1997).
Footnotes
H. Ye's present address: Division of Cellular and Molecular Biology, Toronto Western Research Institute, Toronto, Ontario, Canada M5T 2S8.
D. W. Morton's present address: Premier Medical Imaging, 580 West College Avenue, Marquette, MI 49855.
This work was supported by Whitehall Foundation Grant M97-17, National Science Foundation Grant IBN 0218386, and National Institutes of Health Grant NS047073. We thank Dr. Richard F. Drushel for assistance in illustrating the anatomy of the buccal mass, Jeff McManus for additional analyses of swallowing kinematics, and three anonymous reviewers for helpful comments on a previous draft of this manuscript.
References
- Bässler U, Büschges A (1998). Pattern generation for stick insect walking movements—multisensory control of a locomotor program. Brain Res Rev 27:65–88. [DOI] [PubMed] [Google Scholar]
- Bernstein N (1967). In: The coordination and regulation of movements Oxford: Pergamon.
- Bizzi E, Mussa-Ivaldi FA, Giszter SF (1991). Computations underlying the execution of movement: a biological perspective. Science 253:287–291. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Soechting JF, Flanders M (1997). Postural dependence of muscle actions: implications for neural control. J Neurosci 17:2128–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestek CA, Garverick SL, Tabib-Azar M, Harrison RR, Martin HB, Lu H, Samsukha P, Chiel HJ (2004). Techniques for stimulating and recording wirelessly from a multiaction identified neuron in Aplysia. Soc Neurosci Abstr 30:537–7. [Google Scholar]
- Chiel HJ, Beer RD (1997). The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci 20:553–557. [DOI] [PubMed] [Google Scholar]
- Church PJ, Lloyd PE (1994). Activity of multiple identified motor neurons recorded intracellularly during evoked feeding like motor programs in Aplysia. J Neurophysiol 72:1794–1809. [DOI] [PubMed] [Google Scholar]
- Cohen JL, Weiss KR, Kupfermann I (1978). Motor control of buccal muscles in Aplysia. J Neurophysiol 41:157–180. [DOI] [PubMed] [Google Scholar]
- Cropper EC, Miller MW, Vilim FS, Tenenbaum R, Kupfermann I, Weiss KR (1990). Buccalin is present in the cholinergic motor neuron B16 of Aplysia and it depresses accessory radula closer muscle contractions evoked by stimulation of B16. Brain Res 512:175–179. [DOI] [PubMed] [Google Scholar]
- Curran-Everett D (2000). Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol 279:R1–R8. [DOI] [PubMed] [Google Scholar]
- d’Avella A, Saltiel P, Bizzi E (2003). Combinations of muscle synergies in the construction of natural motor behavior. Nat Neurosci 6:300–308. [DOI] [PubMed] [Google Scholar]
- Dembrow NC, Jing J, Proekt A, Romero A, Vilim FS, Cropper EC, Weiss KR (2003). A newly identified buccal interneuron initiates and modulates feeding motor programs in Aplysia. J Neurophysiol 90:2190–2204. [DOI] [PubMed] [Google Scholar]
- Drushel RF, Neustadter DM, Shallenberger LL, Crago PE, Chiel HJ (1997). The kinematics of swallowing in the buccal mass of Aplysia californica. J Exp Biol 200:735–752. [DOI] [PubMed] [Google Scholar]
- Ekeberg O, Blumel M, Büschges A (2004). Dynamic simulation of insect walking. Arth Struct Develop 33:287–300. [DOI] [PubMed] [Google Scholar]
- Elliott C, Susswein AJ (2002). Comparative neuroethology of feeding control in molluscs. J Exp Biol 205:877–896. [DOI] [PubMed] [Google Scholar]
- Evans CG, Rosen S, Kupfermann I, Weiss KR, Cropper EC (1996). Characterization of a radula opener neuromuscular system in Aplysia. J Neurophysiol 76:1267–1281. [DOI] [PubMed] [Google Scholar]
- Gardner D (1971a). Synaptic organization and bilateral symmetry in the buccal ganglia of Aplysia. PhD thesis New York University. [DOI] [PubMed]
- Gardner D (1971b). Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia Science 173:550–553. [DOI] [PubMed] [Google Scholar]
- Gardner D (1993). Static determinants of synaptic strength. In: The neurobiology of neural networks (Gardner D, ed.) , pp. 21–70. Cambridge, MA: MIT.
- Grillner S (1981). Control of locomotion in bipeds, tetrapods and fish, The nervous system. In: Handbook of physiology (Brookhart JB, Mountcastle VB, eds) , Vol 2: pp. 1179–1236. Bethesda, MD: American Physiological Society. [Google Scholar]
- Grillner S (1985). Neurobiological bases of rhythmic motor acts in vertebrates. Science 228:143–149. [DOI] [PubMed] [Google Scholar]
- Hart CB, Giszter SF (2004). Modular premotor drives and unit bursts as primitives for frog motor behaviors. J Neurosci 24:5269–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Zhurov Y, Orekhova IV, Proekt A, Kupfermann I, Weiss KR, Brezina V (2004). Cycle-to-cycle variability of neuromuscular activity in Aplysia feeding behavior. J Neurophysiol 92:157–180. [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Susswein AJ (1994). Adaptation of feeding sequences in Aplysia oculifera to changes in the load and width of food. J Exp Biol 166:215–235. [Google Scholar]
- Hurwitz I, Neustadter D, Morton DW, Chiel HJ, Susswein AJ (1996). Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J Neurophysiol 75:1309–1326. [DOI] [PubMed] [Google Scholar]
- Jindrich DL, Full RJ (2002). Dynamic stabilization of rapid hexapedal locomotion. J Exp Biol 205:2803–2823. [DOI] [PubMed] [Google Scholar]
- Jing J, Weiss KR (2001). Neural mechanisms of motor program switching in Aplysia. J Neurosci 21:7349–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR (2002). Interneuronal basis of the generation of related but distinct motor programs in Aplysia: Implications for current neuronal models of vertebrate intralimb coordination. J Neurosci 22:6228–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Wu JS, Park JH, Weiss KR (2003). Concerted GABAergic actions of Aplysia feeding interneurons in motor program specification. J Neurosci 23:5283–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Cropper EC, Hurwitz I, Weiss KR (2004). The construction of movement with behavior-specific and behavior-independent modules. J Neurosci 24:6315–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Rome LC (2002). Functional morphology of proximal hindlimb muscles in the frog Rana pipiens. J Exp Biol 205:1987–2004. [DOI] [PubMed] [Google Scholar]
- Kupfermann I (1974). Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol 10:1–26. [DOI] [PubMed] [Google Scholar]
- Levy M, Levy I, Susswein AJ (1997). Respiratory pumping in Aplysia fasciata in natural and artificial tide pools. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 180:81–89. [Google Scholar]
- Loeb GE (1985). Motoneurone task groups: coping with kinematic heterogeneity. J Exp Biol 115:137–146. [DOI] [PubMed] [Google Scholar]
- Lombard WP, Abbott FM (1907). The mechanical effects produced by the contraction of individual muscles of the thigh of the frog. Am J Physiol 20:1–60. [Google Scholar]
- Morton DW, Chiel HJ (1993a). In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 172:17–32. [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ (1993b). The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 173:519–536. [DOI] [PubMed] [Google Scholar]
- Murphy AD (2001). The neuronal basis of feeding in the snail, Helisoma, with comparisons to selected gastropods. Prog Neurobiol 63:383–408. [DOI] [PubMed] [Google Scholar]
- Murray WM, Delp SL, Buchanan TS (1995). Variation of muscle moment arms with elbow and forearm position. J Biomech 28:513–525. [DOI] [PubMed] [Google Scholar]
- Murray WM, Buchanan TS, Delp SL (2000). The isometric functional capacity of muscles that cross the elbow. J Biomech 33:943–952. [DOI] [PubMed] [Google Scholar]
- Neustadter DM, Drushel RF, Chiel HJ (2002). Kinematics of the buccal mass during swallowing based on magnetic resonance imaging in intact, behaving Aplysia californica. J Exp Biol 205:939–958. [DOI] [PubMed] [Google Scholar]
- Orekhova IV, Jing J, Brezina V, Dicaprio RA, Weiss KR, Cropper EC (2001). Sonometric measurements of motorneuron-evoked movements of an internal feeding structure (the radula) in Aplysia. J Neurophysiol 86:1057–1061. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Hedwig B, Wolf H (1989). Are the hindwing chordotonal organs elements of the locust flight pattern generator? J Exp Biol 144:235–255. [Google Scholar]
- Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I (1991). Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci 11:3630–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PSG, Daniels-McQueen S (2004). Variations in motor patterns during fictive rostral scratching in the turtle: knee-related deletions. J Neurophysiol 91:2380–2384. [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Kupfermann I, Weiss KR (1976). The stimulus control of biting in Aplysia. J Comp Physiol 108:75–96. [Google Scholar]
- Susswein AJ, Rosen SC, Gapon S, Kupfermann I (1996). Characterization of buccal motor programs elicited by a cholinergic agonist applied to the cerebral ganglion of Aplysia californica. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 179:509–524. [DOI] [PubMed] [Google Scholar]
- Sutton GP, Macknin JB, Gartman SS, Sunny GP, Beer RD, Crago PE, Neustadter DM, Chiel HJ (2004a). Passive hinge forces in the feeding apparatus of Aplysia aid retraction during biting but not during swallowing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190:501–514. [DOI] [PubMed] [Google Scholar]
- Sutton GP, Mangan EV, Neustadter DM, Beer RD, Crago PE, Chiel HJ (2004b). Neural control exploits changing mechanical advantage and context dependence to generate different feeding responses in Aplysia. Biol Cybern 91:333–345. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, d’Avella A, Bizzi E (2002). Coordination and localization in spinal motor systems. Br Res Brain Res Rev 40:66–79. [DOI] [PubMed] [Google Scholar]
- Warman EN, Chiel HJ (1995). A new technique for chronic single-unit extracellular recording in freely behaving animals using pipette electrodes. J Neurosci Methods 57:161–169. [DOI] [PubMed] [Google Scholar]
- Weiss KR, Chiel HJ, Koch U, Kupfermann I (1986). Activity of an identified histaminergic neuron, and its possible role in arousal of feeding behavior in semi-intact Aplysia. J Neurosci 6:2403–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S-N, Crago PE, Chiel HJ (1999). Biomechanical properties and a kinetic simulation of the smooth muscle I2 in the buccal mass of Aplysia. Biol Cybern 81:505–513. [DOI] [PubMed] [Google Scholar]
- Zajac FE (1989). Muscle and tendon—properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17:359–411. [PubMed] [Google Scholar]
- Zajac FE (1993). Muscle coordination of movement: a perspective. J Biomech 26:109–124. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Neptune RR, Kautz SA (2002). Biomechanics and muscle coordination of human walking. I. Introduction to concepts, power transfer, dynamics and simulations. Gait Posture 16:215–232. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Neptune RR, Kautz SA (2003). Biomechanics and muscle coordination of human walking. II. Lessons from dynamical simulations and clinical implications. Gait Posture 17:1–17. [DOI] [PubMed] [Google Scholar]