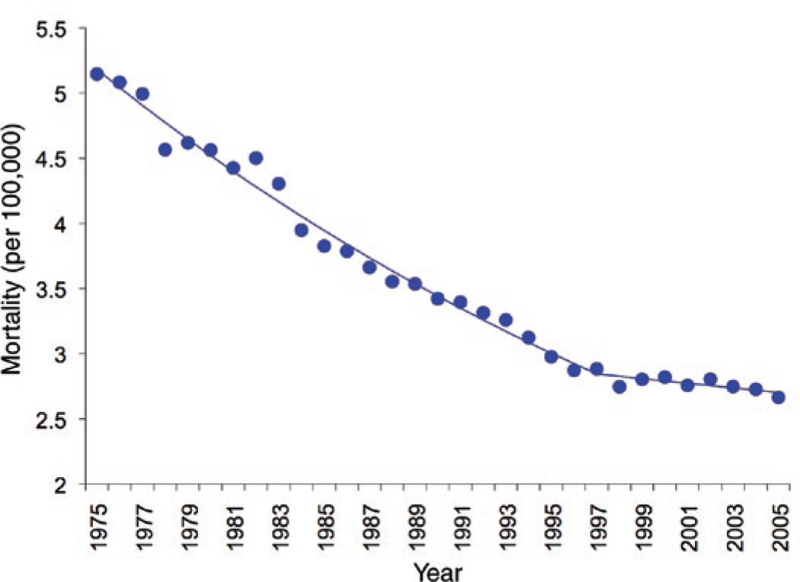
Conventional cytotoxic anticancer drugs have had their greatest impact in the treatment of childhood cancers. Using an integrated multimodality approach, which includes surgery or irradiation to address the primary tumor and combination chemotherapy to eradicate micrometastases, the overall 5-year survival in children diagnosed with solid tumors between 1996 and 2003 is 80%.1 Despite this success, the development of new anticancer drugs continues to be a priority for basic and clinical researchers in pediatric oncology because the rate of decrease in mortality has slowed (Figure 1), because the prognosis remains poor for children with some types of brain tumors and those with solid tumors that present with overt metastatic disease, and because most current cytotoxic anticancer drugs are associated with a high incidence of serious acute and long-term toxicities. Children who receive standard-dose intensive chemotherapy have a greater than 80% chance of having at least one drug-related toxicity that is severe, life threatening, or fatal over the course of their treatment,2 and the late effects of cancer treatment, including permanent organ and tissue damage (e.g., cardiotoxicity), hormonal and reproductive dysfunction, and second cancers, are of special concern in children with cancer because of the high cure rates and potentially long life spans of the survivors.
Figure 1.
Mortality rate per 100,000 in children (under 20 years of age) diagnosed with cancer in the United States between 1975 and 2005. Since 1997 the rate of decline in the mortality has leveled off. (Data from the Surveillance, Epidemiology, and End Results Program, National Cancer Institute.)
All phases of clinical development of new anticancer drugs are conducted separately in children with cancer, starting after initial phase I testing in adults. This need for separate phase I trials in children derives from the potential impact of ontogeny on drug disposition and on normal tissue and organ sensitivity to drugs. Separate pediatric phase II trials are conducted because the tissue of origin, pathogenesis, disease manifestations, and sensitivity to anticancer drugs for childhood cancers differ from those for cancers in adults.
The initial phases of clinical drug development are conducted in children who have received standard treatments and whose cancer has failed to respond or has relapsed after an initial response to front-line treatment. Because of the improving cure rate, the population of individuals with treatment-refractory cancers is shrinking, and cancer types that are less responsive to chemotherapy are overrepresented. In addition, frontline treatment has steadily intensified in terms of the number of drugs included in combination chemotherapy regimens and the dose rate of the drugs used for cancers with a poor prognosis. As a result, patients with treatment-refractory cancers may also be less able to tolerate subsequent investigational drugs.
Dose-finding (phase I) trials of anticancer drugs traditionally define the recommended dose as the highest tolerable dose (maximum tolerated dose, or MTD), and the incidence and severity of acute toxicities are the primary end points used to measure drug effect. The pharmacokinetics of the new drug is also studied separately in children in phase I trials. The starting dose for phase I trials in children is a fraction of the adult MTD (typically 80%), and the dose is escalated in small increments (typically 30%). The design and end points of the pediatric phase I trials are similar to trials in adults, but the dose levels studied, the definition of intolerable toxicity, and the pharmacokinetic sampling times may differ, leading to difficulty in comparing outcomes in children with those in adults.
For recently studied cytotoxic drugs, the MTD defined in pediatric phase I trials exceeded the MTD from adult trials using the same dosing schedule in 70% of trials when comparing doses that are normalized to body surface area.3 The ratio of drug clearance in children to adults across trials ranges from <0.1 to 2.2, but the differences in clearance between children and adults do not uniformly correlate with the differences in the MTD between the two populations.
Using toxicity as the primary measure of drug effect in the dose-finding studies for cytotoxic chemotherapy instead of using a therapeutic end point reflects the nonselective mechanism of action and low therapeutic index of these agents, the difficulty in quantifying an antitumor effect in a timely fashion, and the variable sensitivity of cancers to single-agent treatment. An underlying assumption in defining the MTD as the recommended dose is that the toxicity will become intolerable before the dose that produces the maximum therapeutic (anticancer) effect has been reached. The traditional therapeutic end points for anticancer drugs are response (a measurable decrease in tumor size compared with baseline) and survival, which are drug effects that cannot be measured until months or even years after the start of treatment. In addition, the variable and unpredictable sensitivity to a drug across various histological types of cancer and within a group of individuals with the same type of cancer obscures our attempts to define a single optimal therapeutic dose.
Pediatric phase II trials, which assess the activity of new drugs, tend to include multiple cancer types, each studied as a separate stratum, in a single trial, whereas adult phase II trials tend to study a single cancer type per trial. The traditional primary end point of phase II trials in adults and children is response (typically at least a 30% decrease in tumor size measured in a single dimension relative to pretreatment tumor measurements). Historically, childhood cancers have been considered to be more sensitive to cytotoxic chemotherapy than cancers in adults, as reflected by higher response rates and cure rates; recently, however, there have been anticancer drugs (e.g., taxanes) that are efficacious in a variety of cancers in adults but have failed to demonstrate activity against childhood cancers in single-agent phase II trials. This lack of activity may have a biological basis but may also reflect a multidrug-resistance phenotype resulting from intensive combination chemotherapy regimens received by children before enrollment in the phase II trial. To overcome this problem, a few agents have been tested in children with newly diagnosed cancers before starting standard therapy. These phase II window studies, which evaluate response after one or two treatment cycles of an investigational agent, have detected activity with agents that showed no activity in individuals with relapsed cancers.4
The focus of anticancer drug discovery and development has shifted to newer classes of drugs that selectively target proteins and signal transduction pathways that are directly involved in the development and maintenance of the malignant phenotype in cancer cells. Unlike the situation with conventional cytotoxic agents, the application of these new molecularly targeted drugs to childhood cancers will depend on knowledge of the role of the drug’s target(s) in the pathogenesis of the various types of childhood cancer. The clinical development of these agents for childhood cancers creates new challenges.
Most molecularly targeted drugs that are selected for clinical development block targets found in common cancers in adults. Selection of appropriate agents to develop for children and of specific childhood cancers in which to test these drugs must be based on the role that the drugs’ targets play in the pathogenesis of these cancers. Merely demonstrating that the target is expressed in one or more types of childhood cancer may not provide adequate evidence to predict activity of the agent. Many molecularly targeted drugs, such as the tyrosine kinase receptor inhibitors, block multiple targets, and an “off-target” effect for which the drug is not being clinically developed in adults may be applicable for a childhood cancer. Preclinical testing should focus on understanding the role of drug targets in various childhood cancers and narrowing the range of cancer types in which the agents should be clinically tested.
The greater tolerance of cytotoxic agents in children as compared with adults may not translate to molecularly targeted drugs that have a substantially different mechanism of action and toxicity profile than the conventional anticancer drugs. Many of the receptors and signal transduction pathways (including angiogenesis) that are targeted by these agents play an important role in normal growth and development, and children may therefore be more sensitive to potential toxic effects of these agents than adults, although these effects may be clinically expressed only after prolonged exposure to the drug. Until more safety data are available for these new classes of drugs, consideration should be given to selecting a starting dose that is lower than the traditional 80% of the adult recommended dose for pediatric phase I trials, and patients should be monitored for effects on normal growth and development during chronic administration.
Many of the molecularly targeted drugs are orally administered, and the adult dose is usually fixed rather than normalized to body weight or surface area, which is the traditional dosing method for most cytotoxic anticancer drugs. Fixed dosing is not feasible for children because of the wide range in size and the age-dependent developmental changes that may influence drug disposition. The tablet size is usually tailored for fixed dosing in adults and may be too large to allow for accurate dosing in children in dose-finding studies. Pediatric formulations of these agents will be critical to their clinical development in children.
The traditional end points for phase I (toxicity) and II (response) trials may not be as applicable to molecularly targeted drugs. The more selective mechanism of action may mean that a maximum therapeutic dose can be achieved before dose-limiting toxicity is observed in dose-finding studies, and the cytostatic rather than cytotoxic nature of these agents may mean that delaying tumor growth rather than causing tumor shrinkage is a more realistic measure of a therapeutic effect. New end points should be explored to define the optimal dose and activity of these agents. For example, in the absence of significant toxicity, measurement of target inhibition as a function of the dose may allow for definition of a therapeutic dose without the time delays required when assessing response or survival end points. Direct measurement of target inhibition in tumor tissue in children is not feasible except in those with circulating leukemic cells, but surrogate tissues, such as peripheral blood cells, can often be used.
The final challenge once active agents have been identified will be the integration of these agents into front-line therapy. Because of the high cure rates in many childhood cancers with conventional cytotoxic chemotherapy, it is unlikely that molecularly targeted drugs will supplant our current standard treatment regimens. However, the incorporation of active molecularly targeted drugs could enhance the overall antitumor effect of chemotherapy, and the nonoverlapping mechanism of action and toxicity profile may make these agents ideal for combining with cytotoxic drugs.
The shift in focus from cytotoxic to molecularly targeted anticancer drugs will require new approaches to clinical drug development for childhood cancers, and these new approaches may involve the application of basic pharmacological principles to developing new trial designs and end points.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.National Cancer Institute. SEER Cancer Statistics Review, 1975–2005. <http://seer.cancer.gov/report_to_nation/> (2008).
- 2.Crist WM et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J. Clin. Oncol 19, 3091–3102 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Lee DP, Skolnik JM & Adamson PC Pediatric phase I trials in oncology: an analysis of study conduct efficiency. J. Clin. Oncol 23, 8431–8341 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Pappo AS et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. J. Clin. Oncol 25, 362–369 (2007). [DOI] [PubMed] [Google Scholar]