Abstract
Neurofibromatosis type 1 (NF1) is an autosomal dominant genetic disorder affecting about 1 in 3,500 individuals. Chronic pain is commonly reported among individuals with NF1 and plexiform neurofibroma tumors (PNs). Acceptance and Commitment Therapy (ACT), an empirically supported method for addressing chronic pain, helps individuals re-focus on valued relationships and activities. This pilot study investigated the feasibility and preliminary efficacy of a brief ACT workshop in the NF1 population. Eligible participants included adolescents and young adults (AYA; 12–21 years) with NF1 and chronic pain that interfered with daily functioning and their parents. Patients and parents completed baseline measures of pain interference, pain intensity, functional disability, pain acceptance, depression, and anxiety. Then, AYA and parents participated separately in a 2-day small-group ACT workshop. A telephone booster session occurred 1 month post-intervention. Three-month post-treatment measures were completed by mail. Ten adolescents (4 males; M age = 16.9 years) and seven parents provided baseline and 3-month data. Mean satisfaction with the study was moderate to high (3.9 for patients and 4.6 for parents on a 1–5 scales). Patients and parents reported significant declines in patients’ pain interference at 3 months post-treatment. Patient-reported pain intensity significantly declined from baseline to 3 months. Parents reported marginally greater acceptance of their child’s pain. No changes emerged in functional ability or mood. Preliminary findings suggest that a brief ACT group intervention is feasible and may help AYA with NF1 and PNscope with their chronic pain, although larger randomized studies are needed to confirm treatment efficacy.
Keywords: acceptance, mindfulness, Neurofibromatosis 1, adolescents and young adults, parents
INTRODUCTION
Neurofibromatosis type 1 (NF1) is an autosomal dominant genetic disorder caused by a mutation on chromosome 17 that affects approximately 1 in 3,500 individuals [Tonsgard, 2006]. The clinical presentation varies considerably between patients [Rieley et al., 2011], even in patients carrying the identical germline NF1 mutation. Manifestations and complications include plexiform neurofibromas (PNs), café au lait macules, dermal neurofibromas, optic pathway gliomas, Lisch nodules, scoliosis, malignant peripheral nerve sheath tumors (MPNSTs), as well as learning disabilities, attention problems, and social-emotional problems [Tonsgard, 2006].
Multiple clinical manifestations of NF1 can cause associated pain. For example, PNs are benign, sometimes painful tumors comprised of a proliferation of cells in the nerve sheath present in one-third to over half of people with NF1 [Tucker et al., 2009; Jett and Friedman, 2010]. Individuals with these tumors can have disfigurement, significant functional deficits [Mautner et al., 2010], and reduced activities of daily living [Martin et al., 2014]. Other NF-related manifestations that can cause pain include frequent headaches, scoliosis, and other orthopedic problems, pseudoarthrosis, and gastrointestinal complications [Tonsgard, 2006; Georgescu et al., 2007; Brems et al., 2009].
Despite efforts to develop and investigate chemotherapeutic agents, the first-line treatment for PNs is surgical debulking, but outcomes often are compromised due to the involvement of nearby nerve tissue or other vital structures, and tumor regrowth following surgery is common [Jett and Friedman, 2010]. Pain from PNs and other NF1-related conditions sometimes is managed with medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), anticonvulsants, and narcotics. However, many people taking pain medications experience side effects such as nausea, loss of appetite, and sedation [Swann, 2001; Rodriguez et al., 2008], and may continue to report pain [Wolters et al., 2015].
Acceptance and Commitment Therapy (ACT [Hayes et al., 1999]) is a psychological treatment that has been used to minimize pain interference and maximize quality of life (QOL) among adolescents and adults with chronic pain [Wicksell et al., 2009; Buhrman et al., 2013]. Such individuals tend to spend extensive energy trying to avoid or control their pain, which is often futile. Through processes such as mindfulness and acceptance, individuals can let go of the struggle against pain that cannot be changed, focus more intently on things that matter to them, and engage in behaviors that are consistent with their chosen values [Hayes et al., 1999]. Other ACT processes focus on increasing psychological flexibility through creating distance from unhelpful thoughts (defusion), and enhancing willingness to engage in valued activities even in the presence of painful thoughts or sensations [Hayes et al., 1999; Prevedini et al., 2011]. As such, the goal of ACT is not to eliminate pain, but to change the person’s relationship with their pain so that it is not the defining feature of their life. This shift in perspective resulting from the aforementioned ACT processes ultimately helps the person achieve and maintain a higher QOL [McCracken and Eccleston, 2003; Wicksell et al., 2011].
Although no published studies have utilized ACT in individuals with NF1 to our knowledge, researchers have examined the effectiveness of ACT in various chronic pain populations. Compared to wait-list control groups and usual treatments, ACT has improved pain-related and psychological outcomes among adults with conditions such as fibromyalgia, chronic back pain, and generalized pain [Thorsell et al., 2011; Buhrman et al., 2013; Wicksell et al., 2013], and in youth with idiopathic pain [Wicksell et al., 2009]. A meta-analysis reported moderate effect sizes for acceptance-based interventions on pain intensity, depression, anxiety, and QOL [Veehof et al., 2011]. Overall, research indicates that ACT is at least as helpful as other methodologies (e.g., cognitive behavioral therapy, relaxation) for individuals with various types of pain. However, NF1 is distinct from these other conditions in terms of the probable neuropathic nature of the pain [Ferner et al., 2004] as well as the fact that many individuals with NF1 have learning and attention problems; thus, investigations on the feasibility and efficacy of ACT within this population are needed.
Psychological treatments for youth often involve the parents. In AYA with NF1, this may be particularly important since many of these youth continue living with their parents well after high school graduation [Martin et al., 2014]. Parental involvement in psychological interventions can improve adolescent outcomes [Eccleston et al., 2012]. Further, parents of children with chronic illness may benefit from interventions themselves, since they often report higher levels of stress and depression than parents of healthy children [Patino-Fernandez et al., 2008; Szabo et al., 2010]. ACT therapists help parents shift their focus from alleviating their child’s pain to helping their child live a valued life in the presence of their pain [Wicksell et al., 2009].
Numerous researchers have found ACT to be effective among adolescents and adults when delivered via weekly group sessions [Butryn et al., 2011; Gauntlett-Gilbert et al., 2013] and when delivered in a brief format (e.g., in as little as four 1-hr sessions [Dahl et al., 2004]). To our knowledge, a brief ACT group workshop for pain with parallel parent and youth modules has not been investigated.
Study Objectives
The lack of published studies examining psychological interventions for youth with NF1 and chronic pain points to a noteworthy gap in the literature. To address this gap, this pilot study assessed the feasibility and preliminary efficacy of a 2-day ACT group workshop for AYA with NF1 and chronic pain and a corresponding workshop for their parents. Our primary aim was to assess feasibility by examining adherence to the intervention, rates of missing data, and study satisfaction. Secondary aims were to explore changes in pain interference (i.e., how much pain interferes with daily functioning), pain intensity, functional disability, pain acceptance, pain-related anxiety, depression, and QOL in the patients from baseline to 3 months post-intervention. We also assessed changes in parent mood and acceptance of their child’s pain.
MATERIALS AND METHODS
Eligibility Criteria
Eligible AYA participants were between 12 and 21 years of age and were enrolled on an NF1 natural history or treatment study at a government research institute. All patients must have had a diagnosis of NF1 according to the NIH Consensus Conference criteria [Stumpf et al., 1988] or have had a confirmed germline NF1 mutation with analysis performed in a CLIA-certified laboratory. Patients also must have had a response of three or higher on a pain interference item (1–5 scales) from a self-report or parent-report QOL measure (Impact of Pediatric Illness Scale [Wolters et al., 2010]) assessing the extent to which pain interfered with the patient’s daily functioning over the past month. They could not be participating in other medical or behavioral treatment studies for pain management or scheduled to begin a new treatment protocol for their NF1 during the 3 months on the current study. Finally, patients also had to be living with a primary caregiver who was willing to participate in the parent intervention.
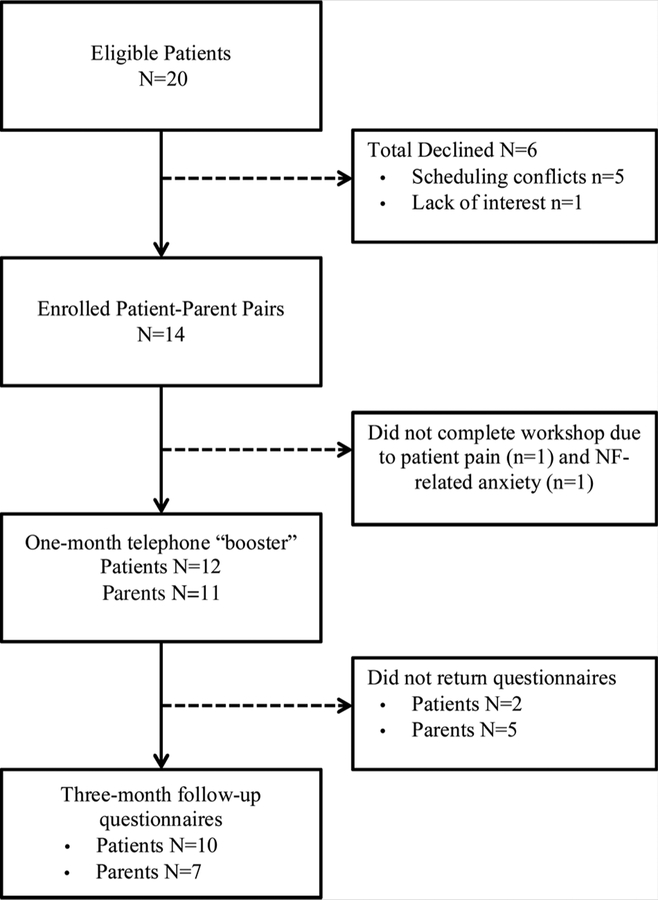
In total, 20 eligible patient–parent pairs were identified (see Fig. 1). Of these pairs, 14 (70%) agreed to participate and were enrolled. The primary reason cited for non-participation was difficulty traveling to the research facility from distant locations. The majority of enrolled patients (86%) took part in all three workshop sessions, and 83% of patients and 56% of parents who completed the workshop provided complete follow-up data.
FIG. 1.
Flowchart of patient recruitment and study completion.
Measures
Questionnaires were administered to patients and parents at baseline and at the 3-month follow-up assessment. These validated measures assessed domains of pain interference, pain intensity, functional disability, pain acceptance, pain-related anxiety, depression, health-related quality of life, parent psychological distress, and disease severity (completed by a nurse practitioner). In addition, two scales developed by the authors for the current study assessed pain management techniques used by the patients (Pain Management Inventory) and treatment adherence and study satisfaction (Post-Treatment Questionnaire). Details on these measures are in Table I.
Table I.
Measures Administered
| Measure | No. of items | Format/scoring | Description | References |
|---|---|---|---|---|
| Modified brief pain inventory | 12 | 1–10 Likert; mean scores | Assesses extent to which pain interfered with activities (e.g., walking, recreational activities, sleep) in past week. | Engel et al. [2009] |
| Pain interference index—self-report and parent report | 6 | 0–6 Likert; mean scores | Assesses how much pain has interfered with sleep, mood, social activities, etc. in past two weeks | Wicksell et al. [2009] and Martin et al., [2015] |
| Pain intensity | 1 | 1–100 visual analogue scale | Patients mark on a 100 mm line to indicate pain severity in past week | Melzack, [1987] and Cohen et al. [2008] |
| Functional disability index—self-report and parent report | 15 | 0–4 Likert; sum of raw scores | Assesses ability to participate in daily activities, (e.g., walking up stairs, doing chores, running) | McGrath et al. [2008] and Palermo, [2009] |
| Chronic pain acceptance questionnaire–adolescent report | 20 | 0–4 Likert; sum of raw scores | Assesses ability to accept one’s pain | McCracken et al. [2010a] and Wallace et al. [2011] |
| Parent acceptance of pediatric illness questionnaire | 31 | 0–6 Likert; sum of raw scores | Assesses parent’s willingness to accept their child’s chronic illness and associated symptoms | Masuda et al. [2011] |
| Pain anxiety symptoms scale-20 | 20 | 0–5 Likert; sum of raw scores | Assesses pain-related anxiety in medical patients | McCracken and Dhingra [2002] |
| Center for epidemiological studies depression scale | 20 | 0–3 Likert; sum of raw scores | Assesses frequently of symptoms (e.g., feeling depressed, enjoying life) in past week | Radloff [1977] and Choi et al. [2010] |
| Impact of pediatric illness scale— self-report and parent report | 46 | 1–5 Likert; mean scores transformed to 0–100 scales | Assesses health-related quality of life (e.g., daily functioning, emotional functioning, medical/physical status, cognitive functioning) | Wolters et al. [2010, 2013] |
| Brief symptom index 18 | 18 | 0–4 Likert; T-scores | Assesses psychological distress (e.g., anxiety, depression, and somatization); administered to parents in our study | Derogatis [2000] and Yagci-Kupeli et al. [2012] |
| Pain management inventory | 2 | Yes/no and Likert | Assesses what pharmacological and non-pharmacological techniques patients employed to manage their pain in past 3 months | N/A; developed by authors for this study |
| NF disease severity | 1 | Multiple choice | Assesses NF disease severity; provides verbal descriptions of symptoms for each category (mild, moderate, severe); takes into account factors such as presence of tumors that impact posture, gait, or vision; completed by nurse-practitioner who conducted physical examinations on patients on this study | Adapted from Ablon [1996] and Martin et al. [2015] |
| Post-treatment questionnaire—self-report and parent versions | 5 | Likert and open-ended | Assesses participants’ use of ACT techniques at home (treatment adherence) and study satisfaction | Adapted from Curran et al. [2009] by authors of current study |
Procedures
The research site’s Institutional Review Board approved study procedures (registered with clinicaltrials.gov, #). Adult patients, or parents of patients younger than 18 years, were contacted by phone if they had indicated the presence of chronic pain to a nurse practitioner during a physical examination at their most recent visit (typically within the past year). If eligible, interested families were scheduled to attend a small group workshop (3–5 patients per group) with approximately same-aged peers. When possible, this visit was scheduled to coincide with a previously scheduled medical visit. A nurse practitioner conducted a history and physical exam, including a thorough pain assessment, and baseline questionnaires were completed. Following baseline procedures, the ACT intervention was implemented in three 2-hr sessions occurring over 2 days. The 2-day workshop format was chosen since most families are referred to our NF1 program from around the United States. Thus, weekly sessions were not practical. One month after the workshop, patients and parents were contacted by telephone for a “booster” session. Three months post-intervention, participants were asked to complete follow-up questionnaires by mail. If forms were not returned within 2 weeks, a researcher called the participant to confirm receipt and to encourage the participant to return them as soon as possible.
ACT Intervention
The ACT workshops consisted of a patient program and corresponding parent program, each focused on helping participants cope with the patient’s pain more effectively. Two therapists conducted each group. Workshop content was adapted from manualized ACT interventions outlined in books [Hayes et al., 1999; Dahl et al., 2005] and articles [Wicksell et al., 2007; Vowles et al., 2009; Masuda et al., 2011]. We modified some techniques to make them more appropriate for teenagers. While the manual content did not differ for younger versus older adolescents, some specific examples of ACT concepts varied. For example, when discussing values, the younger patients were more focused on sports and friends, while the older adolescents often brought up values related to academics, career, and romantic relationships.
During the first workshop session with the AYA, the nurse practitioner briefly discussed physiological aspects of pain in NF1. Patients were informed that if they had any new pain or a significant increase in pain while on study, they should contact their physician. The remainder of the sessions focused on ACT-specific techniques and discussion. Briefly, content included an overview and practice of mindfulness techniques, such as mindful breathing. We also discussed how pain has interfered with the patients’ daily lives, specifically with respect to achieving values-consistent goals. Patients were encouraged to try defusion techniques, such as physicalizing their pain (i.e., picturing their pain as a separate form or shape) [Hayes et al., 1999] and verbalizing fused thought content using a silly or different voice. Acceptance was practiced by engaging in imagery whereby patients visualized their pain inside their body and imagined making space for it instead of trying to get rid of it [Harris, 2008]. Finally, workshop facilitators helped patients outline short- and long-term goals consistent with their identified values, and worked to increase willingness to commit to these goals while living with their pain.
The parent workshop focused on using ACT techniques to support their child in living more consistently with his or her values, and to help themselves cope with feelings about their child’s pain. Parents were encouraged to practice mindfulness, to increase their acceptance of their child’s condition, and to establish goals consistent with their identified parenting values.
All participants were assigned ACT exercises to practice on their own between each session. Patients and parents were also given a workbook to take home that included a summary of the topics discussed and exercises for continuing to work on ACT techniques at home.
One-Month Telephone “Booster” Session
Four to six weeks after the baseline visit, a therapist called all patients and parents individually for a “booster” session. During these 15–20 min calls, participants were asked about the progress they had made toward their goals. The core ACT principles and techniques were reviewed, including strategies for overcoming barriers or challenges the participants reported experiencing. The therapist encouraged them to continue working on their goals. Parents were asked about their child’s progress and their own use of ACT strategies to help them in their parenting goals.
Statistical Analyses
Descriptive statistics were computed on the variables of interest (means, standard deviations). Due to the small sample size, the Wilcoxon signed rank test was used to assess changes on the outcome measures from baseline to 3 months. Spearman correlations were conducted to assess the relationships between primary and secondary measures.
RESULTS
Demographic and Medical Characteristics
Six females and six males completed the patient intervention. Of these, two did not return their 3-month questionnaires, so data for the 10 patients who completed all time points are presented in the sections that follow. Also, we were unable to reach one mother for the 1-month booster session, but included her 3-month data in the analyses. The small sample size precluded statistical examination of pre-intervention differences between those who provided complete data and those who did not. The mean age of the ten patients who completed the study was 16.9 years (SD = 2.9, range 12–20 years). All of the patients were enrolled on an NF1 natural history study at our center; none of the youth were on active treatment for their NF1 (e.g., for tumor shrinkage) during the course of their participation on the current study. Eight of the ten patients were taking pain medication regularly at the baseline evaluation. Nonpharmacological techniques to address pain included yoga (n = 1), massage (n = 1), and hydrotherapy (n = 1). Regarding parents, nine mothers and three fathers completed the workshop. The mean level of parent education was 14.1 years (SD = 1.7, range 12–17 years). Patient age, gender, and parent education were unrelated to primary study variables. Further demographic and medical variables for these patients are shown in Table II.
Table II.
Baseline Demographic and Medical Variables for Participants With Complete Data
| Characteristic | n | % |
|---|---|---|
| Male | 4 | 40 |
| Race | ||
| White | 8 | 80 |
| Hispanic | 1 | 20 |
| Biracial | 1 | 20 |
| Pain medications | ||
| 0 | 2 | 20 |
| 1–2 | 6 | 60 |
| 3–4 | 2 | 20 |
| Disease severity | ||
| Mild | 3 | 30 |
| Moderate | 5 | 50 |
| Severe | 2 | 20 |
| Number of past surgeriesa | ||
| 0 | 4 | 40 |
| 1–2 | 4 | 40 |
| 3–5 | 2 | 20 |
| >5 | 0 | 0 |
| Parent participants | ||
| Mothers | 8 | 80 |
| Fathers | 2 | 20 |
Surgeries are counted if they involved tumor reduction or were otherwise directly related to disease.
Pre-Post Differences
As shown in Table III, patient-reported pain interference on the MBPI was significantly lower at the 3-month follow-up compared to baseline (P = 0.04), although the same results were not found on the self-report PII (P = 0.14). Parent ratings on the PII declined, indicating less pain interference in the patients, from baseline to the 3-month follow-up (P = 0.02).
Table III.
Differences Between Patient and Parent Mean Scores (Baseline to 3 Months)
| Baseline | 3 Months | P* | |
|---|---|---|---|
| Patient measures | M (SD) | M (SD) | |
| MBPI | 1.8 (1.1) | 1.1 (0.9) | 0.04 |
| Pll | 2.1 (1.0) | 1.5 (1.1) | 0.14 |
| McGill VAS | 39.0 (25.1) | 27.5 (25.2) | 0.01 |
| FDI | 12.5 (6.9) | 10.4 (6.3) | 0.37 |
| CPAQ-A | 517 (11.0) | 52.5 (11.1) | 0.55 |
| PASS-20 | 19.8 (10.6) | 20.8 (16.1) | 0.98 |
| CES-D | 13.3 (7.9) | 12.8 (10.1) | 0.52 |
| IPI | 69.0 (11.0) | 70.5 (107) | 0.17 |
| Parent measures | |||
| Pll | 3.4 (0.93) | 2.0 (1.17) | 0.02 |
| PAPIQ | 85.5 (31.9) | 104.0 (23.2) | 0.07 |
| FDI | 15.3 (9.8) | 18.2 (8.0) | 0.69 |
| IPI | 57.8 (9.5) | 59.4 (6.8) | 0.16 |
| BSI global symptoms index | 60.6 (9.4) | 537 (8.8) | 0.99 |
MBPI, Modified Brief Pain Inventory; PII, Pain Interference Index; VAS, visual analogue scale; FDI, Functional Disability Index; CPAQ-A, Chronic Pain Acceptance Questionnaire-Adolescent Version; PASS-20, Pain Anxiety Symptoms Scale; CES-D, Center for Epidemiological Scales, Depression; IPI, Impact of Pediatric Illness Scale; PAPIQ, Parent Acceptance of Pediatric Illness Questionnaire; BSI, Brief Symptom Inventory.
Significance of Wilcoxon signed rank test.
Patient VAS ratings of their pain intensity were significantly lower at the 3-month time point compared to baseline (P = 0.01). Furthermore, parent PAPIQ scores increased, indicating greater acceptance of their child’s pain, from baseline to 3 months, but this difference did not reach statistical significance (P = 0.07). No pre-post changes were evident with respect to functional ability, anxiety, depression, or quality of life by patient or parent report (P values range from 0.17 to 0.99; see Table III).
Six of the ten patients were taking less pain medication at follow-up compared to baseline; of these, three were taking fewer medications, two were taking a lower dose, and one was taking both fewer medications and a lower dose. Two patients were taking more medications and one increased the dose of her current medication. One patient was not taking any medications at either time point.
Adherence to the Intervention
Sixty percent of patients reported using mindfulness at least weekly and 60% reported using defusion techniques at least weekly. Eighty percent of patients endorsed practicing willingness (i.e., engaging in valued activities while in pain) weekly or more. Most parents reported engaging in mindfulness (71%) and defusion (71%) weekly or more. Likewise, 71% reported encouraging their child to do a valued activity while in pain at least once a week.
Overall Satisfaction With Study
At the 3-month follow-up, the mean rating of how much patients liked participating in the study was 3.9 out of 5 (SD = 1.3, range 1–5). The mean parent rating was 4.6 out of 5 (SD = 0.51, range 4–5). Patient satisfaction ratings were not correlated with any outcome measures or adherence variables (all Ps > 0.05). The restricted range in parent satisfaction ratings (i.e., four parents rated satisfaction as 5 out of 5, three parents as 4 out of 5) prevented analysis of how these ratings correlated with study variables. On the open-ended items, patients’ comments regarding what they liked about the study included, “it taught me great strategies to deal with my pain,” and “talking to other kids with NF.” On the other hand, one 15-year old female wrote, “I would have liked to learn more and seen more examples of what we did. Sometimes I feel what I’m doing is wrong.”
Parent responses to the open-ended question about what was most helpful included, “It has helped me deal not only with [my child’s] pain but pain associated with myself and others as well. It has helped out tremendously in our family!” Another parent wrote that it helps her “take time before reacting,” while another indicated that ACT techniques help with “bonding with my daughter [and] helping my daughter deal with pain.” Responses about barriers to practicing ACT techniques included “forgetting to use them” and “reverting back to usual responses.”
DISCUSSION
Based on results of this pilot study, the 2-day, small group ACT workshops seem to be a feasible and effective intervention for AYA with NF1 and chronic pain. Overall, patients and parents liked participating in the study, which is encouraging with respect to feasibility. Eighty-six percent of enrolled families completed all workshop sessions, while 83% of patients and 56% of parents returned their three-month questionnaires by mail. Electronic data collection may have increased our response rates.
The rates of adherence to the intervention were fairly strong, and in line with other acceptance-based treatment protocols [Woods et al., 2006; Goodwin et al., 2012]. Having the parents participate in a parallel workshop so that they could support their child in practicing ACT-related skills may have contributed to the patient’s adherence to the intervention at home (and vice versa). This is consistent with prior research supporting the involvement of parents in interventions geared toward adolescents [Young et al., 2007; Stanger et al., 2013].
Regarding preliminary efficacy, the ACT intervention resulted in less pain interference in the AYA according to patients (on the MBPI) and parents (on the PII parent form). We did not see similar changes on the measure of functional disability (FDI), possibly because the PII specifically asks respondents to consider how much pain has interfered with activities, whereas the FDI asks about actual abilities; thus, limitations may relate less to pain than other factors (e.g., tumor location or size).
Follow-up data also revealed significantly lower patient-reported pain intensity. While this is not a goal of ACT, it is not an uncommon finding [Wicksell et al., 2009; McCracken and Gutierrez-Martinez, 2011; Thorsell et al., 2011]. From an ACT perspective, this may result from increased mindfulness (noticing one’s pain without judging it). A growing body of literature has found that even brief mindfulness-based interventions can reduce reported levels of acute [Zeidan et al., 2012] and chronic pain [Ussher et al., 2014]. Initial research suggests that the mechanisms behind the pain relief may be related to decreased brain activation in areas associated with pain that occurs through repeated mindfulness practice [deCharms et al., 2005; Gard et al., 2012]. In addition, individuals who are engaged in values-based activities tend to report less pain intensity [McCracken et al., 2010b].
It is encouraging that improvements in several pain-related outcomes were found in spite of the brevity of the in-person intervention (4 hr total). On the other hand, results are offered with an emphasis on cautious interpretation in light of our small sample size. Larger studies with additional patients and comparison groups are needed to confirm our findings.
As mentioned previously, the NF1 phenotype commonly includes learning problems. Previous studies examining ACT interventions in youth with chronic pain have not examined the participants’ level of cognitive functioning. Among the patients in our study with complete data, most had been diagnosed either with a learning disability (20%), attention deficit hyperactivity disorder (ADHD; 20%), or both (30%). These data were obtained by parental report (n = 10) and review of neuropsychological testing results (n = 9). Despite this fact, most participants seemed to understand the concepts introduced and were able to benefit from the intervention, as evidenced by lower group mean scores on the pain interference and pain intensity measures at the follow-up assessment.
Although changes in parents’ acceptance of their child’s pain did not reach statistical significance, the raw pre-post differences may be viewed with cautious optimism. Caregivers of pediatric medical patients have been found to have poorer psychosocial outcomes and QOL compared to population norms [Klassen et al., 2008], whereas parental acceptance of their adolescent’s cancer pain has been linked to better psychological wellbeing [Gauthier et al., 2009]. Even a small change in acceptance could be impactful for caregivers struggling to support a child with NF1.
Limitations and Future Directions
As mentioned previously, results of this pilot study should be interpreted in the context of our small sample size. This prevented our ability to do subgroup analyses, so we do not know if the intervention worked differently for patients with varying levels of pain or of different ages (younger vs. older adolescents), or regarding maternal versus paternal participation. Further, because this pilot study lacked a control group, we cannot be sure that the improvements noted were not due to placebo effects. Larger, randomized trials with longer follow-up intervals and inclusion of potential mediating variables (e.g., baseline anxiety and depression) will be essential to expand on our preliminary results. Since all 10 of our patients had PNs, results may not be representative of the entire population of AYA with NF1. Moreover, our cohort should be considered unique with respect to the fact that these families travel from all over the country in order to participate in a research study at this institution; as such, they may be more motivated (possibly due to more severe disease) or may be higher functioning than the average family with a child with NF1. Finally, only 64% of eligible families agreed to participate, mainly due to difficulty scheduling a trip to the research facility. This intervention might have had a higher enrollment rate if implemented in a facility where patients are recruited locally.
CONCLUSIONS
Treatment options for painful NF1-related symptoms are limited. This pilot study is the first to examine feasibility and efficacy of a brief, ACT group workshop for both patients with chronic pain and their parents. Adherence to the intervention and study satisfaction rates support the feasibility of this program. Moreover, preliminary results suggest that ACT may offer an effective tool for decreasing pain interference and pain intensity in AYA with NF1 and chronic pain. It also may help parents increase their acceptance of their child’s pain, although randomized studies with larger samples are needed to confirm these tentative findings. Importantly, even a brief ACT intervention with minimal follow-up may lead to improved pain outcomes, and mental health clinicians are encouraged to implement ACT techniques within NF1 clinics. Thus, this pilot study offers initial support for the use of ACT as a viable alternative to pain medication or as a supplement to medical treatment in youth with NF1 and chronic pain.
ACKNOWLEDGMENTS
The authors are thankful to the families who participated in this study. We also are grateful for the support of Katherine Burns, PhD and Ethan Eisen, MPhil, both of whom assisted with administering the intervention. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. In addition, this project was funded in part by the NCI, NIH, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Conflicts of interest: Dr. Martin received sponsored travel from the Children’s Tumor Foundation in 2013 and 2015, and has funding from the Neurofibromatosis Therapeutics Acceleration Program for a separate project. Ms. Toledo-Tamula is employed by Leidos Biomedical Research Inc. through a contract with the National Cancer Institute. Dr. Wolters was awarded grants from the Childhood Brain Tumor Foundation (2012) and the Neurofibromatosis Therapeutics Acceleration Program (2013). For remaining authors, none declared.
REFERENCES
- Ablon J 1996. Gender response to neurofibromatosis 1. Soc Sci Med 42:99–109. [DOI] [PubMed] [Google Scholar]
- Brems H, Park C, Maertens O, Pemov A, Messiaen L, Upadhyaya M, Claes K, Beert E, Peeters K, Mautner V, Sloan JL, Yao L, Lee CC, Sciot R, De Smet L, Legius E, Stewart DR. 2009. Glomus tumors in neurofibromatosis type 1: Genetic, functional, and clinical evidence of a novel association. Cancer Res 69:7393–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrman M, Skoglund A, Husell J, Bergstrom K, Gordh T, Hursti T, Bendelin N, Furmark T, Andersson G. 2013. Guided internet-delivered acceptance and commitment therapy for chronic pain patients: A randomized controlled trial. Behav Res Ther 51:307–315. [DOI] [PubMed] [Google Scholar]
- Butryn ML, Forman E, Hoffman K, Shaw J, Juarascio A. 2011. A pilot study of acceptance and commitment therapy for promotion of physical activity. J Phys Act Health 8:516–522. [DOI] [PubMed] [Google Scholar]
- Choi S, Kim DY, Whang SH, Lee JH, Hann SK, Shin YJ. 2010. Quality of life and psychological adaptation of Korean adolescents with vitiligo. J Eur Acad Dermatol Venereol 24:524–529. [DOI] [PubMed] [Google Scholar]
- Cohen LL, Lemanek K, Blount RL, Dahlquist LM, Lim CS, Palermo TM, McKenna KD, Weiss KE. 2008. Evidence-based assessment of pediatric pain. J Pediatr Psychol 33:939––955; discussion 956–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran CA, Williams ACD, Potts HWW. 2009. Cognitive-behavioral therapy for persistent pain: Does adherence after treatment affect outcome? Eur J Pain 13:178–188. [DOI] [PubMed] [Google Scholar]
- Dahl J, Wilson KG, Luciano C, Hayes SC. 2005. Acceptance and commitment therapy for chronic pain. Reno, NV: Context Press. [Google Scholar]
- Dahl J, Wilson KG, Nilsson A. 2004. Acceptance and commitment therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: A preliminary randomized trial. Behav Therapy 35:785–801. [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. 2005. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci USA 102:18626–18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L 2000. Brief symptom inventory 18 Minneapolis, MN:National Computer Systems, Inc. [Google Scholar]
- Eccleston C, Palermo TM, Fisher E, Law E. 2012. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev 8:CD009660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JM, Kartin D, Carter GT, Jensen MP, Jaffe KM. 2009. Pain in youths with neuromuscular disease. Am J Hosp Palliat Care 26:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner RE, Hughes RA, Hall SM, Upadhyaya M, Johnson MR. 2004. Neurofibromatous neuropathy in neurofibromatosis 1 (NF1). J Med Genet 41:837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T, Holzel BK, Sack AT, Hempel H, Lazar SW, Vaitl D, Ott U. 2012. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex 22:2692–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntlett-Gilbert J, Connell H, Clinch J, McCracken LM. 2013. Acceptance and values-based treatment of adolescents with chronic pain: Outcomes and their relationship to acceptance. J Pediatr Psychol 38:72–81. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Rodin G, Zimmermann C, Warr D, Moore M, Shepherd F, Gagliese L. 2009. Acceptance of pain: A study in patients with advanced cancer. Pain 143:147–154. [DOI] [PubMed] [Google Scholar]
- Georgescu EF, Stanescu L, Georgescu AC, Dumitrescu D, Foarfa C, Calin G. 2007. Bone abnormalities occurring in the follow-up of the patients with neurofibromatosis type 1. Rom J Morphol Embryol 48:249–256. [PubMed] [Google Scholar]
- Goodwin CL, Forman EM, Herbert JD, Butryn ML, Ledley GS. 2012. A pilot study examining the initial effectiveness of a brief acceptance-based behavior therapy for modifying diet and physical activity among cardiac patients. Behav Modif 36:199–217. [DOI] [PubMed] [Google Scholar]
- Harris R 2008. The happiness trap: How to stop struggling and start living. Boston, MA: Shambhala Publications, Inc. [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. 1999. Acceptance and commitment therapy: An experiential approach to behavior change. New York, NY: The Guilford Press. [Google Scholar]
- Jett K, Friedman JM. 2010. Clinical and genetic aspects of neurofibromatosis 1. Genet Med 12:1–11. [DOI] [PubMed] [Google Scholar]
- Klassen AF, Klaassen R, Dix D, Pritchard S, Yanofsky R, O’Donnell M, Scott A, Sung L. 2008. Impact of caring for a child with cancer on parents’ health-related quality of life. J Clin Oncol 26:5884–5889. [DOI] [PubMed] [Google Scholar]
- Martin S, Nelson Schmitt S, Wolters PL, Abel B, Toledo-Tamula MA, Baldwin A, Wicksell RK, Merchant M, Widemann B. 2015. Development and validation of the english pain interference index and pain interference index-Parent report. Pain Med 16:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Wolters P, Toledo-Tamula MA, Young R, Baldwin A, Gillespie A, Widemann B. 2014. Adolescents and young adults (AYA) with neurofibromatosis type 1 and plexiform neurofibromas (PNs): A cross-sectional profile of adaptive skills. Philadelphia, PA: Society of Pediatric Psychology Conference. [Google Scholar]
- Masuda A, Cohen LL, Wicksell RK, Kemani MK, Johnson A. 2011. A case study: Acceptance and commitment therapy for pediatric sickle cell disease. J Pediatr Psychol 36:398–408. [DOI] [PubMed] [Google Scholar]
- Mautner VF, Kluwe L, Friedrich RE, Roehl AC, Bammert S, Hogel J, Spori H, Cooper DN, Kehrer-Sawatzki H. 2010. Clinical characterisation of 29 neurofibromatosis type-1 patients with molecularly ascertained 1.4 Mb type-1 NF1 deletions. J Med Genet 47:623–630. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Dhingra L. 2002. A short version of the pain anxiety symptoms scale (PASS-20): Preliminary development and validity. Pain Res Manag 7:45–50. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Eccleston C. 2003. Coping or acceptance: What to do about chronic pain? Pain 105:197–204. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Gauntlett-Gilbert J, Eccleston C. 2010a. Acceptance of pain in adolescents with chronic pain: Validation of an adapted assessment instrument and preliminary correlation analyses. Eur J Pain 14:316–320. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Gutierrez-Martinez O. 2011. Processes of change in psychological flexibility in an interdisciplinary group-based treatment for chronic pain based on Acceptance and Commitment Therapy. Behav Res Ther 49:267–274. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Vowles KE, Zhao-O’Brien J. 2010b. Further development of an instrument to assess psychological flexibility in people with chronic pain. J Behav Med 33:346–354. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. 2008. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain 9:771–783. [DOI] [PubMed] [Google Scholar]
- Melzack R 1987. The short-form McGill pain questionnaire. Pain 30:191–197. [DOI] [PubMed] [Google Scholar]
- Palermo TM. 2009. Assessment of chronic pain in children: Current status and emerging topics. Pain Res Manag 14:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino-Fernandez AM, Pai AL, Alderfer M, Hwang WT, Reilly A, Kazak AE. 2008. Acute stress in parents of children newly diagnosed with cancer. Pediatr Blood Cancer 50:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevedini AB, Presti G, Rabitti E, Miselli G, Moderato P. 2011. Acceptance and commitment therapy (ACT): The foundation of the therapeutic model and an overview of its contribution to the treatment of patients with chronic physical diseases. G Ital Med Lav Ergon 33:A53–A63. [PubMed] [Google Scholar]
- Radloff LS. 1977. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401. [Google Scholar]
- Rieley MB, Stevenson DA, Viskochil DH, Tinkle BT, Martin LJ, Schorry EK. 2011. Variable expression of neurofibromatosis 1 in monozygotic twins. Am J Med Genet A 155A:478–485. [DOI] [PubMed] [Google Scholar]
- Rodriguez RF, Castillo JM, Castillo MP, Montoya O, Daza P, Rodriguez MF, Restrepo JM, Leon ME, Angel AM. 2008. Hydrocodone/acetaminophen and tramadol chlorhydrate combination tablets for the management of chronic cancer pain: A double-blind comparative trial. Clin J Pain 24:1–4. [DOI] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Delhey LM, Thrailkill K, Li Z, Li Z, Budney AJ. 2013. A multicomponent motivational intervention to improve adherence among adolescents with poorly controlled type 1 diabetes: A pilot study. J Pediatr Psychol 38:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf DA, Alksne JF, Annegers JF, Brown SS, Conneally M, Housman D, Leppert MF, Miller JP, Moss ML, Pileggi AJ, Rapin I, Strohman RC, Swanson LW, Zimmerman A. 1988. Neurofibromatosis conference statement National Institutes of Health Consensus Development Conference Bethesda, MD: Archives of Neurology; pp 575–578. [PubMed] [Google Scholar]
- Swann AC. 2001. Major system toxicities and side effects of anticonvulsants. J Clin Psychiatry 62:16–21. [PubMed] [Google Scholar]
- Szabo A, Mezei G, Kovari E, Cserhati E. 2010. Depressive symptoms amongst asthmatic children’s caregivers. Pediatr Allergy Immunol 21: e667–e673. [DOI] [PubMed] [Google Scholar]
- Thorsell J, Finnes A, Dahl J, Lundgren T, Gybrant M, Gordh T, Buhrman M. 2011. A comparative study of 2 manual-based self-Help interventions, acceptance and commitment therapy and applied relaxation, for persons with chronic pain. Clin J Pain 27:716–723. [DOI] [PubMed] [Google Scholar]
- Tonsgard JH. 2006. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol 13:2–7. [DOI] [PubMed] [Google Scholar]
- Tucker T, Friedman JM, Friedrich RE, Wenzel R, Funsterer C, Mautner VF. 2009. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J Med Genet 46:81–85. [DOI] [PubMed] [Google Scholar]
- Ussher M, Spatz A, Copland C, Nicolaou A, Cargill A, Amini-Tabrizi N, McCracken LM. 2014. Immediate effects of a brief mindfulness-based body scan on patients with chronic pain. J Behav Med 37:127–134. [DOI] [PubMed] [Google Scholar]
- Veehof MM, Oskam MJ, Schreurs KMG, Bohlmeijer ET. 2011. Acceptance-based interventions for the treatment of chronic pain: A systematic review and meta-analysis. Pain 152:533–542. [DOI] [PubMed] [Google Scholar]
- Vowles KE, Wetherell JL, Sorrell JT. 2009. Targeting acceptance, mindfulness, and values-based action in chronic pain: Findings of two preliminary trials of an outpatient group-based intervention. Cogn Behav Pract 16:49–58. [Google Scholar]
- Wallace DP, Harbeck-Weber C, Whiteside SP, Harrison TE. 2011. Adolescent acceptance of pain: Confirmatory factor analysis and further validation of the chronic pain acceptance questionnaire, adolescent version. J Pain 12:591–599. [DOI] [PubMed] [Google Scholar]
- Wicksell RK, Kemani M, Jensen K, Kosek E, Kadetoff D, Sorjonen K, Ingvar M, Olsson GL. 2013. Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial. Eur J Pain 17:599–611. [DOI] [PubMed] [Google Scholar]
- Wicksell RK, Melin L, Lekander M, Olsson GL. 2009. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain—A randomized controlled trial. Pain 141:248–257. [DOI] [PubMed] [Google Scholar]
- Wicksell RK, Melin L, Olsson GL. 2007. Exposure and acceptance in the rehabilitation of adolescents with idiopathic chronic pain—A pilot study. Eur J Pain 11:267–274. [DOI] [PubMed] [Google Scholar]
- Wicksell RK, Olsson GL, Hayes SC. 2011. Mediators of change in acceptance and commitment therapy for pediatric chronic pain. Pain 152:2792–2801. [DOI] [PubMed] [Google Scholar]
- Wolters PL, Burns KM, Martin S, Baldwin A, Dombi E, Toledo-Tamula MA, Dudley WN, Gillespie A, Widemann BC. 2015. Pain interference in youth with neurofibromatosis type 1 and plexiform neurofibromas and relation to disease severity, social-emotional functioning, and quality of life. Am J Med Genet A 167A:2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PL, Martin S, Toledo-Tamula MA, Gillespie A, Baldwin A, Widemann B. 2013. Quality of life of adolescents with neurofibromatosis type 1 (NF1) and plexiform neurofibromas (PNs): Reliability and validity of the impact of pediatric illness (IPI) Scale self-report form. New Orleans, LA: The National Conference in Pediatric Psychology. [Google Scholar]
- Wolters PL, Martin S, Walker K, Widemann BC. 2010. Impact of Illness (IPI) Scale parent form: Validity and reliability data for children with neurofibromatosis type 1 (NF1) and plexiform neurofibromas (PNs). Baltimore, MD: Children’s Tumor Foundation NF Conference. [Google Scholar]
- Woods DW, Wetterneck CT, Flessner CA. 2006. A controlled evaluation of acceptance and commitment therapy plus habit reversal for trichotillo-mania. Behav Res Ther 44:639–656. [DOI] [PubMed] [Google Scholar]
- Yagci-Kupeli B, Akyuz C, Kupeli S, Buyukpamukcu M. 2012. Health-related quality of life in pediatric cancer survivors: A multifactorial assessment including parental factors. J Pediatr Hematol Oncol 34:194–199. [DOI] [PubMed] [Google Scholar]
- Young KM, Northern JJ, Lister KM, Drummond JA, O’Brien WH. 2007. A meta-analysis of family-behavioral weight-loss treatments for children. Clin Psychol Rev 27:240–249. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. 2012. Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett 520:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]