Abstract
While persons with HIV (PWH) have benefited from significant advances in treatment and resulting longevity, mental health problems remain elevated in this population. Adverse childhood experiences (ACEs) are common among PWH and may negatively affect mental health and HIV-related outcomes. We examined the association between ACEs, depression and anxiety symptoms, substance use, and HIV-related outcomes in a sample of 584 PWH at risk for unhealthy alcohol use enrolled in a primary care-based alcohol intervention study. Participants completed a baseline questionnaire that included depression and anxiety symptoms, past-month substance use and hazardous drinking, health-related quality of life, and antiretroviral therapy (ART) adherence; and a 12-month questionnaire that included self-reported exposure to ACEs. HIV-related clinical indicators (HIV RNA levels and CD4 count) were extracted at baseline from the electronic health record. The sample was 96.9% male, 63.0% non-Hispanic white, with an average age of 49.0 years. ACEs were highly prevalent: 82.5% reported ≥1 ACE, including 34.2% reporting 1-2 ACEs, 25.0% reporting 3-4 ACEs, and 23.3% reporting ≥5 ACEs. The most prevalent ACEs were verbal abuse (45.9%) and living with someone with a substance use problem (40.1%). Adjusting for demographics, having 1-2, 3-4 or ≥5 ACEs was significantly associated with anxiety (ORs (95%CI): 3.41 (1.13-10.33), 4.36 (1.42-3.36), and 3.96 (1.28-12.19), respectively) and poorer mental health quality of life (Betas (SE): −3.21 (1.40), −6.23 (1.51), and −7.09 (1.54), respectively), but not with depression, substance use or HIV outcomes. Trauma-informed interventions to reduce anxiety and improve mental health quality of life in PWH may reduce the negative health sequelae associated with ACEs.
Keywords: adverse childhood experiences, quality of life, HIV, alcohol, epidemiology, trauma, anxiety
Introduction
Effective antiretroviral therapies now allow persons with HIV (PWH) to live healthier and longer lives (Hariri and McKenna, 2007; Marcus et al., 2016), but mental health problems remain elevated in this population. PWH suffer disproportionately from hazardous drinking and other substance use, anxiety and mood disorders, and have worse quality of life relative to the general population (Bing et al., 2001; Galvan, Burnam, & Bing, 2003; Miners et al., 2014; Myer et al., 2008; Olley, Seedat, & Stein, 2004; Pence et al., 2007; Satre, DeLorenze, Quesenberry, Tsai, & Weisner, 2013; Williams et al., 2016). Notably, mental health problems are elevated even among PWH who are virally suppressed on antiretroviral treatment (Garey et al., 2015; Heywood and Lyons, 2016). To improve patient care, it is critical to identify factors that contribute to disparities in mental health in this population.
Adverse childhood experiences (ACEs), including sexual, emotional or physical abuse, neglect, parental loss, and exposure to parental mental illness, substance misuse and domestic violence, are increasingly recognized as clinically meaningful predictors of HIV risk and HIV-related disease burden (Brown and Anda, 2009). ACEs are common among PWH (Kalichman, Sikkema, DiFonzo, Luke, & Austin, 2002; Spies et al., 2012; Whetten et al., 2006; Whetten, Reif, Whetten, & Murphy-McMillan, 2008), and contribute to health risk behaviors that increase the transmission of HIV, including hazardous drinking and illicit drug use, intimate partner violence, and risky sexual behaviors (Cohen et al., 2000; Dube et al., 2003; Hillis, Anda, Felitti, & Marchbanks, 2001; Meade, Kershaw, Hansen, & Sikkema, 2009; Mimiaga et al., 2009; Mosack et al., 2010; Wilson and Widom, 2008).
In the general population, ACEs are associated with poorer health-related quality of life (Weber, Jud, & Landolt, 2016) and have a strong, graded relationship with a broad range of psychological and medical problems (Anda et al., 2006; Centers for Disease Control and Prevention, 2016). A growing body of research suggests that ACEs are also related to psychiatric disorders, worse mental quality of life and medication adherence, faster disease progression, and greater mortality rates among PWH (Bekele et al., 2018; Brezing, Ferrara, & Freudenreich, 2015; LeGrand et al., 2015; Leserman et al., 2007; Mugavero et al., 2009; Pence et al., 2012; Pence, et al., 2007; Spies, et al., 2012; Wong, Sarkisian, Davis, Kinsler, & Cunningham, 2007). However, much of the existing research is limited to studies with small sample sizes or has only focused on childhood sexual abuse (Spies, et al., 2012).
ACEs screening is an emerging strategy for understanding potential clinical and social service needs of patients in healthcare settings (Flanagan et al., 2018; Goldstein, Athale, Sciolla, & Catz, 2017; Korotana, Dobson, Pusch, & Josephson, 2016). While ACES may not significantly affect HIV outcomes for patients actively engaged in healthcare, they may still contribute to worse mental health and well-being. Contemporary research, with large sample sizes and inclusion of multiple forms of ACEs, is needed to better understand whether ACEs are associated with mental health and HIV outcomes. The current study was designed to address key gaps in the literature by examining whether ACEs are associated with depression and anxiety, substance use, physical and mental quality of life, and HIV outcomes in a large sample of PWH at risk for unhealthy alcohol use recruited in a primary care setting.
Methods
Study sample
This study uses data from “The Health and Motivation Study,” a randomized, controlled trial testing two interventions for unhealthy alcohol use among PWH in a primary care clinic in Kaiser Permanente Northern California (KPNC), San Francisco Medical Center. The interventions involved either motivational interviews or brief information delivered by secure message, both compared with usual care. The study population comprised adult primary care patients identified as having HIV through the KPNC HIV patient registry, who were at risk for unhealthy alcohol use (≥ 3 drinks in a day for women and ≥4 drinks in a day for men) at least once in the past year. Given the high prevalence of hazardous drinking among PWH (Williams, et al., 2016), this sample of PWH at risk for unhealthy alcohol use was identified as appropriate for examination of effect of ACEs on quality of life and other outcomes. Exclusion criteria included a clinical recommendation from a provider that the patient was not appropriate to be recruited due to acute psychiatric and/or medical problems or likely inability to understand the consent procedures (1.1%). Study methods and additional details on the study population have been described previously (Silverberg et al., 2018). Of the 614 patients enrolled in the Health and Motivation Study, the 584 (95.1%) who completed a follow-up survey with questions about their exposure to ACEs were included. The study was approved by the KPNC and University of California, San Francisco Institutional Review Boards.
Measures
All measures for this cross-sectional study came from the electronic health record (EHR) or interview at baseline with the exception of ACEs, which were measured at the 12-month follow-up. Exposure to ACEs was measured using questions adapted from the original ACE Study (“Got your ACE score?,” 2018) and includes questions that are standard to ACEs research. The 10-item questionnaire includes experiences that happened during the first 18 years of life, including verbal, physical, and sexual abuse, emotional and physical neglect, witnessing physical abuse to mother, parents divorced or separated, family member incarcerated, living with someone with a substance use problem, and living with someone with mental illness. Scores range from 0-10 and the questionnaire has demonstrated good reliability and validity (Dong et al., 2004; Dube, Williamson, Thompson, Felitti, & Anda, 2004). Reliability in our sample was acceptable (0.73). Prior studies with very large samples have categorized ACEs as 0, 1, 2, 3, 4, and 5+ (Chapman et al., 2004; Dube, et al., 2003), however, due to sample size limitations and several relatively uncommon outcomes, we categorized ACEs as 0, 1-2, 3-4, and 5+ ACEs in order to have an adequate sample in each ACE category.
Health-related quality of life was measured using the 12-item Short Form Survey (SF-12), which includes domains of physical quality of life and mental quality of life in the past four weeks (Ware, Kosinski, & Keller, 1996). Scores for each domain range from 0-100, with higher scores indicative of better quality of life. Differences of two to three points on physical quality of life, and three points on mental quality of life, are considered clinically significant. The SF-12 is commonly used in studies of PWH (Drewes, Gusy, & Ruden, 2013) and has demonstrated good reliability and validity (Delate and Coons, 2000).
Depression and anxiety symptoms in the past two weeks were measured via the 9-item Patient Health Questionnaire (PHQ-9) (Kroenke, Spitzer, & Williams, 2001) and the 7-item Generalized Anxiety Disorder (GAD-7) (Spitzer, Kroenke, Williams, & Lowe, 2006). PHQ-9 scores range from 0-27, with scores ≥10 indicative of moderate-to-severe depression (Kroenke, et al., 2001), and were dichotomized as none/mild (0-9) or moderate-to-severe (10-27) as done in prior research of PWH (Silverberg, et al., 2018). The PHQ-9 has demonstrated adequate reliability and validity among PWH.30,31 GAD-7 scores range from 0-21, with scores >10 indicative of moderate-to-severe anxiety (Spitzer, et al., 2006), and were dichotomized as none/mild (0-9) and moderate-to-severe (10-21) as done in prior research of PWH (Silverberg, et al., 2018). The GAD-7 has demonstrated good reliability and validity (Shacham, Morgan, Onen, Taniguchi, & Overton, 2012; Spitzer, et al., 2006).
Past 30-day substance use was measured via a self-administered questionnaire. Participants reported whether they had used tobacco, marijuana or other substances (including prescription drug use other than as prescribed, tranquilizers, stimulants, cocaine, painkillers, heroin, hallucinogens, and ecstasy) in the prior 30 days. Hazardous drinking was defined as ≥4 or ≥5 drinks/day, for ≥1 of the past 30 days, for women and men, respectively.
HIV clinical factors included HIV RNA, categorized as <75 copies/mL or >75 copies/mL, to quantify the lower level of virus detection as done in prior research (Mitsuya et al., 2006; Panel on Antiretroviral Guidelines for Adults and Adolescents, 2018; Silverberg, et al., 2018), and CD4 cell counts/μl (continuous), obtained from the EHR. Adherence to HIV antiretroviral medication was obtained from the following question on the baseline questionnaire, “What is your best guess about how much of your prescribed HIV medications you have taken in the last month?”. Responses were dichotomized to < or ≥ 90% adherence, as prior studies have shown that HIV medication adherence of at least 90-95% is associated with successful viral suppression (E. Machtinger and Bangsberg, 2005).
Age, sex, race/ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, other, unknown), education (≤ high school, some college/college degree, graduate school), employment status, income (≥$50,000, <$50,000 household income), and marital status (married/domestic partner/intimate partner vs. single) were obtained from the questionnaire.
Statistical analysis
We examined baseline characteristics of study participants overall and by ACEs category. We used multivariable linear regression analyses to evaluate whether ACEs were associated with CD4 T-cell count and quality of life, and multivariable logistic regression analyses to evaluate whether ACEs were associated with mental health and substance use, medication adherence, and HIV RNA, adjusting for demographics. Analyses were conducted in SAS (Version 9.4, Cary, NC).
Results
The sample (N=584) had a mean (SD) age of 49.0 (10.9) years, and was 96.9% male, 63.0% white; 9.2% black; 14.2% Hispanic; 4.1% other; and 9.4% unknown (Table 1). Over half had at least some college education (52.1%), and were employed (72.8%), with income ≥ $50,000 (61.5%), and single marital status (54.6%) (Table 1); 14.2% self-reported moderate-to-severe anxiety and 16.3% self-reported moderate-to-severe depression. Moderate-to-severe depression and anxiety were highly correlated (Chi-square=222.93, p <.0001), with 72% of those with anxiety also having depression, and 63% of those with depression also have anxiety. Past-month tobacco use (24.1%), marijuana or other drug use (59.9%), and hazardous drinking (48.3%) were common. HIV was well controlled with only 6.7% having a HIV RNA ≥75 copies/mL and 12.9% reporting <90% HIV medication adherence. Average CD4 counts were 672.1 cells/μl (SD=279.0 cells/μl ).
Table 1.
Sample Characteristics of Persons with HIV (PWH) Overall and by Adverse Childhood Experiences (ACEs)
| Characteristics | Overall | 0 ACEs | 1-2 ACEs | 3-4 ACEs | ≥ 5 ACEs | P |
|---|---|---|---|---|---|---|
| N = 584 | N=102 (17.5%) | N = 200 (34.2%) | N = 146 (25.0%) | N = 136 (23.3%) | ||
| Demographic Characteristics | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Male1 | 566 (96.9) | 101 (99.0) | 192 (96.0) | 144 (98.6) | 129 (94.9) | 0.155 |
| Age (years) | 0.315 | |||||
| 18-39 | 106 (18.2) | 18 (17.6) | 34 (17.0) | 23 (15.8) | 31 (22.8) | |
| 40-49 | 185 (31.7) | 30 (29.4) | 65 (32.5) | 45 (30.8) | 45 (33.1) | |
| 50-59 | 187 (32.0) | 33 (32.4) | 56 (28.0) | 56 (38.4) | 42 (30.9) | |
| ≥60 | 106 (18.2) | 21 (20.6) | 45 (22.5) | 22 (15.1) | 18 (13.2) | |
| Race/ethnicity | 0.108 | |||||
| Non-Hispanic White | 368 (63.0) | 78 (76.5) | 124 (62.0) | 88 (60.3) | 78 (57.4) | |
| Non-Hispanic Black | 54 (9.2) | 5 (4.9) | 19 (9.5) | 14 (9.6) | 16 (11.8) | |
| Hispanic | 83 (14.2) | 11 (10.8) | 23 (11.5) | 25 (17.1) | 24 (17.6) | |
| Other | 24 (4.1) | 3 (2.9) | 13 (6.5) | 3 (2.1) | 5 (3.7) | |
| Unknown | 55 (9.4) | 5 (4.9) | 21 (10.5) | 16 (11.0) | 13 (9.6) | |
| Education | <.001 | |||||
| ≤ High school | 151 (25.9) | 14 (13.7) | 36 (18.0) | 50 (34.2) | 51 (37.5) | |
| Some college/college degree | 304 (52.1) | 61 (59.8) | 103 (51.5) | 76 (52.1) | 64 (47.1) | |
| Graduate school | 129 (22.1) | 27 (26.5) | 61 (30.5) | 20 (13.7) | 21 (15.4) | |
| Employed | 425(72.8) | 78 (76.5) | 145 (72.5) | 110 (75.3) | 92 (67.6) | 0.391 |
| Income ≥ $50,0002 | 346 (61.5) | 65 (67.7) | 129 (66.8) | 76 (54.3) | 76 (56.7) | 0.041 |
| Single | 319 (54.6) | 46 (45.1) | 103 (51.5) | 82 (56.2) | 88 (64.7) | 0.017 |
| Mental Health Characteristics | ||||||
| Moderate-to-severe anxiety | 83 (14.2) | 6 (5.9) | 26 (13.0) | 26 (17.8) | 25 (18.4) | 0.023 |
| Moderate-to-severe depression | 95 (16.3) | 10 (9.8) | 30 (15.0) | 29 (19.9) | 26 (19.1) | 0.135 |
| Hazardous drinking3 | 282 (48.3) | 45 (44.1) | 99 (49.5) | 73 (50.0) | 65 (47.8) | 0.798 |
| Tobacco use4 | 141 (24.1) | 22 (21.6) | 47 (23.5) | 38 (26.0) | 34 (25.0) | 0.861 |
| Marijuana or other drug use4,5 | 350 (59.9) | 60 (58.8) | 115 (57.5) | 86 (58.9) | 89 (65.4) | 0.507 |
| HIV-Related Variables | ||||||
| HIV RNA ≥ 75 copies/mL6 | 39 (6.7) | 4 (3.9) | 8 (4.0) | 15 (10.3) | 12 (8.9) | 0.056 |
| HIV medication adherence < 90%7 | 73 (12.9) | 14 (14.1) | 19 (9.7) | 18 (12.9) | 22 (16.5) | 0.329 |
| CD4 count cells/μL (Mean (SD))8,9 | 672.1 (279.0) | 657.9 (258.5) | 661.8 (267.8) | 680.5 (298.5) | 689.1 (290.0) | 0.763 |
| Quality of Life (Mean (SD))9 | ||||||
| Physical quality of life | 49.6 (9.6) | 50.7 (8.0) | 50.3 (9.5) | 49.7(10.3) | 47.6 (9.7) | 0.043 |
| Mental quality of life | 44.5 (11.8) | 48.8 (10.5) | 45.8 (11.1) | 42.9 (12.0) | 41.2 (12.3) | <.001 |
Notes:
P-value from Fisher’s exact test.
21 patients were missing income information.
Drinking at the ≥4/5 level for ≥1 day in past month.
In prior 30 days.
Prescription drug use other than as prescribed, tranquilizers, stimulants, cocaine, painkillers, heroin, hallucinogens, and ecstasy.
2 patients with missing HIV RNA information.
18 patients with missing adherence information.
2 Patients with missing CD4 count information.
P-values from ANOVA comparing means.
Bold = significant at P<.05.
ACES were highly prevalent, with 82.5% of participants reporting ≥1 ACE; 34.2% 1-2 ACEs, 25.0% 3-4 ACEs, and 23.3% ≥5 ACEs. The prevalence of individual ACEs is provided in Figure 1. The most common adverse childhood exposures were verbal abuse (45.9%) and living with someone with a substance use problem (40.1%). Having a greater number of ACEs was associated with lower education and income, single marital status, and moderate-to-severe anxiety (Table 1). Age, race, substance use, and HIV-related outcomes did not differ significantly by ACE score, although there was a non-significant trend with greater ACEs associated with a higher percentage of HIV RNA ≥75 copies/mL. Mean physical and mental quality of life scores were significantly lower among PWH with greater ACEs (Table 1).
Figure 1.
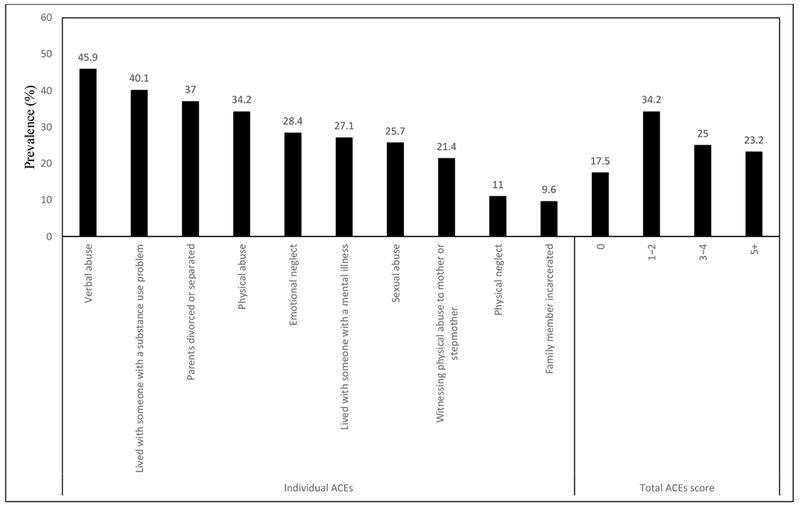
Prevalence of ACEs among Persons with HIV (PWH) (N = 584)
In adjusted regression models (Table 2), PWH with 1-2 ACEs (OR=3.41, 95%CI=1.13-10.33, P=.030), 3-4 ACEs (OR=4.36, 95%CI=1.42-13.36, P=.010), and ≥5 ACEs (OR=3.96, 95%CI=1.28-12.19, P=.017) had significantly higher odds of anxiety relative to those with 0 ACEs. PWH with 1-2 ACEs (β=−3.21, SE=1.40, P=.023), 3-4 ACEs (β=−6.23, SE=1.51, P<.001), and ≥5 ACEs (β=−7.09, SE=1.54, P<.001) had lower mean mental quality of life relative to those with 0 ACEs, suggesting that mental health quality decreases as ACEs increase. PWH with ≥5 ACEs (but not 1-2 or 3-4 ACEs) had significantly poorer physical quality of life relative to those with 0 ACEs (β=−2.53, SE=1.21, P=.037). ACEs were not significantly related to depression, substance use, or HIV-related outcomes.
Table 2.
Adverse Childhood Experiences (ACEs) and Mental Health, Substance Use, and HIV-related Variables in Persons with HIV (PWH) (N = 584)
| Outcome of interest | 0 ACEs N=101 |
1-2 ACEs N = 192 |
3-4 ACEs N = 144 |
≥ 5 ACEs N = 129 |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Moderate-to-severe anxiety | Reference | - | 3.41 (1.13, 10.33) | 0.030 | 4.36 (1.42, 13.36) | 0.010 | 3.96 (1.28, 12.19) | 0.017 |
| Moderate-to-severe depression | Reference | - | 1.60 (0.70, 3.65) | 0.262 | 1.89 (0.81, 4.40) | 0.141 | 1.57 (0.67, 3.69) | 0.304 |
| Hazardous drinking | Reference | - | 1.29 (0.76, 2.18) | 0.344 | 1.20 (0.68, 2.11) | 0.523 | 0.96 (0.54, 1.70) | 0.878 |
| Tobacco use | Reference | - | 0.91 (0.49, 1.68) | 0.756 | 0.88 (0.46, 1.69) | 0.707 | 0.71 (0.37, 1.39) | 0.321 |
| Marijuana or other drug use | Reference | - | 0.89 (0.53, 1.49) | 0.654 | 0.86 (0.49, 1.49) | 0.579 | 1.13 (0.63, 2.00) | 0.686 |
| HIV RNA ≥ 75 copies/mL | Reference | - | 0.73 (0.20, 2.62) | 0.632 | 2.12 (0.64, 7.01) | 0.218 | 1.52 (0.44, 5.19) | 0.507 |
| HIV medication adherence <90% | Reference | - | 0.56 (0.26, 1.20) | 0.136 | 0.72 (0.33, 1.59) | 0.415 | 0.91 (0.42, 1.97) | 0.804 |
| B (SE) | P | B (SE) | P | B (SE) | P | B (SE) | P | |
| CD4 count cells/μl | Reference | - | 7.99 (35.14) | 0.820 | 31.65 (37.66) | 0.401 | 27.07 (38.47) | 0.482 |
| Physical quality of life | Reference | - | −0.17 (1.10) | 0.878 | −0.05 (1.18) | 0.966 | −2.53 (1.21) | 0.037 |
| Mental quality of life | Reference | - | −3.21 (1.40) | 0.023 | −6.23 (1.51) | <.001 | −7.09 (1.54) | <.001 |
Note: OR = Odds Ratio, CI = Confidence Interval. OR and 95% CI were obtained from multivariable regression analyses adjusting for gender, age, race/ethnicity, education, income, employment and marital status.
Discussion
This study used contemporary data obtained from a large, primary care-based sample of PWH at risk for unhealthy alcohol use to examine whether ACEs were associated with mental health and HIV outcomes. We found that the prevalence of ACEs was alarmingly high among PWH, with the majority of the sample exposed to at least one ACE and nearly half exposed to three or more ACEs. The most prevalent ACEs were verbal abuse and living with someone who had a substance use problem. Adjusting for socio-demographic characteristics, having a greater number of ACEs was associated with higher odds of clinically significant anxiety and poorer mental health.
HIV morbidity and mortality are increasingly related to mechanisms other than successful treatment with ART (Smith et al., 2014), and mental health and quality of life may be significant contributors. With increasing longevity among PWH, HIV primary care now includes a focus on aging well and reducing disparities in mental health and quality of life. ACEs can affect the physiologic stress response and alter neurological, immune, hormonal, and cardiovascular systems, mediating increased vulnerability to anxiety and poor mental health in adulthood (Ridout et al., 2015). Our results suggest that with advances in HIV treatment, ACEs may not have a measurable effect on HIV outcomes for patients engaged in healthcare, yet may still contribute to worse mental health.
Few studies have examined whether ACEs are associated with quality life among PWH. Prior research has found inconsistent associations between ACEs and physical quality of life (Bekele, et al., 2018; Pence, et al., 2012), and ACEs were only associated with poorer physical quality of life in this study among PHW with ≥5 ACEs. In contrast, our findings of worse mental quality of life associated with ACEs are consistent with a small but growing body of research indicating that ACEs may have a significant and long-lasting impact on mental health among PWH. In this study, ACEs were strongly related to anxiety. Anxiety among PWH is common but has been under-investigated relative to depression (Brandt et al., 2017; Heywood and Lyons, 2016). However, like depression, it is associated with poor ART adherence and HIV outcomes (Willie, Overstreet, Sullivan, Sikkema, & Hansen, 2016) and cognitive behavioral treatment models have been developed to address anxiety among PWH (Spies, Asmal, & Seedat, 2013). Our results add to growing evidence suggesting that it is important to have a trauma-informed approach to primary care and to consider the impact that ACEs may have on anxiety and mental health symptoms when developing treatment plans.
In contrast to prior research, ACEs were not significantly associated with depression symptoms, substance use, or HIV medication adherence (Bekele, et al., 2018; Pence, et al., 2012). The lack of association with depression symptoms is notable and may be attributable to low power. Our measure of depression combined moderate and severe depression scores, due to low sample size for severe depression when stratifying by ACE categories. While results were not significant, the pattern of findings was in the expected direction, with greater ACEs associated with higher odds of depression. Further, the majority of PWH with anxiety also had depression (72%), and ACEs may contribute to the co-occurrence of both conditions. Our sample comprised PWH who were at risk for unhealthy alcohol use, which may partially explain differences in results relative to prior studies that have found an association between ACEs and substance use in PWH (Bekele, et al., 2018; Spies, et al., 2012). Finally, unlike prior studies, we did not find an association between ACEs and medication adherence or HIV RNA levels (Bekele, et al., 2018; Spies, et al., 2012). However, the lack of association between ACEs and CD4 counts has been previously found (Bekele, et al., 2018). ACEs may not impact adherence to HIV medication among PWH receiving care in integrated healthcare systems like KPNC, where access to quality HIV care and high rates of HIV medication adherence and HIV RNA control are the norm (Satre et al., 2016).
Some healthcare systems and clinicians have proposed screening patients for exposure to ACEs as one approach to better understand their medical and mental health needs (Flanagan, et al., 2018; Goldstein, et al., 2017; Korotana, et al., 2016). ACEs screening may increase the likelihood that clinicians provide trauma-informed care, and patients screened for ACEs may benefit from an improved relationship with their healthcare provider (Flanagan, et al., 2018). Education about ACEs may help PWH to understand the connection between their early childhood experiences and mental health, and could serve as a motivator to seek out resources for stress management. Notably, the stigma and shame associated with mental health problems may also be reduced if these problems are understood by PWH as an attempt to cope with ACEs.
However, while our study shows that ACEs are associated with anxiety and poorer mental health among PWH, additional research is needed to determine whether routine ACEs screening in primary care is appropriate and beneficial for PWH. It may be more important that clinicians understand what to do when they learn that patients have history of ACEs or other childhood and adult traumatic exposures, and that healthcare organizations have systems in place to connect patients with effective tools and resources to understand and manage their symptoms (E. L. Machtinger et al., 2018). Curriculums have been developed to provide clinicians with education and training in screening for and addressing ACEs and posttraumatic stress disorder in persons with HIV (Tavakkoli et al., 2014), but more studies are needed to understand how to improve mental health and quality of life among PWH who have trauma histories (Willie, et al., 2016). Relatively few PWH with mental health disorders access psychiatric specialty treatment (Satre, et al., 2013), and the presence of ACEs could signal a need for additional assistance in linkage to care. Results from our study suggest that clinicians could benefit by recognizing the high prevalence of ACEs among HIV-positive patients, understanding the potential health impacts of ACEs, and providing a safe and sensitive environment, while being aware of potential trauma triggers (Whetten, et al., 2008).
This study has several limitations. First, our sample was primarily male and was limited to PWH in the San Francisco Bay Area who were at risk for unhealthy alcohol use, and results may not be generalizable to the uninsured or PWH in the general US population. Second, PWH who didn’t participate in the study may have unique risk factors; however, the sample was demographically similar to PWH in Northern California (California Department of Public Health, 2016). Third, there are likely important gender and sex differences in the relation between ACEs, mental health, quality of life, and HIV outcomes, which we were unable to investigate due to the small number of women in our sample. Future studies with larger samples are needed to test the potential role of sex differences in the mechanisms and impact of ACEs and other forms of trauma on health and mental health (Croce-Galis, Gay, & Hardee, 2015). Fourth, our measures of ACEs, quality of life, depression and anxiety, substance use and adherence to HIV antiretroviral medication were self-reported and may be subject to social desirability bias and recall bias, which would lead to an underestimation of the association between ACEs and outcomes. Fifth, while our ACEs measure was more comprehensive than many prior studies, we did not include all childhood adversities (e.g., living in foster care, bullying) or details on ACEs severity, timing, frequency or duration, and we did not access trauma exposure in adulthood, which may have also impacted study outcomes. Finally, our study was cross-sectional and longitudinal research is necessary to understand the mechanisms underlying the association between ACEs and mental health among PWH.
Conclusion
Our study contributes to increasing evidence that ACEs are prevalent and may constitute a risk factor for anxiety and poorer overall mental health among PWH, even in the era of highly successful HIV treatment. Trauma-informed interventions to reduce anxiety and improve mental health quality of life in PWH with childhood exposure to adversity may reduce negative health sequelae associated with ACEs. Further research is needed to identify and test these interventions for PWH in primary care with the goal of improving mental health outcomes in this population.
Acknowledgments
Funding: This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (U01 AA021997 and K24 AA025703) and a grant from the National Institute on Drug Abuse (K01 DA043604).
References
- 1.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Giles WH (2006). The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bekele T, Collins EJ, Maunder RG, Gardner S, Rueda S, Globerman J, Ohtn Cohort Study Team, T. (2018). Childhood adversities and physical and mental health outcomes in adults living with HIV: Findings from the Ontario HIV Treatment Network Cohort Study. AIDS Research and Treatment, 2018, 2187232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Shapiro M (2001). Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry, 58, 721–728. [DOI] [PubMed] [Google Scholar]
- 4.Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, & O’Cleirigh CM (2017). Anxiety symptoms and disorders among adults living with HIV and AIDS: A critical review and integrative synthesis of the empirical literature. Clinical Psychology Review, 51, 164–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brezing C, Ferrara M, & Freudenreich O (2015). The syndemic illness of hiv and trauma: Implications for a trauma-informed model of care. Psychosomatics, 56, 107–118. [DOI] [PubMed] [Google Scholar]
- 6.Brown DW, & Anda RF (2009). Adverse childhood experiences: origins of behaviors that sustain the HIV epidemic. AIDS, 23, 2231–2233. [DOI] [PubMed] [Google Scholar]
- 7.California Department of Public Health. (2016). California HIV Surveillance Report - 2014. Retrieved from https://www.cdph.ca.gov/Programs/CID/DOA/CDPH%20Document%20Library/California%20HIV%20Surveillance%20Report%20-%202014_ADA.pdf.
- 8.Centers for Disease Control and Prevention. (2016). Adverse Childhood Experiences (ACEs). Retrieved from https://www.cdc.gov/violenceprevention/acestudy/index.html.
- 9.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, & Anda RF (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82, 217–225. [DOI] [PubMed] [Google Scholar]
- 10.Cohen M, Deamant C, Barkan S, Richardson J, Young M, Holman S, Melnick S (2000). Domestic violence and childhood sexual abuse in HIV-infected women and women at risk for HIV. American Journal of Public Health, 90, 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croce-Galis M, Gay J, & Hardee K (2015). Gender considerations along the HIV treatment cascade: An evidence review with priority actions. Treatment brief. What Works Association. Retrieved from http://www.whatworksforwomen.org/system/attachments/76/original/Gender_Considerations_Along_the_HIV_Treatment_Cascade.pdf?1442623311.
- 12.Delate T, & Coons SJ (2000). The discriminative ability of the 12-item short form health survey (SF-12) in a sample of persons infected with HIV. Clinical Therapeutics, 22, 1112–1120. [DOI] [PubMed] [Google Scholar]
- 13.Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Giles WH (2004). The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse and Neglect, 28, 771–784. [DOI] [PubMed] [Google Scholar]
- 14.Drewes J, Gusy B, & Ruden U (2013). More than 20 years of research into the quality of life of people with HIV and AIDS--a descriptive review of study characteristics and methodological approaches of published empirical studies. Journal of the International Association of Providers of AIDS Care, 12, 18–22. [DOI] [PubMed] [Google Scholar]
- 15.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, & Anda RF (2003). Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics, 111, 564–572. [DOI] [PubMed] [Google Scholar]
- 16.Dube SR, Williamson DF, Thompson T, Felitti VJ, & Anda RF (2004). Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abuse and Neglect, 28, 729–737. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan T, Alabaster A, McCaw B, Stoller N, Watson C, & Young-Wolff KC (2018). Feasibility and acceptability of screening for Adverse Childhood Experiences in prenatal care. Journal of Womens Health, 27, 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvan FH, Burnam MA, & Bing EG (2003). Co-occurring psychiatric symptoms and drug dependence or heavy drinking among HIV-positive people. Journal of Psychoactive Drugs, 35 Suppl 1, 153–160. [DOI] [PubMed] [Google Scholar]
- 19.Garey L, Bakhshaie J, Sharp C, Neighbors C, Zvolensky MJ, & Gonzalez A (2015). Anxiety, depression, and HIV symptoms among persons living with HIV/AIDS: the role of hazardous drinking. AIDS Care, 27, 80–85. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein E, Athale N, Sciolla AF, & Catz SL (2017). Patient preferences for discussing childhood trauma in primary care. The Permanente Journal, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Got your ACE score? (2018). ACES Too High News. Retrieved from https://acestoohigh.com/got-your-ace-score/.
- 22.Hariri S, & McKenna MT (2007). Epidemiology of human immunodeficiency virus in the United States. Clinical Microbiology Reviews, 20, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heywood W, & Lyons A (2016). HIV and elevated mental health problems: diagnostic, treatment, and risk patterns for symptoms of depression, anxiety, and stress in a national community-based cohort of gay men living with HIV. AIDS and Behavior, 20, 1632–1645. [DOI] [PubMed] [Google Scholar]
- 24.Hillis SD, Anda RF, Felitti VJ, & Marchbanks PA (2001). Adverse childhood experiences and sexual risk behaviors in women: a retrospective cohort study. Family Planning Perspectives, 33, 206–211. [PubMed] [Google Scholar]
- 25.Kalichman SC, Sikkema KJ, DiFonzo K, Luke W, & Austin J (2002). Emotional adjustment in survivors of sexual assault living with HIV-AIDS. Journal of Traumatic Stress, 15, 289–296. [DOI] [PubMed] [Google Scholar]
- 26.Korotana LM, Dobson KS, Pusch D, & Josephson T (2016). A review of primary care interventions to improve health outcomes in adult survivors of adverse childhood experiences. Clinical Psychology Review, 46, 59–90. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeGrand S, Reif S, Sullivan K, Murray K, Barlow ML, & Whetten K (2015). A review of recent literature on trauma among individuals living with HIV. Curr HIV/AIDS Rep, 12, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leserman J, Pence BW, Whetten K, Mugavero MJ, Thielman NM, Swartz MS, & Stangl D (2007). Relation of lifetime trauma and depressive symptoms to mortality in HIV. American Journal of Psychiatry, 164, 1707–1713. [DOI] [PubMed] [Google Scholar]
- 30.Machtinger E, & Bangsberg DR (2005). Adherence to HIV antiretroviral therapy In Peiperl L & Volberding PA (Eds.), HIV InSite. Retrieved from http://hivinsite.ucsf.edu/InSite?page=kb-03-02-09 [Google Scholar]
- 31.Machtinger EL, Davis KB, Kimberg LS, Khanna N, Cuca YP, Dawson-Rose C, McCaw B (2018). From treatment to healing: Inquiry and response to recent and past trauma in adult health care. Women’s Health Issues. [DOI] [PubMed] [Google Scholar]
- 32.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP Jr., Klein DB, … Silverberg MJ. (2016). Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. Journal of Acquired Immune Deficiencies Syndrome, 73, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meade CS, Kershaw TS, Hansen NB, & Sikkema KJ (2009). Long-term correlates of childhood abuse among adults with severe mental illness: adult victimization, substance abuse, and HIV sexual risk behavior. AIDS and Behavior, 13, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimiaga MJ, Noonan E, Donnell D, Safren SA, Koenen KC, Gortmaker S, Mayer KH (2009). Childhood sexual abuse is highly associated with HIV risk-taking behavior and infection among MSM in the EXPLORE Study. Journal of Acquired Immune Deficiencies Syndrome, 51, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miners A, Phillips A, Kreif N, Rodger A, Speakman A, Fisher M, Study A (2014). Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: A cross-sectional comparison with the general population. The Lancet. HIV, 1, e32–40. [DOI] [PubMed] [Google Scholar]
- 36.Mitsuya Y, Winters MA, Fessel WJ, Rhee SY, Slome S, Flamm J, Shafer RW (2006). HIV-1 drug resistance genotype results in patients with plasma samples with HIV-1 RNA levels less than 75 copies/mL. Journal of Acquired Immune Deficiencies Syndrome, 43, 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosack KE, Randolph ME, Dickson-Gomez J, Abbott M, Smith E, & Weeks MR (2010). Sexual risk-taking among high-risk urban women with and without histories of childhood sexual abuse: mediating effects of contextual factors. Journal of Child Sexual Abuse, 19, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mugavero MJ, Raper JL, Reif S, Whetten K, Leserman J, Thielman NM, & Pence BW (2009). Overload: impact of incident stressful events on antiretroviral medication adherence and virologic failure in a longitudinal, multisite human immunodeficiency virus cohort study. Psychosomatic Medicine, 71, 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myer L, Smit J, Roux LL, Parker S, Stein DJ, & Seedat S (2008). Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care and STDS, 22, 147–158. [DOI] [PubMed] [Google Scholar]
- 40.Olley BO, Seedat S, & Stein DJ (2004). Psychopathology and coping in recently diagnosed HIV/AIDS patients. South African Medical Journal, 94, 720, 722. [PubMed] [Google Scholar]
- 41.Panel on Antiretroviral Guidelines for Adults and Adolescents. (2018). Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV Washington, DC: Department of Health and Human Services; Retrieved from http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Google Scholar]
- 42.Pence BW, Mugavero MJ, Carter TJ, Leserman J, Thielman NM, Raper JL, Whetten K (2012). Childhood trauma and health outcomes in hiv-infected patients: An exploration of causal pathways. Journal of Acquired Immune Deficiencies Syndrome, 59, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pence BW, Reif S, Whetten K, Leserman J, Stangl D, Swartz M, Mugavero MJ (2007). Minorities, the poor, and survivors of abuse: HIV-infected patients in the US deep South. Southern Medical Journal, 100, 1114–1122. [DOI] [PubMed] [Google Scholar]
- 44.Ridout SJ, Ridout KK, Kao HT, Carpenter LL, Philip NS, Tyrka AR, & Price LH (2015). Telomeres, early-life stress and mental illness. Advances in Psychosomatic Medicine, 34, 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satre DD, Altschuler A, Parthasarathy S, Silverberg MJ, Volberding P, & Campbell CI (2016). Implementation and operational research: Affordable Care Act implementation in a California health care system leads to growth in HIV-positive patient enrollment and changes in patient characteristics. Journal of Acquired Immune Deficiencies Syndrome, 73, e76–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satre DD, DeLorenze GN, Quesenberry CP, Tsai A, & Weisner C (2013). Factors associated with treatment initiation for psychiatric and substance use disorders among persons with HIV. Psychiatric Services, 64, 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shacham E, Morgan JC, Onen NF, Taniguchi T, & Overton ET (2012). Screening anxiety in the HIV clinic. AIDS and Behavior, 16, 2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverberg MJ, Leyden WA, Leibowitz A, Hare CB, Jang HJ, Sterling S, Satre DD (2018). Factors associated with hazardous alcohol use and motivation to reduce drinking among HIV primary care patients: Baseline findings from the Health & Motivation study. Addictive Behaviors, 84, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, Group DADS (2014). Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet, 384, 241–248. [DOI] [PubMed] [Google Scholar]
- 50.Spies G, Afifi TO, Archibald SL, Fennema-Notestine C, Sareen J, & Seedat S (2012). Mental health outcomes in HIV and childhood maltreatment: a systematic review. Systematic Reviews, 1, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spies G, Asmal L, & Seedat S (2013). Cognitive-behavioural interventions for mood and anxiety disorders in HIV: a systematic review. Journal of Affective Disorders, 150, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spitzer RL, Kroenke K, Williams JB, & Lowe B (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine, 166, 1092–1097. [DOI] [PubMed] [Google Scholar]
- 53.Tavakkoli M, Cohen MA, Alfonso CA, Batista SM, Tiamson-Kassab ML, & Meyer P (2014). Caring for persons with early childhood trauma, PTSD, and HIV: a curriculum for clinicians. Academic Psychiatry, 38, 696–700. [DOI] [PubMed] [Google Scholar]
- 54.Ware J Jr., Kosinski M, & Keller SD (1996). A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care, 34, 220–233. [DOI] [PubMed] [Google Scholar]
- 55.Weber S, Jud A, & Landolt MA (2016). Quality of life in maltreated children and adult survivors of child maltreatment: A systematic review. Quality of Life Research, 25, 237–255. [DOI] [PubMed] [Google Scholar]
- 56.Whetten K, Leserman J, Lowe K, Stangl D, Thielman N, Swartz M, Van Scoyoc L (2006). Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. American Journal of Public Health, 96, 1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whetten K, Reif S, Whetten R, & Murphy-McMillan LK (2008). Trauma, mental health, distrust, and stigma among hiv-positive persons: Implications for effective care. Psychosomatic Medicine, 70, 531–538. [DOI] [PubMed] [Google Scholar]
- 58.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, & Samet JH (2016). Alcohol use and Human Immunodeficiency Virus (HIV) infection: current knowledge, implications, and future directions. Alcoholism, Clinical and Experimental Research, 40, 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willie TC, Overstreet NM, Sullivan TP, Sikkema KJ, & Hansen NB (2016). Barriers to HIV medication adherence: Examining distinct anxiety and depression symptoms among women living with HIV who experienced childhood sexual abuse. Behavioral Medicine, 42, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson HW, & Widom CS (2008). An examination of risky sexual behavior and HIV in victims of child abuse and neglect: a 30-year follow-up. Health Psychology, 27, 149–158. [DOI] [PubMed] [Google Scholar]
- 61.Wong MD, Sarkisian CA, Davis C, Kinsler J, & Cunningham WE (2007). The association between life chaos, health care use, and health status among HIV-infected persons. Journal of General Internal Medicine, 22, 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]


