Abstract
Background:
Osteoporosis (OP) results in weak bone and can ultimately lead to fracture. Drugs such as glucocorticoids can also induce OP (glucocorticoid-induced osteoporosis [GIO]). Bone marrow adipose tissue composition and quantity may play a role in OP pathophysiology, but has not been thoroughly studied in GIO compared to primary OP.
Purpose/Hypothesis:
Chemical shift-encoded (CSE) MRI allows detection of subregional differences in bone marrow adipose tissue composition and quantity in the proximal femur of GIO compared to OP subjects and has high agreement with the reference standard of magnetic resonance spectroscopy (MRS).
Study Type:
Prospective.
Subjects:
In all, 18 OP and 13 GIO subjects.
Fields Strength:
3T.
Sequence:
Multiple gradient-echo, stimulated echo acquisition mode (STEAM).
Assessment:
Subjects underwent CSE-MRI in the proximal femurs, and for each parametric map regions of interest (ROIs) were assessed in the femoral head (fHEAD), femoral neck (fNECK), Ward’s triangle (fTRIANGLE), and the greater trochanter (GTROCH). In addition, we compared CSE-MRI against the reference standard of MRS performed in the femoral neck and Ward’s triangle.
Statistical Tests:
Differences between OP/GIO were investigated using the Mann-Whitney nonparametric test. Bland-Altman methodology was used to assess measurement agreement between CSE-MRI and MRS.
Results:
GIO compared with OP subjects demonstrated: decreased monounsaturated fat fraction (MUFA) (−2.1%, P < 0.05) in fHEAD; decreased MUFA (−3.8%, P < 0.05), increased saturated fat fraction (SFA) (5.5%, P < 0.05), and decreased T*2 (−3.8 msec, P <0.05) in fNECK; decreased proton density fat fraction (PDFF) (−15.1%, P < 0.05), MUFA (−9.8%, P < 0.05), polyunsaturated fat fraction (PUFA) (−1.8%, P < 0.01), increased SFA (11.6%, P < 0.05), and decreased T*2 (−5.4 msec, P < 0.05) in fTRIANGLE; and decreased T*2 (−1.5 msec, P < 0.05) in GTROCH. There was high measurement agreement between MRI and MRS using the Bland–Altman test.
Data Conclusion:
3T CSE-MRI may allow reliable assessment of subregional bone marrow adipose tissue (bMAT) quantity and composition in the proximal femur in a clinically reasonable scan time. Glucocorticoids may alter the lipid profile of bMAT and potentially result in reduced bone quality.
Level of Evidence:
2
Technical Efficacy:
Stage 2
OSTEOPOROSIS (OP) is a disease associated with low bone mass and microarchitectural deterioration of bone tissue, leading to bone fragility and higher fracture risk, especially in the hip or proximal femur.1 Glucocorticoids (GCs) are hormones that can influence surrounding osteoblasts, osteoclasts, bone marrow stromal cells, and adipocytes. For osteocytes and osteoblasts, GCs decrease differentiation and function and increase apoptosis, and for osteoclasts, GCs increase their formation and activity and decrease apoptosis.2,3 Glucocorticoid-induced osteoporosis (GIO) is the most common of secondary OP, and the primary cause of OP in young adults. Fragility fracture risk is increased in the case of long-term GC intake (>3 months with 7.5 mg/day prednisolone equivalent), which motivated the inclusion of GC use (yes or no) in the calculation of an individual’s fracture risk assessment score (FRAX4.
Bone marrow adipose tissue (bMAT) plays an important role in energy metabolism and is the site of osteocyte differentiation and production.5,6 Studies of bMAT in terms of adiposity, amount and composition in OP has revealed increased total fat content and decreased unsaturated fatty acids in the proximal femur of OP subjects compared with controls, and increased total bMAT content in the vertebrae of subjects with OP.7–9 Methods for monitoring bMAT content and composition in vivo consist mainly of magnetic resonance spectroscopy (MRS)10–12 and imaging methods based on phase differences between water and fat protons called chemical shift-encoded magnetic resonance imaging (CSEMRI), which allows assessment of fat and water in acquired images.13–16 MRS has limited spatial resolution due to its single-voxel acquisition compared with CSE and requires longer acquisition times.
Recent advances in water-fat imaging methods13,16–19 allows discrimination between different types of fat in terms of their amount of saturation (saturated fat fraction, SFA), poly-unsaturation (poly-unsaturated fat fraction, PUFA) and mono-unsaturation (mono-unsaturated fat fraction, MUFA). This approach has been validated using MRS in liver, adipose tissue, muscle, bone marrow,13,16–18,20,21 and more recently in abdominal adipose tissue using gas chromatography.22
The purposes of our study were: 1) to use CSE-MRI to assess differences in the quantity and composition of bMAT in the proximal femur of OP compared with GIO patients, and 2) to assess the relationship between CSE-MRI and MRS for fat quantification in bone marrow studies.
Materials and Methods
Subjects
This study had Institutional Review Board approval and written informed consent was obtained from all subjects. We recruited a total of 35; 18 with a diagnosis of primary OP (OP, bone mineral density [BMD] T-score < −2.5 in the hip), and 18 GIO subjects (GIO, defined as 10 to 40 mg prednisolone equivalent during at least 24 months). Thirty-two subjects underwent CSE-MRI acquisition and were used for comparing measurements using the Bland-Altman method of agreement. An exclusion criteria based on body mass index (BMI <30 kg.m−2) was used to select 18 OP and 13 GIO for subregional analysis of bMAT.
MRI Acquisition
All MRI acquisitions were performed on a clinical 3T MRI system (Skyra System, Siemens Healthineers, Erlangen, Germany) using an 18-channel flexible coil overlying the pelvis. A 3D spoiled-multiple echo gradient echo sequence with a flyback readout gradient was used. The repetition time (TR) and flip angle (FA) were chosen to avoid T1 weighting: TR: 16 msec; FA 5° 12 echo times (TEs) giving an echo train length of n = (1:12) × 1.2 msec; receiver bandwidth = 2000 Hz.pixel−1; signal averages = 4; the field of view (FOV) was chosen to cover both hips from the level of the femoral head to the femoral shaft with in-plane resolution of 1.5 ×1.5 mm2; matrix = 160 × 160 interpolated to 192 × 192; 52 coronal slices; slice thickness = 2.4 mm, scan time: 5:15 minutes. Phase and magnitude images were systematically saved for postprocessing reconstruction workflow.
MRS Acquisition and Quantification
A localized STEAM (STimulated Echo Acquisition Mode)sequence was used for MRS. No water suppression was applied for acquisition. We use a high-resolution 3D FLASH sequence (TR/TE = 37/4.92 msec, FA = 25°, bandwidth [BW] = 130 Hz/pixel, FOV = 100 mm, matrix = 512 × 512, in-plane resolution =0.234 × 0.234 mm2, 60 coronal slices, slice thickness = 1.5 mm, scan time = 15 min 18 sec) for localization and voxels of 10 × 10 × 10 mm3 were placed in the femoral neck and Ward’s triangle. (The authors note that such a high-resolution sequence is not necessarily needed for voxel placement; a shorter, lower-resolution scan can be used. However, since these data were acquired as part of a bone microarchitecture study, it was also used for voxel placement for MRS.) STEAM parameters were: TE = 20 msec, TR = 2500 msec, TM = 10 msec, BW = 4000 Hz, nPts = 2048, number of average (NA) = 28. Each of the averages were saved separately and inspected for quality control (frequency shift, frequency drift, and phase) and processed through homemade MatLab R2016b (MathWorks, Natick, MA) routines for phase, frequency correction, and then averaged. MRS quantification was performed using jMRUI 5.2 software23 with the AMARES method.24 Values from previous studies in femoral bone marrow were used as fitting starting values, and knowledge about triglyceride properties25,26 were used to soften constraint amplitude and frequency of each triglyceride resonance. Indices for fat fraction and fat composition were derived according to ratios for each amplitude characteristic of triglycerides.19
Image Reconstruction
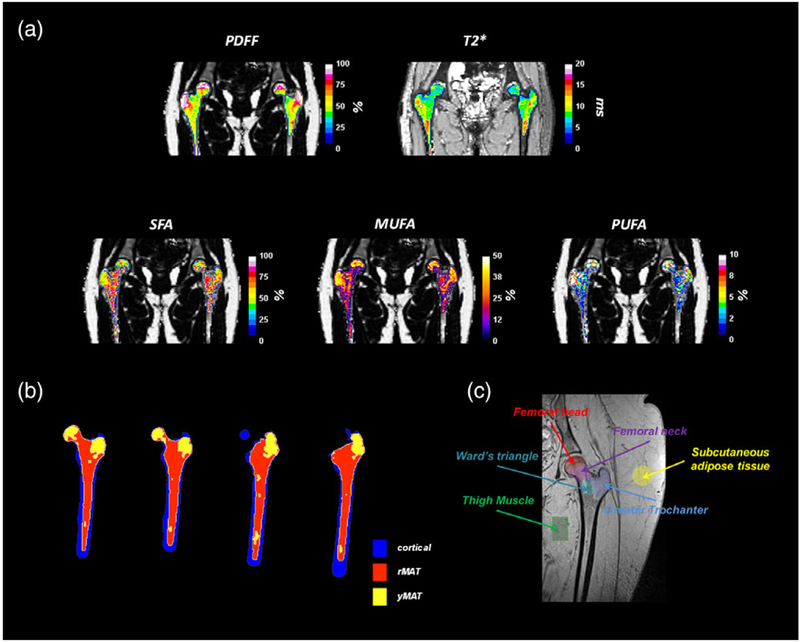
Fat content and fatty acid composition were quantified using methods described in prior references17,18 and implemented in MatLab 2016b. Prior quantification phase images were corrected for zero- and first-order phase through a specific algorithm and used to correct the native phase images leading to time-series B0-demodulated real part images. Using a model of fat spectrum with eight components, the number of double bonds (ndb) and methylene-interrupted double bonds (nmidb) were derived voxel-byvoxel by a stepwise data fitting procedure on the real part of the time-series image. This step provided fat- and water-only images as well as several parametric maps such as T*2, B0 field inhomogeneity (ΔB0), proton density fat fraction (PDFF) and saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acid fractions (Fig. 1a). A 3D-k cluster algorithm was used on fat- and water-only images to semiautomatically separate tissues in the image into soft tissue, muscle, cortical bone, and bone marrow.21 Briefly, an active contour was used to segment bMAT from the fat-only central image and used for initializing a second mask to water image to segment cortical bone. From the 3D bMAT-masked a k-means algorithm (with k = 2) was used on PDFF maps to cluster adipose tissues into rMAT (red marrow adipose tissue) and yMAT (yellow marrow adipose tissue).
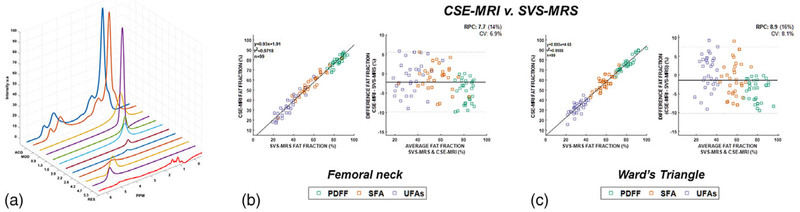
FIGURE 1:
(a) Typical acquired MRS spectrum in proximal femur (ACQ) with quantification (MOD) and residuals (RES). (b) Bland-Altman graph of measurement obtained by CSE-MRI and MRS methods in femoral neck and femoral triangle.
Regional and Subregional Analysis Within the Proximal Femur
From the bMAT mask, whole proximal femur MAT was measured and rMAT fraction and cortical bone fraction were calculated as the ratio, respectively, of rMAT and total bone tissues (rMAT/tB) and cortical bone and total bone tissues (cB/tB).
To analyze marrow fat distribution on a subregional level within the proximal femur and surrounding soft tissues of the hip as internal controls, six regions of interest (ROIs) as depicted in Fig. 1 were manually segmented on acquired and processed parametric maps. These ROIs were chosen in standard anatomic locations that present mostly homogenous fat: the femoral head, femoral neck, Wards triangle, the greater trochanter, as well as subcutaneous adipose tissue in the upper interior area of each subject’s thigh and thigh muscle.
Statistical Analysis
Statistical analysis was performed on each map and segmented region using MatLab R2016b. For each ROI, pixel values were extracted and means and standard deviations were calculated under the assumption that pixels follow a normal distribution within subregions. For each parameter and region, mean values were stratified to the group and differences between OP, GIO were investigated under the assumption that differences between groups is due to a shift of the distribution of each parameter in each subregion using the Mann–Whitney nonparametric test, which does not require a normal distribution of results within each group. Statistical significance was set at P < 0.05. Differences as the percentage difference of fat fraction were analyzed and discussed between OP and GIO subjects in the following. Bland–Altman methodology27 was used to compare measurements obtained from MRI and MRS regarding PDFF, SFA, and UFA in femoral neck and Ward’s triangle; specifically, scatterplots and Pearson correlation were computed to assess the linear relationship between the two methods of measurement, and differences in values vs. average values were plotted with limits of agreement computed as the mean difference between the two methods of measurement ±1.96 times the standard deviation for measuring agreement between methods.
Results
Subject Characteristics
Table 1 gives the cohort characteristics. With the exception of age, no other significant differences between OP and GIO groups were found.
TABLE 1.
Demographics and Characteristics
| OP | GIO | P value | |
|---|---|---|---|
| n = 31 | 18, F | 13, F | |
| Age (years) | 53.3 ± 11.56 | 37.5 ± 12.6 | 0.045a |
| BMI (kg.m−2) | 24.31 ± 3.7 | 26.2 ± 4.8 | 0.253 |
| T-score | −1.12 (−2.6: 1) | −0.67 (−2.8:1.2) | 0.133 |
| FRAX | 7.13 (0.8:16) | 3.21 (1.1:6.4) | 0.127 |
| CBf (%) | 48.69 ± 12.60 | 44.33 ± 7.44 | 0.444 |
| Total rMAT (%) | 34.15 ± 15.16 | 29.24 ± 14.47 | 0.257 |
| Total yMAT (%) | 51.33 ± 12.60 | 55.67 ± 7.44r | 0.444 |
P < 0.05.
cBf: cortical bone fraction (total cortical part of hip bone); rMAT: red marrow adipose tissue; yMAT: yellow marrow adipose tissue.
MRS
Figure 1a show typical spectra obtained in the femoral neck after processing (absorption spectrum). For all subjects and both locations: the range of full-width at half-maximum (FWHM) of methyl proton of fat (located at 1.3 ppm) was from 37–48 Hz. The range of FWHM for the water peak was from 38–59Hz. Figure 1b compares the measurement of PDFF, SFA, and UFA obtained by subregional integration in CSE-MRI and single-voxel MRS using the Bland–Altman methodology. Agreement between CSE-MRI methods to assess bMAT composition and SVS-MRS is assessed in Fig. 3b. The limits of agreement between the methods in the femoral neck and Ward’s triangle were similar.
FIGURE 3:
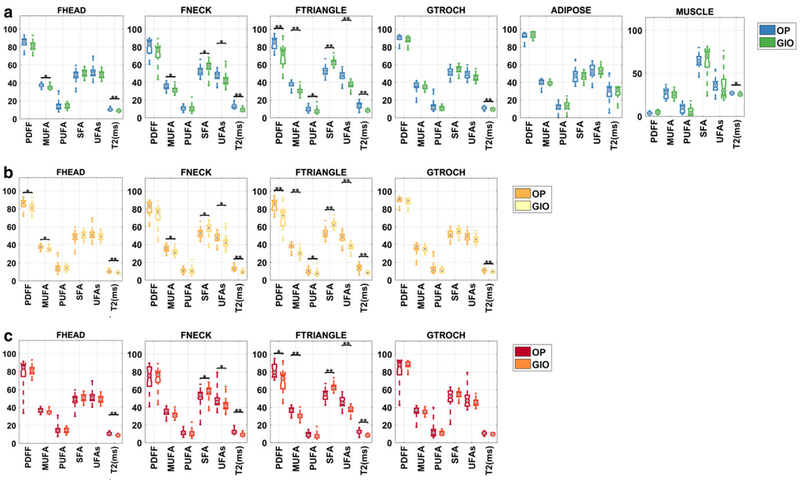
Boxplot of subregional proximal femur marrow fat quantity and composition voxel integrated values for femoral head (fHEAD), femoral neck (fNECK), Ward’s triangle (fTRIANGLE), great trochanter (GTROCH), subcutaneous adipose tissue (ADIPOSE), and muscle (MUSCLE) for (a) bone marrow adipose tissue (bMAT), (b) yellow marrow adipose tissue (yMAT), (c) red marrow adipose tissue (rMAT). On each box, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points. Statistical significance is highlighted by *P <0.05, **P < 0.01.
MRI
Figure 2 show typical MRI images and reconstruction parametric maps obtained (Fig. 2a). Image acquisition allows correct localization of each ROI. Within PDFF of whole hip bone marrow, two distinct distributions were found and were clustered into rMAT and yMAT (Fig. 2b). ROIs used for subregional analysis are depicted in Fig. 2c.
FIGURE 2:
(a) Characteristic parametric maps obtained in proximal femur for PDFF (proton density fat fraction), T*2, SFA (saturated fatty-acids fat fraction), MUFA (mono-unsaturated fatty-acids fat fraction) and PUFA (poly-unsaturated fatty-acids fat fraction). (b)Semiautomatic clustering of red marrow (rMAT), yellow marrow (yMAT), and cortical tissues of proximal femur. (c) Anatomical region of interest in proximal femur used for analysis.
ROIs Fatty Acids Composition
Figure 3 provides a boxplot depicting the significant differences in subregional proximal femur marrow fat quantity and composition in OP compared with GIO subjects.
For bMAT (Fig. 3a), GIO subjects compared with OP subjects demonstrated decreased MUFA (−2.1%, P < 0.05) in fHEAD; decreased MUFA (−3.8%, P < 0.05), increased SFA (5.5%, P < 0.05), and decreased T*2 (−3.8 msec, P < 0.05) in fNECK; decreased PDFF (−15.1%, P < 0.05), MUFA (−9.8%, P < 0.05), PUFA (−1.8%, P < 0.01), increased SFA (11.6%, P < 0.05), and decreased T*2 (−5.4 msec, P < 0.05) in fTRIANGLE; and decreased T*2 (−1.5 msec, P < 0.05) in GTROCH.
For yMAT (Fig. 3b), GIO subjects compared with OP subjects demonstrated decreased MUFA for: fHEAD (−2.1%, P < 0.05); fNECK (−3.8%, P < 0.05); fTRIANGLE (−9.8%, P < 0.05). GIO subjects compared with OP subjects also demonstrated: decreased PUFA (–1.8%, P < 0.05) in fNECK and increased SFA in fNECK (+5.5%, P < 0.05) and fTRIANGLE (+11.6%, P < 0.05). Finally, GIO subjects compared with OP subjects demonstrated decreased T*2 in fHEAD (−1.8 msec, P <0.05); in fNECK (−3.8 msec, P < 0.05); fTRIANGLE (−5.4 msec, P < 0.05), and GTROCH (−1.6 msec, P < 0.05).
For rMAT (Fig. 3c), GIO subjects compared with OP subjects demonstrated decreased T*2 c in the: fHEAD (−3.4,P < 0.01); fNECK (−4.6%, P < 0.01); fTRIANGLE (−4.1%, P < 0.01); GTROCH (−2.8%, P < 0.01). GIO subjects also demonstrated differences in fTRIANGLE compared with OP subjects with regard to PDFF (−12.7%, P < 0.05); MUFA (−9.5%, P < 0.05), and SFA (+10.9%, P < 0.05).
Discussion
In this study we demonstrated the ability of CSE-MRI to detect subregional differences in proximal femur marrow fat composition in subjects with GIO compared with primary OP. We also showed that the CSE-MRI method, which can provide 3D volume information in ~ 5 minutes, has excellent agreement with the reference standard of MRS. Overall, the findings are important because they suggest that while both OP and GIO subjects are at increased fracture risk, the underlying pathophysiology leading to this increased fracture risk in each group may differ. Furthermore, the results suggest that 3D CSE-MRI could be performed as a reliable means to obtain quantitative information about marrow fat tissue composition in vivo for future clinical studies or potentially even clinical practice.
Our results showed that GIO subjects demonstrated lower MUFA and PUFA and higher SFA in the various sub-regions of the proximal femur compared with OP subjects. The majority of the differences in marrow fat composition between groups were found in the femoral neck and Ward’s triangle; these subregions are believed to be the areas of the proximal femur that undergo the most biomechanical changes and thus the most bone remodeling.28,29 Indeed, GC intake in the long term has been associated with bone loss and low BMD in several subregions including the femoral neck, femoral head, and Ward’s triangle.30
In contrast to MUFA, PUFA, and SFA, PDFF or total fat levels were very similar between groups across the various subregions, with the exception of lower PDFF levels in GIO subjects compared with OP subjects within Ward’s triangle. This result suggests that monitoring total marrow fat content via PDFF, compared with monitoring unsaturated and saturated marrow fat levels, may not fully capture the differences in bone biology/bone health in GIO compared with OP subjects.
We believe that the lower MUFA and PUFA and higher SFA marrow fat levels in GIO subjects compared with OP subjects is related to the GC use rather than younger age of the GIO subjects compared with the OP subjects. Recent studies have shown that lower unsaturated lipid content in the femoral neck is associated with higher age and lower BMD T-scores.6,8–10,31,32 We also note that because the two groups in this study did not differ significantly in terms of BMD T-scores or FRAX scores, then the detrimental effects of GC use on bone health may not be fully captured by the standard clinical tests of DXA and FRAX. The use of MRI to assess the composition of marrow fat, which is an integral component of bone tissue, may provide a better method to monitor the negative effects of steroids on bone health. Indeed, prior work has shown that saturated fatty acids secreted by marrow adipocytes can be lipotoxic for bone tissue.33–35
MRS in Ward’s triangle and femoral neck showed similar results to CSE-MRI, with good measurement agreement according to the Bland–Altman method. This result is similar to previous studies, which validated CSE-MRI with: 1) MRS to assess fat quantity and composition in the liver, spine, and bone marrow,17,18,20–22,36 and 2) more recently, gas chromatography of subcutaneous adipose tissue.22 Spectral quality was assessed by measurement of the quality factor, methyl protons, and water peak lineshape (reflecting static field homogeneity in the selected voxel). In our study we used the STEAM sequence, which compared with PRESS has the advantage of being less sensitive to homonuclear scalar coupling and short T2 relaxation times, as explained in Hamilton et al.37
Quantification was processed by the AMARES method and calculated Cramer-Rao lower bounds showed very low values, which indicate very low variances in the case of unbiased estimators. Diallylic methylene protons (2.8 ppm resonance) are a direct measurement of poly-unsaturation, but due to spectral limitation at 3T and spectral overlap it is challenging to resolve such resonance, which can then impact quantification. However, in our study this is compensated by the amount of fat in the subregions, which increase fat spectrum signal-to-noise ratio, and by the use of the short TE STEAM sequence, which reduces the T*2 effect and thus limits global spectral overlap. One limitation regarding our MRS quantification is the use of one short echo time and repetition time to assess T2 or T1 relaxation, respectively, which could induce a bias at low levels of fat (low PDFF). In addition, the limited spatial resolution of MRS (10 × 10 × 10 mm3) compared with CSE-MRI (1.5 × 1.5 × 2.4 mm3) may impede assessment of the bMAT by not being able to fully capture local changes in tissue composition.
Our study is not without limitations. First, the number of subjects was relatively low. However, as an initial feasibility for validating the CSE-MRI method in vivo and against the reference standard of MRS, we believe the number of subjects was sufficient. Second, the GIO and OP subject groups were not matched for age. However, we note the lower age of the GIO subjects would have favored higher T-scores, higher unsaturated fat levels, and lower saturated fat levels based on the literature. Instead, the GIO subjects demonstrated similar T-scores, lower unsaturated fat levels, and higher saturated fat levels compared with the older OP subjects. The results may further evidence that the effects observed may be due to GC use. Third, nutrition and diet could impact fat composition and can also be a possible bias. However, such differences would have been reflected into subcutaneous adipose tissues and muscle, which is not the case in our study.38–40 Finally, while promising, for this approach to become more widely used as a research or clinical tool, the CSE and analysis method should be validated at other skeletal sites (eg, vertebral bodies) and within other regions of interest.
In conclusion, CSE-MRI allows reliable assessment of subregional bMAT composition in vivo and in clinically feasible scan times. Prior studies have shown that bone marrow fat quantity and composition can influence bone health. Our findings provide further evidence that GC even in younger subjects use may alter marrow fat metabolism in the proximal femur, leading to a lipid profile (lower unsaturated, higher saturated) that may have negative consequences for bone health.
Acknowledgments
Contract grant sponsor: National Institutes of Health (NIH); Contract grant numbers: R01-AR070131, R01-AR066008; performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R), a National Institute of Biomedical Imaging and Bioengineering (NIBIB) Biomedical Technology Resource Center (NIH P41 EB017183).
References
- 1.Peck WA, Burckhardt P, Christiansen C, et al. Consensus Development Conference — Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94:646–650. [DOI] [PubMed] [Google Scholar]
- 2.Dempster DW. Bone histomorphometry in glucocorticoid-induced osteoporosis. J Bone Miner Res 1989;4:137–141. [DOI] [PubMed] [Google Scholar]
- 3.Dalle Carbonare L, Bertoldo F, Valenti MT, et al. Histomorphometric analysis of glucocorticoid-induced osteoporosis. Micron 2005;36: 645–652. [DOI] [PubMed] [Google Scholar]
- 4.Lems WF. Glucocorticoids: Bad or safe for the bones? RMD Open 2015; 1(Suppl 1):e000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci 2014;1311: 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheller EL, Doucette CR, Learman BS, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun 2015;6:7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith JF, Yeung DK, Antonio GE, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: Dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology 2005;236:945–951. [DOI] [PubMed] [Google Scholar]
- 8.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. J Magn Reson Imaging 2005;22:279–285. [DOI] [PubMed] [Google Scholar]
- 9.Patsch JM, Li X, Baum T, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res 2013;28:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Pietro G, Capuani S, Manenti G, et al. Bone marrow lipid profiles from peripheral skeleton as potential biomarkers for osteoporosis: A 1H-MR spectroscopy study. Acad Radiol 2016;23:273–283. [DOI] [PubMed] [Google Scholar]
- 11.Pansini V, Monnet A, Salleron J, Hardouin P, Cortet B, Cotten A. 3 Tesla (1) H MR spectroscopy of hip bone marrow in a healthy population, assessment of normal fat content values and influence of age and sex. J Magn Reson Imaging 2014;39:369–376. [DOI] [PubMed] [Google Scholar]
- 12.Karampinos DC, Melkus G, Baum T, Bauer JS, Rummeny EJ, Krug R. Bone marrow fat quantification in the presence of trabecular bone: Initial comparison between water-fat imaging and single-voxel MRS. Magn Reson Med 2014;71:1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson P, Mansson S. Simultaneous quantification of fat content and fatty acid composition using MR imaging. Magn Reson Med 2013;69: 688–697. [DOI] [PubMed] [Google Scholar]
- 14.Dixon WT. Simple proton spectroscopic imaging. Radiology 1984;153: 189–194. [DOI] [PubMed] [Google Scholar]
- 15.Gee CS, Nguyen JT, Marquez CJ, et al. Validation of bone marrow fat quantification in the presence of trabecular bone using MRI. J Magn Reson Imaging 2015;42:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson P, Mansson S. Fat quantification using multiecho sequences with bipolar gradients: Investigation of accuracy and noise performance. Magn Reson Med 2014;71:219–229. [DOI] [PubMed] [Google Scholar]
- 17.Leporq B, Lambert SA, Ronot M, et al. Hepatic fat fraction and visceral adipose tissue fatty acid composition in mice: Quantification with 7.0T MRI. Magn Reson Med 2016;76:510–518. [DOI] [PubMed] [Google Scholar]
- 18.Leporq B, Lambert SA, Ronot M, Vilgrain V, Van Beers BE. Quantification of the triglyceride fatty acid composition with 3.0 T MRI. NMR Biomed 2014;27:1211–1221. [DOI] [PubMed] [Google Scholar]
- 19.Bydder M, Girard O, Hamilton G. Mapping the double bonds in triglycerides. Magn Reson Imaging 2011;29:1041–1046. [DOI] [PubMed] [Google Scholar]
- 20.Leporq B, Lambert SA, Ronot M, Vilgrain V, Van Beers BE. Simultaneous MR quantification of hepatic fat content, fatty acid composition, trans-verse relaxation time and magnetic susceptibility for the diagnosis of non-alcoholic steatohepatitis. NMR Biomed 2017;30:e3766. [DOI] [PubMed] [Google Scholar]
- 21.Martel D, Leporq B, Bruno M, Regatte RR, Honig S, Chang G. Chemical shift-encoded MRI for assessment of bone marrow adipose tissue fat composition: Pilot study in premenopausal versus postmenopausal women. Magn Reson Imaging 2018;53:148–155. [DOI] [PubMed] [Google Scholar]
- 22.Nemeth A, Segrestin B, Leporq B, et al. 3D chemical shift-encoded MRI for volume and composition quantification of abdominal adipose tissue during an overfeeding protocol in healthy volunteers. J Magn Reson Imaging 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 2001;31:269–286. [DOI] [PubMed] [Google Scholar]
- 24.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997;129:35–43. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton G, Schlein AN, Middleton MS, et al. In vivo triglyceride composition of abdominal adipose tissue measured by (1) H MRS at 3T. J Magn Reson Imaging 2017;45:1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed 2011;24:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bland MJ, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;327: 307–310. [PubMed] [Google Scholar]
- 28.Stiehl JB, Jacobson D, Carrera G. Morphological analysis of the proximal femur using quantitative computed tomography. Int Orthop 2007;31: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bitsakos C, Kerner J, Fisher I, Amis AA. The effect of muscle loading on the simulation of bone remodelling in the proximal femur. J Biomech 2005;38:133–139. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook P, Birmingham J, Kempler S, et al. Corticosteroid effects on proximal femur bone loss. J Bone Miner Res 1990;5:1211–1216. [DOI] [PubMed] [Google Scholar]
- 31.Machann J, Stefan N, Wagner R, et al. Intra- and interindividual variability of fatty acid unsaturation in six different human adipose tissue compartments assessed by (1) H-MRS in vivo at 3 T. NMR Biomed 2017;30(9). [DOI] [PubMed] [Google Scholar]
- 32.Griffith JF, Yeung DK, Ahuja AT, et al. A study of bone marrow and sub-cutaneous fatty acid composition in subjects of varying bone mineral density. Bone 2009;44:1092–1096. [DOI] [PubMed] [Google Scholar]
- 33.Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid bio-synthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med 2010;14:982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillet C, Dalla Valle A, Gaspard N, et al. Osteonecrosis of the femoral head: Lipotoxicity exacerbation in MSC and modifications of the bone marrow fluid. Endocrinology 2017;158:490–502. [DOI] [PubMed] [Google Scholar]
- 35.Dhayal S, Welters HJ, Morgan NG. Structural requirements for the cyto-protective actions of mono-unsaturated fatty acids in the pancreatic beta-cell line, BRIN-BD11. Br J Pharmacol 2008;153:1718–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemeth A, Segrestin B, Leporq B, et al. Comparison of MRI-derived vs. traditional estimations of fatty acid composition from MR spectroscopy signals. NMR Biomed 2018;31:e3991. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton G, Middleton MS, Bydder M, et al. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging 2009;30:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian L, Yu X. Fat, sugar, and bone health: A complex relationship. Nutrients 2017;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cawthorn WP, Scheller EL, Parlee SD. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology 2015; 157:508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 2014;20:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]