Abstract
Background
Adherence to a combination of healthy lifestyle factors has been related to a considerable reduction of cardiovascular risk in white populations; however, little is known whether such associations persist in non-white populations.
Objectives
The present study aimed to examine the associations of a combination of modifiable, healthy lifestyle factors with the risks of ischemic cardiovascular diseases and estimate the proportion of diseases that could potentially be prevented by adherence to the healthy lifestyle patterns.
Methods
We examined the associations of six lifestyle factors with ischemic heart disease and ischemic stroke (IS) in the China Kadoorie Biobank of 461,211 participants aged 30-79 years without cardiovascular diseases, cancer, or diabetes at baseline. Low-risk lifestyle factors were defined as non-smoking or having stopped for reasons other than illness, alcohol consumption of <30 g/day, median or higher level of physical activity, diet rich in vegetables and fruits and limited in red meat, body mass index of 18.5-23.9 kg/m2, and waist-to-hip ratio <0.90 for men and <0.85 for women.
Results
During a median of 7.2 years (3.3 million person-years) of follow-up, we documented 3,331 incident major coronary events (MCE) and 19,348 incident IS. In multivariable-adjusted analyses, current non-smoking, light-to-moderate alcohol, high physical activity, diet rich in vegetables and fruits and limited in red meat, and low adiposity were independently associated with reduced risks of MCE and IS. Compared with participants without any low-risk factors, the hazard ratio (95% confidence interval) for those with ≥4 low-risk factors was 0.42 (0.34, 0.52) for MCE and 0.61 (0.56, 0.66) for IS. Approximately 67.9% (46.5% to 81.9%) of the MCE and 39.1% (26.4% to 50.4%) of the IS were attributable to poor adherence to healthy lifestyle.
Conclusions
Our data indicate that adherence to healthy lifestyle may substantially lower the burden of cardiovascular diseases in Chinese.
Keywords: cardiovascular diseases, cohort studies, health behavior, life style
Introduction
Ischemic heart disease (IHD) and ischemic stroke (IS) are posing major burdens to global health,(1) and the leading causes of mortality in China.(2) The pharmacologic treatment, though has shown considerable effectiveness on improved therapy of these diseases, is costly and may lead to side effects. By contrast, adherence to a healthy lifestyle has become a mainstream approach to lower cardiovascular burden through primary prevention.(3)
In epidemiological studies, modifiable lifestyle factors, such as non-smoking,(4) moderate alcohol consumption,(5) physical activity,(6) healthy diets,(7,8) and low adiposity,(9,10) have been consistently linked to a reduced cardiovascular risk. Several previous studies have shown that adherence to a healthy lifestyle defined by a combination of these modifiable factors was related to up to roughly an 80% reduction in coronary heart disease (CHD) incidence (11–14) and a 50% reduction in IS incidence (15) in white populations from developed countries. However, little is known whether such protective effects persist in other non-white populations.
We thus aimed to examine the associations of a combination of modifiable, healthy lifestyle factors with the risks of IHD and IS in a large cohort of 0.5 million of adult Chinese – the China Kadoorie Biobank (CKB) study.(16) In addition, we estimated the proportion of ischemic cardiovascular diseases (CVDs) that could potentially be prevented by adherence to the healthy lifestyle patterns.
Methods
Study population
The CKB cohort was established in ten study areas geographically spread across China during 2004-2008, when all non-disabled, permanent residents of each area who were 35 to 74 years of age were invited to participate in the study. Of the total of approximately 1.8 million eligible adults in these areas, almost 1 in 3 (33% in rural areas and 27% in urban areas) responded.(17) Overall, 512,891 adults aged 30-79 years were enrolled, including a few who were just outside the targeted age range, and all had completed questionnaire, physical measurements, and a written informed consent form. The Ethical Review Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (UK) approved the study. Further details of the CKB cohort have been described in previous publications.(16,17)
In the present analysis, we excluded participants who reported prior medical histories of heart disease (n=15,472), stroke (n=8,884), or cancer (n=2,577), had prevalent diabetes (n=30,300) based on self-reported or glucose testing at baseline, had missing data for body mass index (BMI; n=2), or were lost to follow-up shortly after baseline (n=3). After these exclusions, a total of 461,211 participants remained for the current analysis.
Assessment of lifestyle factors
Participants reported on a range of lifestyle factors in the baseline questionnaire. Questions about tobacco smoking included frequency, type and amount of tobacco smoked per day for ever smokers, and years since quitting and the reason for quitting for former smokers. Questions about alcohol consumption included typical drinking frequency, type of alcoholic beverage drunk habitually, and volume of alcohol drunk on a typical drinking day in the past 12 months. Questions about physical activity included the usual type and duration of activities in occupational, commuting, domestic, and leisure-time related domains in the past 12 months. The daily level of physical activity was calculated by multiplying the metabolic equivalent tasks (METs) value for a particular type of activity by hours spent on that activity per day and summing the MET-hours for all activities. A short qualitative food frequency questionnaire was used to assess the habitual intakes of 12 conventional food groups in the past 12 months (see Appendix).
In a subsample of 1,300 participants who completed the same questionnaire twice at an interval of fewer than 1.5 years (median, 1.4 years), we observed moderate to excellent reproducibility for most of the lifestyle variables. The weighted kappa coefficient was 0.83 for tobacco smoking, 0.66 for alcohol consumption, 0.10 for vegetable intake, 0.40 for fruit intake, and 0.42 for meat intake. The Spearman correlation coefficient was 0.60 for physical activity level. Seasonal availability of fresh vegetables may result in its poor reproducibility.
Trained staff measured weight, height, and circumference of waist and hip using calibrated instruments. BMI was calculated as weight in kilograms divided by height in meters squared. Waist-to-hip ratio (WHR) was the ratio of waist circumference to hip circumference.
Assessment of covariates
Covariate information was inquired by baseline questionnaire including sociodemographic characteristics, personal and family medical history, and women’s reproductive information. Trained staff measured blood pressure at least twice using a UA-779 digital monitor, with the mean of two satisfactory measurements used for analyses. A participant was considered as having a family history of a particular disease if he reported at least one first-degree relative with that disease. Prevalent hypertension was defined as measured systolic blood pressure ≥140 mmHg, measured diastolic blood pressure ≥90 mmHg, self-reported diagnosis of hypertension, or self-reported use of antihypertensive medication at baseline.
Definition of low-risk lifestyle
Six dietary and lifestyle factors were considered to define a low-risk lifestyle, namely smoking, alcohol consumption, physical activity, diet, BMI, and WHR, according to previous studies.(11–15,19) For smoking, the low-risk group was defined as nonsmokers or those who had stopped smoking for reasons other than illness for at least six months. In CKB cohort, about half of former smokers quit because of illness.(20) We included former smokers who stopped smoking for illness in the current smoker category to avoid misleadingly elevated risk. For alcohol consumption, the low-risk group was defined as those who drank greater than zero but less than 30 g alcohol per day. For physical activity, the low-risk group was defined as those who engaged in a sex-specific median or higher level of physical activity.
For diet, we included three food items which are particularly addressed in recent AHA/ACC guideline on lifestyle management to reduce cardiovascular risk.(21) The low-risk group was defined as those who ate vegetables and fruits every day and red meat 1 to 6 days a week, which was consistent with current recommendation that emphasizes intakes of vegetables and fruits and limits intake of red meats. For general adiposity measured by BMI, the low-risk group was defined as those who had a BMI of 18.5-23.9 kg/m2, the standard classification of normal weight specific for Chinese.(22) For central adiposity measured by WHR, the low-risk group was defined as those who had a WHR <0.90 in men and <0.85 in women.(23) Adiposity measures were used to assess energy balance, a critical aspect of cardiovascular-healthy diet.(24)
Ascertainment of outcomes
Incident outcome cases since the participants’ enrollment into the study at baseline were identified using linkage with local disease and death registries, with the recently established national health insurance system, and by active follow-up.(16) The 10th revision of the International Classification of Diseases (ICD-10) was used to code all cases by trained staff “blinded” to baseline information. The primary outcomes were incident major coronary events (MCE, including IHD [I20-I25] death and nonfatal myocardial infarction [I21-I23]) and IS [I63]. We also used a broader IHD outcome, which included incident fatal and nonfatal IHD [I20-I25], in the analysis.
The outcome adjudication process of incident IHD and IS cases has been started since 2014. The medical records of cases were retrieved, and the diagnosis was adjudicated centrally by qualified cardiovascular specialists blinded to study assay. By August 2015, of 12,923 incident IHD cases and 13,744 incident IS cases reported since baseline and whose medical records have been retrieved, the diagnosis was confirmed in 82.4% IHD cases and 91.8% IS cases.
Statistical analysis
Person-years at risk were calculated from the baseline date to the diagnosis of outcomes, death, loss to follow-up, or December 31, 2013, whichever came first. Loss to follow-up in the CKB study referred to a participant whose permanent registered residence had moved out of the study area, could not be contacted after at least three times reasonable efforts within one year, or could be contacted but their new residence was out of the jurisdiction of the Regional Coordinating Center. By December 31, 2013, 2,411 (0.5%) participants were lost to follow-up. Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI), with age as the underlying time scale, and stratified jointly by study area and age at baseline in 5-year interval.
In the analysis considering individual lifestyle factors, the models included all the lifestyle factors simultaneously, as well as age, sex, education, marital status, family histories of heart attack or stroke (only adjusted for in corresponding analysis), prevalent hypertension at baseline, and menopausal status (for women only). The same adjustment was made in the analysis of combined lifestyle factors. The linear trend test for individual factors was performed by assigning the sex-specific median to each category and then modeling this as a continuous variable in a separate model; for combined lifestyle factors by treating the number of low-risk factors as a continuous variable. The test for interaction with sex or residence was performed by using likelihood ratio test comparing models with and without cross-product term.
We calculated population attributable risk percent (PAR%),(25) an estimate of the percentage of incident cases in this population during follow-up that would not have occurred if all participants had been in the low-risk group, assuming a causal relation. In these analyses, we used a single binary variable and compared participants in the low-risk group for each factor with all other participants, following a method previously suggested by Wacholder et al.(26) We further estimated the PAR% according to sex, residence, age, family histories of heart attack or stroke, and the presence of hypertension; and repeated the analysis among never-regular smokers never-regular drinkers, participants who were not underweight, and diabetic participants at baseline.
The statistical analyses were performed using Stata (version 13.1, StataCorp). The calculation of PAR% was performed using SAS (version 9.4, SAS Institute Inc). All P values were two-sided, and statistical significance was defined as P<0.05.
Results
The mean age of the participants was 50.7 ± 10.5 years. Of 461,211 participants, 1.0%, 13.7%, and 41.3% had at least 5, 4, and 3 low-risk lifestyle factors, respectively. Participants of women, younger age, being more educated, and urban residents were more likely to adhere to a healthy lifestyle (eTable 1).
During a median of 7.2 years (3.3 million person-years) of follow-up, we documented 3,331 incident MCE (including 2,179 IHD death and 1,152 nonfatal myocardial infarction), 21,857 IHD cases, and 19,348 IS cases. All lifestyle factors were associated with the risks of MCE, IHD, and IS (Table 1 and 2; and eTable 2). Multivariable-adjusted analysis showed that smoking, underweight, and central adiposity were associated with increased risk of MCE; and light-to-moderate alcohol consumption, high physical activity, and a diet rich in vegetables and fruits and limited in red meat were associated with a reduced risk of MCE. For most of the lifestyle factors, similar associations, but smaller in magnitude, were observed with IHD and IS. Different from MCE, overweight and obesity defined by BMI were associated with increased risks of IHD and IS; and underweight was also related to an increased risk of IHD. All associations of lifestyle factors with the risks of incident MCE, IHD, and IS were consistently observed in both men and women (P > 0.05 for interaction with sex), except for the associations of MCE with smoking (Pinteraction = 0.002) and dietary pattern (Pinteraction = 0.010), and the association of IS with WHR (Pinteraction = 0.030) (eTable 3 and 4).
Table 1. Multivariable-adjusted HRs (95% CIs) for incident ischemic heart disease by lifestyle factors among 461,211 participants.
| Major coronary events (ncase = 3,331) | Ischemic heart disease (ncase = 21,857) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Cases/PYs (/1,000) |
HR (95% CI) | Ptrend* | Cases | Cases/PYs (/1,000) |
HR (95% CI) | Ptrend* | |
| Smoking | ||||||||
| Never | 1,669 | 0.7 | 1.00 | 14,060 | 6.3 | 1.00 | ||
| Former | 154 | 1.7 | 1.21 (1.01, 1.44) | 855 | 9.5 | 1.09 (1.01, 1.18) | ||
| Current (cigarettes/day) | ||||||||
| < 15 | 670 | 1.9 | 1.55 (1.39, 1.74) | 0.017 | 2,970 | 8.6 | 1.23 (1.18, 1.30) | <0.001 |
| 15 - | 598 | 1.4 | 1.69 (1.49, 1.91) | 2,850 | 6.5 | 1.30 (1.23, 1.37) | ||
| 25 - | 240 | 1.4 | 1.90 (1.62, 2.23) | 1,122 | 6.6 | 1.43 (1.34, 1.54) | ||
| Alcohol consumption | ||||||||
| Never | 2,585 | 1.0 | 1.00 | 17,706 | 6.7 | 1.00 | ||
| Former | 249 | 2.1 | 1.14 (0.99, 1.31) | 1,168 | 10.2 | 1.18 (1.11, 1.25) | ||
| Current weekly | 171 | 0.9 | 0.86 (0.73, 1.01) | 1,201 | 6.1 | 0.91 (0.85, 0.97) | ||
| Current daily (g/day) | ||||||||
| < 15 | 19 | 1.2 | 0.57 (0.36, 0.90) | 0.011 | 139 | 8.9 | 0.79 (0.66, 0.93) | 0.211 |
| 15 - | 61 | 1.0 | 0.64 (0.50, 0.83) | 392 | 6.9 | 0.78 (0.70, 0.86) | ||
| 30 - | 111 | 1.1 | 0.80 (0.66, 0.98) | 603 | 6.2 | 0.80 (0.74, 0.87) | ||
| 60 - | 135 | 1.0 | 0.97 (0.81, 1.17) | 648 | 5.0 | 0.83 (0.77, 0.91) | ||
| Physical activity (MET-hours/day) | ||||||||
| < 11.0 | 1,708 | 2.1 | 1.00 | <0.001 | 8,671 | 11.1 | 1.00 | <0.001 |
| 11.0 - | 840 | 1.0 | 0.81 (0.75, 0.89) | 6,198 | 7.4 | 0.94 (0.91, 0.97) | ||
| Men 20.0 -, Women 18.0 - | 474 | 0.6 | 0.69 (0.61, 0.77) | 4,024 | 4.9 | 0.90 (0.86, 0.94) | ||
| Men 33.5 -, Women 29.5 - | 309 | 0.4 | 0.67 (0.58, 0.76) | 2,964 | 3.6 | 0.85 (0.81, 0.89) | ||
| Dietary pattern | ||||||||
| Daily FV, weekly but not daily meat | 163 | 0.8 | 1.00 | -- | 1,717 | 8.1 | 1.00 | -- |
| Less than daily FV, daily meat | 14 | 0.9 | 0.99 (0.57, 1.73) | 96 | 6.4 | 1.20 (0.97, 1.47) | ||
| Other | 3,154 | 1.0 | 1.18 (1.00, 1.39) | 20,044 | 6.6 | 1.07 (1.01, 1.13) | ||
| BMI (kg/m2) | ||||||||
| < 18.5 | 284 | 2.0 | 1.43 (1.26, 1.64) | 0.054 | 1,203 | 8.5 | 1.16 (1.09, 1.23) | <0.001 |
| 18.5 - | 1,642 | 0.9 | 1.00 | 10,010 | 5.7 | 1.00 | ||
| 24.0 - | 1,039 | 1.0 | 1.00 (0.92, 1.09) | 7,547 | 7.2 | 1.10 (1.06, 1.14) | ||
| 28.0 - | 366 | 1.1 | 1.03 (0.91, 1.18) | 3,097 | 9.8 | 1.28 (1.22, 1.34) | ||
| WHR | ||||||||
| Men < 0.90, women < 0.85 | 1,213 | 0.8 | 1.00 | <0.001 | 8,248 | 5.8 | 1.00 | <0.001 |
| Men 0.90 -, women 0.85 - | 915 | 1.0 | 1.13 (1.03, 1.24) | 5,983 | 6.5 | 1.05 (1.01, 1.09) | ||
| Men 0.95 -, women 0.90 - | 1,203 | 1.3 | 1.28 (1.16, 1.41) | 7,626 | 8.4 | 1.15 (1.11, 1.20) | ||
HR indicates hazard ratio; CI, confidence interval; PYs, person-years; MET, metabolic equivalent task; FV: fruits and vegetables; BMI, body mass index; and WHR, waist-to-hip ratio. Multivariable model was adjusted for age, sex, education, marital status, family history of heart attack, and prevalent hypertension at baseline. All six lifestyle factors were included simultaneously in the same model.
Linear trend test for smoking was only performed in current smokers and alcohol consumption in current daily drinkers.
Table 2. Multivariable-adjusted HRs (95% CIs) for incident ischemic stroke by lifestyle factors among 461,211 participants.
| (ncase = 19,348) | Cases | Cases/PYs (/1,000) |
HR (95% CI) | Ptrend* |
|---|---|---|---|---|
| Smoking | ||||
| Never | 12,141 | 5.5 | 1.00 | |
| Former | 839 | 9.3 | 0.98 (0.91, 1.06) | |
| Current (cigarettes/day) | ||||
| < 15 | 2,756 | 7.9 | 1.17 (1.11, 1.23) | 0.018 |
| 15 - | 2,655 | 6.1 | 1.22 (1.16, 1.29) | |
| 25 - | 957 | 5.6 | 1.22 (1.13, 1.31) | |
| Alcohol consumption | ||||
| Never | 15,230 | 5.7 | 1.00 | |
| Former | 1,111 | 9.7 | 1.24 (1.16, 1.32) | |
| Current weekly | 1,079 | 5.5 | 0.90 (0.84, 0.96) | |
| Current daily (g/day) | ||||
| < 15 | 170 | 11.0 | 0.94 (0.81, 1.10) | 0.029 |
| 15 - | 422 | 7.4 | 0.90 (0.81, 0.99) | |
| 30 - | 680 | 7.0 | 1.00 (0.92, 1.09) | |
| 60 - | 656 | 5.1 | 1.06 (0.97, 1.15) | |
| Physical activity (MET-hours/day) | ||||
| < 11.0 | 8,713 | 11.2 | 1.00 | <0.001 |
| 11.0 - | 5,482 | 6.5 | 0.92 (0.89, 0.96) | |
| Men 20.0 -, Women 18.0 - | 2,994 | 3.6 | 0.89 (0.85, 0.93) | |
| Men 33.5 -, Women 29.5 - | 2,159 | 2.6 | 0.84 (0.79, 0.88) | |
| Dietary pattern | ||||
| Daily FV, weekly but not daily meat | 1,397 | 6.6 | 1.00 | -- |
| Less than daily FV, daily meat | 133 | 8.9 | 1.35 (1.12, 1.61) | |
| Other | 17,818 | 5.9 | 1.13 (1.07, 1.20) | |
| BMI (kg/m2) | ||||
| < 18.5 | 786 | 5.5 | 0.94 (0.88, 1.02) | <0.001 |
| 18.5 - | 8,756 | 5.0 | 1.00 | |
| 24.0 - | 7,136 | 6.7 | 1.09 (1.05, 1.13) | |
| 28.0 - | 2,670 | 8.4 | 1.14 (1.09, 1.20) | |
| WHR | ||||
| Men < 0.90, women < 0.85 | 6,712 | 4.7 | 1.00 | <0.001 |
| Men 0.90 -, women 0.85 - | 5,690 | 6.2 | 1.12 (1.08, 1.16) | |
| Men 0.95 -, women 0.90 - | 6,946 | 7.6 | 1.15 (1.11, 1.20) |
HR indicates hazard ratio; CI, confidence interval; PYs, person-years; MET, metabolic equivalent task; FV: fruits and vegetables; BMI, body mass index; and WHR, waist-to-hip ratio. Multivariable model was adjusted for age, sex, education, marital status, family history of stroke, and prevalent hypertension at baseline. All six lifestyle factors were included simultaneously in the same model.
Linear trend test for smoking was only performed in current smokers and alcohol consumption in current daily drinkers.
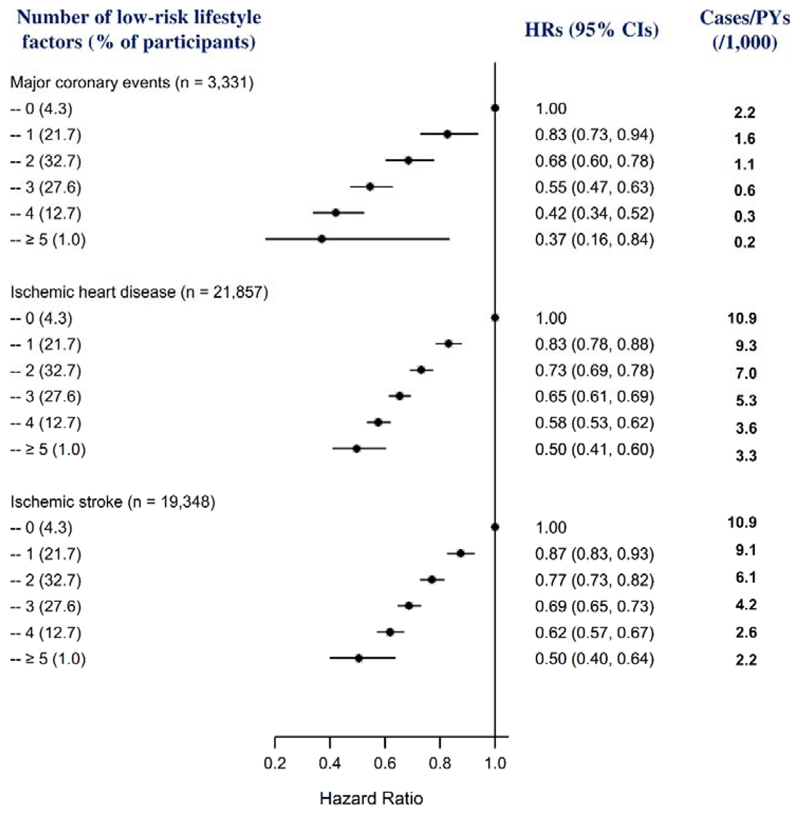
When the six lifestyle factors were collapsed into binary categories, all the low-risk groups were associated with reduced risks of MCE, IHD, and IS (Table 3; and eTable 5); most of these associations were consistently observed between men and women (eTable 6), and between urban and rural residents (eTable 7). The risks of MCE, IHD, and IS decreased significantly with an increasing number of any low-risk factors in the whole cohort (Fig 1; and eTable 8) and both men and women (eTable 9) (all P for linear trend <0.001). Compared with participants who were not in the low-risk group of any factors, the adjusted HRs (95% CIs) of those who had four or more low-risk factors was 0.42 (0.34, 0.52) for MCE, 0.57 (0.53, 0.61) for IHD, and 0.61 (0.56, 0.66) for IS.
Table 3. Multivariable-adjusted HRs (95% CIs) and PAR% (95% CIs) for incident ischemic cardiovascular diseases by low-risk lifestyle factors* among 461,211 participants.
| Cases in low-risk group | Cases/PYs (1,000) in low-risk group | HR (95% CI) | PAR% (95% CI) | |
|---|---|---|---|---|
| Major coronary events (ncase = 3,331) | ||||
| Smoking | 1,823 | 0.8 | 0.63 (0.57, 0.69) | 16.9 (14.5, 19.2) |
| Alcohol consumption | 80 | 1.1 | 0.64 (0.51, 0.80) | 33.9 (18.7, 47.5) |
| Physical activity | 783 | 0.5 | 0.74 (0.68, 0.82) | 21.6 (15.9, 27.1) |
| Dietary pattern | 163 | 0.8 | 0.84 (0.72, 0.99) | 15.2 (1.7, 28.1) |
| BMI | 1,642 | 0.9 | 0.89 (0.83, 0.95) | 5.8 (2.1, 9.4) |
| WHR | 1,213 | 0.8 | 0.89 (0.83, 0.96) | 6.4 (1.8, 11.0) |
| Ischemic heart disease (ncase = 21,857) | ||||
| Smoking | 14,915 | 6.5 | 0.80 (0.77, 0.83) | 6.5 (5.5, 7.5) |
| Alcohol consumption | 531 | 7.3 | 0.80 (0.73, 0.87) | 19.5 (12.4, 26.3) |
| Physical activity | 6,988 | 4.3 | 0.90 (0.87, 0.93) | 8.0 (5.8, 10.1) |
| Dietary pattern | 1,717 | 8.1 | 0.94 (0.89, 0.99) | 6.2 (1.6, 10.7) |
| BMI | 10,010 | 5.7 | 0.86 (0.84, 0.89) | 7.8 (6.2, 9.3) |
| WHR | 8,248 | 5.8 | 0.91 (0.88, 0.94) | 5.2 (3.5, 7.0) |
| Ischemic stroke (ncase = 19,348) | ||||
| Smoking | 12,980 | 5.6 | 0.83 (0.80, 0.87) | 5.5 (4.4, 6.6) |
| Alcohol consumption | 592 | 8.2 | 0.90 (0.82, 0.97) | 8.6 (1.1, 16.1) |
| Physical activity | 5,153 | 3.1 | 0.90 (0.87, 0.93) | 9.2 (6.7, 11.7) |
| Dietary pattern | 1,397 | 6.6 | 0.89 (0.84, 0.94) | 10.8 (6.0, 15.6) |
| BMI | 8,756 | 5.0 | 0.92 (0.89, 0.95) | 4.5 (2.8, 6.1) |
| WHR | 6,712 | 4.7 | 0.86 (0.83, 0.89) | 8.8 (6.8, 10.7) |
HR indicates hazard ratio; CI, confidence interval; PAR%, population attributable risk percent; PYs, person-years; BMI, body mass index; and WHR, waist-to-hip ratio. Multivariable model was adjusted for age, sex, education, marital status, family histories of heart attack or stroke (adjusted for in the corresponding analysis), and prevalent hypertension at baseline. All six lifestyle factors were included simultaneously in the same model.
Low-risk lifestyle factors were defined as: non-smoking or having stopped for reasons other than illness; drinking greater than zero but less than 30 g of alcohol per day; engaging in a sex-specific median or higher level of physical activity; eating fruits and vegetables everyday and red meat 1 to 6 days a week; having a BMI between 18.5 and 23.9 kg/m2; and having a WHR < 0.90 in men and < 0.85 in women.
Figure 1. Multivariable-adjusted HRs (95% CIs) for incident ischemic cardiovascular diseases by number of low-risk lifestyle factors among 461,211 participants.
HR indicates hazard ratio; CI, confidence interval; and PYs, person-years. Horizontal lines represent 95% CI. “n” in parentheses indicates the number of new cases. Multivariable model was adjusted for age, sex, education, marital status, family histories of heart attack or stroke, and prevalent hypertension at baseline.
To test the robustness of the findings, we examined potential confounding of socioeconomic status by adding occupation and household income to the model, or including participants who had diabetes at baseline in the analysis and adjusting for diabetes in the model, or adjusting for systolic blood pressure and use of cardiovascular medications. To minimize potential bias due to subclinical conditions, we performed analyses by further excluding participants whose cardiovascular outcomes occurred in the first two years of follow-up or excluding underweight participants (BMI<18.5 kg/m2). These sensitivity analyses did not substantially alter the risk estimates (data not shown).
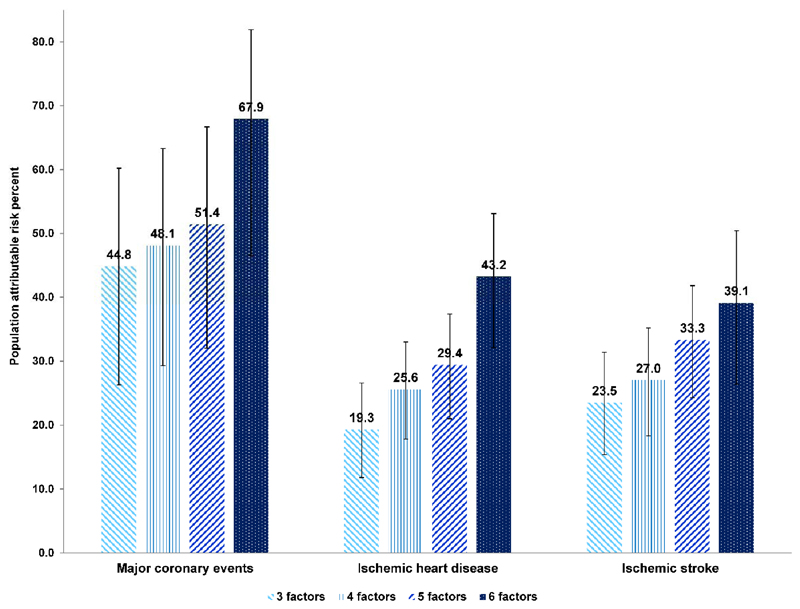
Table 3 (and eTable 6 and 7) presents the PAR% for each lifestyle factor. The combined PAR% (95% CI) of MCE in relation to smoking, lack of physical activity, and unhealthy diet was 44.8% (26.3%, 60.2%), which increased to 51.4% (32.0%, 66.7%) when additionally considering general and central adiposity (Central Illustration; and eTable 10). The PAR% (95% CI) for all six factors was 67.9% (46.5%, 81.9%) for MCE, suggesting that approximately two-thirds of the incident MCE in this population during follow-up period might have been prevented if all participants had been in the low-risk group for six factors. The risk attributable to these modifiable lifestyle factors was lower for IHD and IS. The PAR% (95% CI) for six factors was 43.2% (32.1%, 53.1%) for IHD and 39.1% (26.4%, 50.4%) for IS.
Central Illustration. Multivariable-adjusted PARs% (95% CIs) for incident ischemic cardiovascular diseases by specific combination of low-risk lifestyle factors among 461,211 participants.
PAR% indicates population attributable risk percent; CI, confidence interval; BMI, body mass index; and WHR, waist-to-hip ratio. Specific combinations of low-risk lifestyle factors are: 3 lifestyle factors indicating smoking, physical activity, and dietary pattern; 4 factors additionally including BMI; 5 factors additionally including WHR; 6 factors additionally including alcohol consumption. Multivariable model was adjusted for age, sex, education, marital status, family histories of heart attack or stroke, and prevalent hypertension at baseline. All six lifestyle factors were included simultaneously in the same model.
The PAR% estimates appeared to be similar for men and women, for urban and rural residents, for different age groups, for participants with or without a family history of heart attack or stroke, and for participants with or without hypertension (eTable 10). The potential reduction in risks of MCE, IHD, and IS among never-regular smokers or never-regular drinkers, though with wider CIs, were generally consistent with those observed in the whole study population (eTable 11). Exclusion of underweight participants from the analysis did not substantially alter the PAR% estimates. When we estimated the PAR% in the participants with diabetes at baseline, who were excluded from the primary analysis, larger reduction in risk of IHD was observed in relation to smoking, physical activity, dietary pattern, and adiposity.
Discussion
In this large prospective cohort of 0.5 million middle-to-older aged Chinese, adhering to a healthy lifestyle, that is, never smoking or stopping smoking not for illness, consuming alcohol lightly or moderately, physically active, eating a diet rich in vegetables and fruits and limited in red meat, and maintaining a normal BMI and a lower WHR, was associated with a significantly reduced risk of ischemic CVDs. Compared with participants without any of the low-risk lifestyle factors, participants who had at least four low-risk factors showed a 58%, 43%, and 39% reduction in relative risk of MCE, IHD, and IS, respectively. If observed associations are causal, two-thirds of MCE, two-fifths of IHD, and two-fifths of IS in this population during a median 7.2 years of follow-up could have been avoided by adherence to a healthy lifestyle.
Our findings are consistent with previous cohort studies conducted in the American (11,14,15,19) and European populations,(12,13,27) indicating that the reduction in relative risk of CVD incidence or death is proportional to the increased number of healthy lifestyle factors. Findings from the Nurses’ Health Study (NHS) of 15 to 20 years follow-up data showed that the PAR% (95% CI) for the combination of smoking, alcohol consumption, physical activity, diet, and BMI was 82% (58%, 93%) for CHD incidence,(11) 54% (15%, 78%) for IS incidence,(15) and 74% (55%, 86%) for CVD incidence.(11) Similar PARs% were estimated in other cohorts from the U.S. and Swedish.(12–15) A further analysis of 24-year follow-up data of NHS showed that 75.2% (60.9%, 84.7%) of CVD death could be attributed to above five factors.(19) In a study conducted in elderly European aged 70 to 90 years, lack of adherence to the low-risk pattern of smoking, alcohol consumption, physical activity, and diet was associated with a 64% increase of CHD death and a 61% increase of CVD death during a ten-year period.(27) Longer duration of follow-up and population characterized by higher education and socioeconomic status may partly explain the observed higher PAR% for CVDs in the US cohorts than those observed in our Chinese population.
Only one prospective study from the Shanghai Women’s Health Study quantified the combined impact of a healthier lifestyle pattern, including normal BMI, lower WHR, participation in physical exercise, nonexposed to spousal smoking, and higher fruit and vegetable intake, on CVD death in lifetime nonsmoking and nondrinking Chinese women aged 40 to 70 years.(28) The PAR% for having 4 to 5 unhealthy lifestyle factors was 58.7% for CVD death during 9-year follow-up. However, this study only included women from one of the most developed cities of China, and the small number of incident cases precluded further analyses on different types of CVDs. To the best of our knowledge, the present study was the first that comprehensively assessed the relation between a combination of multiple lifestyle factors and various CVD outcomes in Chinese.
In the present study, we observed that the PAR% was higher for IHD than IS, consistent with observations reported in the NHS.(11,15) A possible explanation is that IS has risk factors partly different from those of IHD.
It is worth mentioning that light-to-moderate alcohol consumption was shown to have a particularly important protective effect on IHD in our population. Nevertheless, it should be noted that even light-to-moderate drinking might increase the risk of other outcomes such as cancer.(29,30) Therefore, we would be cautious in the recommendation of consuming alcohol regarding overall human health.(31,32) In the present population, there were still one-half of MCE and one-third of IS that might have been prevented by compliance with the remaining components of the low-risk lifestyle irrespective of alcohol consumption.
To the best of our knowledge, this is by far the largest prospective study quantifying the burden of ischemic CVDs that could be prevented through adherence to a set of well-studied modifiable lifestyle factors. A large number of incident cases provides more reliable estimates than previous studies. Our study for the first time provided evidence for the joint beneficial effects of multiple lifestyle factors on prevention of ischemic CVDs in the nationally representative general population of Chinese. The inclusion of a geographically spread population living in urban and rural areas, with different socio-demographic characteristics such as gender, education, income, and occupation, makes our results broadly applicable. We carefully controlled for potential confounding factors and sought to minimize the reverse causation bias by excluding participants with major chronic diseases at baseline that might lead to lifestyle changes. We further excluded the participants whose cardiovascular outcomes occurred in the first two years of follow-up or underweight participants to address the concern of subclinical disease; the results remained virtually unchanged. In addition, the anthropometric information was measured rather than self-reported in our cohort, providing more accurate estimates of BMI and WHR.
This study acknowledges a few limitations. The lifestyle behaviors were self-reported, potentially leading to some misclassification. The questionnaire on lifestyle factors used in the CKB study has not yet been validated directly; however, these questions were adapted from validated questionnaires used in several other studies, with some additional modifications after a pilot study. Such measurement errors, however, may be non-differential on subsequent disease status and tend to attenuate the association. The lifestyle factors were measured once at baseline and might not necessarily reflect the long-term patterns. Residual confounding by other unmeasured or unknown factors, particularly socioeconomic status, was still possible. However, adjustment for education, occupation, and household income had little influence on the findings. In addition, lack of detailed dietary information and quantitative measure of food consumption in this study limited our ability to capture the complexity of the dietary patterns comprehensively. Nevertheless, the limited food items included in our study have shown consistent associations with CVD outcomes of interest; and the guidelines for which is also easy to follow. Lack of further classification of IS subtypes may undervalue the role of lifestyle factors in some specific subtypes of IS.
Conclusions
In summary, this thus far the largest prospective cohort of Chinese adults has provided convincing epidemiologic evidence that adherence to a healthy lifestyle, that is, abstinence from or cessation of smoking, light-to-moderate alcohol consumption, eating a healthy diet, being physically active, and maintenance of a healthy weight without central adiposity, would prevent approximately two-thirds of MCE and two-fifths of IS over a period of fewer than ten years. A Larger reduction in CVD risk can be expected with additionally added preventable factors. This study provides critical quantitative estimates of the potential effect of a population-based lifestyle intervention on the growing burden of ischemic CVDs in China. Extended follow-up of this cohort would provide further evidence of the longer-term impact of overall lifestyle modification in disease prevention.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Adherence to a healthy lifestyle could substantially lower the burden of cardiovascular diseases in China. The population-based lifestyle intervention remains a mainstream approach to address the cardiovascular burden worldwide.
Translational Outlook
Effective strategies and measurements are needed to encourage people to adopt and maintain a healthy lifestyle.
Acknowledgments
The most important acknowledgment is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford and the 10 regional centres.
Funding/Support: This work was supported by grants (81390544, 81390541) from the National Natural Science Foundation of China. The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z); by a grant from the Chinese Ministry of Science and Technology (2011BAI09B01). Dr Lv is supported by the State Scholarship Fund of China Scholarship Council (201506015053). Dr Qi is supported by NIH grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024, HL132254), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States – Israel Binational Science Foundation Grant 2011036. Dr Qi was a recipient of the American Heart Association Scientist Development Award (0730094N). The funders had no role in the study design, data collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
Role of Sponsor: The sponsors had no role in study design, data collection, data analysis and interpretation, writing of the report, or decision to submit the article for publication.
Abbreviations and acronyms
- BMI
body mass index
- CHD
coronary heart disease
- CKB
China Kadoorie Biobank
- CVD
cardiovascular diseases
- IHD
ischemic heart disease
- IS
ischemic stroke
- MCE
major coronary events
- METs
metabolic equivalent tasks
- WHR
waist-to-hip ratio
Footnotes
Financial Disclosures: None reported.
Contributor Information
Jun Lv, Email: lvjun@bjmu.edu.cn.
Canqing Yu, Email: yucanqing@bjmu.edu.cn.
Yu Guo, Email: guoyu@kscdc.net.
Zheng Bian, Email: bianzheng@kscdc.net.
Ling Yang, Email: ling.yang@ctsu.ox.ac.uk.
Yiping Chen, Email: yiping.chen@ctsu.ox.ac.uk.
Xuefeng Tang, Email: sccdctxf@163.com.
Weiyuan Zhang, Email: 64536229@qq.com.
Yijian Qian, Email: txcdcqyj@163.com.
Yuelong Huang, Email: 13908483840@126.com.
Xiaoping Wang, Email: maiji-KSCDC@163.com.
Junshi Chen, Email: chenjunshi@cfsa.net.cn.
Zhengming Chen, Email: zhengming.chen@ctsu.ox.ac.uk.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–90. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 4.United States. Public Health Service. Office of the Surgeon General. The health consequences of smoking--50 years of progress: a report of the Surgeon General: executive summary. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2014. [Google Scholar]
- 5.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122:743–52. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 7.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases--incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–8. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol. 2014;25:20–6. doi: 10.1097/MOL.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 9.Prospective Studies Collaboration. Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–29. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 12.Akesson A, Weismayer C, Newby PK, Wolk A. Combined effect of low-risk dietary and lifestyle behaviors in primary prevention of myocardial infarction in women. Arch Intern Med. 2007;167:2122–7. doi: 10.1001/archinte.167.19.2122. [DOI] [PubMed] [Google Scholar]
- 13.Akesson A, Larsson SC, Discacciati A, Wolk A. Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol. 2014;64:1299–306. doi: 10.1016/j.jacc.2014.06.1190. [DOI] [PubMed] [Google Scholar]
- 14.Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65:43–51. doi: 10.1016/j.jacc.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118:947–54. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Chen J, Collins R, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–66. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du H, Li L, Bennett D, et al. Fresh Fruit Consumption and Major Cardiovascular Disease in China. N Engl J Med. 2016;374:1332–43. doi: 10.1056/NEJMoa1501451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu C, Shi Z, Lv J, et al. Major Dietary Patterns in Relation to General and Central Obesity among Chinese Adults. Nutrients. 2015;7:5834–49. doi: 10.3390/nu7075253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Peto R, Zhou M, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 2015;386:1447–56. doi: 10.1016/S0140-6736(15)00340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960–84. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Lu FC, Department of Disease Control Ministry of Health PRC The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 23.World Health Organization. Waist circumference and waist–hip ratio. Report of a WHO expert consultation, Geneva, 8-11 December 2008. Geneva: World Health Organization; 2011. [Google Scholar]
- 24.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18:571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 26.Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;140:303–9. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]
- 27.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–9. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 28.Nechuta SJ, Shu XO, Li HL, et al. Combined impact of lifestyle-related factors on total and cause-specific mortality among Chinese women: prospective cohort study. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–9. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Bagnardi V, Rota M, Botteri E, et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol. 2013;24:301–8. doi: 10.1093/annonc/mds337. [DOI] [PubMed] [Google Scholar]
- 31.Colhoun H, Ben-Shlomo Y, Dong W, Bost L, Marmot M. Ecological analysis of collectivity of alcohol consumption in England: importance of average drinker. BMJ. 1997;314:1164–8. doi: 10.1136/bmj.314.7088.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth A, Teo KK, Rangarajan S, et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet. 2015;386:1945–54. doi: 10.1016/S0140-6736(15)00235-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.