ABSTRACT
Background
At rest, omission of breakfast lowers daily energy intake, but also lowers energy expenditure, attenuating any effect on energy balance. The effect of breakfast omission on energy balance when exercise is prescribed is unclear.
Objectives
The aim of this study was to assess the effect on 24-h energy balance of omitting compared with consuming breakfast prior to exercise.
Methods
Twelve healthy physically active young men (age 23 ± 3 y, body mass index 23.6 ± 2.0 kg/m2) completed 3 trials in a randomized order (separated by >1 week): a breakfast of oats and milk (431 kcal; 65 g carbohydrate, 11 g fat, 19 g protein) followed by rest (BR); breakfast before exercise (BE; 60 min cycling at 50 % peak power output); and overnight fasting before exercise (FE). The 24-h energy intake was calculated based on the food consumed for breakfast, followed by an ad libitum lunch, snacks, and dinner. Indirect calorimetry with heart-rate accelerometry was used to measure substrate utilization and 24-h energy expenditure. A [6,6-2H2]glucose infusion was used to investigate tissue-specific carbohydrate utilization.
Results
The 24-h energy balance was −400 kcal (normalized 95% CI: −230, −571 kcal) for the FE trial; this was significantly lower than both the BR trial (492 kcal; normalized 95% CI: 332, 652 kcal) and the BE trial (7 kcal; normalized 95% CI: −153, 177 kcal; both P < 0.01 compared with FE). Plasma glucose utilization in FE (mainly representing liver glucose utilization) was positively correlated with energy intake compensation at lunch (r = 0.62, P = 0.03), suggesting liver carbohydrate plays a role in postexercise energy-balance regulation.
Conclusions
Neither exercise energy expenditure nor restricted energy intake via breakfast omission were completely compensated for postexercise. In healthy men, pre-exercise breakfast omission creates a more negative daily energy balance and could therefore be a useful strategy to induce a short-term energy deficit. This trial was registered at clinicaltrials.gov as NCT02258399.
Keywords: breakfast, carbohydrate; exercise, energy balance; fasting; metabolism; physical activity; substrate metabolism
Introduction
Obesity is an escalating global epidemic, and is a consequence of a chronic positive energy imbalance. In addition to reducing calorie intake, regular exercise is a commonly proposed strategy for facilitating weight loss or weight maintenance (1). Exercise increases energy expenditure and thus alters energy balance, thereby potentially favoring the conditions for reductions in body and fat mass. Despite this, exercise training interventions often report smaller than expected fat and body mass losses (2, 3). This modest response can be explained by compensation of energy balance behaviors (either by the activity stimulating energy intake, or decreasing physical activity outside of the prescribed exercise, or a combination of these factors), and this can reduce the energy deficit created by the energy expended through exercise (4). A similar example of this compensation is that breakfast omission at rest (i.e., reduced energy intake) decreases morning nonexercise physical activity in lean and obese humans (5, 6).
In particular, altering carbohydrate balance may have implications for subsequent energy balance (7). Endogenous carbohydrate stores (liver and muscle glycogen) have a smaller capacity than lipid stores [<3000 kcal in a lean 75-kg male compared with >100,000 kcal for lipids (8, 9)]. Due to this limited storage capacity, the glycogenostatic theory (7) proposes that endogenous carbohydrate stores are closely regulated, and because of this, glycogen depletion may increasingly stimulate compensatory energy intake in order to favor the replenishment of these stores. For example, in humans there is some (albeit limited) evidence that individuals who display higher rates of carbohydrate utilization when exercising are more likely to compensate with a higher postexercise energy intake (8, 10, 11). Although short-term (1–3 d) alterations in glycogen availability with exercise or diet do not always result in a measurable compensation in energy intake (8), mice overexpressing hepatic protein targeted at glycogen (which increases liver glycogen concentrations) also display reduced energy intake and increased energy expenditure (12). If carbohydrate metabolism is indeed a driver of subsequent energy intake, any strategies that attenuate carbohydrate use during exercise may help protect against compensation through postexercise energy intake (thereby reducing any erosion of the exercise-induced energy deficit). One strategy that reduces the rate of whole-body carbohydrate utilization during exercise is pre-exercise fasting (i.e. breakfast omission) (13). Furthermore, because breakfast omission under resting conditions does not result in energy intake compensation at lunch (14, 15), any potential protection against a subsequent higher energy intake (by a lower carbohydrate utilization during exercise when fasting) is unlikely to be compromised due to the lower pre-exercise food intake (i.e., with the omission of breakfast).
If moderate-intensity (55% VO2 peak) endurance-type exercise is performed in the overnight fasting state, whole-body carbohydrate utilization is lower (and fat utilization is higher) than with exercise after breakfast (13). Interestingly, humans do not fully compensate with energy intake at a subsequent meal if breakfast is omitted before exercise (15, 16), which extends observations made about breakfast consumption during resting conditions (5). These findings are consistent with a role for carbohydrate balance in the regulation of energy balance. However, an objective assessment of daily energy balance (when energy intake and energy expenditure are assessed in a free-living setting) after exercise with prior breakfast consumption compared with omission has never been conducted. This limitation is especially important in light of findings that the omission of breakfast at rest decreases light intensity (i.e., non-exercise) physical activity energy expenditure (5, 6). Whether the carbohydrate status (content or rate of utilization) of a specific tissue (liver or muscle glycogen) is a more potent regulator of postexercise energy balance also remains unknown. Indeed, no study has specifically assessed a relationship between muscle or liver carbohydrate utilization and postexercise energy balance in humans. Investigating this response would provide mechanistic insights into the regulation of postexercise energy balance, and this information could then be applied to refine exercise and nutritional strategies (e.g., pre-exercise fasting to lower liver glycogen concentrations compared with exercise to deplete muscle glycogen).
Therefore, the main aim of this experiment was to investigate the role of carbohydrate availability during exercise on net energy balance over 24 h. In addition, the application of glucose tracer methods (to assess hepatic glucose output and utilization) combined with indirect calorimetry was implemented to address a secondary aim of exploring the tissue-specific regulation of energy balance.
Methods
Ethical approval
These results were collected as part of a wider study (17), but none of the outcome measures reported here have been previously published. The study was completed at the University of Bath (UK) as per the Declaration of Helsinki. Ethical approval was given by the National Health Service South-West Research Ethics Committee (15/SW/0006). The trial was registered at clinicaltrials.gov as NCT02258399. Prior to participation, written and informed consent was obtained from all participants.
Study design
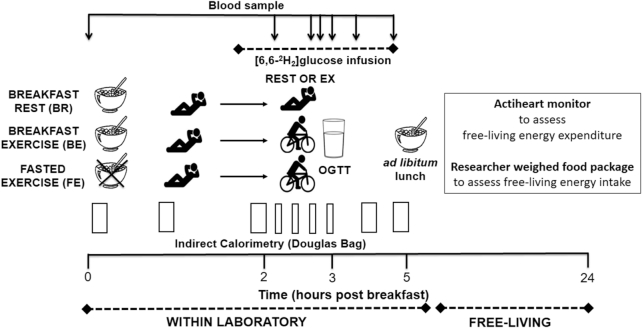
This study adopted a randomized cross-over design (randomization performed by JTG with Research Randomizer version 3.0, http://www.randomizer.org/). Preliminary testing was followed by 3 trials (7–30 d apart), which were breakfast followed by rest (BR), breakfast followed by exercise (BE), and overnight fasting followed by exercise (FE), in a random and counterbalanced order. A protocol schematic is shown in Figure 1. For all trials, participants arrived at the laboratory at 0800 ± 1 h having fasted overnight (12–14 h). In BR, upon arrival at the laboratory, a porridge breakfast was consumed, followed by 3 h of rest, and a 2-h oral glucose tolerance test (OGTT). In BE, the breakfast was consumed, before 2 h rest and 60 min of cycling, and the OGTT. In FE, breakfast was omitted but the trial otherwise replicated BE. After the OGTT, participants were provided an ad libitum lunch (within the laboratory) as well as a researcher-weighed food package for consumption over the remaining 24-h trial (free-living). Daily energy expenditure was assessed via indirect calorimetry (for within-laboratory components) and heart-rate with accelerometry (free-living after leaving the laboratory). All trials were completed in similar laboratory conditions as previously described (17).
FIGURE 1.
Schematic. Twelve healthy physically active young men completed 3 trials in randomly assigned order: breakfast followed by rest (BR), breakfast followed by exercise (BE), or overnight fasting followed by exercise (FE). Daily energy intake was determined based on an ad libitum lunch, snacks, and dinner meals. Indirect calorimetry (within-lab) and heart-rate accelerometry (free-living) were used to assess substrate use and daily energy expenditure. A [6,6-2H2]glucose infusion was used to assess tissue-specific carbohydrate utilization.
Participants
Twelve healthy and physically active men were recruited from Bath and the surrounding area, between May and November 2015. Participant characteristics are shown in Table 1. The main exclusion criteria for participants were a history of metabolic disease, or any condition that may have posed undue personal risk to the participant or introduced bias to the study. Participants were also asked to confirm that they were not taking any medication that may have influenced any of the reported outcomes.
TABLE 1.
Participant Characteristics1
| Age, y | 23 ± 3 |
| Stature, cm | 179.8 ± 4.4 |
| Body mass, kg | 76.3 ± 7.9 |
| BMI, kg/m2 | 23.6 ± 2.0 |
| Fat mass, kg2 | 10.6 ± 3.7 |
| Fat mass index,2 kg/m2 | 3.26 ± 1.12 |
| Body fat,2 % | 14 ± 4 |
| Fat-free mass,2 kg | 65.5 ± 6.4 |
| Resting metabolic rate, kcal/day | 2091 ± 101 |
| V̇O2 peak,3 L/min | 4.00 ± 0.72 |
| V̇O2 peak,3 mL · kg · min−1 | 53 ± 10 |
| Peak power output, W | 317 ± 67 |
| Max heart rate, beats/min | 189 ± 10 |
1Values are means ± SDs; n = 12 healthy physically active young men. V̇O2 peak, peak oxygen uptake.
2Obtained by DXA.
3 n = 11 because of technical difficulties with the breath-by-breath analysis during a preliminary testing session.
Preliminary testing
Each participant's stature was measured to the nearest 0.1 cm with a stadiometer (Seca Ltd) and their body mass was recorded to the nearest 0.1 kg with electronic weighing scales (BC543 Monitor; Tanita). A whole-body DXA scan was completed to quantify fat and fat-free mass (Discovery; Hologic). An incremental cycling exercise test was completed on an electronically braked ergometer (Excalibur Sport; Lode BV) as previously described, to quantify peak power output and peak oxygen uptake (V̇O2 peak) (17).
Main trials
Participants abstained from alcoholic and caffeinated drinks for 24 h prior to all trials. Food intake ceased at 2000 on the evening before trials and participants then fasted overnight (≥12 h), consuming only water (ad libitum) during this period. The final meal consumed by the participants on the evening before all trials was provided (spinach and ricotta cannelloni; Tesco) to ensure the energy and macronutrient intake across participants was standardized [592 kcal of energy (2479 kJ); 51 g carbohydrate, 32 g fat, 25 g protein]. Participants were also asked to refrain from strenuous physical activity for 24 h prior to trials, but were allowed to otherwise maintain their normal physical activity behaviors (replicated for subsequent trials). To help ensure this standardization, participants recorded an activity diary and wore a physical activity monitor (Actiheart; Cambridge Neurotechnology). There were no differences between trials in pretrial physical activity energy expenditure as previously reported (17).
Participants arrived at the laboratory at 0800 ± 1 h and this time was replicated for subsequent trials. They voided, and all further urine samples were collected for measurement of urine urea concentrations and estimates of urinary nitrogen excretion. An intravenous catheter (Venflon Pro; BD) was placed retrograde into a dorsal hand vein that had been heated for 20 min with a heated-air box set to 55°C. A baseline blood sample (10 mL) was drawn, before a 5-min expired gas sample was collected. In BE and BR, a porridge breakfast was then consumed, but FE participants were only permitted water. This was followed by 2 h of rest (activities such as reading while semirecumbent were allowed), with 5-min expired gas samples collected hourly. During this period, a catheter was inserted into an antecubital vein and a primed variable-rate infusion of [6,6-2H2]glucose was initiated. After the 2-h rest period (in BE and FE only), participants began 60 min of cycling at 50% peak power output on an ergometer (Corival; Lode BV). In BR, participants continued to rest in this period. Expired gases were collected every 15 min (for 2 min) and blood was sampled at 40 and 50 min of exercise (rest in BR). Then a 2-h OGTT was completed, with blood sampled every 10 min and 5-min expired gas samples collected hourly. Muscle biopsy samples were collected pre- and post-OGTT as detailed elsewhere (17). Participants were then given a researcher-weighed ad libitum lunch. After lunch, participants left the laboratory with a researcher-weighed food package, and wore the Actiheart for the remainder of the 24-h trial.
Test meals
Prior to participation participants confirmed they had no allergies or aversions to any of the foods provided. The breakfast was 72 g of instant refined oats (Oatso Simple Golden Syrup; Quaker Oats) and 360 mL of semiskimmed milk (Tesco), providing 431 kcal of energy (1803 kJ; 65 g carbohydrate, 11 g fat, 19 g protein). Lunch comprised oats (Everyday Value; Tesco), whole milk (Tesco), maltodextrin, whey protein isolate (both Myprotein), olive oil (Tesco), and water, designed to limit the degree of palatability, with the aim of preventing overconsumption (18). The meal provided 150 kcal of energy per 100 g of cooked food (626 kJ; 20 g carbohydrate; 5 g fat; 5 g protein) and was terminated when participants said that they felt “comfortably full.” Fresh, warmed portions were continually provided to ensure that the participant finishing a portion was not responsible for meal termination. Participants ate the lunch meal in isolation and every attempt was made to avoid cues that may have influenced their eating behaviors (the television was switched off and their mobile phones were not present during the meal). Remaining food was removed and weighed out of the sight of participants. The free-living food package comprised the following: 1) a pasta meal, containing pasta, tomato sauce, cheddar cheese, and olive oil (Tesco, prepared by the researchers), providing 151 kcal of energy per 100 g of cooked food (632 kJ; 20 g carbohydrate; 6 g fat; 5 g protein); 2) four 35-g snack bars [GoAhead; 367 kcal (1536 kJ) per 100 g; 74 g carbohydrate, 8 g fat; 3 g protein]; and 3) two 180-mL chocolate milk flavor drinks [Mars Milk; 63 kcal (264 kJ) per 100 mL; 10 g carbohydrate; 2 g fat; 3 g protein]. Participants were instructed to eat until they were “comfortably full,” not to eat or drink anything not provided by this food package (although ad libitum water consumption was allowed), and to bring any remaining food back to the laboratory the following morning. The carbohydrate, fat, and protein intake was taken as grams provided (cooked weight) minus grams remaining.
Blood sampling and analysis
Whole blood was dispensed into EDTA-coated tubes (BD) which were centrifuged (4°C and 3500 × g) for 10 min (Heraeus Biofuge Primo R; Kendro Laboratory Products Plc) to obtain plasma. This was dispensed into 0.5-mL aliquots and frozen at −20°C, before longer-term storage at −80°C. Plasma glucose concentrations (intra-assay CV, 3.2%; interassay CV, 3.8%) were measured on an automated analyzer (Daytona; Randox Lab). Plasma leptin and fibroblast growth factor 21 (FGF-21) concentrations were measured with commercially available ELISAs (Mercodia AB; intra-assay CV, 5.8%; interassay CV, 7.1% for leptin; BioVendor Research & Diagnostic Products). For blood analysis, samples were analyzed in batches after sample collection had been completed, and for a given participant all samples were run on the same plate. Plasma [2H2]glucose enrichments were determined via GC-MS as described elsewhere (17) (GC, Agilent 6890 N; MS, Agilent 5973 N; Agilent Technologies). Radziuk's 2-compartment non-steady-state model was used to assess plasma glucose flux (19, 20).
Energy expenditure and substrate utilization
Indirect calorimetry was used to assess energy expenditure and substrate utilization from expired gas samples (21). An average of the baseline expired gas sample from the trials was taken for the resting metabolic rate. Urinary nitrogen excretion was estimated from urine urea concentrations, which were measured on an automated analyzer (Daytona; Randox Lab). This allowed protein utilization to be accounted for in calculations of carbohydrate and lipid utilization. Free-living physical activity energy expenditure was assessed [from when the participant left the laboratory until 24 h after breakfast consumption (or omission)] with the use of an Actiheart, which integrates accelerometer and heart-rate signals (22). Physical activity energy expenditure was calculated through the use of branched-equation modeling (23) with measured energy expenditure and heart-rate values from exercise and from rest entered into the Actiheart software for an individually calibrated model (24, 25). Data were considered to be of a usable quality if >90% of the activity trace was “not lost” with <30% of the heart-rate trace extrapolated by the software. Daily energy expenditure was calculated from both indirect calorimetry (for the within-laboratory period), and the Actiheart data (for free-living periods).
Calculations and statistical analysis
A sample size calculation was completed based on previous research, and gave a mean ± SD difference in energy balance (within-lab) between an FE and BE trial of 1580 ± 1410 kJ (15). With an α-level of 0.05, 12 participants were required to provide an 80% chance of detecting a statistically significant difference in energy balance with a repeated-measures ANOVA and 3 trials. The total and incremental area under the concentration-time curves for plasma FGF-21 and plasma leptin were calculated with the trapezoid rule. This was divided by the observation time period (i.e., 300 min) for a time-averaged value (mmol/L).
Whole-body fat and carbohydrate utilization rates were calculated via stoichiometric equations (21), with adjustments made to account for protein utilization and the utilization of muscle glycogen during exercise (21). Within-lab energy expenditure was determined, and lipids, glucose, and glycogen were found to produce 40.81, 15.64 and 17.36 kJ/g of energy, respectively (21). At these exercise intensities, plasma glucose utilization during exercise can be assumed to be equivalent to the plasma glucose rate of disposal (26). Muscle glycogen utilization was calculated as total carbohydrate utilization minus plasma glucose utilization during exercise (this estimate therefore includes the utilization of all nonglucose carbohydrates, e.g., lactate). To account for excess postexercise oxygen consumption in the BE and FE trials, estimates of substrate utilization and energy expenditure were adjusted based on similar research (27). To assess whether the carbohydrate status of specific tissues (i.e., liver or muscle) were predictors of postexercise energy intake, plasma glucose and muscle glycogen utilization during exercise in the FE trial was correlated with compensation in energy intake at lunch (energy intake on FE minus energy intake on BR, normalized to resting metabolic rate, as this measure best reflects the energy requirements of rest). Only data from the FE trial were included in this analysis because, when fasting, plasma glucose utilization during exercise primarily represents the utilization of glucose from hepatic sources, whereas during the BE trial, plasma glucose utilization during exercise reflects liver glucose utilization and glucose from the pre-exercise breakfast.
All data were first tested for normality with a Shapiro-Wilk test. One-way repeated-measures ANOVAs were used to detect differences between trials at baseline and for summary measures. For multiple comparisons, 2-factor repeated-measures ANOVAs (time × trial) were employed. Degrees of freedom were Greenhouse-Geisser corrected for ε < 0.75, with Huynh-Feldt corrections used for less severe asphericity. If time × trial interaction effects were identified, multiple paired t tests (with Holm-Bonferroni stepwise adjustments) were used to locate the variance between trials. Pearson rs were used to explore correlations. Unless otherwise stated, data are means ± normalized (n) 95% CIs (28). Statistical analyses were performed with commercially available packages (Graph Pad Prism 7, Microsoft Excel 2013, and IBM SPSS version 22 for Windows). For 3 participants, the Actiheart data did not satisfy the inclusion criteria and these people were then excluded from certain analyses (e.g., daily energy expenditure).
Results
Energy intake
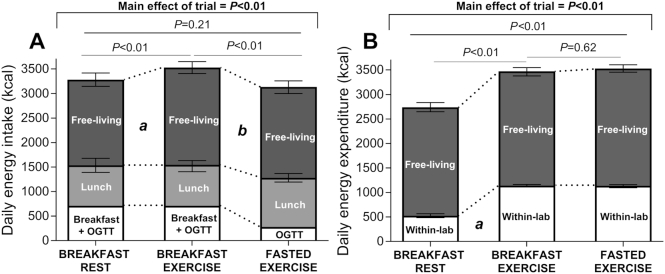
There was no meaningful compensation through energy intake at lunch for the energy deficit created by exercise as the mean difference in energy intake between BR and BE was 11 kcal (n95% CI: −182, 203 kcal; P > 0.05) (Figure 2A). The omission of breakfast pre-exercise was partially compensated for by lunch energy intake in FE compared with BE (Figure 2A), but this difference of 166 kcal (n95% CI: −27, 308 kcal) was less than the energy provided in the breakfast (∼430 kcal). Thus, when accounting for breakfast, within-lab energy intake did not significantly differ in BR and BE, but was significantly higher in BE compared with FE (265 kcal; n95% CI: 123, 407 kcal; P < 0.01). There was some compensation for the energy deficit created by exercise via free-living energy intake in BE, but the omission of breakfast prior to exercise did not result in any further compensation with energy intake in a free-living environment (Figure 2A). Thus, daily energy intake was significantly higher in BE than in BR (245 kcal; n95% CI: 81, 362 kcal; P < 0.01) and in BE than in FE (393 kcal; n95% CI: 276, 510 kcal; P < 0.01).
FIGURE 2.
Daily energy intake (A) and energy expenditure (B). Data are means ± normalized (n) 95% CIs for n = 12 (9 for free-living energy expenditure) healthy young men. In panel A, "a" represents a difference in free-living energy intake with breakfast rest and breakfast exercise with P < 0.05, and "b" a difference in lunch energy intake in breakfast exercise compared with fasted exercise with P < 0.05. In panel B, "a" represents a difference for within-lab energy expenditure with breakfast rest and both exercise trials with P < 0.05.
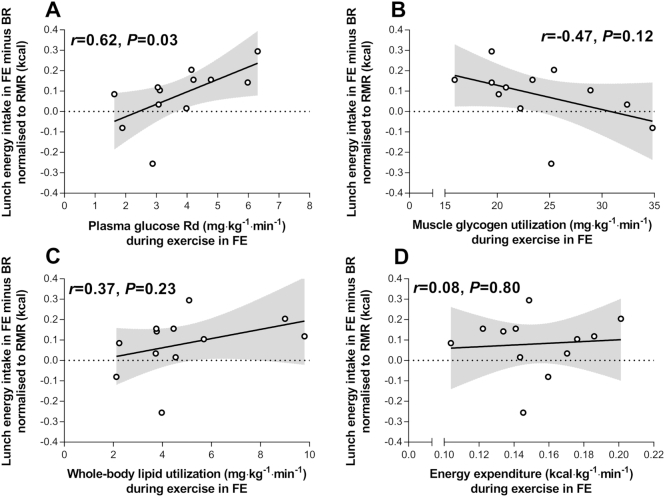
The total plasma glucose rate of disposal during FE (representing plasma glucose—primarily from hepatic sources—utilization during exercise) was positively correlated with compensation in lunch energy intake in FE compared with BR when normalized to resting metabolic rate (Figure 3A). The total plasma glucose rate of appearance during exercise in FE was also correlated (r = 0.67, P = 0.02) with compensation in lunch energy intake (normalized to resting metabolic rate). A similar relationship was apparent when the plasma glucose rate of disposal during FE was correlated with compensation in lunch energy intake when energy intake data were not normalized (r = 0.61, P = 0.04) or normalized to DXA-derived fat-free mass (r = 0.57, P = 0.05). Muscle glycogen utilization, whole-body lipid utilization, and energy expenditure during exercise in FE were not correlated with compensation in lunch energy intake (Figures 3B–D).
FIGURE 3.
The plasma glucose rate of disappearance (Rd) (A), muscle glycogen utilization (B), whole-body lipid utilization (C), and energy expenditure (D) during fasted exercise compared with lunch energy intake compensation (lunch energy intake after fasted exercise minus lunch energy intake after rest) normalized to resting metabolic rate (RMR). Data are Pearson's r. n = 12 healthy young men.
Energy expenditure and substrate utilization
The exercise was completed as prescribed and the mean ± SD intensity was 61 ± 3% V̇O2 peak. Energy expenditure during cycling was 683 kcal in BE (n95% CI: 664, 702 kcal) and 697 kcal in FE (n95% CI: 678, 715 kcal; P = 0.28 compared with BE). Carbohydrate utilization was significantly higher in BE (536 kcal; n95% CI: 510, 561 kcal) than in FE (478 kcal; n95% CI: 452, 503 kcal; P < 0.01), but whole-body lipid utilization was significantly lower in BE (138 kcal; n95% CI: 102, 175 kcal) than in FE (212 kcal; n95% CI: 176, 248 kcal; P < 0.01). During the OGTT, no difference between the trials in energy expenditure or substrate use was detected. There was no detectable difference between any trials for free-living physical activity (Figure 2B). Thus, daily energy expenditure was significantly lower with BR than with BE and FE, but did not significantly differ between BE and FE (Figure 2B).
Energy and substrate balance
Consuming breakfast but performing exercise (BE) resulted in a significantly lower within-lab energy balance compared with rest (BR), which was primarily driven by a difference in carbohydrate balance (Figure 4A). The omission of breakfast prior to exercise (FE) resulted in a significanly lower within-lab energy balance compared with BE, primarily via the induction of a significantly lower fat balance, as no significant difference in carbohydrate balance was apparent between FE and BE (Figure 4A). The same pattern between trials was still apparent over 24 h (Figure 4B), where a significantly higher energy balance was observed in BR than in BE, and in BE than in FE.
FIGURE 4.
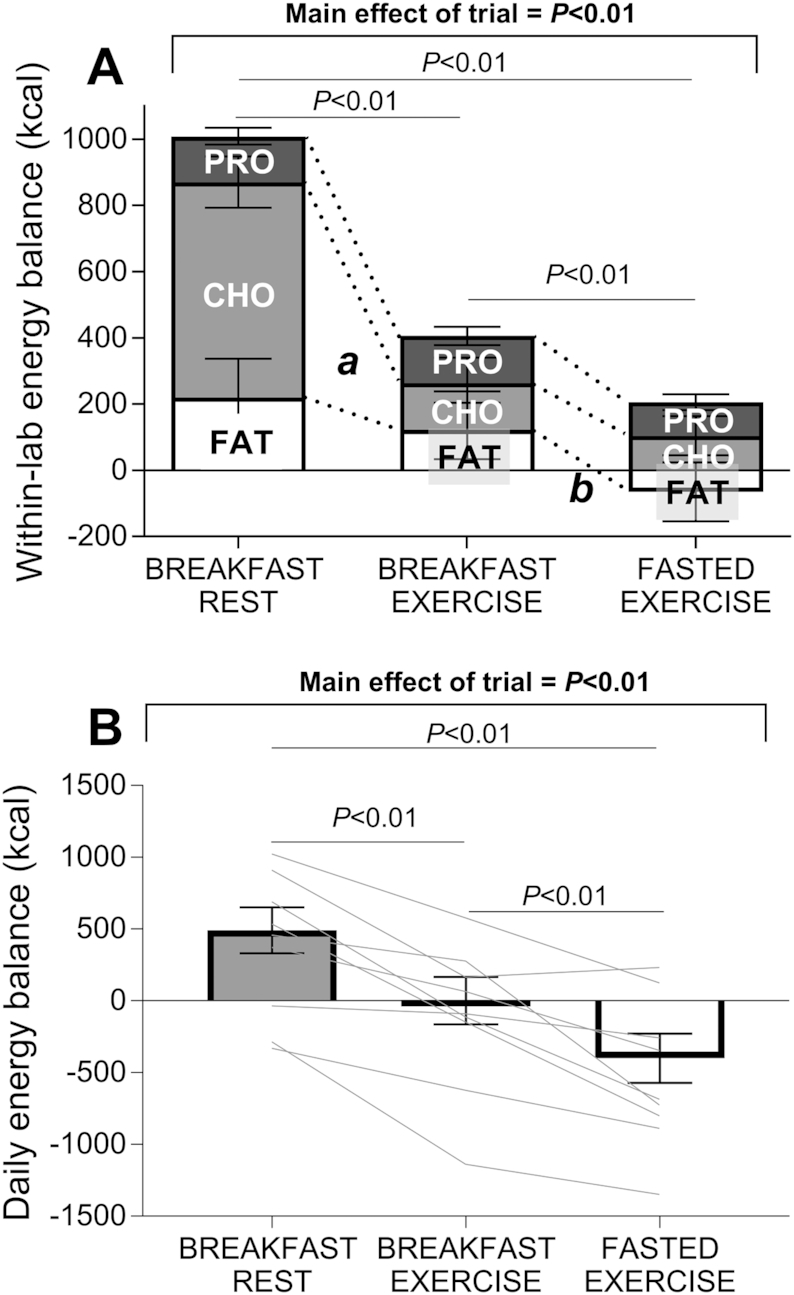
Within-lab (A) and daily energy balance (B). Data are means ± normalized (n) 95% CIs for n = 12 (9 for daily energy balance) healthy young men. In panel A, "a" represents a difference in CHO balance between breakfast rest and breakfast exercise with P < 0.05, and "b" a difference in FAT balance for breakfast exercise and fasted exercise with P < 0.05. PRO, protein; CHO, carbohydrate.
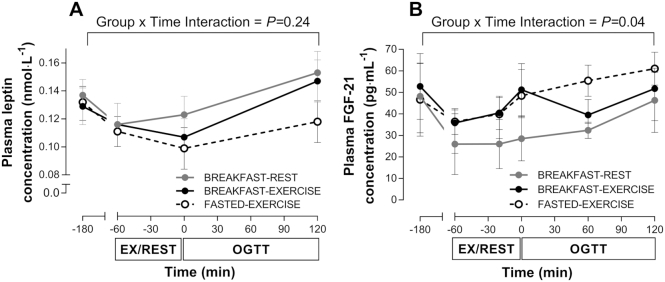
Plasma leptin and FGF-21 concentrations
At baseline there were no differences for plasma leptin or FGF-21 concentrations (Figure 5). A time × trial interaction effect was detected for plasma leptin, but with post-hoc adjustment there was no significant difference at any time point, and no main effect of trial was apparent for the leptin AUC (P = 0.21). No time × trial interaction effect was observed for FGF-21, and there was no main effect for the FGF-21 AUC (P = 0.07).
FIGURE 5.
Plasma leptin (A) and FGF-21 (B) concentrations. Data are means ± normalized (n) 95% CIs for n = 12 healthy young men. EX, exercise; FGF-21, fibroblast growth factor 21; OGTT, oral glucose tolerance test.
Discussion
This is the first study to assess the effect of pre-exercise feeding compared with fasting on all components of energy balance over 24 h, with inclusion of both within-lab and free-living periods. We showed that breakfast omission before exercise (FE) is not fully compensated for with postexercise energy intake, and not compensated for at all with subsequent free-living energy expenditure, creating a more negative daily energy balance compared with breakfast consumption prior to exercise (BE). Our results also demonstrate that plasma glucose utilization during FE demonstrated a stronger relationship with energy intake compensation than muscle glycogen utilization, whole-body lipid utilization, or exercise energy expenditure. Plasma glucose utilization was also the only carbohydrate source to demonstrate a positive relationship with energy intake compensation. Because plasma glucose during exercise when fasting is primarily derived from hepatic sources, this result supports a potential role for liver carbohydrate status in the regulation of postexercise energy balance. These data offer new insights into responses to feeding and exercise that are readily applicable to typical daily living (29, 30).
We showed that the energy deficit created by exercise was not compensated for through a higher energy intake at an ad libitum lunch in an exercise compared with a rest trial (BE compared with BR), but was partially compensated for with a higher energy intake later in the day (in a free-living setting). In contrast, breakfast omission before exercise (FE) was partially compensated for at lunch, but not further compensated for later in the day. These findings suggest that the compensation for the energy deficits created by exercise and pre-exercise breakfast omission likely occur over different time periods. The energy intake responses we report with pre-exercise breakfast omission are in line with previous observations that when healthy men perform 60 min of running after breakfast, their evening and 24-h energy intakes are higher than if they exercised before breakfast (16). As fasting prior to exercise reduces carbohydrate utilization during exercise (13), these results are consistent with a theory that the status of endogenous carbohydrate stores (i.e., liver and muscle glycogen) may play a role in energy balance regulation (7). Further evidence supporting the role of carbohydrate metabolism in regulating energy balance comes from our current, and prior (15), observations that the more negative within-lab energy balance in the FE trial was attributable to a negative fat balance, because carbohydrate balance was similar to that observed in the BE trial.
The ability to fully apply these energy intake findings to daily living is, however, incomplete without an assessment of free-living energy expenditure. Here, we also demonstrate that pre-exercise breakfast omission is not fully compensated for via nonexercise physical activity energy expenditure when this is based on objective measures of physical activity in a free-living environment. Thus, we provided a more complete picture of energy balance after breakfast compared with fasting before exercise. Indeed, the physical activity monitor we used has been validated in laboratory and free-living settings (including against doubly-labeled water) (25, 31, 32). Overall, our results show the following: 1) even with the inclusion of a free-living component, fasting prior to exercise creates a more negative daily energy balance compared with when a pre-exercise breakfast is consumed; and 2) whole-body carbohydrate balance may contribute to energy balance regulation (at least in the postexercise period).
Previously, it was unclear if a specific carbohydrate store was a primary regulator of postexercise energy balance. Here, we show that plasma glucose disposal rates and plasma glucose appearance rates during exercise in FE (which primarily represents the mobilization and utilization of glucose from hepatically derived sources, because no carbohydrate was ingested before or during exercise in the FE trial) were the only carbohydrate-related outcomes (i.e., not due to muscle glycogen utilization) to demonstrate a positive relation with energy intake compensation. This is the first evidence of a link between tissue-specific carbohydrate utilization during exercise and energy balance in humans. In our fasting exercise trial, participants with higher rates of plasma glucose disposal consumed more energy at lunch than during their rest trial. This finding that a more rapid utilization of plasma glucose during exercise in FE (and a likely higher rate of utilization of carbohydrate from hepatic sources) may increase postexercise energy intake is consistent with research showing that a higher liver glycogen content in mice is associated with a lower energy intake and a lower body and fat mass (33). That result suggests that the liver glycogen content may be a regulator of energy balance, and is consistent with the correlation we report because higher liver glucose utilization rates would be expected to deplete the liver glycogen content during exercise to a greater extent. Future studies should now confirm this relationship through the use of a direct measure of net hepatic glycogen utilization, such as 13C magnetic resonance spectroscopy.
A possible link between the liver carbohydrate status and postexercise energy intake regulation may be explained by concentrations of circulating hormones. For example, exercise increases liver FGF-21 secretion (34), which may influence subsequent energy intake (35). It has also been suggested that blood leptin concentrations may help to regulate carbohydrate metabolism and energy balance during periods of fasting (36). During rest, higher plasma leptin concentrations after a lunch meal have been previously observed in a breakfast compared with morning fasting trial (14). This observation may be accounted for by a slow release of leptin in response to breakfast, a diurnal shift in leptin release with morning fasting, or a combination of both these effects (14). However, although we have confirmed that exercise increases plasma FGF-21 concentrations, we showed that neither plasma FGF-21 nor plasma leptin concentrations were altered by pre-exercise breakfast omission (FE) compared with pre-exercise breakfast consumption (BE). The postprandial leptin response we observed after exercise was, however, similar to the response observed with breakfast omission at rest. Alternatively, a higher rate of liver carbohydrate utilization may influence energy balance via a liver-brain neural network (the hepatic branch of the vagus nerve) to the central nervous system, as has been demonstrated in rodents (12). Although future research needs to further investigate any mechanisms linking overall and liver carbohydrate metabolism to energy balance in humans, the evidence presented here is the first to demonstrate that plasma glucose utilization during fasted exercise (and thus carbohydrate use from hepatic sources) is positively correlated with postexercise energy intake in humans. Our results are most applicable to young, physically active men. However, as untrained individuals show increased liver glucose utilization during endurance-type exercise (compared to trained individuals) (37), it is possible that these people may be even more susceptible to a higher postexercise energy intake, which would (in theory) make it more difficult for these individuals to lose weight through exercise.
Although the regulatory mechanisms remain unclear, this study provides novel insights into the regulation of all behavioral components of daily energy balance after pre-exercise breakfast omission (FE) compared with breakfast consumption (BE). Our findings must, however, be interpreted in light of the study design. Firstly, although the protocol included a free-living period, participants were constrained to a laboratory environment during the morning, and it is possible that behaviors that alter energy balance may have changed in a free-living environment during this time after fasting compared with fed exercise. This restriction of physical activity likely contributed to the positive 24-h energy balance observed in the BR trial. In addition, participants also had a limited choice of food during the study and it is possible that energy intake may have differed under more free-living conditions. Our results also only relate to breakfast omission and not altered breakfast timing. Despite this, under energy-balanced conditions (with the consumption of breakfast either pre- or postexercise) energy intake at an ad libitum lunch does not differ, despite alterations in substrate balance (38). In light of that result and our current findings, future research should now investigate all components of energy balance with altered timing of breakfast and in free-living settings (rather than breakfast omission and the time-restricted feeding with fasting prior to exercise). Investigating energy balance responses to regular exercise in the fasting compared with fed state and with overweight populations should be another focus for future work. This would provide insights as to whether the 24-h energy balance responses we report here translate into enduring changes in body mass or composition with repeated bouts of exercise in the fasting compared with the fed state. Finally, although this study lacks characterization of other gut hormones that have been associated with energy intake, it has been shown that acylated ghrelin and glucagon-like peptide 1 responses to exercise in the fasting compared with fed states do not differ to any meaningful degree (15, 39, 40). Plasma insulin concentrations during and after exercise were also not altered to any great extent by pre-exercise feeding in our participants (17).
To conclude, pre-exercise breakfast omission is not fully compensated for by energy intake, and is not compensated for at all by nonexercise energy expenditure postexercise, creating a more negative 24-h energy balance compared with when breakfast is consumed before exercise. We also show that postexercise energy intake compensation is positively correlated with plasma glucose utilization when exercise is performed when fasting, highlighting a possible role for liver carbohydrate status in energy-balance regulation. These results have important implications for the regulation of postexercise energy balance, and suggest that for healthy young men a short-term energy deficit may be more easily attained if breakfast is omitted before exercise.
Acknowledgments
The authors’ responsibilities were as follows—JTG, KDT, DLH, EJS, JAB, and DT: designed the research; RME, JTG AH, HAS, RLT, and JPW: conducted the research; RME, JTG, AH, HS, and GAW: analyzed the data; RME and JTG: performed the statistical analysis; RME and JTG: primarily wrote the paper; and all authors: contributed to earlier versions of the manuscript, and read and approved the final version.
Notes
Funding for this work was provided by the European Society for Clinical Nutrition and Metabolism and the Rank Prize Funds. DT, JTG, and JAB are funded by the MRC (MR/P002927/1) and the BBSRC (BB/R018928/1).
Author disclosures: none of the authors reported a conflict of interest related to the study.
Abbreviations used: BE, breakfast before exercise; BR, breakfast followed by rest; FE, overnight fasting before exercise; FGF-21, fibroblast growth factor 21; OGTT, oral glucose tolerance test.
References
- 1. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. [DOI] [PubMed] [Google Scholar]
- 2. King NA, Hopkins M, Caudwell P, Stubbs R, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes. 2008;32:177–84. [DOI] [PubMed] [Google Scholar]
- 3. Turner JE, Markovitch D, Betts JA, Thompson D. Nonprescribed physical activity energy expenditure is maintained with structured exercise and implicates a compensatory increase in energy intake. Am J Clin Nutr. 2010;92:1009–16. [DOI] [PubMed] [Google Scholar]
- 4. Thompson D, Peacock OJ, Betts J. Substitution and compensation erode the energy deficit from exercise interventions. Med Sci Sports Exerc. 2014;46:423. [DOI] [PubMed] [Google Scholar]
- 5. Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, Thompson D. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr. 2014;100:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chowdhury EA, Richardson JD, Holman GD, Tsintzas K, Thompson D, Betts JA. The causal role of breakfast in energy balance and health: a randomized controlled trial in obese adults. Am J Clin Nutr. 2016;103:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flatt JP. Macronutrient composition and food selection. Obes Res. 2001;9:256–62. [DOI] [PubMed] [Google Scholar]
- 8. Hopkins M, Jeukendrup A, King NA, Blundell JE. The relationship between substrate metabolism, exercise and appetite control. Sports Med. 2011;41:507–21. [DOI] [PubMed] [Google Scholar]
- 9. Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152:1718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alméras N, Lavallée N, Després J-P, Bouchard C, Tremblay A. Exercise and energy intake: effect of substrate oxidation. Physiol Behav. 1995;57:995–1000. [DOI] [PubMed] [Google Scholar]
- 11. Hopkins M, Blundell JE, King NA. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br J Sports Med, 2014;48:1472–6., (Epub; DOI: 10.1136/bjsports-2012-091721). [DOI] [PubMed] [Google Scholar]
- 12. López-Soldado I, Fuentes-Romero R, Duran J, Guinovart JJ. Effects of hepatic glycogen on food intake and glucose homeostasis are mediated by the vagus nerve in mice. Diabetologia. 2017;60:1076–83. [DOI] [PubMed] [Google Scholar]
- 13. Enevoldsen L, Simonsen L, Macdonald I, Bülow J. The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. J Physiol. 2004;561:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chowdhury EA, Richardson JD, Tsintzas K, Thompson D, Betts JA. Carbohydrate-rich breakfast attenuates glycaemic, insulinaemic and ghrelin response to ad libitum lunch relative to morning fasting in lean adults. Br J Nutr. 2015;114:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzalez JT, Veasey RC, Rumbold PL, Stevenson EJ. Breakfast and exercise contingently affect postprandial metabolism and energy balance in physically active males. Br J Nutr. 2013;110:721–32. [DOI] [PubMed] [Google Scholar]
- 16. Bachman JL, Deitrick RW, Hillman AR. Exercising in the fasted state reduced 24-hour energy intake in active male adults. J Nutr Metab. 2016, (Epub; DOI: 10.1155/2016/1984198). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edinburgh RM, Hengist A, Smith HA, Travers RL, Koumanov F, Betts JA, Thompson D, Walhin J-P, Wallis GA, Hamilton DL et al.. Pre-Exercise breakfast ingestion versus extended overnight fasting increases postprandial glucose flux after exercise in healthy men. Am J Physiol Endocrinol Metab. 2018;315:1062–74. (Epub ahead of print; DOI: 10.1155/2016/1984198). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deighton K, Frampton J, Gonzalez J. Test-meal palatability is associated with overconsumption but better represents preceding changes in appetite in non-obese males. Br J Nutr. 2016;116:935–43. [DOI] [PubMed] [Google Scholar]
- 19. Radziuk J. An integral equation approach to measuring turnover in nonsteady compartmental and distributed systems. Bull Math Biol. 1976;38:679–93. [DOI] [PubMed] [Google Scholar]
- 20. Radziuk J, Norwich KH, Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol Endocrinol Metab. 1978;234:E84. [DOI] [PubMed] [Google Scholar]
- 21. Jeukendrup A, Wallis G. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med. 2005;26:28–37. [DOI] [PubMed] [Google Scholar]
- 22. Brage S, Brage N, Franks P, Ekelund U, Wareham N. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59:561–70. [DOI] [PubMed] [Google Scholar]
- 23. Brage S, Brage N, Franks PW, Ekelund U, Wong M-Y, Andersen LB, Froberg K, Wareham NJ. Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. J Appl Physiol. 2004;96:343–51. [DOI] [PubMed] [Google Scholar]
- 24. Brage S, Ekelund U, Brage N, Hennings MA, Froberg K, Franks PW, Wareham NJ. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol. 2007;103:682–92. [DOI] [PubMed] [Google Scholar]
- 25. Brage S, Westgate K, Franks PW, Stegle O, Wright A, Ekelund U, Wareham NJ. Estimation of free-living energy expenditure by heart rate and movement sensing: a doubly-labelled water study. PLoS One. 2015; (Epub; DOI: 10.1371/journal.pone.0137206). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeukendrup AE, Wagenmakers AJM, Stegen JHCH, Gijsen AP, Brouns F, Saris WHM. Carbohydrate ingestion can completely suppress endogenous glucose production during exercise. Am J Physiol Endocrinol Metab. 1999;276:672–83. [DOI] [PubMed] [Google Scholar]
- 27. Edinburgh R, Hengist A, Smith HA, Betts JA, Thompson D, Walhin J-P, Gonzalez JT. Prior exercise alters the difference between arterialised and venous glycaemia: implications for blood sampling procedures. Br J Nutr. 2017;10:1414–21. [DOI] [PubMed] [Google Scholar]
- 28. Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychon Bull Rev. 1994;1:476–90. [DOI] [PubMed] [Google Scholar]
- 29. de Castro JM. The time of day of food intake influences overall intake in humans. J Nutr. 2004;134:104–11. [DOI] [PubMed] [Google Scholar]
- 30. Ruge T, Hodson L, Cheeseman J, Dennis AL, Fielding BA, Humphreys SM, Frayn KN, Karpe F. Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab. 2009;94:1781–8. [DOI] [PubMed] [Google Scholar]
- 31. Villars C, Bergouignan A, Dugas J, Antoun E, Schoeller DA, Roth H, Maingon A-C, Lefai E, Blanc S, Simon C. Validity of combining heart rate and uniaxial acceleration to measure free-living physical activity energy expenditure in young men. J Appl Physiol. 2012;113:1763–71. [DOI] [PubMed] [Google Scholar]
- 32. Thompson D, Batterham AM, Bock S, Robson C, Stokes K. Assessment of low-to-moderate intensity physical activity thermogenesis in young adults using synchronized heart rate and accelerometry with branched-equation modeling. J Nutr. 2006;136:1037–42. [DOI] [PubMed] [Google Scholar]
- 33. López-Soldado I, Zafra D, Duran J, Adrover A, Calbó J, Guinovart JJ. Liver glycogen reduces food intake and attenuates obesity in a High-Fat Diet–Fed mouse model. Diabetes. 2015;64:796–807. [DOI] [PubMed] [Google Scholar]
- 34. Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces FGF21 expression in mice and in healthy humans, PLoS One. 2013;8:(5), (Epub; DOI: 10.1371/journal.pone.0063517). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B et al.. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016;23:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perry RJ, Wang Y, Cline GW, Rabin-Court A, Song JD, Dufour S, Zhang XM, Petersen KF, Shulman GI. Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell. 2018;234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gonzalez J, Betts J. Dietary sugars, exercise and hepatic carbohydrate metabolism: sugars and liver metabolism. Proc Nutr Soc. 2018,; (Epub ahead of print; DOI: 10.1017/S0029665118002604). [DOI] [PubMed] [Google Scholar]
- 38. Farah NM, Gill JM. Effects of exercise before or after meal ingestion on fat balance and postprandial metabolism in overweight men. Br J Nutr. 2013;109:2297–307. [DOI] [PubMed] [Google Scholar]
- 39. Clayton DJ, Barutcu A, Machin C, Stensel DJ, James LJ. Effect of breakfast omission on energy intake and evening exercise performance. Med Sci Sports Exerc. 2015;47:2645–52. [DOI] [PubMed] [Google Scholar]
- 40. Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Effect of fasting versus feeding on the bone metabolic response to running. Bone. 2012;51:990–9. [DOI] [PubMed] [Google Scholar]