Abstract
BACKGROUND:
Previous studies indicate that cardiorespiratory fitness (CRF) and sleep are each favorably associated with Alzheimer’s disease (AD) pathophysiology, including reduced amyloid-beta (Aβ) and tau pathology. However, few studies have examined CRF and sleep in the same analysis.
OBJECTIVE:
To examine the relationship between sleep and core AD cerebrospinal fluid (CSF) biomarkers among at-risk healthy late-middle-aged adults and determine whether CRF modifies this association.
METHODS:
Seventy-four adults (age=64.38±5.48, 68.9% female) from the Wisconsin Registry for Alzheimer’s Prevention participated. Sleep was evaluated using the Medical Outcomes Study Sleep Scale, specifically the Sleep Problems Index I (SPI), which incorporates domains of sleep disturbance, somnolence, sleep adequacy, and shortness of breath. Higher scores indicate greater sleep problems. To assess CRF, participants underwent a graded exercise test. CSF was collected via lumbar puncture, from which Aβ42, total-tau (t-tau) and phosphorylated-tau (p-tau) were immunoassayed. Regression analyses examined the association between SPI and CSF biomarkers, and the interaction between SPI and CRF on these same biomarkers, adjusting for relevant covariates.
RESULTS:
Higher SPI scores were associated with greater p-tau (p=.027) and higher t-tau/Aβ42 (p=.021) and p-tau/Aβ42 (p=.009) ratios. Analyses revealed significant SPI*CRF interactions for t-tau (p=.016), p-tau (p=.008), and p-tau/Aβ42 (p=.041); with a trend for t-tau/Aβ42 (p=.061). Specifically, the relationship between poorer sleep and these biomarkers was significant among less fit individuals, but not among those who were more fit.
CONCLUSION:
In a late-middle-aged at-risk cohort, CRF attenuated the association between poor sleep and levels of select CSF biomarkers. This suggests fitness may play an important role in preventing AD by protecting against pathology, even in impaired sleep.
Keywords: Cardiorespiratory fitness, Alzheimer’s disease, beta-amyloid protein, biomarkers, cerebrospinal fluid, sleep, tau protein
INTRODUCTION
Alzheimer’s disease (AD) is developing into one of the world’s most pressing public health concerns, currently affecting more than 47 million individuals and projected to affect as many as 131 million by the year 2050 [1]. As such, research into possible preventative measures to delay onset of the disease is becoming increasingly urgent. Of particular interest are interventions targeted to modifiable lifestyle factors during the preclinical phase of AD, in which pathophysiological brain changes, such as development of amyloid-beta (Aβ) plaques, neurofibrillary tangles, and neurodegeneration may occur without any outward cognitive symptoms [2].
Two such interventions, improved sleep and increased physical activity (PA), have previously been associated with these preclinical brain alterations, among other AD outcomes, and may have the potential to alter the trajectory of disease progression [3, 4]. Measures of sleep quality—including increased sleep efficiency, greater adequacy, reduced latency, greater duration, fewer sleep problems, and less somnolence—have been associated with reduced amyloid burden, as measured by positron emission tomography [5–7] and cerebrospinal fluid (CSF) markers [8, 9]. Poorer sleep has also been associated with greater cortical atrophy [5, 10] and cognitive decline [11]. These studies suggest sleep may play an important role in the evolution of AD pathophysiology.
Studies examining the relationship between PA and AD pathologies indicate that increased PA is associated with lower Aβ and tau burden [12, 13]. PA also demonstrates a capacity for attenuating the deleterious effect of age [14] and the apolipoprotein E (APOE) ε4 allele [15, 16] on Aβ deposition. For this particular study, we indexed PA using cardiorespiratory fitness (CRF), a measure of habitual PA. Increased CRF has been associated with favorable neurological outcomes, including improved cognition, increased gray matter volumes, and reduced white matter hyperintensities [17–19], as well as a lower risk of dementia [20] and dementia mortality [21]. Few studies have examined CRF and its association with Aβ and tau markers, though a recent study by our group found CRF may lessen the adverse effect of genetic risk factors on Aβ and tau burden [16].
Although considerable data exist on the bidirectional relationship between sleep and PA [22–27], to our knowledge, only few studies [28–30] have concurrently examined both exposures with respect to AD-relevant outcomes biomarkers. As such, our objective with this study was to i) examine the association between sleep and CSF biomarkers in a cohort of healthy, late-middle-aged adults with risk factors for AD, and ii) determine whether CRF modifies this association.
MATERIALS AND METHODS
Participants
This study included 74 participants from the Wisconsin Registry for Alzheimer’s Prevention (WRAP). WRAP is a longitudinal registry of over 1500 cognitively healthy, late-middle-aged adults predominantly between the ages of 40 and 65 at study entry [3, 31]. All participants were deemed to be cognitively healthy based on comprehensive cognitive assessment, clinical examination, and a multi-disciplinary consensus conference [3]. With respect to the cognitive assessment, each participant completed a comprehensive neuropsychological battery [3] that included psychometric measures spanning conventional cognitive domains of memory, attention, executive function, language, and visuospatial ability. Previous factor analytic studies [32, 33] of these tests within the full WRAP cohort found that they map onto six cognitive factors, i.e., Immediate Memory, Verbal Learning & Memory, Working Memory, Speed & Flexibility, Visuospatial Ability, and Verbal Ability. These cognitive factor scores are standardized z-scores (~N (0,1)). Prior work from our lab [17, 34] has primarily focused on the first four factors because they are comprised of cognitive tests that tap into episodic memory and executive function, which are the cognitive abilities with established links to aerobic fitness and Alzheimer’s disease [16–19]. Participants’ scores on these four factors are included in Table 1. Participants for the present study were selected based on participation in WRAP-linked studies that involved completion of a graded exercise test (GXT), self-report sleep questionnaires, and lumbar puncture for collection of CSF. The sample was enriched with persons with risk factors for AD, specifically individuals with a parental family history (79.7%) and/or carrying ≥ 1 apolipoprotein E ε4 (APOE ε4) allele (40.5%). Table 1 displays the participants’ relevant background characteristics. All study procedures were approved by the University of Wisconsin Institutional Review Board and each subject provided informed consent prior to participation.
Table 1.
Participant Characteristics (N=74)
| Characteristic | Value* |
|---|---|
| Demographics | |
| Age†, years | 64.38 (5.48) |
| Age† ≥ 65 years, % | 52.7 |
| Female, % | 68.9 |
| Race, % | |
| Non-Hispanic White | 95.9 |
| African American | 2.7 |
| Asian | 1.4 |
| Education, years | 16.26 (2.11) |
| Family history positive, % | 79.7 |
| APOE ε4 positive, % | 40.5 |
| Time interval between GXT and sleep assessment, years | 1.17 (.60) |
| Time interval between sleep assessment and CSF collection, years | 1.10 (1.08) |
| Time interval between GXT and CSF collection, years | 1.37 (1.06) |
| Cognitive Measures | |
| MMSE§ | 30.00 (29, 30) |
| Immediate Memory | .24 (1.01) |
| Verbal Learning & Memory | .01 (.97) |
| Working Memory | .12 (.92) |
| Speed & Flexibility | −.05 (.98) |
| Medical and Vascular Risk Indices | |
| VO2 peak, mL/kg/min (N=57) | 25.86 (5.51) |
| Body mass index†, kg/m2 | 28.31 (5.42) |
| Total cholesterol†, mg/dL | 208.69 (39.63) |
| HDL cholesterol†, mg/dL | 66.80 (20.39) |
| Systolic blood pressure†, mmHg | 128.64 (17.65) |
| Diastolic blood pressure†, mmHg | 75.32 (9.38) |
| Hypertension, % | 24.3 |
| Diabetes, % | 2.7 |
| Reported use of pharmaceutical sleep aid, % | 12.2 |
Values indicate mean and standard deviation, unless otherwise indicated.
Indicates value at time of GXT
Value indicates median (25th percentile, 75th percentile)
APOE ε4=ε4 allele of apolipoprotein E gene; VO2 peak=peak volume of oxygen consumed during graded exercise test; MMSE=Mini-Mental State Examination; HDL=high-density lipoprotein; GXT=graded exercise test
Sleep Assessment
As part of their participation in WRAP, participants completed the Medical Outcomes Study (MOS) Sleep Scale [35], a questionnaire that assesses 8 domains of sleep over the previous 4 weeks. The first question asks participants how long it takes them to fall asleep, ranging from 1 (0–15 minutes) to 5 (more than 60 minutes). The second question asks the average number of hours slept each night and is free-response style. The last 10 questions assess other qualities of sleep and are rated on a 6-point scale ranging from 1 (all the time) to 6 (none of the time). Responses were then converted to a 0–100 point scale, such that higher values denote more of the characteristic being measured. Scores were then summed to give totals for 8 domains of sleep: sleep disturbance, somnolence, sleep adequacy, snoring, awakening short of breath or with a headache, sleep quantity, and 2 global indices of sleep problems [36]. We specifically focused on the Sleep Problems Index I (SPI) because it incorporates domains of sleep disturbance, somnolence, sleep adequacy, and shortness of breath into a single score [36]. Previous studies have shown that SPI is particularly sensitive to health-related outcomes [37, 38]. Higher SPI scores indicate more sleep problems and thus, worse sleep. Table 2 shows the full questionnaire, and indicates which questions contributed to the SPI score. Table 3 shows participants’ average scores on other measures of sleep quality taken from the MOS Sleep Scale.
Table 2.
Medical Outcomes Study Sleep Scale
| Sleep Problems Index I | |
|---|---|
| During the past 4 weeks… | |
| 1. How long did it usually take for you to fall asleep?a | |
| 2. On the average, how many hours did you sleep each night?b | |
| How often did you…c | |
| 3. Feel that your sleep was not quiet (moving restlessly, feeling tense, speaking, and so forth, while sleeping)? | |
| 4. Get enough sleep to feel rested upon waking in the morning? | o |
| 5. Awaken short of breath or with a headache? | o† |
| 6. Feel drowsy or sleepy during the day? | |
| 7. Have trouble falling asleep? | o† |
| 8. Awaken during your sleep time and have trouble falling asleep again? | o† |
| 9. Have trouble staying awake during the day? | o† |
| 10. Snore during your sleep? | |
| 11. Take naps (5 min or longer) during the day? | |
| 12. Get the amount of sleep you needed? | o |
Responses were converted to a 0–100 scale and summed and averaged to produce the total SPI score. Thus, higher scores indicate greater sleep problems.
o indicates item included in SPI score
indicates item was reversed-scored before computing SPI score
Responses were on 15-minute increments from 1 (0–15 minutes) to 5 (more than 60 minutes).
Responses were free entry.
Responses were on a 6-point scale from 1 (all of the time) to 6 (none of the time).
SPI=Sleep Problems Index I
Table 3.
Additional Sleep Quality Indicators
| Measure of Sleep Quality | Mean (SD) | Contributing MOS Questions |
|---|---|---|
| Sleep Problems Index II, score | 23.96 (13.76) | 1, 3–9, 12 |
| Sleep disturbance, score | 22.97 (18.58) | 1, 3, 7, 8 |
| Sleep adequacy, score | 61.48 (22.55) | 4, 12 |
| Somnolence, score | 20.72 (14.83) | 6, 9, 11 |
| Snoring, score | 29.32 (27.50) | 10 |
| Awakening short of breath or with a headache, score | 5.14 (11.96) | 5 |
| Sleep quantity, hours | 7.00 (1.06) | 2 |
Responses were converted to a 0–100 scale and summed and averaged to produce the overall score for each domain listed (except sleep quantity). Higher scores indicate more of the domain being measured (i.e., more disturbed sleep, more adequate sleep, etc.)
Graded Exercise Testing
As part of a WRAP-linked study, participants underwent GXT using a modified Balke protocol [39]. Comfortable brisk walking speeds were determined prior to testing as a safety precaution and to ensure a valid test. For participants who were capable of walking at 3.5 miles per hour comfortably, this speed was used throughout the test. For participants who found this walking speed uncomfortable, a slower speed was chosen. The grade of the treadmill was increased by 2.5% every two minutes until the participant reached volitional exhaustion. Oxygen uptake (VO2), carbon dioxide production, minute ventilation, HR, and work rate were measured continuously using a metabolic cart and two-way non-rebreathing valve (TrueOne® 2400, Parvomedics, Sandy, UT). The system was calibrated 4 hours prior to each test using standard gases with known concentrations and with a calibrated three-liter syringe. Peak oxygen consumption (VO2 peak, mL/kg/min) during exercise is the canonical method for assessing CRF [40] and was used as the index of CRF in this study. The average time between GXT and sleep assessment was 1.17 ± 0.60 years.
CSF Assessment
Lumbar puncture for collection of CSF was performed the morning after a 12-hour fast, with a Sprotte 24- or 25-gauge spinal needle at L3/4 or L4/5 using gentle extraction into polypropylene syringes. Each sample consisted of 22 mL of CSF, which was then combined, mixed, and centrifuged at 2000g for 10 minutes. Supernatants were frozen in 0.5 mL aliquots in polypropylene tubes and stored at −80°C. The samples were immunoassayed for Aβ42, total tau (t-tau), and phosphorylated tau (p-tau) (phosphorylated at threonine 181) using INNOTEST enzyme-linked immunosorbent assays (Fujirebio, Gent, Belgium) by board-certified laboratory technicians who were blind to clinical data and used protocols accredited by the Swedish Board for Accreditation and Conformity Assessment as previously described [41]. We additionally computed t-tau/Aβ42 and p-tau/Aβ42 ratios. These ratios combine multiple aspects of AD pathology (i.e., amyloidosis and tau burden) in a single measure and have been shown to possess higher predictive and diagnostic power compared to individual biomarkers [42–44]. Elevated t-tau/Aβ42 or p-tau/Aβ42 ratio indicates greater AD pathology. The average time between GXT and CSF collection was 1.37 ± 1.06 years. The average time between sleep assessment and CSF collection was 1.10 ± 1.08 years.
Statistical Analyses
To examine whether sleep problems, as indexed by SPI score, were associated with CSF biomarkers of AD, we fitted a series of linear regression models—one for each CSF biomarker—that were adjusted for age at GXT, sex, APOE ε4 status, and time interval between sleep assessment and CSF collection.
In order to further investigate whether CRF has the capacity to modify the relationship between sleep problems and CSF biomarkers, we additionally refitted the models to incorporate terms for VO2 and a SPI*VO2 peak interaction term, while still adjusting for age at GXT, sex, APOE ε4 status, time interval between GXT and sleep assessment, and time interval between sleep assessment and CSF collection. Where significant, the SPI*VO2 peak interaction term would indicate that the association between SPI and CSF biomarkers varies across levels of aerobic fitness, suggesting that CRF may have the potential to ameliorate the deleterious effect of poor sleep on CSF biomarkers.
Because there is evidence that older adults may, on average, be less likely to give maximal effort on GXT, thus underestimating their CRF [45], the interactive model was restricted to the 57 individuals who were deemed to have attained peak effort based on meeting 2 or more of the following criteria [46]: (i) respiratory exchange ratio (RER) ≥ 1.1, (ii) change in with an increase in work, (iii) rating of perceived exertion of 17 or greater, and (iv) achieving at least 90% of age predicted maximal heart rate (220 - age). All analyses were conducted using IBM SPSS, version 24.0. Only findings with p ≤ 0.05 (two-tailed) were considered significant.
RESULTS
Participant Characteristics
Table 1 details the relevant background characteristics of the participants. The sample had an average age of 64.38 ± 5.48 years at the time of GXT completion and was majority female (68.9%). 79.7% had a parental family history of AD and 40.5% were APOE ε4 positive. Overall, the sample was well-educated, with an average of 16.26 ± 2.11 years of education. Consistent with their cognitive health status, the median MMSE is 30 and performance on all other cognitive domains was well within normal limits (−1 SD would be the threshold for mild impairment). The average body mass index (BMI) was 28.31 ± 5.42 kg/m2. None of the participants reported diagnoses of sleep apnea or restless leg syndrome.
Association between Sleep Problems and CSF Biomarkers
Greater sleep problems (i.e. higher SPI scores) were associated with higher levels of p-tau (p=.027), as well as higher t-tau/Aβ42 (p=.021) and p-tau/Aβ42 (p=.009) ratios. However, SPI scores were not associated with CSF Aβ42 levels, and only revealed a trend for t-tau (p=.099). These findings are reported in Table 4.
Table 4.
Association between sleep problems and CSF biomarkers
| CSF Biomarker | SPI Score | |
|---|---|---|
| β (SE) | p | |
| Aβ42 | −5.23 (29.95) | .862 |
| t-tau | 26.30 (15.71) | .099 |
| p-tau | 4.49 (1.98) | .027 |
| t-tau/Aβ42 | .11 (.05) | .021 |
| p-tau/Aβ42 | .02 (.01) | .009 |
Models were adjusted for age at GXT, sex, APOE ε4 status, and time interval between sleep and CSF assessments.
CSF=cerebrospinal fluid; SPI=Sleep Problems Index I; β=regression estimate; SE=standard error; Aβ42=amyloid-beta 42; t-tau=total tau; p-tau=phosphorylated tau; GXT=graded exercise test; APOE ε4= the ε4 allele of the apolipoprotein E gene
Cardiorespiratory Fitness and Sleep-Related Alterations in CSF Biomarker Levels
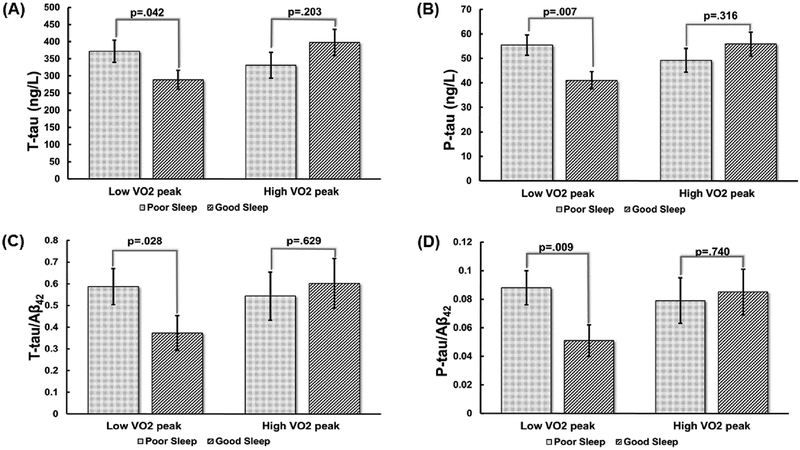
There were significant SPI*VO2 peak interactions for t-tau (p=.016), p-tau (p=.008), and p-tau/Aβ42 (p=.041), with a trend for t-tau/Aβ42 (p=.061) (Table 5). To display this graphically we followed standard procedure for generating plots for interactions between two continuous variables, which entails solving the regression equation at specific “anchor points” for each of the continuous variables. In our case, we solved the equation at ±1 standard deviation away from the mean for both SPI score (−.03 ± .86) and VO2 peak (25.86 ± 5.51), representing Good vs. Poor Sleep and Low vs. High VO2 peak, respectively. These graphs, shown in Figure 1A–D, revealed that poor sleep was associated with a worse CSF biomarker profile in the Low VO2 peak group but not in the High VO2 peak group. Specific regression estimates (i.e., simple main effects) for the influence of poor sleep on tau levels among those with Low vs. High VO2 peak are as follows: for t-tau, β (SE)=83.08 (39.84), p=.042 in the Low VO2 peak group vs. β (SE)=−66.43 (51.44), p=.203 in the High VO2 peak group; for p-tau, β (SE)=14.39 (5.06), p=.007 in the Low VO2 peak group vs. β (SE)=−6.62 (6.54), p=.316 in the High VO2 peak group; for t-tau/Aβ42, β (SE)=.21 (.09), p=.028 in the Low VO2 peak group vs. β (SE)=−.06 (.12), p=.629 in the High VO2 peak group; for p-tau/Aβ42, β (SE)=.04 (.01), p=.009 in the Low VO2 peak group vs. β (SE)=−.01 (.01), p=.740 in the High VO2 peak group.
Table 5.
CRF attenuates the adverse effect of poor sleep on CSF biomarkers
| CSF Biomarker | SPI × VO2 peak§ | SPI (Low VO2 peak)† | SPI (High VO2 peak)‡ | |||
|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | |
| Aβ42 | −6.05 (5.93) | .313 | - | - | - | - |
| t-tau | −7.94 (3.20) | .016 | 83.08 (39.84) | .042 | −66.43 (51.44) | .203 |
| p-tau | −1.12 (.41) | .008 | 14.39 (5.06) | .007 | −6.62 (6.54) | .316 |
| t-tau/Aβ42 | -.02 (.01) | .061 | .21 (.09) | .028 | −.06 (.12) | .629 |
| p-tau/Aβ42 | −.01 (.01) | .041 | .04 (.01) | .009 | −.01 (.01) | .740 |
The regression estimates and associated p values are for the SPI × VO2 peak interactive term in each CSF biomarker’s model. This term assesses whether VO2 peak modifies the effect of sleep on the examined CSF biomarkers.
The regression estimates and associated p values are for the simple main effect for the influence of sleep problems on each CSF biomarker within the Low VO2 peak group.
The regression estimates and associated p values are for the simple main effect for the influence of sleep problems on each CSF biomarker within the High VO2 peak group.
Variables included in the model were age at GXT, sex, APOE ε4 status, time interval between GXT and sleep assessment, time interval between sleep and CSF assessments, SPI score, VO2 peak, and a SPI × VO2 peak interaction, with the SPI × VO2 peak interaction term being the effect of primary interest.
CRF=cardiorespiratory fitness; CSF=cerebrospinal fluid; SPI=Sleep Problems Index I; VO2 peak=peak volume of oxygen consumed during graded exercise test; β=regression estimate; SE=standard error; Aβ42=amyloid-beta 42; t-tau=total tau; p-tau=phosphorylated tau; GXT=graded exercise test; APOE ε4=the ε4 allele of the apolipoprotein E gene
Figure 1. CRF modifies the influence of poor sleep on CSF biomarkers.
Figures display adjusted means and standard errors from analyses that modeled t-tau (A), p-tau (B), t-tau/Aβ42 (C), and p-tau/Aβ42 (D) as a function of age, sex, APOE ε4 status, time interval between GXT and sleep assessment, time interval between sleep assessment and CSF collection, SPI, VO2 peak, and a SPI*VO2 peak interaction. The SPI*VO2 peak interaction term was the effect of primary interest in all models.
Although SPI and VO2 peak were included in the analyses as continuous variables, for the purposes of graphing the study findings, we chose two anchor points (i.e., ±1 standard deviation away from the mean) to represent Poor vs. Good Sleep and High vs. Low VO2 peak.
CRF=cardiorespiratory fitness; CSF=cerebrospinal fluid; t-tau=total tau; VO2 peak=peak volume of oxygen consumed during graded exercise test; p-tau=phosphorylated tau; Aβ42=amyloid-beta 42; APOE ε4=the ε4 allele of the apolipoprotein E gene; GXT=graded exercise test; SPI=Sleep Problems Index I
Supplemental Analyses
In order to further examine potential factors that may influence the findings, we conducted additional sensitivity analyses, interrogating variables possibly associated with our key measures. These included body mass index (BMI), cholesterol, blood pressure, and family history of AD. We only found BMI to be significantly correlated with VO2 peak (R=−.48, p<.001). Therefore, we refit the interaction models while additionally including BMI as a covariate. Results remained essentially the same, with significant SPI*VO2 peak interactions for t-tau (β (SE)=−7.81 (3.23), p=.020), p-tau (β (SE)=−1.10 (.41), p=.010), and p-tau/Aβ42 (β (SE)=−.01 (.01), p=.046), and a trend for t-tau/Aβ42 (β (SE)=−.01 (.01), p=.070).
For completeness’ sake, we also ran the original interaction model (i.e., with terms for SPI, VO2 peak, age at GXT, sex, APOE ε4 status, time interval between GXT and sleep assessment, time interval between sleep assessment and CSF collection, and a SPI*VO2 interaction term) within the full sample of 74 individuals. Results revealed significant SPI*VO2 peak interactions for t-tau (β (SE)=−5.55 (2.54), p=.032) and p-tau (β (SE)=−.69 (.33), p=.039). However, the interaction for p-tau/Aβ42 was no longer significant (p=.452). While we felt this set of results was important to include, we present it as ancillary analysis because of the aforementioned evidence concerning older adults’ tendency to give submaximal effort during GXT, leading to an underestimation of their VO2 peak [45]. Taken together, these additional analyses further reinforce our original findings and suggest that sleep and CRF may synergistically influence AD pathology.
DISCUSSION
In this study, we found increased sleep problems were associated with higher levels of p-tau, as well as higher t-tau/Aβ42 and p-tau/Aβ42. Importantly, we also found that aerobic fitness mitigated this adverse association between poor sleep and CSF biomarkers, such that individuals with higher fitness were protected from the impact of sleep problems on CSF biomarkers.
Our group had previously reported that sleep disturbance is associated with greater AD pathology [9]. Specifically, we had found that self-report of inadequate sleep, somnolence, and sleep problems was associated with higher CSF t-tau/Aβ42 and p-tau/Aβ42, and lower sleep adequacy was associated with higher t-tau. A recent study by Lucey and colleagues found decreased non-rapid eye movement sleep to be associated with greater tau pathology [47]. Another study by the same group found a relationship between sleep deprivation and increased CSF tau [48]. Ju and colleagues also found sleep efficiency, measured via Actigraph, to be correlated with increased tau [8]. Disrupted breathing during sleep has also been associated with greater tau pathology. Osorio and colleagues [49] reported that individuals with sleep-disordered breathing exhibited higher levels of CSF t-tau and p-tau compared to controls. Similarly, in a study by Liguori and colleagues [50], patients with obstructive sleep apnea (OSA) displayed higher ratios of t-tau/Aβ42 compared to patients without OSA; and within the OSA group, sleep impairment was correlated with higher CSF tau. Although the mechanism is not clear, a previous study by Tapia-Rojas and colleagues [51] found voluntary exercise in mice led to fewer phosphorylated tau protein, suggesting physical activity may play a role in reducing tau pathology. Taken together, these findings indicate a link between poor sleep quality and AD pathology, suggesting interventions to improve sleep quality may be a viable pathway to protect against AD pathological changes.
With regard to the interaction between sleep and CRF, our results are novel. To our knowledge, few studies have examined sleep and PA as predictors in the same analysis and most have focused on cognition as an outcome. Wilckens and colleagues [29] assessed PA and sleep in relation to executive function. They found sleep efficiency, but not sleep duration, served as a mediator for the positive association between PA and performance on measures of executive control. This suggested that improved sleep efficiency may serve as one of the mechanisms by which PA improves cognition. Another study [28] investigated the interaction between objectively-measured sleep and PA in adult women. Analogous to our findings, they found that poor sleep efficiency was associated with worse cognitive performance, but only among those who engaged in low levels of PA. Finally, a preliminary study by Brown and colleagues [30] found that, among APOE ε4 carriers, greater self-reported PA was associated with reduced Aβ42 deposition only among those reporting good sleep quality. It should be noted that these studies examined various measures of PA, rather than CRF. Even so, taken together with our findings, these studies underscore the intricate relationship between PA and sleep hygiene.
Limitations of this study include its cross-sectional design, which limits the ability to establish causality. Future studies with a prospective design will be vital in determining whether sleep quality causes the observed differences in biomarker levels, or vice versa, and what role CRF plays in this relationship. The time interval between collection of GXT, sleep, and CSF data poses another limitation. The average time between any two data elements was approximately one year and it is possible that during this time participant behavior may have changed, particularly sleep patterns. Future studies in which all data are collected contemporaneously would be of value. Future studies would also benefit from a more comprehensive cataloguing of medication usage, medical comorbidities, and their potential influence on study findings. In addition, given the number of CSF biomarkers tested, our analyses are potentially vulnerable to multiplicity. Applying a conventional Bonferroni correction would set alpha at .01, leaving only the positive association between SPI and p-tau/Aβ42 and the interaction between SPI and CRF on p-tau as significant. Such a correction was deemed overly restrictive given the study’s modest sample size but, consequently, our findings should be interpreted cautiously. Another potential limitation is the use of self-report measures to assess sleep quality. There is considerable variability in individual interpretations of sleep quality [52] and self-report also limits our ability to identify true sleep disorders, such as sleep apnea or sleep-disordered breathing. Studies utilizing polysomnography or actigraph-measured sleep would be beneficial in providing objective measures of sleep quality. However, it should also be noted that self-reported sleep measures add an important dimension to the analysis, as objective measures may not fully capture sleep quality [53]. Finally, our sample was relatively homogeneous with regard to race and education, being mostly well-educated non-Hispanic whites. This limits the generalizability of our findings to the larger population.
In conclusion, this study reveals novel findings regarding the relationship between sleep, CRF, and CSF biomarkers among late-middle-aged adults at risk for AD. Specifically, sleep problems were associated with greater AD pathology but that relationship was attenuated among high fit individuals. Given the novelty of our study, further studies of a similar design and with larger sample sizes are needed to validate our findings. Overall, these results suggest improving sleep hygiene and increasing PA may be practical targets to protect against AD pathophysiological changes and slow progression of the disease in at-risk individuals.
ACKNOWLEDGMENTS
This work was supported by National Institute on Aging grants K23 AG045957 (OCO), R21 AG051858 (OCO), F31 AG048732 (KES), R56 AG052698 (BBB), R01 AG037639 (BBB), R01 AG031790 (CMC), R01 AG027161 (SCJ), R01 AG021155 (SCJ), and P50 AG033514 (SA); and by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison. Portions of this research were supported by the Extendicare Foundation, Alzheimer’s Association, Wisconsin Alumni Research Foundation, the Veterans Administration, including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI, and the Swedish Research Council, the European Research Council, the Torsten Söderberg Foundation, the Swedish Brain Foundation, and the Wallenberg Academy. We thank the staff and study participants of the Wisconsin Registry for Alzheimer’s Prevention, and the laboratory technicians at the Clinical Neurochemistry Laboratory, Mölndal, Sweden, without whom this work would not be possible.
Footnotes
DISCLOSURE STATEMENT
The authors have no conflict of interest to report.
REFERENCES
- [1].Alzheimer’s Disease International (2016) in World Alzheimer Report 2016. [Google Scholar]
- [2].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr., Kaye, Montine, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, Bendlin BB, Engelman CD, Okonkwo OC, Hogan KJ, Asthana S, Carlsson CM, Hermann BP, Sager MA (2018) The Wisconsin Registry for Alzheimer’s Prevention: A review of findings and current directions. Alzheimers Dement (Amst) 10, 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Branger P, Arenaza-Urquijo EM, Tomadesso C, Mezenge F, Andre C, de Flores R, Mutlu J, de La Sayette V, Eustache F, Chetelat G, Rauchs G (2016) Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging 41, 107–114. [DOI] [PubMed] [Google Scholar]
- [6].Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM (2015) Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging 36, 2568–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM (2013) Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 70, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, Fagan AM, Mignot E, Zempel JM, Claassen J, Holtzman DM (2017) Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain 140, 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Sager MA, Asthana S, Johnson SC, Benca RM, Bendlin BB (2017) Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology 89, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, Fjell AM (2014) Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology 83, 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Waller KL, Mortensen EL, Avlund K, Osler M, Fagerlund B, Lauritzen M, Jennum P (2016) Subjective sleep quality and daytime sleepiness in late midlife and their association with age-related changes in cognition. Sleep Med 17, 165–173. [DOI] [PubMed] [Google Scholar]
- [12].Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D (2010) Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol 68, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brown BM, Peiffer JJ, Taddei K, Lui JK, Laws SM, Gupta VB, Taddei T, Ward VK, Rodrigues MA, Burnham S, Rainey-Smith SR, Villemagne VL, Bush A, Ellis KA, Masters CL, Ames D, Macaulay SL, Szoeke C, Rowe CC, Martins RN (2013) Physical activity and amyloid-beta plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol Psychiatry 18, 875–881. [DOI] [PubMed] [Google Scholar]
- [14].Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik R, Gallagher CL, Dowling NM, Carlsson CM, Bendlin BB, LaRue A, Rowley HA, Christian BT, Asthana S, Hermann BP, Johnson SC, Sager MA (2014) Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology 83, 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC (2012) Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch Neurol 69, 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schultz SA, Boots EA, Darst BF, Zetterberg H, Blennow K, Edwards DF, Koscik RL, Carlsson CM, Gallagher CL, Bendlin BB, Asthana S, Sager MA, Hogan KJ, Hermann BP, Cook DB, Johnson SC, Engelman CD, Okonkwo OC (2017) Cardiorespiratory fitness alters the influence of a polygenic risk score on biomarkers of AD. Neurology 88, 1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boots EA, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, LaRue A, Asthana S, Hermann BP, Sager MA, Johnson SC, Okonkwo OC (2014) Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bugg JM, Shah K, Villareal DT, Head D (2012) COGNITIVE AND NEURAL CORRELATES OF AEROBIC FITNESS IN OBESE OLDER ADULTS. Experimental Aging Research 38, 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, McLemore EC (2007) Cognitive performance in older women relative to ApoE-epsilon 4 genotype and aerobic fitness. Medicine and Science in Sports and Exercise 39, 199–207. [DOI] [PubMed] [Google Scholar]
- [20].Defina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, Weiner MF, Berry JD (2013) The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Ann Intern Med 158, 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu R, Sui X, Laditka JN, Church TS, Colabianchi N, Hussey J, Blair SN (2012) Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med Sci Sports Exerc 44, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kline CE (2014) The bidirectional relationship between exercise and sleep: Implications for exercise adherence and sleep improvement. Am J Lifestyle Med 8, 375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Konecny T, Geske JB, Ludka O, Orban M, Brady PA, Abudiab MM, Albuquerque FN, Placek A, Kara T, Sahakyan KR, Gersh BJ, Tajik AJ, Allison TG, Ommen SR, Somers VK (2015) Decreased exercise capacity and sleep-disordered breathing in patients with hypertrophic cardiomyopathy. Chest 147, 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin CC, Hsieh WY, Chou CS, Liaw SF (2006) Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol 150, 27–34. [DOI] [PubMed] [Google Scholar]
- [25].Mougin F, Simon-Rigaud ML, Davenne D, Renaud A, Garnier A, Kantelip JP, Magnin P (1991) Effects of sleep disturbances on subsequent physical performance. Eur J Appl Physiol Occup Physiol 63, 77–82. [DOI] [PubMed] [Google Scholar]
- [26].Plyley MJ, Shephard RJ, Davis GM, Goode RC (1987) Sleep deprivation and cardiorespiratory function. Influence of intermittent submaximal exercise. Eur J Appl Physiol Occup Physiol 56, 338–344. [DOI] [PubMed] [Google Scholar]
- [27].Shapiro CM, Warren PM, Trinder J, Paxton SJ, Oswald I, Flenley DC, Catterall JR (1984) Fitness facilitates sleep. Eur J Appl Physiol Occup Physiol 53, 1–4. [DOI] [PubMed] [Google Scholar]
- [28].Lambiase MJ, Gabriel KP, Kuller LH, Matthews KA (2014) Sleep and executive function in older women: the moderating effect of physical activity. J Gerontol A Biol Sci Med Sci 69, 1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wilckens KA, Erickson KI, Wheeler ME (2016) Physical Activity and Cognition: A Mediating Role of Efficient Sleep. Behav Sleep Med, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brown BM, Rainey-Smith SR, Villemagne VL, PJ J, Bird S, Laws SM, Taddei K, Macauley L, Rowe CC, Ames D, Masters CL, Martins RN (2015) Investigating the synergistic relationship between sleep quality, physical activity, and levels of brain beta-amyloid. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 11, P451. [Google Scholar]
- [31].Sager MA, Hermann B, La Rue A (2005) Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol 18, 245–249. [DOI] [PubMed] [Google Scholar]
- [32].Koscik RL, La Rue A, Jonaitis EM, Okonkwo OC, Johnson SC, Bendlin BB, Hermann BP, Sager MA (2014) Emergence of mild cognitive impairment in late middle-aged adults in the wisconsin registry for Alzheimer’s prevention. Dement Geriatr Cogn Disord 38, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dowling NM, Hermann B, La Rue A, Sager MA (2010) Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology 24, 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Okonkwo OC, Cohen RA, Gunstad J, Poppas A (2011) Cardiac output, blood pressure variability, and cognitive decline in geriatric cardiac patients. J Cardiopulm Rehabil Prev 31, 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hays R, Stewart A (1992) Sleep measures In: Stewart A, Ware J, editors. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Duke University Press, 235–259. [Google Scholar]
- [36].Spritzer K, Hays R (2003) MOS Sleep Scale: A Manual for Use and Scoring, Version 10.
- [37].Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, Cha BY (2013) Validity of the medical outcomes study sleep scale in patients with painful diabetic peripheral neuropathy in Korea. J Diabetes Investig 4, 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kline CE, Hall MH, Buysse DJ, Earnest CP, Church TS (2018) Poor Sleep Quality is Associated with Insulin Resistance in Postmenopausal Women With and Without Metabolic Syndrome. Metab Syndr Relat Disord 16, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Balke B, Ware RW (1959) An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J 10, 675–688. [PubMed] [Google Scholar]
- [40].Pescatello LS, American College of Sports Medicine. (2014) ACSM’s guidelines for exercise testing and prescription, Wolters Kluwer/Lippincott Williams & Wilkins Health, Philadelphia. [Google Scholar]
- [41].Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, Owenius R, Hagerstrom D, Wollmer P, Minthon L, Hansson O (2014) Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol 71, 1282–1289. [DOI] [PubMed] [Google Scholar]
- [42].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, Alzheimer’s Disease Neuroimaging I (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Racine AM, Koscik RL, Nicholas CR, Clark LR, Okonkwo OC, Oh JM, Hillmer AT, Murali D, Barnhart TE, Betthauser TJ, Gallagher CL, Rowley HA, Dowling NM, Asthana S, Bendlin BB, Blennow K, Zetterberg H, Carlsson CM, Christian BT, Johnson SC (2016) Cerebrospinal fluid ratios with Abeta42 predict preclinical brain beta-amyloid accumulation. Alzheimers Dement (Amst) 2, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM (2007) Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64, 343–349. [DOI] [PubMed] [Google Scholar]
- [45].Dougherty RJ, Lindheimer JB, Stegner AJ, Van Riper S, Okonkwo OC, Cook DB (2018) An Objective Method to Accurately Measure Cardiorespiratory Fitness in Older Adults Who Cannot Satisfy Widely Used Oxygen Consumption Criteria. J Alzheimers Dis 61, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].American College of Sports M (2014) ACSM’s Guidelines for Exercise Testing and Prescription, Lippincott, Williams, & Wilkins. [Google Scholar]
- [47].Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TLS, Holtzman DM (2019) Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM (2019) The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Osorio RS, Pirraglia E, Gumb T, Mantua J, Ayappa I, Williams S, Mosconi L, Glodzik L, de Leon MJ (2014) Imaging and cerebrospinal fluid biomarkers in the search for Alzheimer’s disease mechanisms. Neurodegener Dis 13, 163–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, Placidi F (2017) Obstructive Sleep Apnea is Associated With Early but Possibly Modifiable Alzheimer’s Disease Biomarkers Changes. Sleep 40. [DOI] [PubMed] [Google Scholar]
- [51].Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC (2016) Voluntary Running Attenuates Memory Loss, Decreases Neuropathological Changes and Induces Neurogenesis in a Mouse Model of Alzheimer’s Disease. Brain Pathol 26, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Van Dongen HP, Baynard MD, Maislin G, Dinges DF (2004) Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 27, 423–433. [PubMed] [Google Scholar]
- [53].Krystal AD, Edinger JD (2008) Measuring sleep quality. Sleep Med 9 Suppl 1, S10–17. [DOI] [PubMed] [Google Scholar]