Abstract
Background
Appendiceal cancer is a rare malignancy that exhibits a wide range of histology and treatment response. Given the rarity and heterogeneous nature of the disease, it has been difficult to define optimal treatment strategies. Our goal is to examine the association between use of systemic chemotherapy and survival in patients with metastatic low-grade mucinous appendiceal adenocarcinoma.
Methods
The National Cancer Database (2004–2015) was queried, and patients with mucinous, grade 1, stage IV appendiceal adenocarcinoma were identified. The Kaplan-Meier method was used to calculate survival, and a Cox regression model was used to identify predictors of survival.
Results
639 patients were identified. Five-year overall survival for patients undergoing no chemotherapy versus chemotherapy was 52.9% and 61.3%, respectively. After adjusting with Cox proportional hazards model, chemotherapy was not associated with overall survival (HR:1.1, 95% CI:0.82–1.40, p=0.61). Patients who underwent surgical resection (HR:0.40, 95% CI:0.28–0.57, p<0.001) or were female (HR:0.61, 95% CI:0.5–0.8, p<0.001) had improved survival in adjusted analysis.
Conclusions
There is no association between undergoing chemotherapy and overall survival in this cohort of patients with stage IV low-grade mucinous appendiceal adenocarcinoma. Development of national treatment guidelines is urgently needed for more consistency in the management of patients with appendiceal cancers.
Keywords: Appendix, cancer, surgery, treatment
Introduction
The management of appendiceal cancers presents a unique challenge to clinicians as they exhibit a wide range of histology, behaviors, and varying responses to therapies. It is a rare malignancy with a reported increase in incidence over the past few decades [1, 2]. Adenocarcinoma is the most common type of primary appendiceal cancer, accounting for approximately 60% of cases [3]. It presents with mucinous, non-mucinous, ex-goblet cell, or signet ring histology with mucinous adenocarcinoma being the most frequent subtype [4]. Mucinous histology is further divided into grades, with well (low), moderately, poorly and undifferentiated grades having different clinical behavior and frequently present with peritoneal involvement indicating stage IV disease [5]. Tumor grade in stage IV mucinous appendiceal adenocarcinoma has been linked to significant differences in survival, with five-year survival rates of 57% for well differentiated to 11% for poorly differentiated tumors [6]. Given the rarity and heterogeneous nature of the disease, it has been difficult to define optimal treatment strategies.
Cytoreductive surgery with or without intraperitoneal chemotherapy has been used at specialty centers for patients with low-grade appendiceal mucinous adenocarcinomas and peritoneal involvement [7]. The role of systemic chemotherapy (SC) in treatment of low grade appendiceal mucinous adenocarcinoma with peritoneal spread, however, remains unclear [8]. The National Comprehensive Cancer Network (NCCN) does not have specific guidelines for patients with appendiceal cancer and recommends systemic therapy for appendiceal adenocarcinoma in accord with the NCCN guidelines for colon cancer [9]. One investigation reported that use of SC for stage IV, well differentiated (low-grade) mucinous appendiceal adenocarcinomas was not associated with differences in survival; however this was based on data collected from patients diagnosed between 1985–2006 [6]. The past two decades have seen significant developments in SC, including the additions of oxaliplatin, irinotecan and biologics, and SC appears to be widely utilized in the management of patients with low grade mucinous appendiceal adenocarcinoma despite the unknown benefit [10].
The aim of this study is to evaluate the association of survival with contemporary SC for patients with metastatic low grade mucinous appendiceal adenocarcinoma utilizing the National Cancer Data Base (NCDB), a national, multicenter, prospectively collected database.
Methods
Data Source:
The National Cancer Database Participant Use Data Files from 2004–2015 were used in this investigation. The NCDB is a joint project sponsored by the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The NCDB collects patient data from over 1,500 cancer programs in the United States, which amounts to more than 34 million patient records. The NCDB methodology has been described in previously published reports [11]. Deidentified patient data including diagnosis date, cancer stage, treatment, and survival are reported by local data extractors to the NCDB. The quality of data is continually assessed with audits. The data that support the findings of this study are available from the NCDB. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of the NCDB.
Patient population:
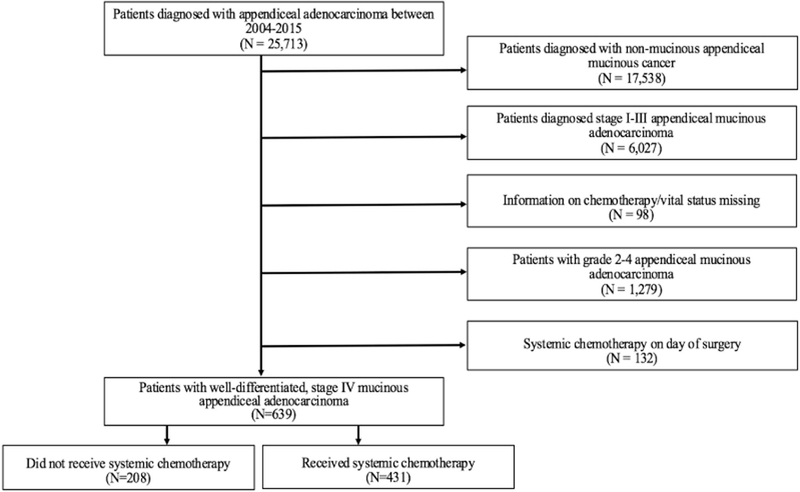
Patients with appendiceal cancers were identified using the International Classification of Diseases for Oncology (ICD-O3) codes (Figure 1). The topographical code was C18.1 indicating the primary site of disease was the appendix. ICD histologic codes of 8470/3 (mucinous cystadenocarcinoma), 8480/3 (mucinous adenocarcinoma), 8481/3 (mucin-producing adenocarcinoma) were used to identify all patients with mucinous appendiceal adenocarcinoma. Low grade appendiceal neoplasms and non invasive carcinoma histologies were not included. Patients were excluded from further analysis if they had unknown tumor stage, unknown treatment status, or unknown follow-up. Further, we excluded 132 patients who received chemotherapy on same day as surgery, under the assumption that these patients underwent intraperitoneal chemotherapy and it is not possible to assess if additional systemic chemotherapy was given. Patients with grade 1 disease and stage IV disease were included in the study. This study was reviewed and deemed exempt by the Institutional Review Board of the Brigham and Women’s Hospital.
Figure 1:
Schematic Representation of Patient Selection
Study Variables:
The study variables included demographics such as age of patient at diagnosis, sex, race, and insurance status; further tumor and operative characteristics such as cancer grade, systemic chemotherapy, immunotherapy status, surgical resection, and American Joint Committee on Cancer stage were analyzed. The primary outcome for this study was overall survival. The secondary outcome was the trend in use of systemic chemotherapy.
Statistical analysis:
All statistical analyses were carried out using Statistical Package for the Social Sciences (SPSS), (SPSS Inc, Chicago, IL, USA). P < 0.05 was considered significant for all tests. Chi-square and Wilcoxon rank-sum tests were carried out for bivariate analysis. Continuous variables are presented as a median with interquartile range (IQR) while categorical variables are presented as proportions. The Kaplan-Meier method was used to calculate survival from date of diagnosis until date of death with censoring of patients who were alive at the last follow-up. Cox regression was utilized to determine independent predictors of death with resulting hazard ratios and 95% confidence intervals. Variables included in the Cox model were age, gender, Charlson comorbidity score, systemic chemotherapy, and surgery as these variables have been shown to influence survival in previous studies [4, 6, 12].
Results
Demographics:
Overall, a total of 639 patients were identified with mucinous, grade 1, stage IV appendiceal adenocarcinoma (Table 1). There were 208 patients in the no chemotherapy group and 431 patients comprised the SC group. The median age at diagnosis was higher for patients who did not undergo chemotherapy (61.0 IQR: 52.25–74) compared to patients who underwent SC (55.0, IQR: 46–65), p <0.001. There were no differences between no chemotherapy and SC patients when comparing patient characteristics of gender, race, Charlson Comorbidity score, or income (p > 0.05). There was also no difference in the surgical resection rate between the two groups of patients (p > 0.05).
Table 1:
Patient characteristics
| Variable | No Chemotherapy | Chemotherapy | |
|---|---|---|---|
| (N = 208) | (N =431) | p-value | |
| Age, years (median, interquartile range) | 61.0 (52.25–74) | 55.0 (46–65) | <0.001 |
| Female sex, n (%) | 128 (61.5%) | 261 (60.6%) | 0.86 |
| Race, n (%) | 1.00 | ||
| White | 176 (88.9%) | 370 (88.7%) | |
| Black | 22 (11.1%) | 47 (11.3%) | |
| Charlson Score, n (%) | 0.16 | ||
| 0–1 | 201 (96.6%) | 424 (98.4%) | |
| ≥2 | 7 (3.4%) | 7 (1.6%) | |
| Insurance Type, n (%) | <0.001 | ||
| None | 10 (5.3%) | 12 (2.9) | |
| Private | 83 (44.4%) | 267 (64.5%) | |
| Medicaid | 7 (3.7%) | 33 (8.0%) | |
| Medicare | 85 (45.5%) | 96 (23.2%) | |
| Other | 2 (1.1%) | 6 (1.4%) | |
| Population, n (%) | 0.74 | ||
| Metro | 175 (85.8%) | 355 (84.5%) | |
| Urban | 25 (12.3%) | 59 (14.0%) | |
| Rural | 4 (2.0%) | 6 (1.4%) | |
| Facility Type | 0.15 | ||
| Community cancer program | 10 (5.1%) | 15 (4.0%) | |
| Comprehensive community cancer program | 66 (33.8%) | 96 (25.8%) | |
| Academic/Research program | 105 (53.8%) | 223 (59.9%) | |
| Integrated network cancer program | 14 (7.2%) | 38 (10.2%) | |
| Median income, n (%) | 0.86 | ||
| <38,000 | 27 (13.1%) | 60 (14.0%) | |
| 38,000–47,999 | 53 (25.7%) | 97 (22.6%) | |
| 48,000–62,999 | 52 (25.2%) | 114 (26.6%) | |
| >63,000 | 74 (35.9%) | 158 (36.8%) | |
| Surgical resection, n (%) | 182 (87.9%) | 392 (91.1%) | 0.21 |
| Immunotherapy, n (%) | 0 (0%) | 22 (5.1%) | <0.001 |
Survival:
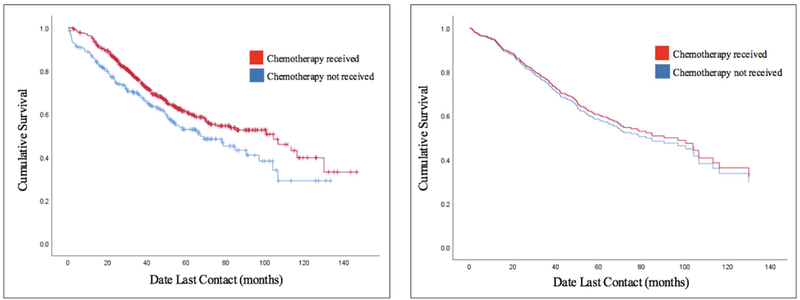
Five-year overall survival for patients undergoing no chemotherapy versus SC was 52.9% and 61.3%, respectively. SC use was associated with decreased mortality (HR: 0.73, 95%CI: 0.56–0.94, p = 0.01). The unadjusted and adjusted overall survival rates are shown in Figures 2a and 2b, respectively. After adjusting with Cox proportional hazards modeling, SC was not associated with overall survival (HR: 1.1, 95% CI: 0.82–1.40, p = 0.61) (Table 2). There was an increased risk of death for patients who had higher Charlson Comorbidity score (HR: 3.8, 95% CI: 2.2–6.7, p <0.001) or were older (HR: 1.03, 95% CI: 1.02–1.04, p < 0.001). Patients who underwent surgical resection (HR: 0.40, 95% CI: 0.28–0.57, p < 0.001) or were female (HR: 0.61, 95% CI: 0.5–0.8, p < 0.001) had a decreased risk of death. We ran a sensitivity analysis including the patients excluded for undergoing chemotherapy on the same day as surgery; the results did not change when adding these patients to the analysis (HR: 0.90, 95% CI: 0.70–1.16, p = 0.43).
Figure 2:
Survival Curves
Figure 2a: Unadjusted Kaplan-Meier Survival Curves
Figure 2b: Adjusted Survival Curves
Table 2:
Cox proportional hazards regression model to predict death
| Predictor | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Chemotherapy | 1.08 | 0.82–1.40 | 0.61 |
| Female sex | 0.61 | 0.47–0.78 | <0.001 |
| Age | 1.03 | 1.02–1.04 | <0.001 |
| Charlson score | 3.81 | 2.15–6.74 | <0.001 |
| Surgery | 0.40 | 0.28–0.57 | <0.001 |
Trend in Chemotherapy Use:
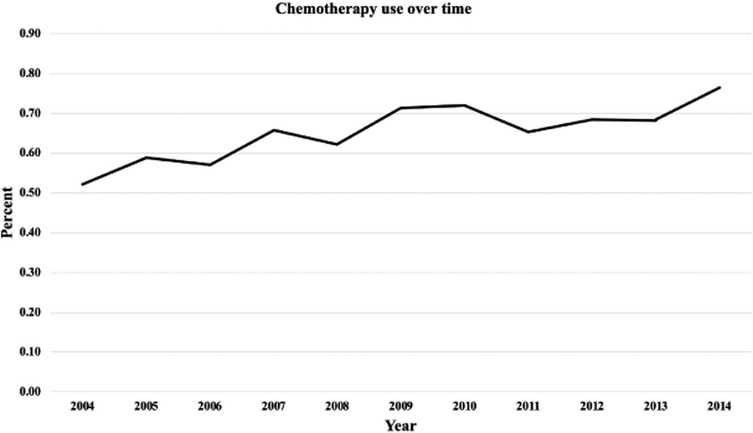
There was a significant trend towards increased use of SC; 52.2% in 2004 to 76.4% in 2014, p = 0.02 (Figure 3). In the SC group, 5.1% of patients were given immunotherapy.
Figure 3:
Trends in Systemic Chemotherapy Use
Discussion
Our study, with a total of 639 patients, is the largest study to date to show that contemporary SC is not associated with prolonged survival in patients with metastatic low grade appendiceal mucinous adenocarcinoma. We also demonstrate that female sex and surgical resection were associated with improved overall survival. SC continues to be widely used in the management of this disease, with a significant trend towards increased use over the past ten years.
Previously, Asare et al utilized the NCDB to evaluate patients from 1985–2006 with appendiceal adenocarcinoma and the association of SC with survival [6]. They found that adjuvant SC had better overall survival for all histology for Stage I-III disease, whereas Stage IV disease response to SC was histology dependent; specifically, patients receiving chemotherapy for low grade mucinous appendiceal adenocarcinomas had no improvement in overall survival [6]. Our results are in accord with this prior study, even though our patients are from a sample which would have received combination therapy after 2004 [13]. Although these regimens have improved survival in metastatic colorectal cancer, our analyses demonstrate that contemporary SC is not associated with overall survival in patients with metastatic low grade mucinous appendiceal adenocarcinoma. The indolent biology and slow growth of low grade appendiceal neoplasms has been hypothesized to contribute to poor response to cell-cycle specific systemic chemotherapy agents [14].
There are mixed results from other small, mostly single institution studies examining the association of SC and survival in stage IV low grade mucinous appendiceal adenocarcinomas. Shaib et al demonstrated that patients with low grade stage IV appendiceal mucinous neoplasms who did not receive SC had longer median OS of 82 months compared to the 32 months of those who did [15]. Similarly, a single institution study by Baratti et al examined a cohort of 104 patients who underwent CRS and HIPEC for low grade mucinous adenocarcinoma, and found that those who had a history of preoperative SC had worse OS [16]. Blackham et al investigated the outcomes associated with perioperative SC by tumor grade, and showed no difference in survival in patients with low-grade disease with median OS 72 months with perioperative SC, compared to 107 months in patients without [8]. Despite a lack of evidence supporting the use of SC in the treatment of stage IV mucinous appendiceal cancer, our analysis shows that not only is it still widely being used, its use has significantly increased between 2004–2014. This may be putting patients unnecessarily at risk for experiencing SC related adverse effects.
Our results indicate that surgical intervention is associated with improved OS. These results support the continued use of surgical debulking in patients with metastatic low grade mucinous appendiceal adenocarcinoma. This is also in accord with prior studies which have shown that one of the most important predictors of survival is completeness of cytoreduction [7].
This study has limitations that must be considered when interpreting our results. The NCDB does not have specific codes for cytoreduction or debulking. For patients who underwent surgery, we included patients who carried a code for any type of colectomy, with or without resection of contiguous organs, in addition to surgery not-otherwise-specified. We are thus unable to determine extent or intent of surgery, however our analysis demonstrated that any surgical intervention was associated with improved survival. There may also be significant selection bias and confounding by indication for SC, as the SC cohort was significantly younger (median age 55 years) than the non-SC group (median age 61 years). Additionally, who underwent SC may have had incomplete surgical cytoreduction or clinical suggestion of more aggressive tumor behavior. Some variables of interest specifically related to appendiceal cancer such as extent of peritoneal disease, commonly measured by the peritoneal carcinomatosis index, completeness of surgical cytoreduction, use of intraperitoneal chemotherapy, or chemotherapy regimen, are not recorded in the database; therefore, they could not be included in the model and may contribute to unmeasured confounding. Furthermore, we can only measure overall survival as no data on recurrence is recorded in the database. Lastly, our analysis has excluded patients who were reported as receiving SC on the same day as surgery as they may represent misclassified intraperitoneal chemotherapy. As there is no separate code for HIPEC, eliminating this cohort of patients who might have undergone HIPEC in the SC group may have skewed our results. For this cohort we are unable to determine if they received other systemic chemotherapy, however sensitivity analysis including this group did affect our conclusions. Despite these limitations, our study has several strengths. The study has a large sample size for a particular histology given the rarity of disease, and the dataset is from a multicenter national database which allows for greater generalizability.
With a disease that is as heterogeneous and rare as appendiceal adenocarcinoma, only small mostly single institution studies exist to assess treatment outcomes. While some investigation in the molecular profiles of appendiceal cancers has been performed, recent studies of targetable molecular mutations in appendiceal cancer show that the heterogeneity of the clinical behavior of this disease is also reflected in the wide variety of mutations present amongst different pathologic specimens [17, 18]. Further investigation into the targetable alterations of mucinous appendiceal adenocarcinomas in effort to individualize treatments may improve outcomes in the future, but the current mainstay of treatment remains surgical cytoreduction. With data from the NCDB, we were unable to identify an association with SC and survival for patients with stage IV low grade mucinous appendiceal adenocarcinoma. These data suggest that despite advances in SC regimens that have resulted in improvements in survival for late stage colorectal cancers, these improvements may not be applicable to patients with metastatic low grade mucinous appendiceal adenocarcinomas. Nevertheless, in patients who are not candidates for surgery or who have more aggressive tumor behavior, such as early recurrence after surgery or metastases outside of the peritoneum, one could consider SC with early assessment of response by imaging, CEA and/or symptoms prior to continuation of SC. Development of national guidelines is urgently needed for more consistency in the management of patients with appendiceal cancers.
Synopsis:
Appendiceal cancer is a rare disease with a wide range of histologies and treatment responses. In evaluating the effect of systemic chemotherapy use in patients with metastatic low grade mucinous appendiceal adenocarcinoma, we find no association with improved survival.
Funding:
Dr. Garrett Nash receives funding from the NCI Grant P30 CA008748.
Footnotes
Data Availability Statement:
The data that support the findings of this study are available from the National Cancer Database [19]. Restrictions apply to the availability of the data, which were used under a data use agreement for this study. The data used in this study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
References
- 1.Gupta S, Parsa V, Adsay V, Heilbrun LK, Smith D, Shields AF, et al. Clinicopathological analysis of primary epithelial appendiceal neoplasms. Med Oncol. 2010;27(4):1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmor S, Portschy PR, Tuttle TM, Virnig BA. The rise in appendiceal cancer incidence: 2000–2009. J Gastrointest Surg. 2015;19(4):743–50. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9(8):730–56. [DOI] [PubMed] [Google Scholar]
- 4.McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer. 2002;94(12):3307–12. [DOI] [PubMed] [Google Scholar]
- 5.Grotz TE, Royal RE, Mansfield PF, Overman MJ, Mann GN, Robinson KA, et al. Stratification of outcomes for mucinous appendiceal adenocarcinoma with peritoneal metastasis by histological grade. World J Gastrointest Oncol. 2017;9(9):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asare EA, Compton CC, Hanna NN, Kosinski LA, Washington MK, Kakar S, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: Analysis of the National Cancer Data Base. Cancer. 2016;122(2):213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaib WL, Assi R, Shamseddine A, Alese OB, Staley C, Memis B, et al. Appendiceal Mucinous Neoplasms: Diagnosis and Management . Oncologist. 2017;22(9):1107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackham AU, Swett K, Eng C, Sirintrapun J, Bergman S, Geisinger KR, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2014;109(7):740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCCN. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Colon Cancer (Version 4.2018) [Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 10.Tejani MA, ter Veer A, Milne D, Ottesen R, Bekaii-Saab T, Benson AB, et al. Systemic therapy for advanced appendiceal adenocarcinoma: an analysis from the NCCN Oncology Outcomes Database for colorectal cancer. J Natl Compr Canc Netw. 2014;12(8):1123–30. [DOI] [PubMed] [Google Scholar]
- 11.Winchester DP, Stewart AK, Phillips JL, Ward EE. The national cancer data base: past, present, and future. Ann Surg Oncol. 2010;17(1):4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bijelic L, Kumar AS, Stuart OA, Sugarbaker PH. Systemic Chemotherapy prior to Cytoreductive Surgery and HIPEC for Carcinomatosis from Appendix Cancer: Impact on Perioperative Outcomes and Short-Term Survival . Gastroenterol Res Pract. 2012;2012:163284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carethers JM. Systemic treatment of advanced colorectal cancer: tailoring therapy to the tumor. Therap Adv Gastroenterol. 2008;1(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietrantonio F, Maggi C, Fanetti G, Iacovelli R, Di Bartolomeo M, Ricchini F, et al. FOLFOX-4 chemotherapy for patients with unresectable or relapsed peritoneal pseudomyxoma. Oncologist. 2014;19(8):845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaib WL, Martin LK, Choi M, Chen Z, Krishna K, Kim S, et al. Hyperthermic Intraperitoneal Chemotherapy Following Cytoreductive Surgery Improves Outcome in Patients With Primary Appendiceal Mucinous Adenocarcinoma: A Pooled Analysis From Three Tertiary Care Centers . Oncologist. 2015;20(8):907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baratti D, Kusamura S, Nonaka D, Langer M, Andreola S, Favaro M, et al. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol. 2008;15(2):526–34. [DOI] [PubMed] [Google Scholar]
- 17.Raghav KP, Shetty AV, Kazmi SM, Zhang N, Morris J, Taggart M, et al. Impact of molecular alterations and targeted therapy in appendiceal adenocarcinomas. Oncologist. 2013;18(12):1270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borazanci E, Millis SZ, Kimbrough J, Doll N, Von Hoff D, Ramanathan RK. Potential actionable targets in appendiceal cancer detected by immunohistochemistry, fluorescent in situ hybridization, and mutational analysis. J Gastrointest Oncol. 2017;8(1):164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.[Dataset] National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb/puf. Accessed June 5, 2019