Abstract
New Findings
-
What is the central question of this study?
What are the differential roles of the mechanosensitive and chemosensitive afferent renal nerves in the reno‐renal reflex that promotes natriuresis, sympathoinhibition and normotension during acute and chronic challenges to sodium homeostasis?
-
What is the main finding and its importance?
The mechanosensitive afferent renal nerves contribute to an acute natriuretic sympathoinhibitory reno‐renal reflex that may be integrated within the paraventricular nucleus of the hypothalamus. Critically, the afferent renal nerves are required for the maintenance of salt resistance in Sprague–Dawley and Dahl salt‐resistant rats and attenuate the development of Dahl salt‐sensitive hypertension.
Abstract
These studies tested the hypothesis that in normotensive salt‐resistant rat phenotypes the mechanosensitive afferent renal nerve (ARN) reno‐renal reflex promotes natriuresis, sympathoinhibition and normotension during acute and chronic challenges to fluid and electrolyte homeostasis. Selective ARN ablation was conducted prior to (1) an acute isotonic volume expansion (VE) or 1 m NaCl infusion in Sprague–Dawley (SD) rats and (2) chronic high salt intake in SD, Dahl salt‐resistant (DSR), and Dahl salt‐sensitive (DSS) rats. ARN responsiveness following high salt intake was assessed ex vivo in response to noradrenaline and sodium concentration (SD, DSR and DSS) and via in vivo manipulation of renal pelvic pressure and sodium concentration (SD and DSS). ARN ablation attenuated the natriuretic and sympathoinhibitory responses to an acute VE [peak natriuresis (µeq min−1) sham 52 ± 5 vs. ARN ablation 28 ± 3, P < 0.05], but not a hypertonic saline infusion in SD rats. High salt (HS) intake enhanced ARN reno‐renal reflex‐mediated natriuresis in response to direct increases in renal pelvic pressure (mechanoreceptor stimulus) in vivo and ARN responsiveness to noradrenaline ex vivo in SD, but not DSS, rats. In vivo and ex vivo ARN responsiveness to increased renal pelvic sodium concentration (chemoreceptor stimulus) was unaltered during HS intake. ARN ablation evoked sympathetically mediated salt‐sensitive hypertension in SD rats [MAP (mmHg): sham normal salt 102 ± 2 vs. sham HS 104 ± 2 vs. ARN ablation normal salt 103 ± 2 vs. ARN ablation HS 121 ± 2, P < 0.05] and DSR rats and exacerbated DSS hypertension. The mechanosensitive ARNs mediate an acute sympathoinhibitory natriuretic reflex and counter the development of salt‐sensitive hypertension.
Keywords: afferent renal nerves, blood pressure, sodium homeostasis
1. INTRODUCTION
The sensory afferent renal nerves (ARNs), comprising mechanosensitive and chemosensitive fibres originating primarily from the renal pelvic wall, contribute to sympathoinhibitory and sympathoexcitatory reno‐renal reflexes that modulate efferent renal sympathetic nerve activity, natriuresis and blood pressure (Johns, 2014; Kopp, 2015). The sympathoinhibitory reno‐renal reflex, likely driven by mechanosensitive ARN fibres, has been implicated in the tonic regulation of renal sympathetic nerve activity in healthy, normotensive animal models (Chien, Chien, Cheng, Chen, & Hsu, 2000; Johns, 2014; Johns & Abdulla, 2013; Kopp, 1993; Kopp, 2015; Ma, Huang, & Chen, 2002a). In this reflex, activation of the ARNs results in the suppression of efferent renal sympathetic nerve activity, promoting a natriuretic response facilitating sodium homeostasis and normotension (Johns, 2014; Johns & Abdulla, 2013; Kopp, 1993; Kopp, 2015). In contrast, the sympathoexcitatory reno‐renal reflex, which increases renal sympathetic outflow and sodium retention, is primarily mediated by chemosensitive ARN fibres that are activated in animal models of disease states, including heart failure and renal failure (Barry & Johns, 2015; Johns, 2014; Johns & Abdulla, 2013; Kopp, 1993; Kopp, 2015).
Salt sensitivity of blood pressure is characterized by an exaggerated pressor response to dietary sodium intake that independently predicts hypertension risk (Appel et al., 2011; Franco & Oparil, 2006). In salt‐resistant individuals, dietary sodium evokes a sympathoinhibitory response that facilitates natriuresis and normotension (Johns, 2014; Lohmeier, Hildebrandt, & Hood, 1999). In contrast, in most salt‐sensitive individuals, sympathoexcitation promotes sodium retention and hypertension in response to dietary sodium intake (Brooks, Haywood, & Johnson, 2005; Stocker, Monahan, & Browning, 2013). It was recently postulated that neurohumoral control of renal excretory function is the first line of defence against salt‐sensitive hypertension (Evans & Bie, 2016); however the role(s) of the ARNs in this process is unknown.
A potential role for ARN regulation of natriuresis and blood pressure is suggested by increased ARN activity during high dietary sodium intake in the salt‐resistant Sprague–Dawley (SD) rat (Kopp et al., 2009, 2011; Kopp, Cicha, & Smith, 2006). The removal of all sensory afferent inputs from multiple end organs, including the ARNs, innervating multiple levels of the spinal cord, surgically via dorsal rhizotomy or pharmacologically by subcutaneous capsaicin evokes salt‐sensitive hypertension in SD rats (Kopp, Cicha, & Smith, 2003; Wang, Li, & Qiu, 1998; Wang, Wu, & Lookingland, 2001). Significantly, direct electrical stimulation of the ARNs, a non‐specific stimulus that does not preferentially target mechanosensitive or chemosensitive ARN terminals, activates hypothalamic paraventricular nucleus (PVN) parvocellular neurons and increases blood pressure (Solano‐Flores, Rosas‐Arellano, & Ciriello, 1997; Xu, Zheng, Liu, & Patel, 2015). These data indicate that the PVN contributes to a sympathoexcitatory reno‐reflex. Other studies raise the possibility that the PVN could also participate in a sympathoinhibitory reno‐renal reflex. For example, lesions of the PVN attenuate the inhibition of renal sympathetic nerve activity associated with volume expansion (Haselton, Goering, & Patel, 1994). This suggests that the PVN integrates visceral sensory information, which includes the ARNs for both sympathoexcitatory and sympathoinhibitory reflexes involved in fluid regulation.
In the current study, we hypothesized that in normotensive salt‐resistant rat phenotypes the mechanosensitive ARN reno‐renal reflex promotes natriuresis, sympathoinhibition and normotension during acute and chronic challenges to fluid and electrolyte homeostasis. To address this hypothesis, we used in vivo and ex vivo preparations to test the impact of dietary salt intake on ARN reflexes. Selective ARN ablation was used to investigate the ARNs’ role in the sympathoinhibitory, natriuretic and blood pressure responses to (1) acute sodium challenges designed to differentially activate the mechanosensitive and chemosensitive ARN and (2) a chronic high sodium diet in salt‐resistant and salt‐sensitive rat models.
2. METHODS
2.1. Ethical approval
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) under protocol number AN15241 in accordance with the guidelines of the Boston University School of Medicine and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All possible steps were taken to minimize pain and suffering and killing was conducted in accordance with approved protocols. Our studies fully comply with the ethical principles and animal ethics checklist of Experimental Physiology.
2.2. Animals
Male Sprague–Dawley (SD), Dahl salt‐resistant (DSR), and Dahl salt‐sensitive (DSS) rats weighing 275–300 g, aged approximately 12 weeks, were purchased from Envigo (Indianapolis, IN, USA) and transported in accordance with all NIH and AALAC guidelines. Rats were pair‐housed prior to surgical intervention and housed separately following survival surgery as described below. Animals were housed in a temperature (range 20–26°C) and humidity (range 30–70%) controlled facility under a 12‐h light–dark cycle and were allowed tap water and standard irradiated rodent diet [Envigo Teklad, Madison, WI, USA; Teklad Global Diet no. 2918, 18% protein, 5% crude fat, 5% fibre, total NaCl content 0.6% (174 mEq Na+ kg−1)] or experimental high sodium diet [Envigo Teklad Diets, WI, TD.03095, 19% protein, 5% crude fat, 3% fibre, total NaCl content 4% (678 mEq Na+ kg−1)] ad libitum. Rats were randomly assigned to experimental groups.
2.3. Surgical procedures
2.3.1. Acute femoral vein, femoral artery, renal artery and bladder cannulation
On the day of the acute study, rats were anaesthetized with sodium methohexital (20 mg kg−1 i.p., supplemented with 10 mg kg−1 i.v. as required). Rats were instrumented with PE‐50 catheters in the left femoral vein, left femoral artery and bladder to allow i.v. administration of isotonic saline and experimental sodium challenges, measurement of mean arterial pressure (MAP) and heart rate (HR), and assessment of renal excretory function, respectively (Carmichael, Carmichael, Kuwabara, Cunningham, & Wainford, 2016; Wainford, Pascale, & Kuwabara, 2013; Walsh, Kuwabara, Shim, & Wainford, 2016). In a subset of rats, the caudal branch of the dorsal division of the left renal artery was cannulated with a heat pulled PE‐10 catheter following cannulation of the vein, artery and bladder to allow renal artery infusion of bradykinin. This surgical approach avoided renal artery occlusion and maintained renal blood flow (verified in pilot studies by the observation of lissamine green dye distribution across the entire kidney) to minimize the risk of renal hypoperfusion. Rats were placed in a Plexiglas rat holder and an i.v. infusion of isotonic saline (20 µl min−1) was maintained during a 2 h surgical recovery period allowing rats to return to full consciousness and stable renal and cardiovascular function prior to study. Inulin (300 mg kg−1 h−1) and para‐amminohippurate (PAH; 40 mg kg−1 h−1) were added to the i.v. infusion for a 90 min equilibration period during the recovery period and maintained during experimentation. MAP and HR were recorded continuously via the femoral artery cannula using computer‐driven BIOPAC data acquisition software (MP150 and AcqKnowledge 3.8.2; BIOPAC Systems In., Goleta, CA, USA) connected to an external pressure transducer (P23XL; Viggo Spectramed Inc., Oxnard, CA, USA).
2.3.2. Renal pelvis cannulation
Cannulation of the femoral vein and femoral artery was performed under pentobarbital sodium anaesthesia (20 mg kg−1 i.p.) and maintained with an i.v. infusion of pentobarbital sodium, 0.04 mmol kg−1 h−1, in isotonic saline at 50 µl min−1. The left renal pelvis was cannulated via the left ureter using PE‐50 tubing containing three heat‐pulled tips of PE‐10 tubing that allowed manipulation of renal pelvic sodium concentration and pelvic pressure, drainage of effluent and continuous recording of renal pelvic pressure, respectively (Kopp, Smith, & Pence, 1994; Lin, Lee, Huang, Chen, & Ma, 2015). The right renal pelvis was cannulated via the ureter for contralateral urine collection. Animals recovered for 90 min to allow collection of stable steady state urine. Renal pelvic pressure was recorded continuously via the surgically implanted pelvic cannula.
2.3.3. Selective afferent renal nerve ablation (ADNX) and sham ADNX
Selective afferent renal nerve ablation (ADNX) was performed via direct application of 33 mm capsaicin to the renal nerves (Foss, Wainford, Engeland, Fink, & Osborn, 2015). Under pentobarbital sodium anaesthesia (20 mg kg−1 i.p.) each kidney was exposed via a dorsal flank incision and the renal artery and vein were gently separated from the surrounding fascia. A cotton‐tipped swab was used to apply capsaicin (33 mm in isotonic saline containing 5% ethanol and 5% Tween‐20), taking care to isolate the dissected renal artery and vein from the surrounding tissue to avoid off‐target capsaicin exposure. Any excess capsaicin was dried before suturing the flank muscle and skin. In the sham group, each kidney was exposed and the renal artery and vein were visualized before suturing.
The efficacy of selective ADNX was confirmed at the end of the 21 day salt‐intake study via (1) the MAP response to intra‐renal bradykinin infusion (40 µg kg−1 min−1) assessed as the average value of the last 2 min of a 5 min infusion period (Foss et al., 2015), (2) enzyme‐linked immunosorbent assay (ELISA) analysis of noradrenaline (NA) content in kidney tissue as per the manufacturer's instructions (IB89537, IBL America, Minneapolis, MN, USA), and (3) ELISA analysis of renal pelvic calcitonin gene related peptide (CGRP) content in kidney tissue as per manufacturer's instructions (no. 589001, Cayman Chemical Co., Ann Arbor, MI, USA) (Foss et al., 2015). ADNX did not alter renal NA content, reduced renal pelvic CGRP to undetectable levels and abolished the pressor response to intra‐renal bradykinin 10 days post‐ADNX (Figure 2). Twenty one days post‐ADNX the pressor response to intra‐renal bradykinin was abolished and CGRP levels were less than 90% of sham animals (Figure 7).
Figure 2.
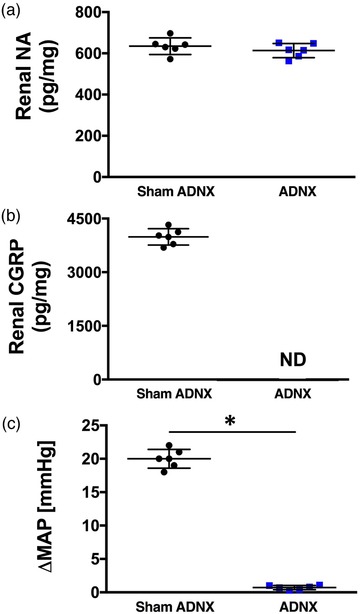
Impact of afferent renal nerve ablation on renal noradrenaline and CGRP content and the blood pressure response to intra‐renal bradykinin. Renal noradrenaline (NA) content (a), renal pelvic calcitonin gene related peptide content (CGRP) (b) and peak change in mean arterial pressure (c) in response to 40 µg kg−1 min−1 intra‐renal bradykinin in subsets of Sprague–Dawley rats that underwent acute volume expansion or 1 m NaCl infusion 10 days after sham surgery or selective afferent renal nerve ablation (ADNX). n = 6/group. *P < 0.05 vs. sham ADNX group value
Figure 7.
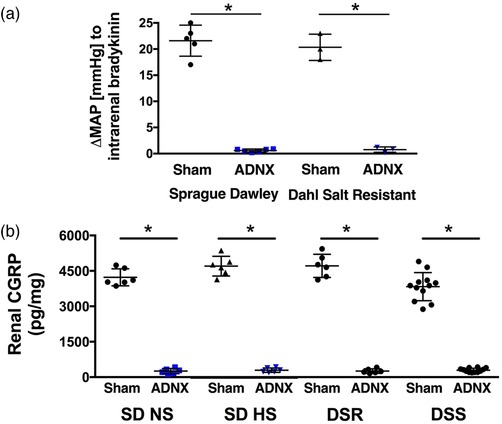
Impact of afferent renal nerve ablation on the blood response to intra‐renal bradykinin and renal CGRP content 21 days post‐surgery. (a) Change in blood pressure in response to intrarenal bradykinin infusion in Sprague–Dawley (SD) (n = 5/group) and Dahl salt‐resistant (DSR) rats (n = 3/group). (b) Renal CGRP content in SD (n = 6/group), DSR (n = 6/group), and Dahl salt‐sensitive (DSS) rats (n = 12/group) maintained on a normal (NS; 0.6% NaCl) or high (HS; 4% NaCl) salt diet following sham surgery or selective afferent renal nerve ablation (ADNX). *P < 0.05 vs. respective sham group
2.3.4. Radiotelemetry probe implantation
Rats were anaesthetized with ketamine combined with xylazine (30 mg kg−1 i.p. ketamine, 3 mg kg−1 i.p. xylazine). A radiotelemetry probe (PA‐C40, DSI, New Brighton, MN, USA) was implanted into the abdominal aorta via the left femoral artery and all animals recovered for 5–7 days prior to collection of baseline blood pressure data (Brouwers, Smolders, Wainford, & Dupont, 2015; Foss et al., 2015; Wainford, Carmichael, Pascale, & Kuwabara, 2015).
2.4. Experimental protocols
2.4.1. Acute volume expansion
SD rats maintained on a normal salt diet (0.6% NaCl) underwent acute femoral vein, femoral artery, renal artery and bladder cannulation 10 days after ADNX or sham ADNX surgery (n = 6/ surgical group) (see ‘Surgical procedures’). Following the 2 h recovery period, conscious rats underwent an acute i.v. volume expansion protocol consisting of a 20 min control period (isotonic saline, 20 µl min−1) follow by a 30 min volume expansion period (isotonic saline, 5% body weight over 30 min) and a 90 min recovery period (isotonic saline, 20 µl min−1) (Kapusta, Pascale, & Wainford, 2012; Wainford & Kapusta, 2010). MAP and HR were monitored continuously via the femoral artery cannula and BIOPAC. Urine was collected in consecutive 10 min increments and blood was collected in the middle of each half hour period. Immediately following the completion of the 120 min volume expansion (VE) protocol the pressor response to intra‐renal bradykinin infusion was assessed and brain and kidney tissue were collected for PVN Fos immunohistochemistry and validation of ADNX efficacy, respectively, as described below. In a separate set of SD rats, tissue was collected for baseline PVN Fos immunohistochemistry immediately after the end of the 2 h surgical recovery period and a 20 min control period (n = 6).
2.4.2. Acute 1 m NaCl sodium infusion
SD rats maintained on a normal salt diet (0.6% NaCl) underwent acute femoral vein, femoral artery, renal artery and bladder cannulation 10 days after ADNX or sham ADNX surgery (n = 6/surgical group) (see ‘Surgical procedures’). Following the 2 h recovery period, conscious rats were challenged with an acute 1 m NaCl infusion protocol consisting of a 1 h control period (isotonic saline, 20 µl min−1, i.v.) followed by a 2 h sodium infusion period (1 m NaCl, 20 µl min−1, i.v.) (Kompanowska‐Jezierska et al., 2008; Wainford et al., 2013). MAP and HR were monitored continuously via the femoral artery cannula and BIOPAC. Urine was collected in consecutive 15 min increments and blood was collected in the middle of each half hour period. Immediately following the completion of the 120 min 1 m NaCl protocol, the pressor response to intrarenal bradykinin infusion was assessed after which brain and kidney tissues were collected for PVN Fos immunohistochemistry and validation of ADNX efficacy, respectively, as described below. In a separate set of SD rats, tissue was collected for baseline PVN Fos immunohistochemistry immediately after the end of the 2 h surgical recovery period and a 20 min control period (n = 6).
2.4.3. Fos immunohistochemistry
Rats that underwent a 120 min acute VE study, a 120 min acute 1 m NaCl infusion, or a 2 h recovery period were deeply anaesthetized with sodium methohexital (10 mg kg−1 i.v.) and underwent transcardiac perfusion with 0.1 m phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Brains were removed and submerged in 4% PFA overnight followed by 30% w/v sucrose for 2 days. The PVN was sectioned into three sets of serial 40 µm coronal sections that were stored free‐floating in cryoprotectant (Watson, Wiegand, Clough, & Hoffman, 1986) at −20°C until immunohistochemistry was performed (Carmichael et al., 2016).
On one set of free‐floating PVN sections from each rat, Fos immunohistochemistry was performed as previously described (Carmichael et al., 2016; Randolph, Li, Curtis, Sullivan, & Cunningham, 1998). Sections stored in cryoprotectant came to room temperature and were rinsed twice in 0.1 m PBS for 30 min. Sections were incubated in 0.3% hydrogen peroxide in dH2O for 30 min and rinsed for 30 min in 0.1 m PBS. Sections were blocked in PBS diluent (0.1 m PBS containing 3% normal horse serum and 0.25% Triton X‐100) for 2 h and then incubated with primary antibody (anti‐Fos Ab‐5, Calbiochem, San Diego, CA, USA; 1:30,000 in PBS diluent) for 48 h at 4°C. Sections were rinsed twice in 0.1 m PBS for 30 min and then incubated with secondary antibody (biotinylated horse anti‐rabbit IgG, Vector Laboratories, Burlingame, CA, USA; 1:200 in PBS diluent) for 2 h at room temperature. Sections were then incubated with an avidin‐peroxidase conjugate (ABC Vectastain Kit; Vector Laboratories) and developed using 0.04% 3,3′‐diaminobenzidene hydrochloride and 0.04% nickel ammonium sulfate in 0.1 m PBS. Sections were mounted onto gelatin‐coated slides and dehydrated using a graded series of alcohols followed by xylenes. Coverslips were placed on the slides using Permount mounting medium. Tissue sections were imaged using an Olympus microscope (BX41) and an Olympus DP70 digital camera with DP MANAGER software (v 2.2.1) (Olympus, Center Valley, PA, USA). The PVN was sampled at three rostral–caudal levels and two sets of tissue from each animal were analysed. The Fos‐positive cell count was quantified by participants blind to the experimental conditions using National Institutes of Health ImageJ software (NIH, Bethesda, MD, USA), and the counts for each PVN subnucleus were averaged for each animal.
2.4.4. In vivo renal pelvic studies
SD and DSS rats underwent renal pelvis cannulation (see ‘Surgical procedures’) on day 21 of normal (0.6% NaCl) or high salt (4% NaCl) intake (n = 6/strain/diet). Anaesthesia (pentobarbital sodium in isotonic saline, 0.04 mmol kg−1 h−1 at 50 µl min−1, i.v.) was maintained during a 90 min rest period and the experimental protocol. During pelvic pressure and sodium concentration studies renal pelvic pressure was recorded continuously via the surgically implanted renal pelvic cannula and BIOPAC.
Manipulation of renal pelvic pressure
A syringe filled with 154 mm isotonic saline was connected to the renal pelvis cannula and elevated to increase renal pelvic pressure across a physiological range (0–10 mmHg). Renal pelvic pressure was increased in 2.5 mmHg increments (0, 2.5, 5.0, 7.5 and 10.0 mmHg, in random order for each rat) for 10 min per increment with a 10 min recovery period (0 mmHg) between increments. During each increment, contralateral urine was collected.
Manipulation of renal pelvic sodium concentration
The renal pelvis cannula was used to deliver a 10 min infusion of 154 mm isotonic saline (20 µl min−1) followed by a 10 min infusion of saline adjusted to contain 450 mm NaCl (solution osmolality 900 mosmol l−1) at the same rate. During each period, contralateral urine was collected. Renal pelvic pressure was not altered during either infusion.
2.4.5. Ex vivo renal pelvis assay
SD, DSR and DSS rats were maintained on a 21 day normal (0.6% NaCl) or high salt (4% NaCl) diet (n = 6/strain/diet) prior to conscious decapitation and dissection of the renal pelvis from each kidney. Direct ARN responsiveness in the isolated renal pelvis, assessed as substance P release, was tested in response to (1) a general ARN stimulus, noradrenaline (1250 pm; which acts at α1‐adrenoceptors to drive an increase in ARN activity; Kopp, Cicha, Smith, Mulder, & Hokfelt, 2007) and (2) a specific chemoreceptor stimulus (450 mm NaCl; the upper range of urinary sodium content). Each renal pelvis was placed individually in a separate well of a 24‐well plate containing 400 µl HEPES medium and maintained at 37°C. The medium was replaced every 10 min during a 2 h equilibration period. During the experiment, medium was collected and replaced every 5 min during four control periods, one treatment period (1250 pm noradrenaline or 450 mm NaCl in HEPES), and four recovery periods. Each rat served as its own internal control, with one renal pelvis incubated with 1250 pm NA and one renal pelvis incubated with 450 mm NaCl during the treatment period. The medium was stored at −80°C for analysis of substance P content via ELISA (Enzo Life Sciences, Farmingdale, NY, USA; cat. no. ADI‐901‐018). Noadrenaline‐ and NaCl‐evoked substance P release was calculated as the difference in substance P release between the treatment period and the average of the control periods.
2.4.6. Metabolic balance studies
Metabolic studies were conducted in separate groups of SD, DSR and DSS rats. In brief, rats were continuously housed individually in metabolic cages with ad libitum access to food and water. Following 48 h acclimatization, all rats underwent a 10 day baseline period on a normal salt (0.6% NaCl) diet prior to random assignment to sham ADNX or ADNX surgery (see ‘Surgical procedures’). Immediately following surgery, sham ADNX and ADNX rats were randomly assigned to a 21 day experimental dietary period of either normal salt (0.6% NaCl; SD rats only) or high salt (4% NaCl) intake (n = 6/strain/intervention/diet). Food and water consumption and urine output were measured daily. Twenty‐four‐hour sodium balance was calculated as the difference between dietary sodium intake and urinary sodium excretion.
2.4.7. Radiotelemetry studies
SD, DSR and DSS rats underwent radiotelemetry probe implantation (see ‘Surgical procedures’) and blood pressure data were recorded by radiotelemetry [Dataquest A.R.T. 4.2 software (DSI)] via scheduled sampling for 10 s every 10 min. Rats were maintained on a normal salt (0.6% NaCl) diet for a 10 day baseline period and were then randomly assigned to sham ADNX or ADNX surgery and placed immediately on a 21 day normal salt (0.6% NaCl; SD rats only) or high salt (4% NaCl) diet (n = 6/strain/intervention/diet) and blood pressure was recorded for a further 21 days. At the end of the protocol, whole kidneys and plasma were collected and stored at −80°C.
2.4.8. Analytical techniques
Urine volume was assessed gravimetrically assuming 1 g = 1 ml. Urine and plasma sodium content was determined by flame photometry (IL‐943; Instrumentation Laboratory, Bedford, MA, USA). Plasma renin activity (PRA), assessed as generated angiotensin I, and urinary angiotensinogen (UAGT) levels were determined using ELISA (PRA Tecan Boston Inc., Medford, MA, USA; Cat. No. DB52011, UAGT IBL America, Minneapolis, MN, USA; no. 27414) as per the manufacturers’ instructions. Inulin and PAH concentrations were determined using standard colorimetric assays (Cervenka, Wang, & Navar, 1998).
2.5. Statistical analysis
All data are expressed as means ± SD. The magnitude of change in cardiovascular and renal excretory parameters at different time points after initiation of acute sodium challenge or chronic dietary sodium intake was compared with the average group control value by a one‐way repeated‐measures analysis of variance (ANOVA) with subsequent Dunnett's test. Differences between treatment groups (e.g. sham ADNX vs. ADNX) were assessed by a two‐way repeated measure ANOVA, with treatment group being one fixed effect and time the other, with the interaction included. The time (min) was used as the repeated factor. Post hoc analysis was performed using Bonferroni's test, to compare variations among the groups. Statistical analysis was carried out using Prism 7 (GraphPad Software Inc., La Jolla, CA, USA). In all studies, statistical significance was defined as P < 0.05.
3. RESULTS
3.1. The ARNs modulate the cardiovascular, renal and parvocellular PVN neuronal responses to an acute volume expansion
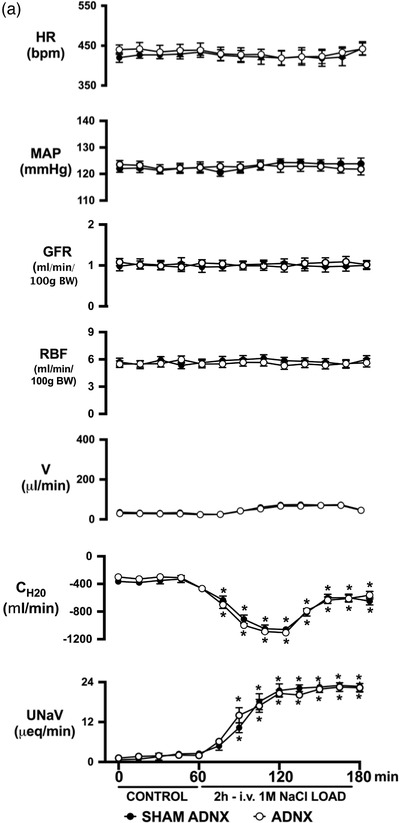
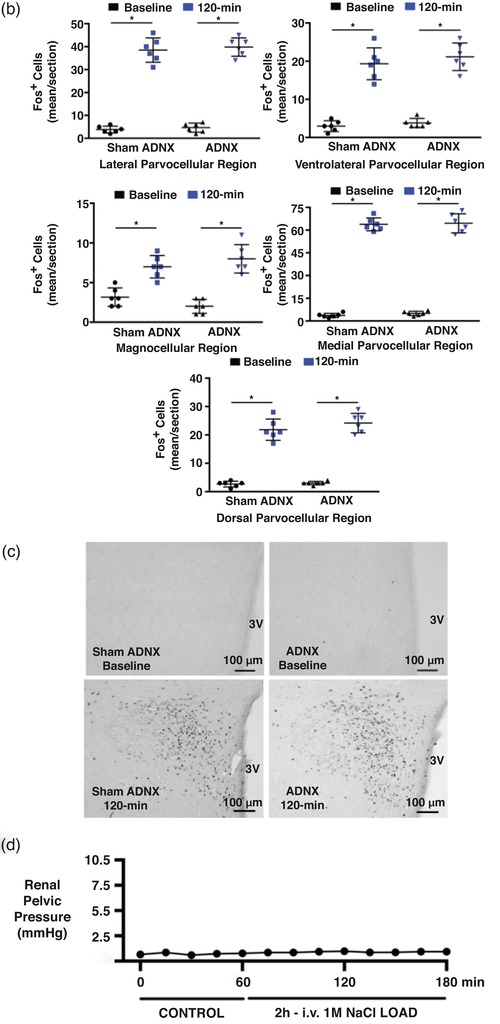
Conscious sham afferent renal denervated (ADNX) SD rats, in which surgery was performed but application of capsaicin was omitted to leave the ARNs intact, exhibited robust natriuretic and diuretic responses with a transient increase in free water clearance and no change in blood pressure, heart rate or renal haemodynamics (glomerular filtration rate, GFR, and renal plasma flow, RPF) during an acute VE (Figure 1a). The acute VE produced minimal increases in urine Na+ and osmolality (peak urine Na+ concentration 105 ± 8 mmol l−1, peak urine osmolality 299 ± 10 mosmol kg−1) and a significant increase in renal pelvic pressure (RPP) in anaesthetized naïve SD rats with intact ARNs (Figure 1d). Further, in sham ADNX rats an acute VE resulted in increased Fos staining in all PVN parvocellular and magnocellular subnuclei (Figure 1b,c).
Figure 1.
Effect of selective afferent renal nerve ablation on the cardiovascular, renal and PVN neuronal responses to an acute volume expansion. (a) Cardiovascular and renal responses to a 30 min 5% body weight isotonic saline volume expansion (VE) followed by a 90 min recovery period in conscious male Sprague–Dawley (SD) rats 10 days after a selective afferent renal nerve ablation (ADNX) or sham ADNX procedure. (b) Neuronal activation (c‐fos‐positive cell count) in the lateral parvocellular, ventrolateral parvocellular, magnocellular, medial parvocellular and dorsal parvocellular regions of the paraventricular nucleus (PVN) of the hypothalamus following VE or a 2 h surgical recovery and control period (baseline group) in conscious male SD rats 10 days after a sham or ADNX procedure. (c) Representative images from level 2 of the PVN. (d) Renal pelvic pressure in a separate group of anaesthetized male SD rats during a control period (denoted C), a 30 min 5% isotonic saline VE period and a 90 min recovery period. n = 6/group. *P < 0.05 vs. group baseline value, denoted C; †P < 0.05 vs. respective sham ADNX value. CH2O, free water clearance; GFR, glomerular filtration rate; HR, heart rate; MAP, mean arterial pressure; RPF, renal plasma flow; U Na V, urinary sodium excretion; V, urinary flow rate
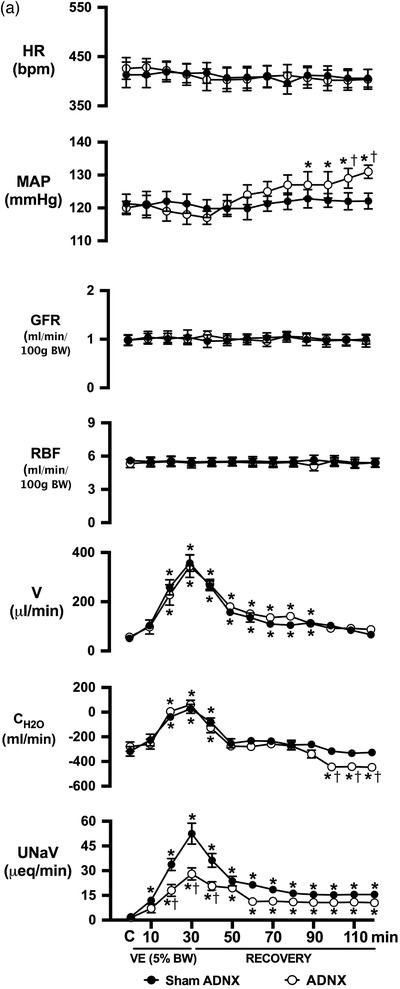
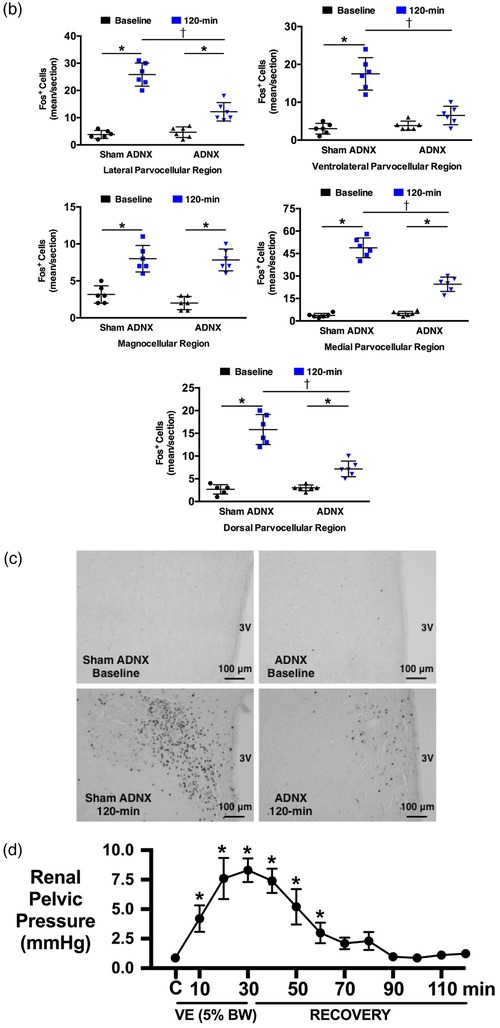
Selective ablation of the ARNs did not alter baseline cardiovascular or renal excretory parameters and had no impact on the baseline Fos staining in any region of the PVN (Figure 1a‐c). In ADNX rats, the natriuretic, but not diuretic or renal haemodynamic response, to the acute VE protocol was significantly blunted and blood pressure was increased (Figure 1a). Similarly, VE‐evoked Fos staining of PVN parvocellular neurons was attenuated in ADNX rats, while Fos activation in magnocellular PVN neurons remained intact (Figure 1b,c). The efficacy and selectivity of ARN ablation was confirmed by (1) the loss of blood pressure response to intra‐renal bradykinin infusion, and (2) the selective elimination of renal pelvic CGRP content, but not renal NA content (Figure 2).
3.2. The ARNs are not required for the cardiovascular, renal and PVN neuronal responses to an acute 1 m NaCl infusion
In conscious sham ADNX SD rats, acute 1 m NaCl infusion evoked a natriuretic response in the absence of diuresis, with a corresponding reduction in free water clearance, and had no impact on blood pressure, heart rate, GFR or RPF (Figure 3a). Further, the acute 1 m NaCl infusion evoked increases in urine Na+ and osmolality approximately 3 times greater than that observed during VE (peak urine Na+ concentration 270 ± 9 mmol l−1, peak urine osmolality 1167 ± 72 mosmol kg−1) and did not alter RPP in naïve intact anaesthetized rats (Figure 3d). Fos staining was increased in all parvocellular and magnocellular PVN regions of sham ADNX rats following infusions of 1 m NaCl (Figure 3b,c). Ablation of the ARNs did not alter cardiovascular, renal excretory or renal haemodynamic responses, or PVN Fos staining assoicated with 1 m NaCl infusion (Figure 3a–c).
Figure 3.
Effect of selective afferent renal nerve ablation on the cardiovascular, renal, and PVN neuronal responses to an acute 1 m NaCl infusion. (a) Cardiovascular and renal responses to a 2 h 1 m NaCl infusion in conscious male Sprague–Dawley (SD) rats 10 days after a selective afferent renal nerve ablation (ADNX) or sham ADNX procedure. (b) Neuronal activation (c‐fos‐positive cell count) assessed after a 120 min sodium load or a 2 h surgical recovery and control period (baseline group) in the lateral parvocellular, ventrolateral parvocellular, magnocellular, medial parvocellular and dorsal parvocellular regions of the paraventricular nucleus of the hypothalamus following 1 m NaCl infusion in conscious male SD rats 10 days after sham or ADNX procedure. (c) Representative images from level 2 of the PVN. (d) Renal pelvic pressure in a separate group of anaesthetized male SD rats during a 1 h control isotonic saline infusion (20µl min−1) and a 2 h 1 m NaCl infusion (20 µl min−1). n = 6/group. *P < 0.05 vs. group baseline value, denoted C. CH2O, free water clearance; GFR, glomerular filtration rate; HR, heart rate; MAP, mean arterial pressure; RPF, renal plasma flow; U Na V, urinary sodium excretion; V, urinary flow rate
3.3. High salt intake increases ARN responsiveness in salt‐resistant but not salt‐sensitive rat models
Ex vivo ARN release of substance P in response to NA stimulation was comparable among SD, DSR and DSS rats on a normal salt diet (Figure 4a). A 21 day high salt (HS) diet enhanced ex vivo NA‐evoked substance P release in salt‐resistant SD and DSR rats, a response that was absent in DSS rats (Figure 4a). In vivo, SD rats on a normal salt diet exhibit a natriuretic response to increased RPP (direct mechanoreceptor stimulus), and a 21 day HS diet dramatically enhanced the natriuretic response to graded increases in RPP in SD rats (Figure 4b,c). In contrast, increased RPP did not alter natriuresis in DSS rats fed a normal salt diet and a HS‐induced increase in natriuresis was only observed at the upper limit of the physiological range of increased RPP (Figure 4b,c).
Figure 4.
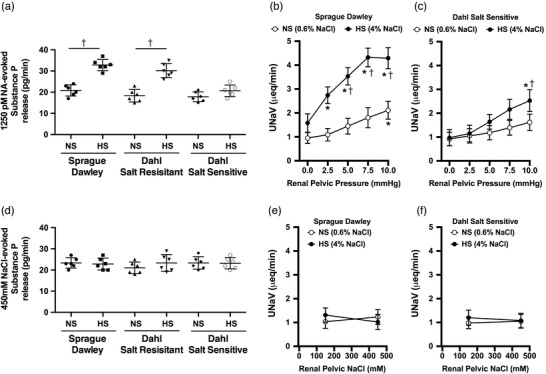
Effect of high salt intake on afferent renal nerve responsiveness. (a) Ex vivo renal pelvis substance P release (pg min−1) in response to 1250 pm noradrenaline (NA) on day 21 of a normal salt (NS; 0.6% NaCl) or high salt (HS; 4% NaCl) diet in male Sprague–Dawley (SD), Dahl salt‐resistant (DSR), and Dahl salt‐sensitive (DSS) rats. (b,c) Urinary sodium excretion (U Na V) in response to graded increases in renal pelvic pressure in male SD (b) and DSS (c) rats following a 21 day NS or HS diet. (d) Ex vivo renal pelvis substance P release in response to 450 mm NaCl on day 21 of a NS or HS diet in male SD, DSR and DSS rats. (e,f) Urinary sodium excretion in response to increased renal pelvic sodium concentration in male SD (e) and DSS (f) rats. n = 6/group. *P < 0.05 vs. group baseline U Na V with renal pelvic pressure at 0 mmHg, †P < 0.05 vs. respective NS group value
Ex vivo ARN release of substance P in response to 450 mm NaCl, a chemoreceptor stimulus, was similar in SD, DSR and DSS rats on a normal salt diet and the response was unaffected by a 21 day HS diet (Figure 4d). Further, the acute manipulation of renal pelvic sodium concentration did not alter natriuresis in SD or DSS rats regardless of dietary sodium intake (Figure 4e,f).
3.4. The ARNs are required for SD and DSR rats to maintain a salt‐resistant phenotype
In SD rats fed a normal salt diet, sham ADNX and ADNX did not impact blood pressure, urine volume, or sodium balance (Figure 5a–c). Similarly on day 21 of normal salt intake, markers of renal and global sympathetic tone and renin–angiotensin system activity were similar in sham ADNX and ADNX SD rats (Figure 5d–g). A 21 day HS diet did not alter blood pressure in sham ADNX SD and DSR rats, which possess intact ARNs (Figures 5a and 6a). In contrast, SD and DSR rats that underwent ADNX to remove the influence of the ARNs immediately prior to HS intake exhibited an increase in blood pressure during the first 3 days of HS intake that was maintained for the remainder of the 21 day experimental period (Figures 5a and 6a). These HS‐fed SD and DSR rats exhibited an increase in urine volume that was similar among sham and ADNX treatment groups (Figures 5b and 6b). Further, HS intake transiently increased daily sodium balance in sham ADNX SD and DSR rats, while this increase was significantly exacerbated in ADNX SD rats (Figures 5c and 6c). The 21 day HS diet evoked a reduction in plasma and renal NA content in sham ADNX SD rats and this reduction was abolished following ADNX (Figure 5d,e). In contrast, both sham ADNX and ADNX SD rats exhibited similar HS‐evoked reductions in plasma renin activity and urinary angiotensinogen (Figure 5f,g). Plasma and renal NA content were increased on day 21 of HS intake in ADNX DSR rats compared to sham ADNX (Figure 6d,e). In both sham and ADNX SD and DSR rats a HS intake did not alter plasma sodium (pNa) [pNa (mEq l−1) SD: NS Sham, 140 ± 1; HS Sham, 139 ± 1; NS ADNX, 139 ± 1; HS ADNX, 141 ± 1; DSR: NS Sham, 141 ± 1; HS Sham, 140 ± 1; NS ADNX, 141 ± 1; HS ADNX, 140 ± 2]. The efficacy of the ADNX procedure was confirmed in subgroups of rats via the absence of a pressor response to intrarenal bradykinin infusion and a significant reduction in renal pelvic CGRP content (Figure 7).
Figure 5.
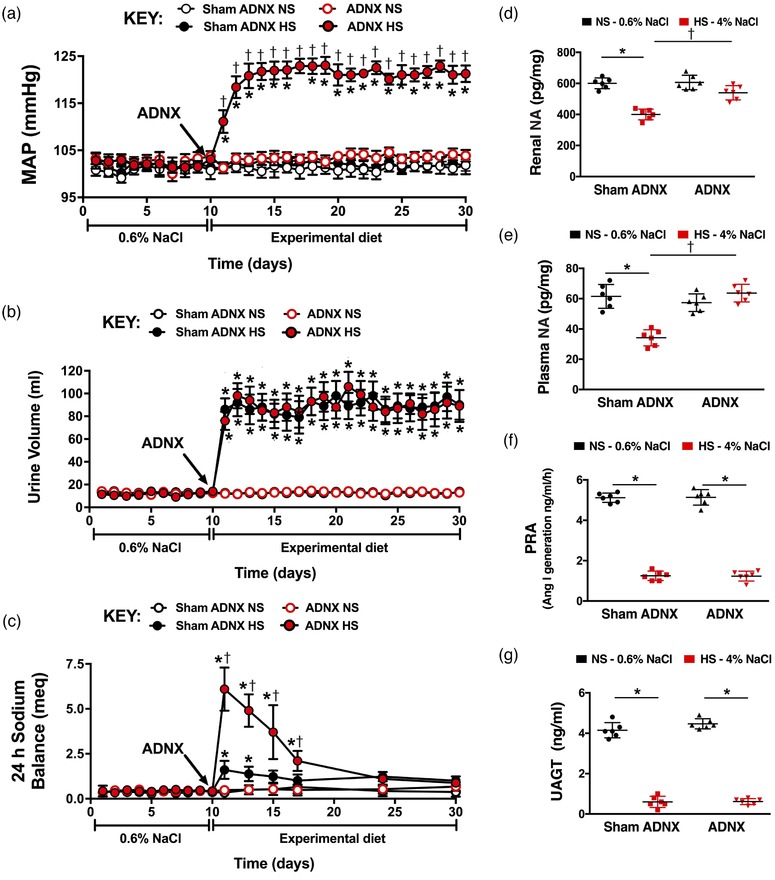
Effect of selective afferent renal nerve ablation on the cardiovascular, renal and sympathetic responses to high salt intake in Sprague–Dawley rats. (a–c) Daily mean arterial pressure (MAP) (a), 24 h urine volume (b), and 24 h sodium balance (meq) (c) in male Sprague–Dawley rats that underwent sham ADNX or ADNX immediately prior to a 21 day experimental normal salt (NS; 0.6% NaCl) or high salt (HS; 4% NaCl) diet. (d–g) Renal noradrenaline (NA) content (d), plasma NA concentration (e), plasma renin activity (PRA; angiotensin I (ang I) generation) (f) and urinary angiotensinogen (UAGT) (g) on day 21 of NS or HS intake. n = 6/group. *P < 0.05 vs. baseline (NS intake); †P < 0.05 vs. respective sham ADNX group
Figure 6.
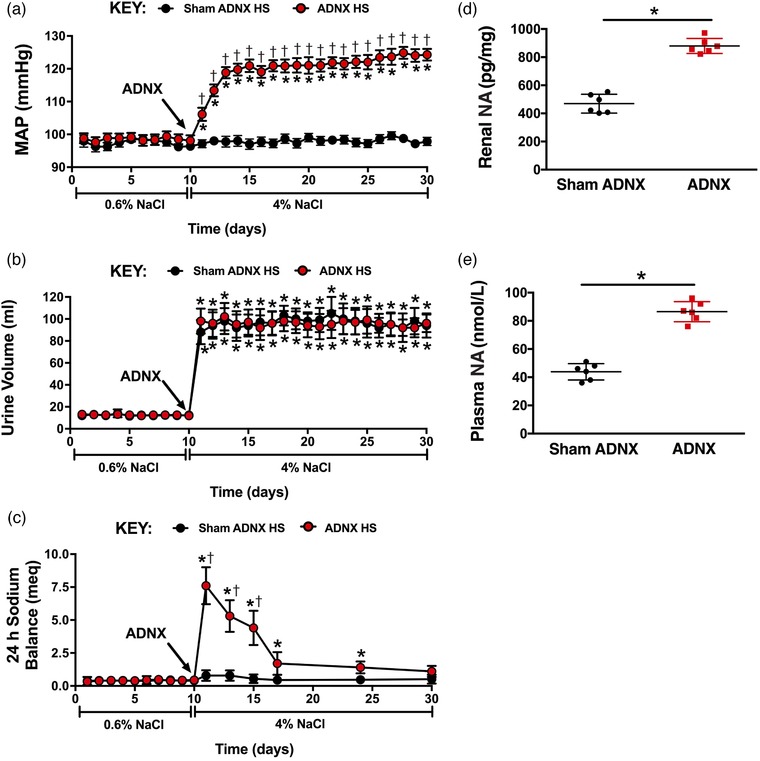
Effect of selective afferent renal nerve ablation on the cardiovascular, renal and sympathetic responses to high salt intake in Dahl salt‐resistant rats. (a–c) Daily mean arterial pressure (MAP) (a), 24 h urine volume (b), and 24 h sodium balance (c) in male Dahl salt‐resistant rats that underwent sham ADNX or ADNX immediately prior to a 21 day experimental high salt (HS; 4% NaCl) diet. (d,e) Renal noradrenaline (NA) content (d) and plasma NA concentration (e) on day 21 of HS intake. n = 6/group. *P < 0.05 vs. baseline (NS intake); †P < 0.05 vs. sham ADNX
3.5. The ARNs attenuate the development of DSS hypertension
Sham ADNX DSS rats placed on a HS diet exhibited a significant increase in blood pressure by day 7 of HS intake (Figure 8a). ARN ablation immediately prior to HS intake exacerbated the increase in blood pressure (Figure 8a). Sham and ADNX DSS rats exhibited similar increases in urine output during HS intake (Figure 8b), with an increase in 24 h sodium balance that was exacerbated in ADNX rats (Figure 8c). Consistent with increased sympathetic tone, plasma and renal NA content was increased in ADNX vs. sham ADNX DSS rats (Figure 8d‐e). In DSS rats HS intake did not alter plasma sodium (pNa) irrespective of sham or ADNX surgery [pNa (mEq l−1), DSS: NS Sham, 141 ± 2; Sham HS, 141 ± 1; NS ADNX, 140 ± 1; HS ADNX, 139 ± 2]. ARN ablation was confirmed via a reduction of renal pelvic CGRP content in all rats (Figure 7). Further, ARN ablation conducted after 21 days’ HS intake in DSS rats with established hypertension had no impact on blood pressure (Figure 9).
Figure 8.
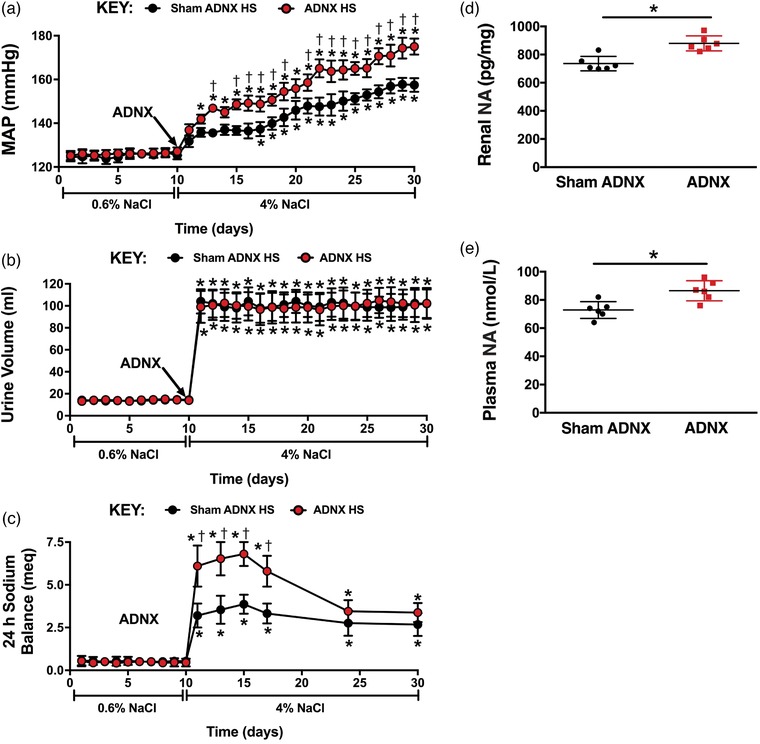
Effect of selective afferent renal nerve ablation on the cardiovascular, renal and sympathetic responses to high salt intake in Dahl salt‐sensitive rats. (a–c) Daily mean arterial pressure (MAP) (a), 24 h urine volume (b), and 24 h sodium balance (c) in male Dahl salt‐sensitive rats that underwent sham ADNX or ADNX immediately prior to a 21 day experimental high salt (HS; 4% NaCl) diet. (d,e) Renal noradrenaline (NA) content (d) and plasma NA concentration (e) on day 21 of HS intake. n = 6/group. *P < 0.05 vs. baseline (NS intake); †P < 0.05 vs. sham ADNX
Figure 9.
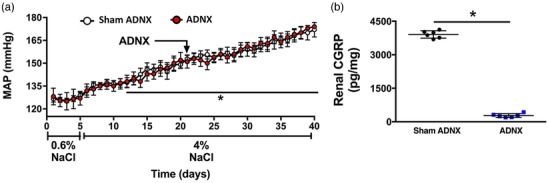
Effect of selective afferent renal nerve ablation on blood pressure in Dahl salt‐sensitive rats with established hypertension. (a) Blood pressure in radiotelemetered Dahl salt‐sensitive (DSS) rats that underwent selective afferent renal nerve ablation (ADNX) after 16 days of high salt (4% NaCl) intake. (b) Renal CGRP content on day 40 of high salt intake. *P < 0.05 vs. baseline, †P < 0.05 vs. sham ADNX
4. DISCUSSION
Our initial studies were designed to determine the role(s) of the sensory ARNs in the regulation of acute natriuresis in conscious normotensive SD rats. Our experimental approach, which employed an acute VE to increase RPP to activate mechanosensitive sensory ARNs supports previous work demonstrating that (1) ARN mechanoreceptors that selectively respond to increased RPP also respond to intravenous VE (Chien et al., 2000), and (2) increased RPP in the range seen during acute VE activates the ARNs (Kopp et al., 1994). Consistent with multiple prior studies demonstrating an acute VE activates sympathoinhibitory parvocellular PVN neurons, (Haselton et al., 1994; Ng, De Matteo, & Badoer, 2004; Randolph et al., 1998) and a robust natriuretic response (Haselton et al., 1994; Ng et al., 2004), we observed an increase in Fos‐positive cell bodies in all parvocellular PVN subnuclei and a strong natriuretic response with robust diuresis and increased free water clearance in sham ADNX SD rats. Capsaicin‐mediated ARN ablation blunted VE‐evoked parvocellular, but not magnocellular, PVN neuronal activation and natriuresis, suggesting a specific role for the ARNs in VE‐evoked parvocellular PVN Fos induction and subsequent natriuresis.
The PVN has been previously implicated as a site of integration for the sympathoexcitatory reno‐renal reflex (Xu et al., 2015). However, our data functionally link mechanosensitive ARNs to the PVN and the sympathoinhibitory reno‐renal reflex. As VE stimulates non‐renal sensory afferent signaling pathways to activate parvocellular PVN neurons (Pyner, Deering, & Coote, 2002), it is possible these non‐renal pathways contribute to the residual VE‐evoked parvocellular PVN neuronal Fos induction in ADNX rats. While our Fos immunostaining and physiological findings clearly suggest that ADNX reduces activation of PVN neurons, we acknowledge that Fos staining does not always equate to neuron activation (Dampney & Horiuchi, 2003) and that our data do not confirm these PVN neurons are functionally activated or sympathoinhibitory in nature.
To assess the role of the chemosensitive ARNs, which respond to changes in the chemical composition of fluid in the renal pelvis including alterations in sodium concentration, we employed a 1 m NaCl infusion (Wainford et al., 2013). In the present studies, we did not assess changes in plasma sodium during a 1 m NaCl infusion, but based on our prior study showing no change in plasma sodium during this challenge (Wainford et al., 2013), we speculate that plasma sodium remained unaltered during the course of this experiment. While a 1 m NaCl infusion has central effects, the data suggest that it did not activate ARN mechanoreceptors as RPP and urine output remained unchanged. Our data demonstrate selective ARN ablation does not alter 1 m NaCl‐evoked natriuresis or PVN parvocellular and magnocellular neuronal activation. This is consistent with our previous finding that the natriuretic response to 1 m NaCl infusion remains intact following bilateral renal denervation (Wainford et al., 2013). These data suggest that mechanisms independent of the chemosensitive and mechanosensitive ARNs, potentially other sodium/osmosensitive visceral afferents or circumventricular organs (Kinsman, Simmonds, Browning, & Stocker, 2017), mediate 1 m NaCl‐evoked PVN neuron activation and natriuresis. A limitation of our acute in vivo studies is that baseline blood pressure is mildly elevated compared to that observed in radiotelemetered rats. However, these values are consistent with previous studies and baseline pressures are identical between sham and ADNX treatment groups. As such, we believe this limitation has not adversely impacted the current data. Further, while ADNX was validated in vivo via renal artery bradykinin infusion, this approach preferentially assesses the function of renal cortical afferents, which are likely sympathoexcitatory, rather than those terminating in the renal pelvis, which are likely sympathoinhibitory. However, combined with the striking reduction in renal pelvic CGRP following ADNX, our data suggest that local application of capsaicin to the renal nerves is a valid ablation approach that prevents all ARN signalling. Additionally, owing to our aim to examine ARN integration and neuronal activation in the PVN, direct ARN recording, which requires cutting of the nerve and prevention of an ARN signal reaching the CNS, was not performed. As such, future studies, outside the focus of the current work, may be conducted to directly assess afferent and efferent renal nerve activity during acute in vivo physiological challenges.
ARN responsiveness, assessed as substance P release, to a general ARN stimulus (noradrenaline – acts at α1‐adrenoceptors to promote ARN activity) (Kopp et al., 2007) is enhanced by a 21 day HS diet in normotensive salt‐resistant SD and DSR rats but not in hypertensive DSS rats. This suggests that enhanced ARN responsiveness during HS intake may be protective and critical for salt resistance. Significantly, a HS diet has no impact on ARN responsiveness to a direct chemoreceptor stimulus (450 mm NaCl/900 mosmol L−1 – the upper range of urinary sodium content) in either salt‐resistant or salt‐sensitive rats. This preparation potentially exposes regions of the isolated pelvis to supra‐physiological NaCl concentrations. However, given that this represents a concentration that occurs physiologically within the renal pelvis and that substance P release remained similar to baseline levels following a 5 min NaCl exposure, we believe this represents a validated approach to test renal chemoreceptor responses. This suggests that enhanced responsiveness to noradrenaline may reflect a selective increase in the responsiveness of the mechanosensitive ARNs during HS intake. Despite the lack of an enhanced response to a HS diet, our data indicate that the ARNs in DSS rats are fully responsive to direct ARN stimuli and may be playing a functional role during normal salt intake. The results also indicate that a HS diet significantly enhances the natriuretic response to increased RPP, which activates the mechanosensitive ARNs (Kopp et al., 2003; Ma et al., 2002a; Ma, Huang, Wu, Chien, & Chen, 2002b), across a full physiological range in SD rats, while in DSS rats RPP‐evoked natriuresis is enhanced only near the upper physiological limit. Consistent with a specific role for the mechanosensitive ARNs in salt resistance, HS intake had no impact on the natriuretic response to a chemoreceptor specific stimulus (Ma et al., 2002a,b) in SD or DSS rats. Given the identical responses observed in the ex vivo renal pelvis preparation in SD and DSR rats, we elected to conduct anaesthetized in vivo renal pelvic studies only in SD and DSS rats. Our in vivo findings, in concert with our ex vivo data, strongly suggest the mechanosensitive sympathoinhibitory ARN reno‐renal reflex is selectively enhanced during HS intake in salt‐resistant rats. In the in vivo renal pelvic chemoreceptor assay, we exclusively manipulate renal pelvic NaCl concentration and not the concentration of other solutes that influence overall pelvic interstitial osmolarity (e.g. urea). As such, there is the potential this resulted in a hypo‐osmotic infusion. However, given the small infusion volume and rate that remained constant throughout the study, and the acute (10 min) nature of the challenge, we believe that this represents a valid chemoreceptor challenge that is not compromised by alterations in renal pelvic osmolarity.
In salt‐resistant SD and DSR rats, removal of the ARNs abolished HS‐evoked global and renal sympathoinhibition and induced salt‐sensitive hypertension. Immediately upon HS intake there was a rapid and transient increase in sodium balance and blood pressure, which remained elevated over the 21 day protocol, suggesting resetting of the set‐point blood pressure to facilitate pressure natriuresis and sodium homeostasis. Based on our acute VE data, ex vivo and in vivo pelvic studies and evidence that there was no change in plasma sodium during HS intake following ARN ablation coupled with a dramatic increase in urine output to approximately 90 ml day−1 – a level that increases RPP to a level that would activate mechanosensitive ARNs – we believe the development of salt‐sensitive hypertension reflects the loss of a protective mechanosensitive ARN reflex. This hypothesis is supported by evidence that attenuated sympathoinhibitory ARN reno‐renal reflex activity correlates with enhanced renal sympathetic outflow in the spontaneously hypertensive rat (Kopp, Smith, & DiBona, 1987). It should be noted that our observations contrast with prior work suggesting the ARNs have no impact on blood pressure during stepped increases in dietary sodium intake that concluded 7 weeks post‐ADNX in SD rats (Foss et al., 2015). However, in the prior study (Foss et al., 2015), (1) ARN‐independent adaptive mechanisms could have been activated to facilitate sodium homeostasis and normotension and (2) physical ARN re‐innervation occurred but was not functionally assessed. To address the potential confounding effects of non‐ARN‐mediated compensatory mechanisms and functional ARN reinnervation, ADNX was performed immediately prior to the start of HS intake, and absence of a pressor response to bradykinin and significantly reduced renal pelvic CGRP was confirmed 21 days post‐ADNX. Our data, validated in the DSR phenotype, suggest that removal of the ARN may be a causal mechanism in the salt sensitivity of blood pressure observed in prior studies following generalized sensory afferent denervation via dorsal rhizotomy or subcutaneous capsaicin treatment in SD rats (Kopp et al., 2003; Wang et al., 1998, 2001).
Intriguingly, our data in DSS rats suggest that the function of the ARNs can change during hypertension. ADNX immediately prior to HS intake, a time point at which our ex vivo data demonstrate the ARNs are functional, evokes a more severe blood pressure phenotype and exacerbated sympathoexcitation, suggesting that the sympathoinhibitory ARN reno‐renal reflex has a minor role in countering the development of DSS hypertension. In contrast ADNX during established DSS hypertension did not impact blood pressure (Figure 9), confirming prior studies that reported the ARNs do not play a role in the maintenance of early or late phase DSS hypertension (Foss, Fink, & Osborn, 2016).
Collectively, these data indicate that the mechanosensitive ARN‐mediated sympathoinhibitory reno‐renal reflex contributes to homeostatic responses to acute sodium challenge in healthy normotensive SD rats. These data also provide evidence that the PVN can be regulated by mechanosensitive ARN stimulation and may be a critical site of central integration of the acute sympathoinhibitory reno‐renal reflex. Additionally, our data support a central role for the mechanosensitive ARN sympathoinhibitory reno‐renal reflex contributing to salt resistance by facilitating sympathoinhibition and sodium balance. Further, our data add to a growing body of literature indicating that variability in ARN activity and function could influence the efficacy of bilateral renal nerve ablation as an anti‐hypertensive intervention (Fudim et al., 2018). We speculate that renal nerve ablation in hypertensive individuals in whom the sympathoinhibitory reno‐renal reflex is intact would remove an essential natriuretic pathway, potentially exacerbating hypertension. As such, the development of diagnostic tests to assess sympathoinhibitory reno‐renal reflexes could guide patient selection for phenotypically targeted renal nerve ablation.
COMPETING INTERESTS
There are no competing interests for any author.
AUTHOR CONTRIBUTIONS
A.A.F, C.Y.C., J.T.C. and R.D.W. conceived and designed research; A.A.F, C.Y.C., J.T.K., J.T.C. and R.D.W performed experiments; A.A.F, C.Y.C., J.T.C. and R.D.W JDM, PC, and RDW analysed data; A.A.F, C.Y.C., J.T.C. and R.D.W interpreted results of experiments; A.A.F. and R.D.W prepared figures; A.A.F, J.T.C. and R.D.W drafted manuscript; A.A.F, J.T.C. and R.D.W edited and revised manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Frame AA, Carmichael CY, Kuwabara JT, Cunningham JT, Wainford RD. Role of the afferent renal nerves in sodium homeostasis and blood pressure regulation in rats. Experimental Physiology. 2019;104:1306–1323. 10.1113/EP087700
Funding information
This work was supported by NIH R56 AG057687, R01 HL139867, R01 HL141406, R01 HL107330 and K02 HL112718 and AHA 16MM32090001 and 17GRNT33670023 to R.D.W. and NIH F31 DK116501 to A.A.F. The work of J.T.C. was supported by NIH R01 HL141406 to R.D.W.
Edited by: Kate Denton
REFERENCES
- Appel, L. J. , Frohlich, E. D. , Hall, J. E. , Pearson, T. A. , Sacco, R. L. , Seals, D. R. , … Van Horn, L. V. (2011). The importance of population‐wide sodium reduction as a means to prevent cardiovascular disease and stroke: A call to action from the American Heart Association. Circulation, 123, 1138–1143. 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- Barry, E. F. , & Johns, E. J. (2015). Intrarenal bradykinin elicits reno‐renal reflex sympatho‐excitation and renal nerve‐dependent fluid retention. Acta Physiologica, 213, 731–739. 10.1111/apha.12420 [DOI] [PubMed] [Google Scholar]
- Brooks, V. L. , Haywood, J. R. , & Johnson, A. K. (2005). Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clinical and Experimental Pharmacology & Physiology, 32, 426–432. 10.1111/j.1440-1681.2005.04206.x [DOI] [PubMed] [Google Scholar]
- Brouwers, S. , Smolders, I. , Wainford, R. D. , & Dupont, A. G. (2015). Hypotensive and sympathoinhibitory responses to selective central AT2 receptor stimulation in spontaneously hypertensive rats. Clinical Science, 129, 81–92. 10.1042/CS20140776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael, C. Y. , Carmichael, A. C. , Kuwabara, J. T. , Cunningham, J. T. , & Wainford, R. D. (2016). Impaired sodium‐evoked paraventricular nucleus neuronal activation and blood pressure regulation in conscious Sprague‐Dawley rats lacking central Gαi2 proteins. Acta Physiologica, 216, 314–329. 10.1111/apha.12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka, L. , Wang, C. T. , & Navar, L. G. (1998). Effects of acute AT1 receptor blockade by candesartan on arterial pressure and renal function in rats. American Journal of Physiology, 274, F940–F945. 10.1152/ajprenal.1998.274.5.F940 [DOI] [PubMed] [Google Scholar]
- Chien, C. T. , Chien, H. F. , Cheng, Y. J. , Chen, C. F. , & Hsu, S. M. (2000). Renal afferent signaling diuretic response is impaired in streptozotocin‐induced diabetic rats. Kidney International, 57, 203–214. 10.1046/j.1523-1755.2000.00826.x [DOI] [PubMed] [Google Scholar]
- Dampney, R. A. , & Horiuchi, J. (2003). Functional organisation of central cardiovascular pathways: Studies using c‐fos gene expression. Progress in Neurobiology, 71, 359–384. 10.1016/j.pneurobio.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Evans, R. G. , & Bie, P. (2016). Role of the kidney in the pathogenesis of hypertension: Time for a neo‐Guytonian paradigm or a paradigm shift? American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 310, R217–R229. 10.1152/ajpregu.00254.2015 [DOI] [PubMed] [Google Scholar]
- Foss, J. D. , Fink, G. D. , & Osborn, J. W. (2016). Differential role of afferent and efferent renal nerves in the maintenance of early‐ and late‐phase Dahl S hypertension. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 310, R262–R267. 10.1152/ajpregu.00408.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss, J. D. , Wainford, R. D. , Engeland, W. C. , Fink, G. D. , & Osborn, J. W. (2015). A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 308, R112–122. 10.1152/ajpregu.00427.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, V. , & Oparil, S. (2006). Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. Journal of the American College of Nutrition, 25, 247S–255S. 10.1080/07315724.2006.10719574 [DOI] [PubMed] [Google Scholar]
- Fudim, M. , Sobotka, A. A. , Yin, Y. H. , Wang, J. W. , Levin, H. , Esler, M. , … Sobotka, P. A. (2018). Selective vs. global renal denervation: A case for less is more. Current Hypertension Reports, 20, 37 10.1007/s11906-018-0838-2. [DOI] [PubMed] [Google Scholar]
- Haselton, J. R. , Goering, J. , & Patel, K. P. (1994). Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. Journal of the Autonomic Nervous System, 50, 1–11. 10.1016/0165-1838(94)90117-1 [DOI] [PubMed] [Google Scholar]
- Johns, E. J. (2014). The neural regulation of the kidney in hypertension and renal failure. Experimental Physiology, 99, 289–294. 10.1113/expphysiol.2013.072686. [DOI] [PubMed] [Google Scholar]
- Johns, E. J. , & Abdulla, M. H. (2013). Renal nerves in blood pressure regulation. Current Opinion in Nephrology and Hypertension, 22, 504–510. 10.1097/MNH.0b013e3283641a89. [DOI] [PubMed] [Google Scholar]
- Kapusta, D. R. , Pascale, C. L. , & Wainford, R. D. (2012). Brain heterotrimeric Gαi2‐subunit protein‐gated pathways mediate central sympathoinhibition to maintain fluid and electrolyte homeostasis during stress. FASEB Journal, 26, 2776–2787. 10.1096/fj.11-196550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsman, B. J. , Simmonds, S. S. , Browning, K. N. , & Stocker, S. D. (2017). Organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension, 69, 163–170. 10.1161/HYPERTENSIONAHA.116.08372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompanowska‐Jezierska, E. , Wolff, H. , Kuczeriszka, M. , Gramsbergen, J. B. , Walkowska, A. , Johns, E. J. , & Bie, P. (2008). Renal nerves and nNOS: Roles in natriuresis of acute isovolumetric sodium loading in conscious rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 294, R1130–R1139. 10.1152/ajpregu.00908.2007. [DOI] [PubMed] [Google Scholar]
- Kopp, U. C. (1993). Renorenal reflexes in hypertension. Journal of Hypertension, 11, 765–773. [DOI] [PubMed] [Google Scholar]
- Kopp, U. C. (2015). Role of renal sensory nerves in physiological and pathophysiological conditions. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 308, R79–R95. 10.1152/ajpregu.00351.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp, U. C. , Cicha, M. Z. , & Smith, L. A. (2003). Dietary sodium loading increases arterial pressure in afferent renal‐denervated rats. Hypertension, 42, 968–973. 10.1161/01.HYP.0000097549.70134.D8 [DOI] [PubMed] [Google Scholar]
- Kopp, U. C. , Cicha, M. Z. , & Smith, L. A. (2006). Differential effects of endothelin on activation of renal mechanosensory nerves: Stimulatory in high‐sodium diet and inhibitory in low‐sodium diet. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 291, R1545–R1556. 10.1152/ajpregu.00878.2005. [DOI] [PubMed] [Google Scholar]
- Kopp, U. C. , Cicha, M. Z. , Smith, L. A. , Mulder, J. , & Hokfelt, T. (2007). Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2‐dependent activation of α1‐ and α2‐adrenoceptors on renal sensory nerve fibers. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 293, R1561–R1572. 10.1152/ajpregu.00485.2007 [DOI] [PubMed] [Google Scholar]
- Kopp, U. C. , Cicha, M. Z. , Smith, L. A. , Ruohonen, S. , Scheinin, M. , Fritz, N. , & Hokfelt, T. (2011). Dietary sodium modulates the interaction between efferent and afferent renal nerve activity by altering activation of α2‐adrenoceptors on renal sensory nerves. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 300, R298–R310. 10.1152/ajpregu.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp, U. C. , Grisk, O. , Cicha, M. Z. , Smith, L. A. , Steinbach, A. , Schluter, T. , … Hokfelt, T. (2009). Dietary sodium modulates the interaction between efferent renal sympathetic nerve activity and afferent renal nerve activity: Role of endothelin. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 297, R337–R351. 10.1152/ajpregu.91029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp, U. C. , Smith, L. A. , & DiBona, G. F. (1987). Impaired renorenal reflexes in spontaneously hypertensive rats. Hypertension, 9, 69–75. 10.1161/01.HYP.9.1.69 [DOI] [PubMed] [Google Scholar]
- Kopp, U. C. , Smith, L. A. , & Pence, A. L. (1994). Na+‐K+‐ATPase inhibition sensitizes renal mechanoreceptors activated by increases in renal pelvic pressure. American Journal of Physiology, 267, R1109–R1117. 10.1152/ajpregu.1994.267.4.R1109 [DOI] [PubMed] [Google Scholar]
- Lin, C. S. , Lee, S. H. , Huang, H. S. , Chen, Y. S. , & Ma, M. C. (2015). H2O2 generated by NADPH oxidase 4 contributes to transient receptor potential vanilloid 1 channel‐mediated mechanosensation in the rat kidney. American Journal of Physiology. Renal Physiology, 309, F369–F376. 10.1152/ajprenal.00462.2014. [DOI] [PubMed] [Google Scholar]
- Lohmeier, T. E. , Hildebrandt, D. A. , & Hood, W. A. (1999). Renal nerves promote sodium excretion during long‐term increases in salt intake. Hypertension, 33, 487–492. 10.1161/01.HYP.33.1.487. [DOI] [PubMed] [Google Scholar]
- Ma, M. C. , Huang, H. S. , & Chen, C. F. (2002a). Impaired renal sensory responses after unilateral ureteral obstruction in the rat. Journal of the American Society of Nephrology, 13, 1008–1016. [DOI] [PubMed] [Google Scholar]
- Ma, M. C. , Huang, H. S. , Wu, M. S. , Chien, C. T. , & Chen, C. F. (2002b). Impaired renal sensory responses after renal ischemia in the rat. Journal of the American Society of Nephrology, 13, 1872–1883. 10.1097/01.ASN.0000022009.44473.56 [DOI] [PubMed] [Google Scholar]
- Ng, C. W. , De Matteo, R. , & Badoer, E. (2004). Effect of muscimol and L‐NAME in the PVN on the RSNA response to volume expansion in conscious rabbits. American Journal of Physiology. Renal Physiology, 287, F739–F746. 10.1152/ajprenal.00431.2003 [DOI] [PubMed] [Google Scholar]
- Pyner, S. , Deering, J. , & Coote, J. H. (2002). Right atrial stretch induces renal nerve inhibition and c‐fos expression in parvocellular neurones of the paraventricular nucleus in rats. Experimental Physiology, 87, 25–32. 10.1113/eph8702279 [DOI] [PubMed] [Google Scholar]
- Randolph, R. R. , Li, Q. , Curtis, K. S. , Sullivan, M. J. , & Cunningham, J. T. (1998). Fos expression following isotonic volume expansion of the unanesthetized male rat. American Journal of Physiology, 274, R1345–R1352. 10.1152/ajpregu.1998.274.5.R1345 [DOI] [PubMed] [Google Scholar]
- Solano‐Flores, L. P. , Rosas‐Arellano, M. P. , & Ciriello, J. (1997). Fos induction in central structures after afferent renal nerve stimulation. Brain Research, 753, 102–119. 10.1016/S0006-8993(96)01497-7 [DOI] [PubMed] [Google Scholar]
- Stocker, S. D. , Monahan, K. D. , & Browning, K. N. (2013). Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Current Hypertension Reports, 15, 538–546. 10.1007/s11906-013-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainford, R. D. , Carmichael, C. Y. , Pascale, C. L. , & Kuwabara, J. T. (2015). Gαi2‐protein‐mediated signal transduction: Central nervous system molecular mechanism countering the development of sodium‐dependent hypertension. Hypertension, 65, 178–186. 10.1161/HYPERTENSIONAHA.114.04463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainford, R. D. , & Kapusta, D. R. (2010). Hypothalamic paraventricular nucleus Gαq subunit protein pathways mediate vasopressin dysregulation and fluid retention in salt‐sensitive rats. Endocrinology, 151, 5403–5414. 10.1210/en.2010-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainford, R. D. , Pascale, C. L. , & Kuwabara, J. T. (2013). Brain Gαi2‐subunit protein‐gated pathways are required to mediate the centrally evoked sympathoinhibitory mechanisms activated to maintain sodium homeostasis. Journal of Hypertension, 31, 747–757. 10.1097/HJH.0b013e32835ebd54. [DOI] [PubMed] [Google Scholar]
- Walsh, K. R. , Kuwabara, J. T. , Shim, J. W. , & Wainford, R. D. (2016). Norepinephrine‐evoked salt‐sensitive hypertension requires impaired renal sodium chloride cotransporter activity in Sprague‐Dawley rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 310, R115–R124. 10.1152/ajpregu.00514.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. H. , Li, J. , & Qiu, J. (1998). Salt‐sensitive hypertension induced by sensory denervation: Introduction of a new model. Hypertension, 32, 649–653. 10.1161/01.HYP.32.4.649 [DOI] [PubMed] [Google Scholar]
- Wang, D. H. , Wu, W. , & Lookingland, K. J. (2001). Degeneration of capsaicin‐sensitive sensory nerves leads to increased salt sensitivity through enhancement of sympathoexcitatory response. Hypertension, 37, 440–443. [DOI] [PubMed] [Google Scholar]
- Watson, R. E., Jr , Wiegand, S. J. , Clough, R. W. , & Hoffman, G. E. (1986). Use of cryoprotectant to maintain long‐term peptide immunoreactivity and tissue morphology. Peptides, 7, 155–159. 10.1016/0196-9781(86)90076-8 [DOI] [PubMed] [Google Scholar]
- Xu, B. , Zheng, H. , Liu, X. , & Patel, K. P. (2015). Activation of afferent renal nerves modulates RVLM‐projecting PVN neurons. American Journal of Physiology. Heart and Circulatory Physiology, 308, H1103–1111. 10.1152/ajpheart.00862.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


