Abstract
Novel materials with unique or enhanced properties relative to conventional materials are being developed at an increasing rate. These materials are often referred to as advanced materials (AdMs) and they enable technological innovations that can benefit society. Despite their benefits, however, the unique characteristics of many AdMs, including many nanomaterials, are poorly understood and may pose environmental safety and occupational health (ESOH) risks that are not readily determined by traditional risk assessment methods. To assess these risks while keeping up with the pace of development, technology developers and risk assessors frequently employ risk-screening methods that depend on a clear definition for the materials that are to be assessed (e.g., engineered nanomaterial) as well as a method for binning materials into categories for ESOH risk prioritization. The term advanced material lacks a consensus definition and associated categorization or grouping system for risk screening. In this study, we aim to establish a practitioner-driven definition for AdMs and a practitioner-validated framework for categorizing AdMs into conceptual groupings based on material characteristics. Results from multiple workshops and interviews with practitioners provide consistent differentiation between AdMs and conventional materials, offer functional nomenclature for application science, and provide utility for future ESOH risk assessment prioritization. The definition and categorization framework established here serve as a first step in determining if and when there is a need for specific ESOH and regulatory screening for an AdM as well as the type and extent of risk-related information that should be collected or generated for AdMs and AdM-enabled technologies.
Keywords: Advanced materials, definition and categorization, environmental safety and occupational health, grouping, risk prioritization
1. INTRODUCTION
Emerging technologies are being developed at a rapid pace as technology developers continue to discover and use materials that exhibit novel or enhanced properties that improve performance over conventional products and processes. These innovative materials are often referred to as advanced materials (AdMs), a term that has been in use for over three decades (Advanced Materials Scientific Journal, 2018) but has become more common in recent years as a descriptor for nano-enabled materials. However, the field of environmental safety and occupational health (ESOH) has no unified process or protocol for describing these materials, nor determining the necessity for risk characterization of such AdMs with emergent properties. In many ways, the challenge of defining AdMs is similar to the challenge of defining nanomaterials. In the case of nanomaterials, we know that a definition based on size-dependent unique properties has proven to be a functional, albeit imperfect, strategy to address ESOH concerns (EU Commission, 2011; Hill, Kennedy, Warner, & Hull, 2018; Hull, 2017). However, all definitions of nanomaterials fail to adequately cover the gamut of AdMs that exhibit novel or enhanced properties that are not dependent on the consensus definition of nanoscale size (1–100 nm). Furthermore, outside of an expected increase in surface area that accompanies shrinking particle size (assuming mass is kept constant), many nanoscale materials do not exhibit novel or enhanced properties relative to their bulk form. The term advanced material resolves this issue by encapsulating the subset of engineered nanomaterials (ENMs) that demonstrate unique behaviors attributable to a size dimension in the nanoscale (1–100 nm) (U.S. National Nanotechnology Initiative [U.S. NNI], 2018), as well as the broader set of materials that derive novel or enhanced properties from size-independent characteristics. However, this term is not adequately defined to allow grouping of materials into categories for technology development or for ESOH risk prioritization, as illustrated by comprehensive efforts to group ENMs beyond their simple nanoscale definition in context of risk assessment (e.g., Arts et al., 2015).
While AdMs are imperative to numerous technological innovations that offer clear benefits to society, the unique characteristics of many AdMs are poorly understood and may pose potential ESOH risks (National Research Council, 2012). For example, ENMs have demonstrated significant benefits in several applications (Dang, Zhang, Fan, Chen, & Roco, 2010; Khot, Sankaran, Maja, Ehsani, & Schuster, 2012; Pelaz et al., 2017; Zhang, Uchaker, Candelaria, & Cao, 2013), but some forms have also been linked to potential adverse health outcomes in humans and animals (Coll et al., 2016; Hansen, Jensen, & Baun, 2014; Rycroft, Trump, Poinsatte-Jones, & Linkov, 2018; Shvedova, Pietroiusti, & Kagan, 2016). Often, these ENM hazards could not be predicted despite an in-depth understanding of the bulk (or dissolved) counterpart’s toxicological profile.
A similar challenge with AdMs beyond the 1–100 nm scale is that hazards cannot always be predicted using surrogates, as these proxies are not entirely indicative of an AdM’s potential behavior. In order to fully and accurately characterize the risk of AdMs throughout their lifecycles, a detailed risk assessment is required for each specific material (or class of material) and intended use scenario. Such a timeand resource-intensive effort, however, is not practical given the rapid increase in the number of AdM-enabled applications. Additionally, an in-depth risk assessment may not be necessary for all AdMs and use scenarios, since many are relatively benign, may not exhibit novel properties after transformation in the environment, or will not result in exposure and therefore pose a low ESOH risk (Collier et al., 2015; Malloy et al., 2017). As a substitute, when an in-depth risk assessment cannot be performed due to resource constraints and there is inadequate ESOH information to make risk-informed decisions, there is a tendency for risk managers to equate novel behavior to novel risk and take a precautionary approach to managing that risk (Calliess & Stockhaus, 2012; Chapman, Fairbrother, & Brown, 1998; Justo-Hanani & Dayan, 2016). Such precautionary approaches may include the application of conservative uncertainty factors or stringent restrictions or prohibitions on a material’s production or use (Dourson, Felter, & Robinson, 1996; REACH, 2006; Schmidt, 2016). While advantageous and protective against harmful AdMs, precautionary measures implemented in the absence of risk information may inadvertently prevent safe and useful AdMs from entering the marketplace, or stifle future innovation by deterring technology developers from utilizing materials that are poorly understood or perceived (Rogers, 2001; Van den Belt, 2003).
A useful intermediary between the resource-intensive risk assessment approach (decisions made with lower uncertainty) and the precautionary risk assessment approach (decisions made with higher uncertainty) is the risk-screening method in which materials are examined for potential risk in a stepwise manner and with increasing rigor, and limited resources are only allocated to the materials that warrant further scrutiny based on sound scientific reasoning (Bates, Keisler, Zussblatt, Plourde, Wender, & Linkov, 2016; Frank R. Lautenberg Chemical Safety for the 21st Century Act, 2016; Mitchell et al., 2013). An example of this type of approach is the Nano Guidance for Risk Informed Deployment (NanoGRID) framework (Collier et al., 2015). Each tier within the NanoGRID framework requires a technology developer or risk manager/assessor to collect incrementally more information about the technology using protocols and methods applicable to ENMs and ENM-enabled products. The type of risk information collected in the tiered framework includes whether: (1) the in-use ENM meets applicable definitions of “nano,” (2) the relevant use of the ENM-enabled product releases nanoscale constituents, (3) the released material results in ecological exposures to nano-constituents after fate, transport, and transformation in the environment, and (4) the released, transformed, ecologically exposed material results in toxicological impact. In progressing through these tiers, the NanoGRID framework helps identify when a new technology requires additional risk testing or when it can be addressed within traditional regulatory and safety frameworks.
An expansion of the NanoGRID framework that incorporates the broader set of AdMs beyond the 1–100 nm size definition would be valuable to preempt the allocation of resources for an in-depth risk assessment that may not be warranted or the premature application of precautionary risk management strategies for AdMs. In order to realize this expanded application, a straightforward and consensus definition of what constitutes an AdM is required. However, while it can easily be argued that the term ENM is overly general, applying to a diverse group of materials of seemingly limitless chemical compositions and surface functionalities, the term advanced materials is far more general and wide reaching, making it difficult to define which materials do or do not fit the AdM mold. Therefore, relying on a definition alone is inadequate in practice; a clear and user-friendly categorization method is also required to operationalize this definition that technology developers and risk assessors can use to unambiguously categorize materials. A consensus definition and associated categorization regime would identify whether a technology is an AdM or is AdM-enabled and, if it is, the categorization method would identify what functional category the material fits within for risk assessment. Several AdM definitions have been offered (Table I) (Featherston & O’Sullivan, 2014; Maine & Garnsey, 2006; NIST, 2011; South Africa DTI, 2018; UK Technology Strategy Board, 2011) but have not garnered widespread endorsement from the AdM community, and no categorization scheme has been proposed for AdM risk assessment beyond the categorization framework specific to ENM hazard identification (Hansen, Larsen, Olsen, & Baun, 2007).
Table I.
Existing AdM Definitions
| Definition | Reference |
|---|---|
| Radical advanced materials technologies: product and process improvements that significantly enhance the cost-performance frontier of functional materials. | Maine and Garnsey (2006) |
| Advanced materials: materials, and their associated process technologies, with the potential to be exploited in high value-added products. | UK Technology Strategy Board (2011) |
| Innovative advanced materials technologies: [technologies that] make a direct and positive impact on economic growth, the environment and quality of life, via improved processes and products, throughout their life cycle. | |
| Advanced materials: materials that have been developed to the point that unique functionalities have been identified and these materials now need to be made available in quantities large enough for innovators and manufacturers to test and validate in order to develop new products. | NIST (2011); Featherston and O’Sullivan (2014) |
| Advanced materials: all materials that represent advances over the traditional materials that have been used for hundreds or even thousands of years … advanced materials refer to all new materials and modifications to existing materials to obtain superior performance in one or more characteristics that are critical for the application under consideration. They can also exhibit completely novel properties. | South Africa DTI (2018) |
In this study, we reduce the complexity of the term “advanced material” by establishing a practitioner-driven definition for AdMs and a practitioner-validated categorization process for organizing AdMs into conceptual categories based on material characteristics. The benefits of this effort are to: (1) enable consistent differentiation between AdMs and conventional materials, (2) offer functional nomenclature for application science, and (3) provide the foundation for a risk prioritization framework that provides expeditious binning of AdMs into high-and low-risk categories, thereby releasing certain materials from heightened scrutiny and providing a faster pathway to safe and rapid acquisition and commercialization. The definition and categorization scheme proposed and validated here serves as a first step in determining the type and extent of risk-related information that should be collected for AdMs and AdM-enabled technologies.
2. METHODS
Two workshops were held for initial method development and derivation of a preliminary definition of AdMs to disseminate to practitioners involved in the web survey that guided the main study results. The workshops included material scientists, geochemists, physicists, toxicologists, social scientists, and risk assessors from the U.S. Army Corps of Engineers and the National Institute for Occupational Safety and Health (hereafter referred to as “workshop participants”). Workshops took place in March and July 2017 and included 11 and 20 participants, respectively. Information was collected through a moderator-led discussion and a web voting system. The workshop-derived preliminary definition of AdMs then evolved into a material categorization method that could be validated through a web survey of practitioners in AdM research, development, and governance. Workshop participants identified the attributes that qualify materials as either “advanced” or “conventional” and used these attributes to develop the following consensus definition for AdMs. The first part of the definition focuses exclusively on the material attributes, while the second part addresses how these unique attributes may impact ESOH profiles:
Advanced Materials are materials that are specifically engineered to exhibit novel or enhanced properties that confer superior performance relative to conventional materials. As a result of their unique characteristics, advanced materials have a highly uncertain hazard profile and the potential to require special testing procedures and methods to assess potential for adverse environmental health and safety impacts.
Of critical importance in our method development process was an understanding of the term “uncertain” as used above. The hazard and risk profile of these materials is uncertain due to lack of information and test methods. However, this uncertain risk profile does not indicate a predisposition (or assumption) of hazard or risk. Uncertainty is an important and recurring challenge in the emerging technology risk assessment arena (e.g., ENMs and synthetic biology) but can be leveraged to inform—rather than restrict—risk-based decisions (Finkel & Gray, 2018; Trump, Hristozov, Malloy, & Linkov, 2018). It was equally important to understand that a material’s categorization as “conventional” does not denote absence of risk given that many conventional materials have been evaluated in the past.
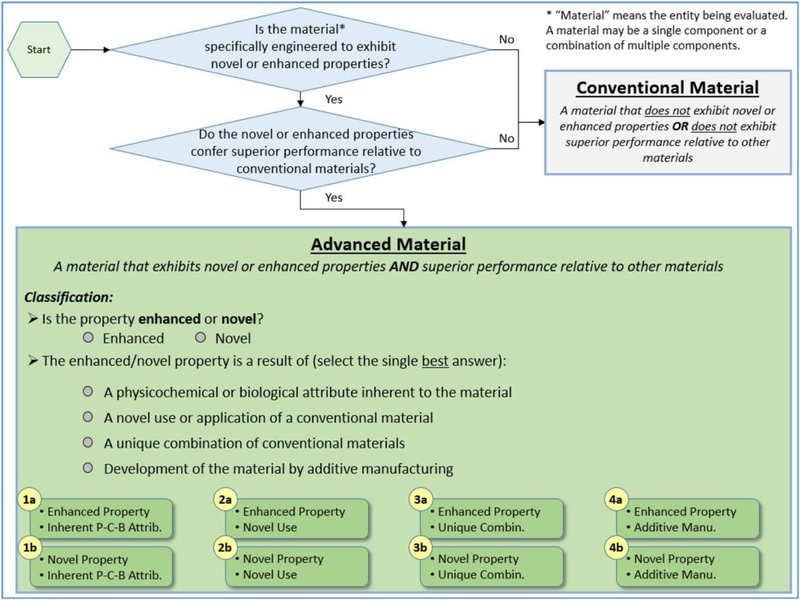
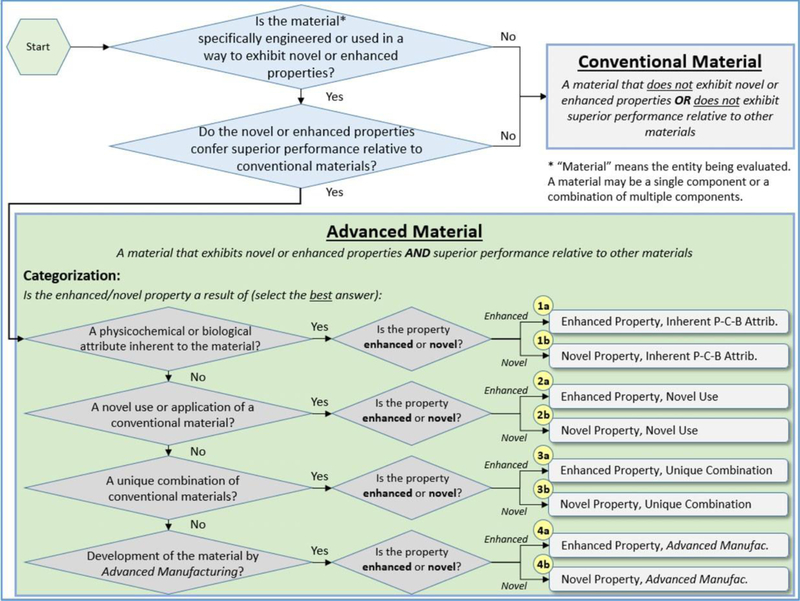
To frame the web survey sent to the practitioners and organize the study results, the workshop participants also identified methods for grouping AdMs into categories that have distinct implications for risk assessment and agreed that the most effective categorization scheme was to organize AdMs according to the source of their advanced behavior. This categorization scheme enabled a clear division between the risk implications for each AdM category while maintaining simplicity by limiting the number of categories to four. An illustration of the categorization approach used in this study, which was derived from the workshop-driven definition for AdMs, is depicted in Fig. 1. The four sources of advanced behavior were identified as:
a physicochemical or biological attribute inherent to the material
a novel use or application of a conventional material
a unique combination of conventional materials
development of the material by additive manufacturing
Fig. 1.
Categorization method for AdMs initially developed from workshops that were tailored to guide the web survey of practitioners and the main study results, which are presented in Fig. 6.
The consensus categorization scheme was then translated into a series of questions, divided into three question sets. Set A aligns with the decision logic of the categorization method, Set B delineates the four categories of AdMs identified as part of the workshop-driven definition, and Set C asks for a qualitative description of whether a material should be considered conventional or advanced and the reason for that selection. These questions served as the basis for a practitioner elicitation described below (Supporting Information 1).
The web survey (for complete survey, see Supporting Information 2) was conducted to validate the AdM categorization method by:
Assessing the extent to which the categorization method represents a current consensus model of the state of AdMs from a risk management perspective.
Using prototype materials in a case study approach to confirm that the decision space enforced by the categorization method questions easily differentiates case materials into the categories that they are intended to represent.
Practitioners in AdM research, development, and governance (hereafter referred to as “participants”) were contacted to participate in a web survey for a project conducted by the authors. A total of 92 practitioners were invited to complete the web survey. Participants were sent materials explaining the goals of the project, the process of elicitation, and instructions on how to complete the survey. A total of 17 practitioners completed the web survey (19% response rate). Functional roles of the cohort tended to be AdM researchers, many of whom also self-reported as AdM developers. Eleven participants self-reported as AdM researchers, six as AdM developers, three as AdM regulators, and three listed an affiliation-specific functional role. The median number of years that participants reported working with AdMs was 13, with a range of 0–33 years.
The survey asked practitioners a combination of YES/NO, multiple choice, and free-response questions (Supporting Information 1) about six real-world materials to determine if they considered the materials to be “conventional” or “advanced” and elicited the reason for their choice. The six materials were selected such that four were classified as AdMs and two were classified as conventional according to workshop participants and the workshop-derived consensus definition of AdMs. The four AdMs were Chitosan Graphene Oxide Composite (CSGO), 3D-Printed Cobalt-Chromium Alloy (3D C-C Alloy), Sapphire Glass (a form of aluminum oxide; Al2O3), and Glass Reinforced Aluminum (GLARE), and the two conventional materials were 3D-Printed Stainless Steel (3D S-Steel) and Cold-Water Fish Skin. The question cascade for the survey (Supporting Information 1) was translated directly from the categorization method established in the workshops (Fig. 1). All questions—those applicable to AdMs only and both AdMs and conventional materials— were displayed to the participants.
Participants were presented the six materials one at a time and provided a picture of each material along with its name, description, application, manufacturing method, and a comparator material (Supporting Information 2). They were instructed that their responses should be based on their professional judgment using the information provided for each material, and informed that previous familiarity with the material was not required. Participants were also instructed to respond to all questions for all materials in order to break any path dependencies between material properties and potential categorization outcomes.
Throughout the web survey, participants were shown a sidebar with a list of definitions for important terms used within the survey. They were:
Advanced Material: “Advanced Materials are materials that are specifically engineered to exhibit novel or enhanced properties that confer superior performance relative to conventional materials. As a result of their unique characteristics, advanced materials have a highly uncertain hazard profile and the potential to require special testing procedures and methods to assess potential for adverse environmental health and safety impacts.”
Specifically Engineered: “Intentionally and knowingly designed for a particular purpose.”
Novel: “New and not resembling something formerly known or used, such as a physical phenomenon not previously known for a given material or application, or an entirely new physical phenomenon.”
Enhanced: “Increased, intensified, or further improved in quality, value, or extent.”
Superior: “Better than average, or better than others of the same type.”
Additive Manufacturing: “Additive Manufacturing is a layer-by-layer process of producing 3-dimensional objects directly from a digital model, unlike conventional or subtractive manufacturing processes.”
NOTE: Materials or applications developed using additive manufacturing techniques (e.g., 3-D printing) must meet the above definition of an Advanced Material in order to be considered an Advanced Material.
Participants were also provided a brief hypothetical example of a material that satisfied the definition of an AdM as well as justification for this conclusion using the definitions provided (Supporting Information 2). After entering responses for the six materials, participants were asked a final freeresponse question to gauge the usability of the categorization method. Participants were given two weeks to complete the survey.
3. RESULTS AND DISCUSSION
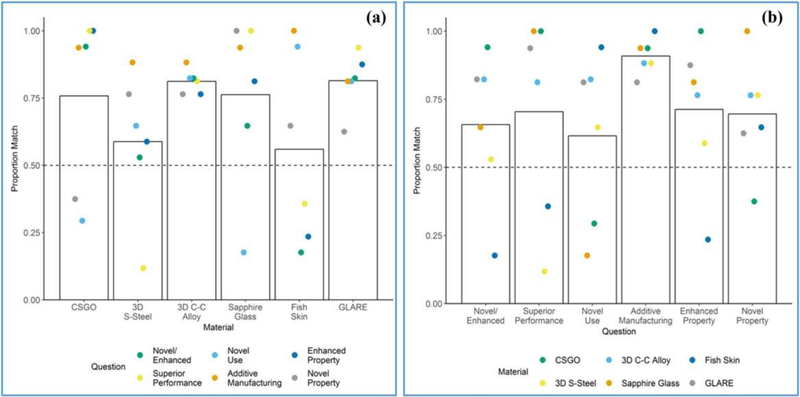
In question Set A, which aligns with the section of the workshop-driven categorization method where “advanced” or “conventional” is determined according to the consensus definition for AdMs, participant responses were generally consistent with the authors’ a priori assessments across all six materials (Fig. 2). In Fig. 2(a), colored dots correspond to the six questions (abbreviated) in Set A, and the top of the bar represents the mean proportion match across all six questions for a given material. In Fig. 2(b), colored dots correspond to the six materials, and the top of the bar represents the mean proportion match across all six materials for a given question in Set A.
Fig. 2.
Responses to questions related to categorization of the material (Set A). Bars represent the extent to which participants’ responses agreed with the authors’ responses. A proportion match of 1.00 represents complete agreement.
Participants diverged most from the authors in their judgments of the two assumed conventional materials, 3D S-Steel and Fish Skin (proportion match 0.59 and 0.56, respectively), as well as in their judgments of novel use across materials and whether materials demonstrated a novel/enhanced property (0.61 and 0.66, respectively). Participants diverged least from the authors in their judgments of 3D C-C Alloy and GLARE (both 0.81).
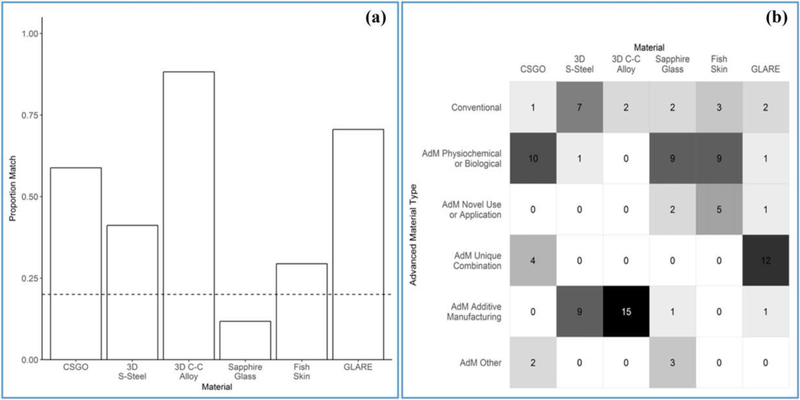
In question Set B, which aligns with the section of the workshop-driven categorization method where an AdM is categorized according to the source of its advanced behavior, participant designations tended to align with the authors’ a priori categorizations (Fig. 3). In Fig. 3(a), bars represent the extent to which participants’ responses agreed with the authors’ responses for a given material. A proportion match of 1.00 represents complete agreement. In Fig. 3(b), shading and associated response counts indicate the number of participants who selected each source of advanced behavior for each material.
Fig. 3.
Responses to the categorization method where an AdM is categorized according to the source of its advanced behavior (Set B).
Sapphire Glass presented an exception, as nine out of 17 participants cited “a physicochemical or biological attribute inherent to the material” as the source of its novel/enhanced properties, whereas the authors felt that the best attribution for the novel/enhanced properties in the study example was “novel use or application of a conventional material” (selected by two out of 17 participants). This inclina-tion by the participants is reasonable, however, and in qualitative entries several respondents argued that novel/enhanced properties will always be a result of inherent physicochemical or biological attributes of the AdM. While the authors agree with this rationale in general, there are many cases where the foremost reason for an AdM’s advanced behavior is one of the three alternative categorizations: “novel use or application of a conventional material,” “unique combination of conventional materials,” or “development of the material by additive manufacturing.” Attempts to preclude this misalignment by asking participants for the best answer from Set B as it pertains to the use scenario were insufficient and should be augmented in follow-up efforts.
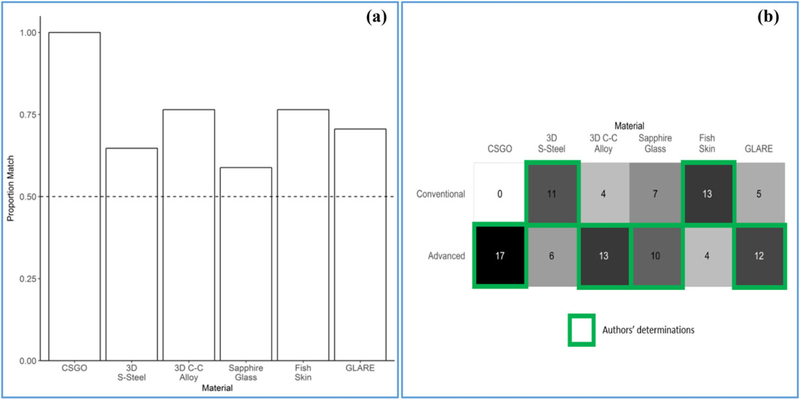
In question Set C, where participants were asked whether a material was conventional or advanced and to present their reasons for their selection, participant responses aligned well with the authors’ determinations for most materials (Fig. 4). In Fig. 4(a), bars represent the extent to which participants’ responses agreed with the authors’ responses for each material. A proportion match of 1.00 represents complete agreement. In Fig. 4(b), shading and response counts depict the number of participants who selected each designation (conventional, advanced) for each material.
Fig. 4.
Advanced or conventional judgments on materials compared to the authors’ determination (Set C).
Misalignment was greatest for 3D S-Steel and Sapphire Glass, though more than half of participants’ judgments matched the authors’ judgments for these materials (0.65 and 0.59 proportion match, respectively). Notably, and in contradiction with the authors’ consensus, two respondents commented that because the Sapphire Glass was based on a naturally occurring material it must be conventional, regardless of the fact that it had been intentionally engineered into a sheet of glass. Additionally, one respondent disagreed with the authors’ determina-tion that GLARE is an AdM based on the argument that the use of conventional materials to form a multi-material complex requires that the final material is conventional. A counterexample to refute that argument is gun powder, which consists of a unique combination of conventional materials (e.g., sulfur, charcoal, KNO3) that individually do not behave as an energetic but in combination provide a new property to induce a stable reaction capable of propelling a projectile.
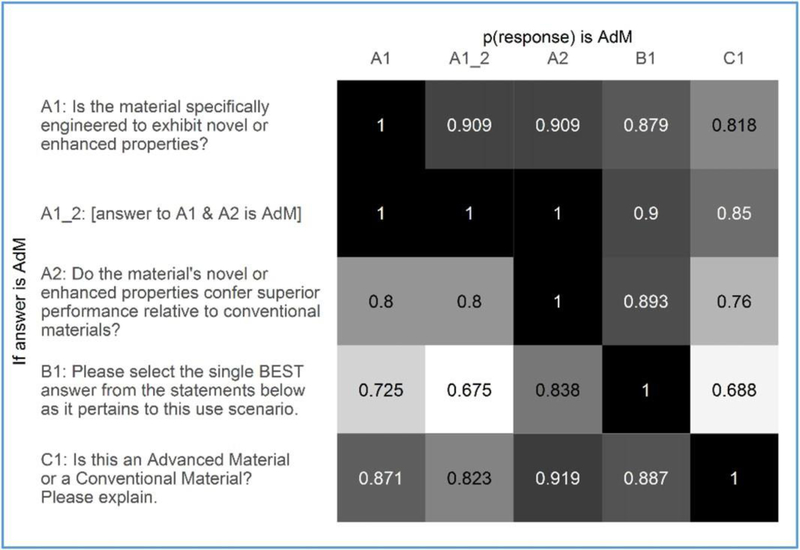
Validation of our workshop-driven definition for AdMs was provided in part by respondent comments that were generally consistent with our AdM definition. Additionally, the majority of respondents agreed with the “advanced” or “conventional” designations that we assigned to each material (Fig. 4) and, importantly, they followed a process for arriving at that designation that was consistent with the proposed definition (Fig. 5). Fig. 5 shows that of the respondents who answered “Yes” to questions 1 and 2 in Set A, 85% concluded that the material was “advanced” when asked directly about its designation in Set C (row 2, column 5), thereby following the logic depicted by the two definition-aligned decision points in Fig. 1. Reciprocally, of the respondents who concluded in Set C that the material was “advanced,” 82.3% answered “Yes” to questions 1 and 2 in Set A (row 5, column 2).
Fig. 5.
Contingency matrix for selected questions from Sets A, B, and C. Shading and proportions indicate the frequency with which participants provided a response for x-axis questions consistent with an advanced material assessment assuming y-axis questions were answered in an advanced-material-consistent manner.
Responses to Set B and the final free-response question provided validation that we had identified in our categorization method four highly relevant parameters to which AdMs can attribute their advanced behavior. In comments provided in question Set B, respondents did not provide any additional organizational classes to use for categorization of AdMs, so it is possible that the four we identified represent the complete list. In the limited instances where a respondent selected “the novel/enhanced property is a result of something that is not mentioned above,” the explanations attributed the property to a source that was either redundant with the existing four categories or a combination of existing categories.
In the final free-response question, 82.4% (N 14) of respondents said the four categories were inclusive of all AdMs, with one stating “it seems to cover any new invention or engineering application.” The 17.6% (N 3) who said the categorization system was not sufficiently encompassing of all AdMs did not offer examples of AdMs where the advanced attributes could not be attributed to the four categories. One respondent recommended broadening the category “the novel/enhanced property is a result of development of the material by additive manufacturing” to a wider range of manufacturing methods, commenting that additive manufacturing “is a useful method but not the only new or advanced method for processing materials into “advanced” form factors or achieving unique multi-material end products, especially with advances in synthetic biology, manipulation of high energy (e.g., plasma or magnetic fields) and other routes.” We agree with this viewpoint and put forward “advanced manufacturing” as a more inclusive term for these manufacturing methods. This term also aligns with nomenclature used by the U.S. National Science and Technology Council’s Subcommittee for Advanced Manufacturing (U.S. NSTC-SAM, 2016).
Several of the preconceptions revealed in the qualitative responses were inconsistent with our thinking, the most prevalent being the notion that materials developed using additive manufacturing are automatically considered AdMs. Six respondents held this belief despite our statement of the contrary made in the sidebar of the web survey. This preconception may be attributable to the respondents’ prior notion that many materials being developed for use in additive manufacturing are considered AdMs, which may or may not be true. Additionally, three respondents overlooked the “enhanced” component of the AdM definition entirely and noted that in the case where a material lacked any novel property the material should not be classified as advanced. Two respondents also felt that if a material was being used in a novel way, then that material had to be advanced. Of note, two respondents claimed that thresholds for “enhanced” and “superior performance” were necessary to label a material advanced, where materials that do not have sufficiently enhanced properties or offer only incremental performance improvements over conventional materials should be classified as conventional. This consideration arose in the workshops but it was decided that a quantitative metric by which to measure enhanced properties or superior performance could not be made at this time, as almost every material would require its own unique metric for comparison, and even proportional representations of improvement are not always useful. For example, in some fields a 1% improvement in functional performance is a significant achievement, while in other fields an improvement of at least an order of magnitude is required to disrupt the status quo. Also debated in the workshops was the idea that a product can be advanced without its constituent material being advanced; one respondent made a similar statement regarding the 3D C-C Alloy, claiming that the alloy itself was not advanced but the resulting fuel nozzle product was advanced. Lastly, one participant commented that Fish Skin was not used in sufficient volume so it could not be conventional, implying that scale of production plays a role in classification of AdMs. However, while the volume of a material produced may relate to regulatory reporting rules and impact risk management decisions, it cannot impact the inherent definition of what the material is. These findings from the validation study shaped small modifications to the initial workshop-driven categorization method (Fig. 1) that served as the foundation for the web survey. Fig. 1 was adapted to a workflow format (Fig. 6) to enable a user to more intuitively follow the categorization process in support of a decision. Additionally, Category 4 was broadened to “development of the material by advanced manufacturing” (replacing “additive” with “advanced”) to incorporate a wider range of novel and enhanced manufacturing methods. Also, the wording “used in a way” was added to the question in the first decision point to help ensure that users that might end up in Categories 2 or 3 do not exit the workflow prematurely. Findings from the validation study revealed that the AdM definition derived via the workshops was suitable for assigning consistent “advanced” or “conventional” designations and was therefore left unchanged.
Fig. 6.
Practitioner-validated categorization workflow for AdMs.
To facilitate accurate and consistent results using the practitioner-validated categorization workflow (Fig. 6) and to eliminate preconceptions and biases toward or against certain categories as much as possible (which may result in inaccurate categorization), we recommend the AdM community adhere to four important guidelines. First, the same definitions as the web survey described herein should be used for the terms “specifically engineered,” “novel,” “enhanced,” and “superior.” Improved agreement on these selected terms and their corresponding definitions will help to limit subjective interpretations. Second, a conventional comparator material should be identified for the purpose of assessing relative “superior” performance. Third, the foremost reason for an AdM’s advanced behavior should be considered rather than acceding to the generalization that novel or enhanced properties are always a result of inherent physicochemical or biological attributes of an AdM. And fourth, a material should not automatically be categorized as: (1) “advanced” solely because it was developed using advanced (or additive) manufacturing, (2) “advanced” solely because it is used in a novel way, (3) “conventional” solely because it utilizes a naturally occurring material (e.g., sapphire), and (4) “conventional” solely because conventional materials were used to form a multi-material complex.
4. CONCLUSION
In this study, we sought to provide clarity and reduce the ambiguity of the term “advanced material” by establishing a practitioner-driven definition for AdMs and a practitioner-validated categorization workflow for organizing AdMs into conceptual categories based on material characteristics. The work-shops and practitioner elicitation conducted in this study offer a definition and categorization scheme that can assist in defining, assessing, and managing the ESOH risks associated with AdMs.
The definition and categorization framework proposed and validated here serve as a first step in determining if and when there is a need for specific ESOH and regulatory screening for an AdM as well as the type and extent of risk-related information that should be collected for AdMs and AdM-enabled technologies. This risk-screening approach may facilitate efforts to determine potential risks or impacts of products incorporating specific AdMs or certain classes of AdMs. Such guidance should be incorporated into an existing decision support tool for ENMs (e.g., NanoGRID [Collier et al., 2015]) to expand the focus to all AdMs. Additionally, Stone et al. identify “[a]dvanced tools to facilitate risk-based decision making, including an assessment of the needs of users regarding risk assessment, mitigation, and transfer” as one of the three essential elements required to generate an effective risk governance framework for nanomaterials (Stone et al., 2018); a shared consensus definition and categorization workflow for AdMs—which leaves room for integration of quantitative experimental information alongside qualitative expert insight (Linkov et al., 2018)—can help facilitate risk-based decision making and may contribute to improved governance of AdMs by stakeholders in the United States, Europe, and elsewhere. Future work to augment the AdM definition and categorization workflow developed in this study should focus on establishing a consensus definition for a recognized standard term “advanced manufacturing” that encompasses advanced and additive manufacturing, including when AdMs are used as additives, as well as techniques such as synthetic biology and methods that employ or manipulate high energy (Hill et al., 2018). Further discussion of the nuance between an AdM and an “advanced technology”—which may focus more on a product’s use characteristics—would also be beneficial. Additionally, key performance indicators or thresholds for “enhanced” and “superior” performance are needed so materials that display incremental performance improvements are not automatically categorized as AdMs. Guidance on identifying appropriate performance measures will help to improve consensus of the conventional or advanced designations of materials like those highlighted in this study. Future exploration of whether all eight categories are necessary for AdM classification would also be beneficial in order to refine the framework to the simplest system possible and to develop the simplest possible standardized definition.
This study was limited in the sample of practitioners who completed the web survey. While the intention was to have a much larger sample size of practitioners (N 92), the realized sample size of respondents was smaller (N 17), and while the results provided validation of the definition and categorization workflow, a larger sample from the pool would have provided more weight to the validation. Additionally, web surveys are constrained in the amount of detail that they can collect; even though opportunity for thorough responses was provided in question Set C and the final free-response question, some informative nuance from the participants may have been lost due to the selection of a survey as the elicitation instrument.
Despite these limitations, it is important to recognize progress made toward practical, near-term definitions, nomenclature, and categorization strategies for AdMs even as idealized, longer-term solutions may be years or perhaps even decades away. Preliminary working solutions like the AdM definition and categorization workflow developed in this study must be more thoroughly tested and applied, and can be revised and perfected through iteration as AdMs and their associated risk assessment and management requirements evolve. Increased adoption and use of this AdM definition and categorization workflow can be supported through broader engagement and education of practitioners in materials science and engineering regarding the concepts and selected terminology required for risk assessment.
It must also be recognized that the categorization tools and strategies needed to perform informed risk assessments of AdMs and AdM-enabled products may not necessarily be the same ones required by physicists, chemists, materials scientists, and chemical engineers, for example, to design, develop, apply, and manufacture them. An important next step is to put this AdM definition and categorization workflow in the hands of multidisciplinary practitioners where it can be applied to real-world cases that require risk screening and decision support and where iterative, experience-informed improvements can be proposed.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank NanoSafe, Inc. (Blacksburg, VA) for its assistance in administering the web-based validation survey under U.S. Army Research Office contract W91NF-14–2-0090. The authors thank David Martin, Aimee Poda, Robert Moser, Emily Asenath-Smith, Jessica Coleman, and Mark Chappell from the U.S. Army Engineer Research and Development Center (Vicksburg, MS and Hanover, NH) for technical assistance in conducting this study. This work was funded by the Army Environmental Quality and Installations Research Program, Military Materials in the Environment (Dr. Elizabeth Ferguson was the technical director). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Advanced Materials Scientific Journal. (2018). Overview: Aims and scope. Retrieved from https://onlinelibrary.wiley.com/page/journal/15214095/homepage/productinformation.html. [Google Scholar]
- Arts JH, Hadi M, Irfan MA, Keene AM, Kreiling R, Lyon D, … Warheit D (2015). A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regulatory Toxicology and Pharmacology, 71(2), S1–S27. [DOI] [PubMed] [Google Scholar]
- Bates ME, Keisler JM, Zussblatt NP, Plourde KJ, Wender BA, & Linkov I (2016). Balancing research and funding using value of information and portfolio tools for nanomaterial risk classification. Nature Nanotechnology, 11, 198–203. [DOI] [PubMed] [Google Scholar]
- Calliess C, & Stockhaus H (2012). Precautionary principle and nanomaterials: REACH revisited. Journal for European Environmental & Planning Law, 9(2), 113–135. [Google Scholar]
- Chapman PM, Fairbrother A, & Brown D (1998). A critical evaluation of safety (uncertainty) factors for ecological risk assessment. Environmental Toxicology and Chemistry, 17(1), 99–108. [Google Scholar]
- Coll C, Notter D, Gottschalk F, Sun T, Som C, & Nowack B (2016). Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology, 10(4), 436–444. [DOI] [PubMed] [Google Scholar]
- Collier ZA, Kennedy AJ, Poda AR, Cuddy MF, Moser RD, MacCuspie RI, … Steevens JA (2015). Tiered guidance for risk-informed environmental health and safety testing of nanotechnologies. Journal of Nanoparticle Research, 17(3), 155. [Google Scholar]
- Dang Y, Zhang Y, Fan L, Chen H, & Roco MC (2010). Trends in worldwide nanotechnology patent applications: 1991 to 2008. Journal of Nanoparticle Research, 12(3), 687–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourson ML, Felter SP, & Robinson D (1996). Evolution of science-based uncertainty factors in noncancer risk assessment. Regulatory Toxicology and Pharmacology, 24(2), 108–120. [DOI] [PubMed] [Google Scholar]
- EU Commission. (2011). Commission recommendation of 18 October 2011 on the definition of nanomaterial (2011/696/EU). Official Journal of the European Communities: Legis, 275, 38. [Google Scholar]
- Featherston CR, & O’Sullivan E (2014). A review of international public sector strategies and roadmaps: A case study in advanced materials. Retrieved from https://www.ifm.eng.cam.ac.uk/uploads/Resources/FeatherstonOSullivan_2014_-_A_review_of_international_public_sector_roadmaps-_advanced_materials_full_report.pdf.
- Finkel AM, & Gray G (2018). Taking the reins: How regulatory decision-makers can stop being hijacked by uncertainty. Environment Systems and Decisions, 38(2), 230–238. [Google Scholar]
- Frank R Lautenberg Chemical Safety for the 21st Century Act. (2016). Public Law No. 114–182. 15 USC 2601. Retrieved from https://www.congress.gov/bill/114th-congress/house-bill/2576.
- Hansen SF, Jensen KA, & Baun A (2014). NanoRiskCat: A conceptual tool for categorization and communication of exposure potentials and hazards of nanomaterials in consumer products. Journal of Nanoparticle Research, 16(1), 2195. [Google Scholar]
- Hansen SF, Larsen BH, Olsen SI, & Baun A (2007). Categorization framework to aid hazard identification of nanomaterials. Nanotoxicology, 1(3), 243–250. [Google Scholar]
- Hull MS (2017). National security and the nano factor. Journal of Homeland Defense & Security Information Analysis. Special Nanotechnology Issue, 4, 16–21. [Google Scholar]
- Hill WC, Kennedy AJ, Warner CM, & Hull MS (2018). Beyond nano and the environment: The implications of 21st century innovation on safety and security In Hull MS & Bowman DM (Eds.), Nanotechnology environmental health and safety: Risks, regulation, and management (3rd ed., pp. 497–513). Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Justo-Hanani R, & Dayan T (2016). Explaining transatlantic policy divergence: The role of domestic politics and policy styles in nanotechnology risk regulation. Global Environmental Politics, 16(1), 79–98. [Google Scholar]
- Khot LR, Sankaran S, Maja JM, Ehsani R, & Schuster EW (2012). Applications of nanomaterials in agricultural production and crop protection: A review. Crop Protection, 35, 64–70. [Google Scholar]
- Linkov I, Trump BD, Anklam E, Berube D, Boisseasu P, Cummings C, … Jensen KA (2018). Comparative, collaborative, and integrative risk governance for emerging technologies. Environment Systems and Decisions, 38(2), 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine E, & Garnsey E (2006). Commercializing generic technology: The case of advanced materials ventures. Research Policy, 35(3), 375–393. [Google Scholar]
- Malloy TF, Zaunbrecher VM, Batteate C, Blake A, Carroll WF, Corbett C, Foss Hansen S, Lempert R, Linkov I, McFadden R, Moran KD, Olivetti E, Ostrom N, Romero M, Schoenung J, Seager T, Sinsheimer P, & Thayer K (2017). Advancing alternative analysis: Integration of decision science. Environmental Health Perspectives, 125, 066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Pabon N, Collier ZA, Egeghy PP, Cohen-Hubal E, Linkov I, & Vallero DA (2013). A decision analytic approach to exposure-based chemical prioritization. PLoS ONE, 8, e70911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Standards and Technology (NIST). (2011). Manufacturing and biomanufacturing: Materials advances and critical processes. Gaithersburg, MD: Department of Commerce. [Google Scholar]
- National Research Council. (2012). A research strategy for environmental, health, and safety aspects of engineered nanomaterials. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S, … Bosi S (2017). Diverse applications of nanomedicine. ACS Nano, 11(3), 2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REACH. (2006). Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency. Retrieved from http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20140410.
- Rogers MD (2001). Scientific and technological uncertainty, the precautionary principle, scenarios and risk management. Journal of Risk Research, 4(1), 1–15. [Google Scholar]
- Rycroft T, Trump B, Poinsatte-Jones K, & Linkov I (2018). Nanotoxicology and nanomedicine: Making development decisions in an evolving governance environment. Journal of Nanoparticle Research, 20(2), 52. [Google Scholar]
- Schmidt CW (2016). TSCA 2.0: A new era in chemical risk management. Environmental Health Perspectives, 124(10), A182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova A, Pietroiusti A, & Kagan V (2016). Nanotoxicology ten years later: Lights and shadows. Toxicology and Applied Pharmacology, 299, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South Africa Department of Trade and Industry (DTI). (2018). Advanced materials—Background. Retrieved from http://www.dti.gov.za/industrial_development/Advanced_Materials.jsp.
- Stone V, Führ M, Feindt PH, Bouwmeester H, Linkov I, Sabella S, … Fito C (2018). The essential elements of a risk governance framework for current and future nanotechnologies. Risk Analysis, 38(7), 1321–1331. [DOI] [PubMed] [Google Scholar]
- Trump BD, Hristozov D, Malloy T, & Linkov I (2018). Risk associated with engineered nanomaterials: Different tools for different ways to govern. Nano Today, 21, 9–13. [Google Scholar]
- UK Technology Strategy Board. (2011). Advanced materials key technology area 2008–2011. Retrieved from http://www.nibec.ulster.ac.uk/uploads/documents/advanced_materials_strategy.pdf.
- U.S. National Nanotechnology Initiative (U.S. NNI). (2018). What it is and how it works. Retrieved from http://www.nano.gov/nanotech-101/what.
- U.S. National Science and Technology Council (NSTC) Sub-committee on Advanced Manufacturing (SAM). (2016). Advanced manufacturing: A snapshot of priority technology areas across the federal government. Retrieved from https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Blog/NSTC%20SAM%20technology%20areas%20snapshot.pdf.
- Van den Belt H (2003). Debating the precautionary principle: “Guilty until proven innocent” or “innocent until proven guilty?” Plant Physiology, 132(3), 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Uchaker E, Candelaria SL, & Cao G (2013). Nano-materials for energy conversion and storage. Chemical Society Reviews, 42(7), 3127–3171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.