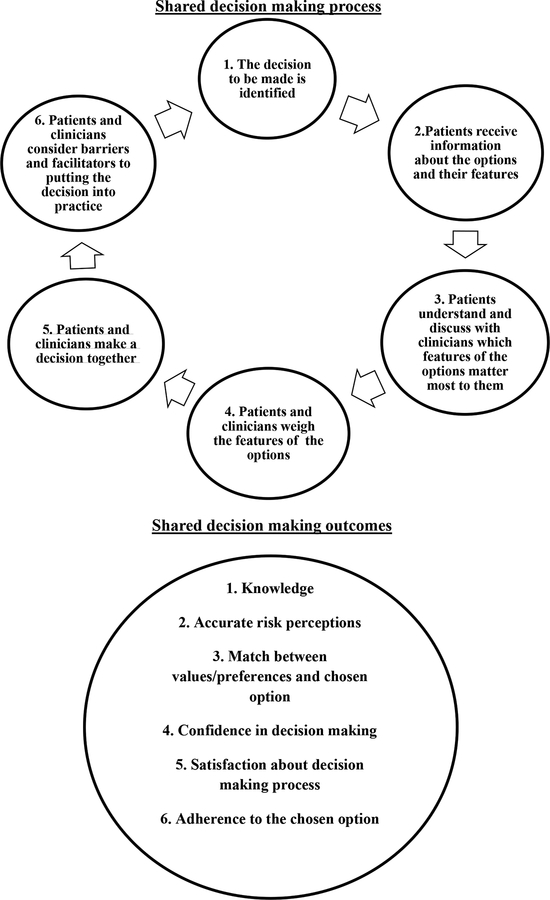
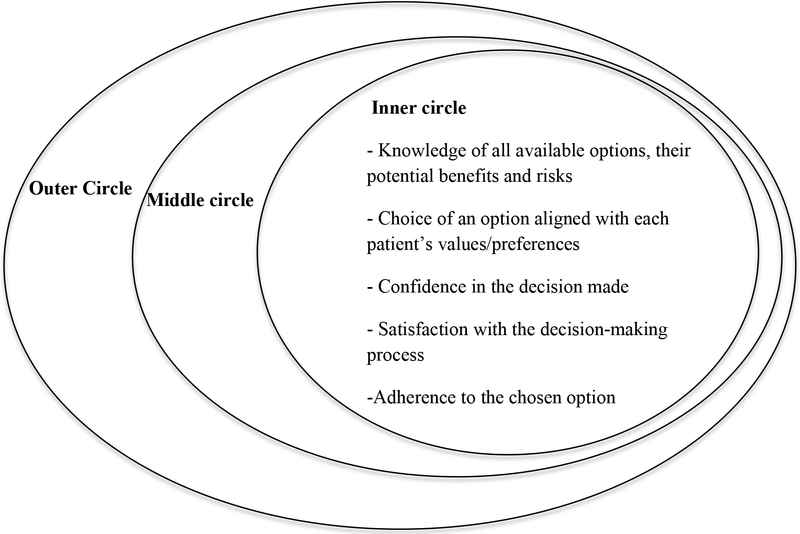
Karine Toupin April
Karine Toupin April
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Jennifer L Barton
Jennifer L Barton
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Liana Fraenkel
Liana Fraenkel
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Alexa Meara
Alexa Meara
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Linda C Li
Linda C Li
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Peter Brooks
Peter Brooks
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Maarten de Wit
Maarten de Wit
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Dawn Stacey
Dawn Stacey
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
France Légaré
France Légaré
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Beverley Shea
Beverley Shea
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Anne Lyddiatt
Anne Lyddiatt
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Cathie Hofstetter
Cathie Hofstetter
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Robin Christensen
Robin Christensen
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Marieke Scholte Voshaar
Marieke Scholte Voshaar
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Maria E Suarez-Almazor
Maria E Suarez-Almazor
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Annelies Boonen
Annelies Boonen
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Tanya Meade
Tanya Meade
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Lyn March
Lyn March
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Janet Elizabeth Jull
Janet Elizabeth Jull
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Willemina Campbell
Willemina Campbell
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Rieke Alten
Rieke Alten
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Suvi Karuranga
Suvi Karuranga
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Esi M Morgan
Esi M Morgan
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Ayano Kelly
Ayano Kelly
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Jessica Kaufman
Jessica Kaufman
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Sophie Hill
Sophie Hill
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Lara J Maxwell
Lara J Maxwell
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Dorcas Beaton
Dorcas Beaton
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Yasser El-Miedany
Yasser El-Miedany
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Shikha Mittoo
Shikha Mittoo
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Susan J Bartlett
Susan J Bartlett
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Jasvinder A Singh
Jasvinder A Singh
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1,
Peter Tugwell
Peter Tugwell
1K. Toupin-April, PhD, Associate Scientist, Children’s Hospital of Eastern Ontario Research Institute, Assistant Professor, Department of Pediatrics and School of Rehabilitation Sciences, University of Ottawa, Ottawa, Canada; J.L. Barton, MD, VA Portland Health Care System, Associate Professor, Oregon Health & Science University, Portland, USA; L. Fraenkel, MD, Professor of Medicine, Department of Internal Medicine, Yale University, New Haven, USA; A. Meara, MD, The Ohio State University, Columbus, USA; L. C. Li, PT, PhD, Professor, Department of Physical Therapy, University of British Columbia; Senior Scientist, Arthritis Research Canada, Vancouver, Canada; P. Brooks, MD, Professor, School of Population and Global Health, University of Melbourne, Melbourne, Australia; M. de Wit, PhD, Patient Research Partner, Amsterdam University Medical Centre, Dept. Medical Humanities, Amsterdam, The Netherlands; D. Stacey, RN, PhD, Full Professor, School of Nursing, University of Ottawa, Senior Scientist, The Ottawa Hospital Research Institute, Ottawa, Canada; F. Légaré, MD, PhD, Full Professor, Department of Family Medicine and Emergency Medicine, Université Laval, Quebec City, Canada; B. Shea, PhD, Clinical Scientist, Bruyère Research Institute, Senior Methodologist, Ottawa Hospital Research Institute, Adjunct Professor, Department of Epidemiology and Community Medicine, University of Ottawa, Ottawa, Canada; A. Lyddiatt, Patient Research Partner, Canada; C. Hofstetter, Patient Research Partner, Canada; R. Christensen, MSc, PhD, Professor, Musculoskeletal Statistics Unit, The Parker Institute, Bispebjerg and Frederiksberg Hospital, Copenhagen, & Department of Rheumatology, Odense University Hospital, Denmark; M. Scholte-Voshaar, MSc, Patient Research Partner, Department of Psychology, Health and Technology, University of Twente, Enschede, The Netherlands; M.E. Suarez-Almazor MD, PhD, Professor, Department of General Internal Medicine, Section of Rheumatology and Clinical Immunology, University of Texas MD Anderson Cancer Center, Houston, USA; A. Boonen, MD, PhD, Professor of Rheumatology, Department of Internal Medicine, Division of Rheumatology, Maastricht University Medical Center and Caphri Research Institute, Maastricht University, Maastricht, The Netherlands; T. Meade, PhD, Professor, Western Sydney University and Faculty of Medicine, University of Sydney, Sydney, Australia; L. March, MD, PhD, Professor, Department of Medicine, University of Sydney, Institute of Bone and Joint Research and Department of Rheumatology, Royal North Shore Hospital, Sydney, Australia; J. E. Jull, OT, PhD, Assistant Professor, School of Rehabilitation Therapy, Queen’s University, Kingston, Canada; W. Campbell, LLB, Patient Research Partner, Toronto Western Hospital, University Health Network, Canada; R. Alten, MD, PhD, Professor of Medicine, Head of Department of Internal Medicine II, Director of Rheumatology Research Center, Rheumatology, Clinical Immunology, Osteology, Physical Therapy and Sports Medicine, Schlosspark-Klinik, Charité, University Medicine Berlin, Berlin, Germany; S. Karuranga, Researcher, Access to Medicine Foundation, Haarlem, The Netherlands; E. M. Morgan, MD, Associate Professor, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, USA; A. Kelly, MD, PhD student, Canberra Rheumatology, Department of Rheumatology, Canberra Hospital, and College of Health and Medicine, Australian National University; J. Kaufman, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; S. Hill, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia; L. J. Maxwell, PhD, Ottawa Hospital Research Institute, Centre for Practice-Changing Research and University of Ottawa, Ottawa, Canada; D. Beaton, PhD, Senior Scientist, Institute for Work & Health, Associate Professor, Institute Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada; Y. El-Miedany, MD, Professor, Rheumatology and Rehabilitation Ain Shams University, Egypt; Honorary senior clinical lecturer, King’s college London, United Kingdom; S. Mittoo, MD, Rheumatologist, Involved Medicine and Adjunct Professor University of Toronto, Toronto, Canada, S. Bartlett, Professor, McGill University, Montreal, Canada; J. A. Singh, MD Professor, University of Alabama at Birmingham, Birmingham, USA; P. Tugwell, MD, Professor, Division of Rheumatology, Department of Medicine, and School of Epidemiology and Public Health,Faculty of Medicine, University of Ottawa; Senior Scientist, Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, Canada.
1