Abstract
Background & Aims:
Renal dysfunction increases risk of death for patients with cirrhosis. We investigated whether mortality differs significantly among patients with acute kidney injury (AKI), chronic kidney disease (CKD), and both.
Methods:
We performed a retrospective analysis of all non-status 1 adults on the waitlist for liver transplantation for at least 90 days, collected from the Organ Procurement and Transplantation Network registry from July 1, 2007 through July 1, 2014. We assigned patients to groups of AKI (an increase of ≥0.3 mg/dL or ≥50% in serum creatinine in the last 7 days or fewer than 72 days of hemodialysis), CKD (an estimated glomerular filtration rate <60 ml/min/1.73 m2 for 90 days with a final rate ≥30 ml/min/1.73 m2 or ≥72 days of hemodialysis), AKI and CKD (meet both definitions), or normal (meet neither definition). We performed competing risk analyses to associate patterns of renal dysfunction with waitlist mortality, accounting for liver transplantation, with renal pattern as a time-dependent covariate. Logistic regression for 6-month mortality determined the added benefit of including renal function pattern in the assessment.
Results:
There were 22,680 patients in the cohort; they spent a median 1.6 years (range, 0.7–3.1 years) on the waitlist and a median 5 years (range, 2–9 years) undergoing assessments of renal function. In competing risk analysis, even after adjusting for confounders including final model for end-stage liver disease sodium (MELD-Na) scores, the pattern of renal function was significantly associated with waitlist mortality: AKI and CKD (subhazard ratio [SHR], 2.86; 95% CI, 2.65–3.10), AKI (SHR, 2.42; 95% CI 2.22–2.64), CKD (SHR, 1.56; 95% CI, 1.45–1.67) compared with normal. The area under the curve values, based on MELD-Na score at time of placement on the waitlist, were 0.80 with renal function pattern and 0.71 without (P<.001).
Conclusion:
In competing risk analysis, even after adjusting for confounders including final MELD-Na score, we found the pattern of renal dysfunction to associate with mortality in patients with cirrhosis. Including information on type of renal dysfunction could improve risk analysis.
Keywords: Renal Dysfunction, Liver Disease, Waitlist Mortality, Liver Transplantation
INTRODUCTION
Renal dysfunction in patients with cirrhosis is common, affecting 20% of patients who are hospitalized and 37% of patients who are followed as outpatients (1,2). Renal dysfunction is also a key determinant of outcomes, with 50% of patients with cirrhosis dying within 30 days of developing renal failure (3,4). However, renal dysfunction in the context of cirrhosis arises from a broad spectrum of pathologies, including acute kidney injury (AKI) from alterations in perfusion (e.g., pre-renal, hepatorenal syndrome) to chronic kidney disease (CKD) from renal parenchymal damage (e.g., diabetic nephropathy, hypertensive nephrosclerosis). It is not well-established if each of these pathologies impacts survival equally (5,6).
To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI (2, 7, 8). While AKI is a critical determinant of mortality in patients with cirrhosis, the data regarding CKD and AKI on CKD are limited. The one study that focused on the cause of renal failure in patients with cirrhosis and prognosis reported a statistically different association between the etiology of renal dysfunction and survival outcomes. However, this study was limited by subjective definitions of renal failure as well as relatively short follow-up that could not address the long-term natural progression of renal dysfunction in patients with cirrhosis (6).
Herein, we present a study focused on determining the association between the pattern of renal function and mortality in patients with cirrhosis. We hypothesized that the pattern of renal function would have varying impact on survival, specifically that patients with AKI or AKI on CKD are at greater risk than those with CKD or normal renal function.
METHODS
Patients
All patients listed for liver transplantation in the UNOS/OPTN registry from July 1st 2007 through July 1st 2014, which represents a time frame of relatively stable liver allocation policy, were evaluated for inclusion in this study. Patients who were less than 18 years old or listed as Status 1, including those with fulminant hepatic failure were excluded. Those who received exceptions points or underwent a living donor liver transplantation were also excluded, as these patients have a likelihood of receiving a liver transplant that is independent of their liver function. Because we could not know whether patients listed for < 90 days had acute or chronic kidney disease, we excluded those who spent < 90 days on the waitlist.
Covariates
Data were obtained from the UNOS/OPTN registry as of June 17th, 2016. Demographic data and transplant date were collected at listing. The following data were collected at listing and at the end of follow-up: total bilirubin, international normalized ratio (INR), presence of hepatic encephalopathy (HE), and presence of ascites. Cutoffs deemed to be implausible were as follows: total bilirubin ≤ 0 mg/dL, INR ≤ 0, and creatinine ≤ 0 mg/dL (9). Observations with implausible values or missing data were censored - this represented <3% of the cohort. The Model for End-Stage Liver Disease including Serum Sodium (MELDNa) score (10) was calculated and capped at 6 and 40, per current liver allocation policy. Listing diagnoses were grouped into the following common diagnostic categories: hepatitis C virus (HCV), nonalcoholic fatty liver disease (NAFLD, including cryptogenic cirrhosis and nonalcoholic steatohepatitis), alcohol-related cirrhosis (ALD), autoimmune etiologies (including primary biliary cirrhosis, primary sclerosing cholangitis and autoimmune hepatitis), and other etiologies of cirrhosis (any other listing code that met inclusion criteria). Listing and receipt of a simultaneous-liver kidney transplant (SLKT) was determined at the time of last follow up, as outlined in UNOS protocol these patients received no prioritization for liver transplantation.
Renal Function
Serum creatinine and estimated glomerular filtration rate (eGFR) were determined longitudinally from the time of listing for liver transplantation to removal from the liver transplant waitlist. Renal function was assessed every 7 days or longer. When a patient had more than one serum creatinine for the same 7-day period, the first test result was used. Observations with missing creatinine values were excluded. For the assessment of AKI, each serum creatinine was compared to the serum creatinine from 7 or more days earlier; a cut-off of ≥ 0.3 mg/dL or ≥ 50% from previous value was chosen as outlined by the International Club of Ascites (11). When the testing interval was > 48 hours we assumed that the increase in sCr happened in the 48 hours prior to testing. We defined CKD as outlined by the UNOS/OPTN: an eGFR < 60 ml/min/1.73 m2 for ≥ 90 days with a current eGFR of ≤ 30 ml/min/1.73 m2 (12). We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine based equation (13). We chose this equation, because understanding the limitations of the GFR calculators that can be used with the data available in the UNOS/OPTN registry, the CKD-EPI creatinine based equation most closely estimates GFR relative to GFR as measured by iothalamate clearance in patients with cirrhosis (14–16). Those on hemodialysis were treated as having an eGFR <15 ml/min/1.73 m2 and were not included in the descriptive statistics for serum creatinine and eGFR. Based on current UNOS/OPTN criteria for CKD if patients received hemodialysis during follow-up, we defined this as AKI if they were on hemodialysis for <72 days and CKD if they were on hemodialysis for ≥ 72 days (12). Patients with two or fewer observations of renal function were treated as having normal renal function, unless they were on hemodialysis, which was treated as AKI or CKD, as above.
Renal Function Pattern Definitions
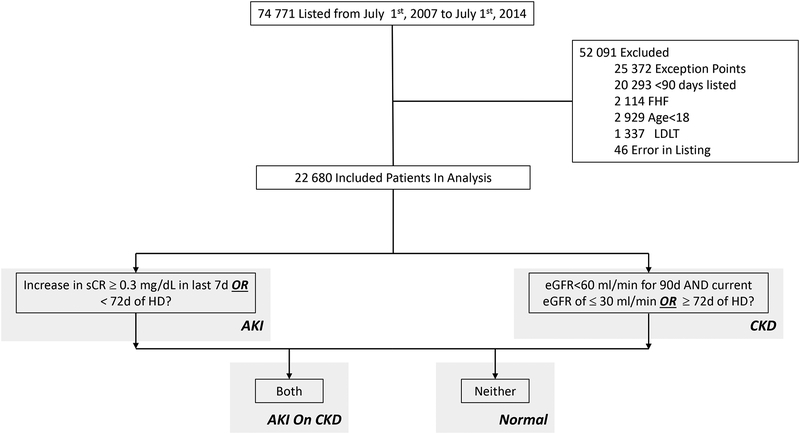
We defined renal function patterns as follows (Figure 1):
Figure 1.
Flow Diagram with Definitions of Renal Patterns
AKI: an increase in serum creatinine by ≥ 0.3 mg/dL or ≥ 50% in the last 7 days or < 72 days of hemodialysis
CKD: an eGFR<60 ml/min/1.73 m2 for 90 days with a final eGFR of ≤ 30 ml/min/1.73 m2 or ≥ 72 days of hemodialysis
AKI on CKD: meeting both AKI and CKD definitions
Normal Renal Function: meeting neither definition
Outcomes
The primary outcome was waitlist mortality defined as death or removal from the waitlist for sickness. Patient follow-up began on the date of listing for liver transplantation and ended at the time of death, removal from the waitlist, or transplant. Patients removed from the waitlist for adequate hepatic reserve or social reasons were censored at the time of their removal.
Statistical analysis
Continuous variables were compared between groups by Wilcoxon rank-sum or Kruskall-Wallis. Categorical variables were compared between groups by chi-squared test.
Unadjusted models were used to assess the association of covariates with the outcomes of interest. All covariates with a p<0.2 in univariate analysis were considered for inclusion in multivariate models. Sequential backward selection was used to eliminate those not reaching significance of p<0.05.
For the analysis of waitlist mortality, competing risk was used in assessing the association between the pattern of renal function with the primary outcome. In this analysis using the Fine-Gray model, removal from the waitlist for transplantation was treated as a competing risk (17). Further, renal function pattern was treated as a time-dependent covariate to allow the pattern of renal function to vary over time. For example, if a patient had normal renal function, then developed AKI that progressed to CKD, this patient would contribute time to the normal, AKI, and CKD groups during follow-up. Postestimation analysis of the final multivariate model included determining the adjusted mean subhazard ratio (SHR) for each pattern of function pattern at 2 MELDNa point intervals from a MELDNa of 6 to 40 (Figure 1).
To determine the added benefit of including renal function pattern in the prediction of waitlist mortality, but not overall mortality risk, bivariate logistic regression was completed. In this analysis, the MELDNa at listing and the renal function pattern at day 90 (the earliest timepoint where patients could have developed CKD or AKI on CKD) were used as predictors to estimate odds ratios and assess the independent association of renal function pattern and waitlist mortality at 6 months. The timepoint of 6 months was specifically chosen to assess the 3-month mortality from the earliest timepoint where renal function pattern can be assessed. The c-statistics for listing laboratory MELDNa score with and without renal function pattern were compared using nonparametric methods (18).
Two-sided p-values <0.05 were considered statistically significant. Analyses were performed using Stata 15.0 statistical software (College Station, TX). This study was approved by the institutional review board at the University of California, San Francisco.
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
RESULTS
Patient Characteristics
From July 1st 2007 through July 1st 2014, 74,771 patients were listed for liver transplantation. A total of 52,091 patients were excluded from this study: 25,372 who received exception points, 1,337 who received a living donor liver transplant, 46 who were listed in error, 2,929 who were less than 18 years old, 2,114 who were listed with fulminant hepatic failure, 20,293 who were listed for less than 90 days. After excluding these patients, a total of 22,680 patients formed the study cohort (Figure 1).
Of the 22,680 patients in the cohort, patients spent a median of 1.6 (IQR 0.7 – 3.1) years on the waitlist, with a median of 5 (IQR 2 – 9) assessments of renal function. The study cohort had a median age of 57 (IQR 51 – 63) years and was comprised of 8,805 (39%) women, 16,572 (73%) Caucasians, and 5,665 (25%) patients with diabetes mellitus. The etiology of cirrhosis was HCV in 8,762 (39%), nonalcoholic steatohepatitis (NASH) in 4,840 (21%), ALD in 4,613 (20%), autoimmune-related in 3,142 (14%), and other in 1,323 (6%) patients. The median MELDNa score at listing was 16 (IQR 13 – 20), with a final or most recent median MELDNa score of 20 (IQR 14 – 29). There were 19,816 episodes of AKI (13% of total follow-up visits). There were 6,965 (31%) patients who met the criteria for CKD at the end of follow up. A total of 2,522 (11%) patients received any hemodialysis during follow up and 2,333 (10%) of patients were listed for SLKT. These patients received a median of 50 (IQR 0 – 259) days of hemodialysis. At the end of follow up, 8,582 (38%) were still waiting for liver transplantation, 7,460 (33%) received a liver transplant and 6,638 (29%) either died on the waitlist or were removed for sickness (Table 1).
Table 1.
Baseline Demographics
| Characteristic | Entire Cohort (n = 22 680) |
|---|---|
| Years on waitlist, m(IQR) | 1.6 (0.7 – 3.1) |
| Age at delisting, m(IQR) | 57 (51 – 63) |
| Female sex, no (%) | 8805 (39) |
| Listing diagnosis, no. (%) | |
| Alcohol | 4613 (20) |
| HCV | 8762 (39) |
| NAFLD/NASH | 4840 (21) |
| Autoimmune1 | 3142 (13) |
| Other | 1323 (6) |
| Non-Hispanic white, no. (%) | 16572 (73) |
| Ascites, no. (%) | 7302 (32) |
| Hepatic encephalopathy, no. (%) | 2997 (13) |
| Diabetes Mellitus, no. (%) | 5665 (25) |
| MELDNa at listing, m(IQR) | 16 (13 – 20) |
| MELDNa at delisting, m(IQR) | 20 (14 – 29) |
| Met CKD, no. (%) | 6965 (31) |
| Hemodialysis during follow-up, no. (%) | 2522 (11) |
| Listed for SLKT, no. (%) | 2333 (10) |
| Waitlist Outcome, no. (%) | |
| Still Waiting | 8582 (38) |
| “Too Sick” or Death | 7460 (33) |
| DDLT | 6638 (29) |
Combined Autoimmune Hepatitis, Primary Sclerosing Cholangitis, and Primary Biliary Cholangitis; Hepatitis C (HCV); Non-Alcoholic Steatohepatitis (NASH); Model for End-Stage Liver Disease with Serum Sodium (MELDNa)
Time-Dependent Competing Risk Survival Analysis for Waitlist Mortality
In univariable analysis, the pattern of renal function was significantly associated with waitlist mortality: CKD compared to normal (SHR 1.82, 95CI 1.70 – 1.95); AKI compared to normal (SHR 3.25, 95CI 3.00 – 3.52); AKI on CKD compared to normal (SHR 4.03, 95CI 3.75 – 4.34). Further, even when treating CKD as the reference group, there remained a significant association with waitlist mortality: Normal compared to CKD (SHR 0.55, 95CI 0.51 – 0.59); AKI compared to CKD (SHR 1.78, 95CI 1.63 – 1.95); AKI on CKD compared to CKD (SHR 2.21, 95CI 2.03 – 2.41).
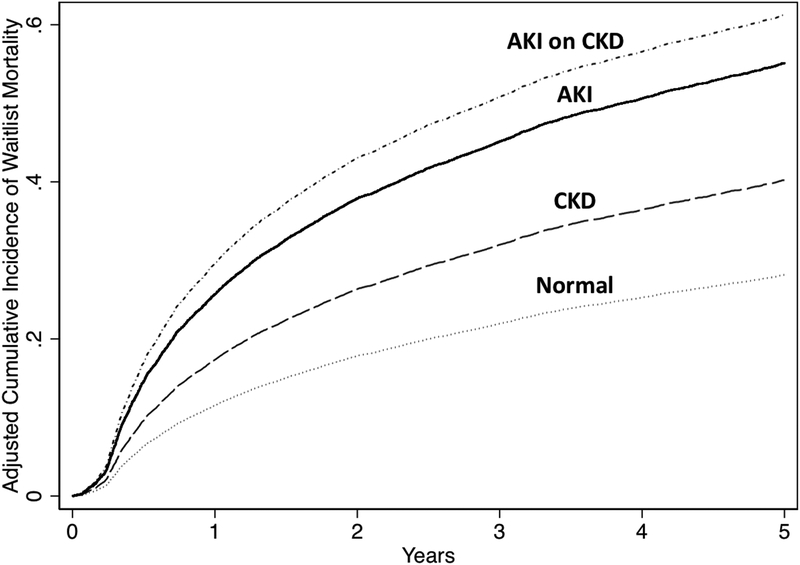
The other factors that were significantly associated with waitlist mortality in univariable analysis after accounting for liver transplantation were: final age (SHR 1.02, 95CI 1.02 – 1.03); female sex (SHR 1.09, 95CI 1.04 – 1.15); HCV compared to ALD (SHR 1.22, 95CI 1.14 – 1.31); NASH compared to ALD (SHR 1.17, 95CI 1.09 – 1.27); autoimmune-related compared to ALD (SHR 0.88, 95CI 0.81 – 0.97); Caucasian race (SHR 0.94, 95CI 0.89 – 0.99); presence of ascites (SHR 1.56, 95CI 1.48 – 1.63); presence of HE (SHR 2.80, 95CI 2.65 – 2.96); presence of diabetes mellitus (SHR 1.30, 95CI 1.23 – 1.37); final MELDNa (SHR 1.05 per 1 point, 95CI 1.05 – 1.06); and final albumin (0.63 per 1 g/dL, 95CI 0.61 – 0.66). In the final multivariate model even after adjusting for confounders, including final MELDNa, final albumin, age, female sex, etiology of cirrhosis, BMI, presence of ascites, presence of HE, presence of diabetes mellitus, region, and blood type the pattern of renal function was significantly associated with waitlist mortality: AKI compared to normal (SHR 2.42, 95CI 2.22 – 2.64), CKD compared to normal (SHR 1.56, 95CI 1.45 – 1.67); AKI on CKD compared to normal (SHR 2.86, 95CI 2.65 – 3.10) (Table 2). Further even after adjustment, both the AKI (SHR 1.56, 95CI 1.43 – 1.72) and the AKI on CKD (SHR 1.85, 95CI 1.70 – 2.02) groups had significantly higher rates of waitlist mortality when compared to the CKD group. The cumulative incidence of waitlist mortality by pattern of renal function is demonstrated in Figure 2.
Table 2.
Time-Dependent Competing Risk Regression Analysis for Waitlist Mortality
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
| SHR | 95% CI | p-value | SHR | 95% CI | p-value | |
| Renal Group | ||||||
| Normal | - | - | - | - | - | - |
| AKI | 3.25 | 3.00 – 3.52 | <0.001 | 2.42 | 2.22 – 2.64 | <0.001 |
| CKD | 1.82 | 1.70 – 1.95 | <0.001 | 1.56 | 1.45 – 1.67 | <0.001 |
| AKI on CKD | 4.03 | 3.75 0 4.34 | <0.001 | 2.86 | 2.65 – 3.10 | <0.001 |
| Age per Year | 1.02 | 1.02 – 1.03 | <0.001 | 1.02 | 1.02 – 1.03 | <0.001 |
| Female Sex | 1.09 | 1.04 – 1.15 | <0.001 | 1.11 | 1.05 – 1.18 | <0.001 |
| Etiology | ||||||
| Alcohol | - | - | - | - | - | - |
| HCV | 1.22 | 1.14 – 1.31 | <0.001 | 1.03 | 0.95 – 1.11 | 0.48 |
| NASH | 1.17 | 1.09 – 1.27 | <0.001 | 0.93 | 0.85 – 1.02 | 0.13 |
| Autoimmune1 | 0.88 | 0.81 – 0.97 | 0.006 | 0.85 | 0.76 – 0.94 | 0.002 |
| Other | 0.96 | 0.85 – 1.08 | 0.48 | 1.01 | 0.88 0 1.16 | 0.85 |
| Non-Hispanic White | 0.94 | 0.89 – 0.99 | 0.02 | |||
| BMI per 1 kg/m2 | 1.00 | 1.00 – 1.01 | 0.08 | 0.99 | 0.98 – 0.99 | <0.001 |
| Ascites | 1.56 | 1.48 – 1.63 | <0.001 | 0.86 | 0.81 – 0.92 | <0.001 |
| HE | 2.80 | 2.65 – 2.96 | <0.001 | 1.91 | 1.79 – 2.05 | <0.001 |
| Diabetes Mellitus | 1.30 | 1.23 – 1.37 | <0.001 | 1.09 | 1.02 – 1.17 | 0.007 |
| KPS at last follow-up per 10 points | 0.94 | 0.93 – 0.95 | <0.001 | |||
| Ventilator Use at Last Follow-Up | 0.96 | 0.55 – 1.67 | 0.88 | |||
| Final MELDNa per point | 1.05 | 1.05 – 1.06 | <0.001 | 1.03 | 1.02 – 1.04 | <0.001 |
| Final Albumin per 1g/dL | 0.63 | 0.61 – 0.66 | <0.001 | 0.68 | 0.65 – 0.70 | <0.001 |
Combined Autoimmune Hepatitis, Primary Sclerosing Cholangitis, and Primary Biliary Cholangitis; Hepatitis C (HCV); Non-Alcoholic Steatohepatitis (NASH); Body Mass Index (BMI); Hepatic Encephalopathy (HE); Karnofosky Performance Score (KPS); Model for End-Stage Liver Disease with Serum Sodium (MELDNa);
adjusted for region and blood type
Figure 2.
Cumulative Incidence Curve for Waitlist Mortality by Renal Function Pattern
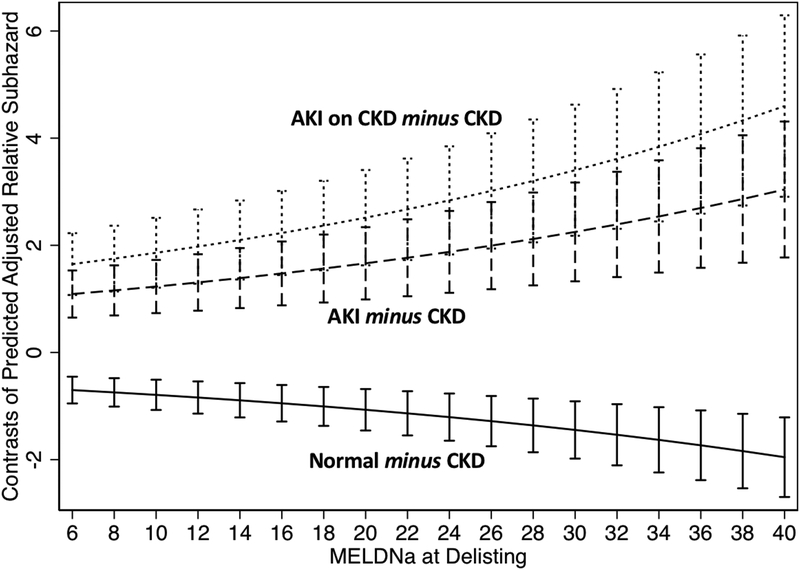
Next, we wanted to explore if this association was present at all levels of the final MELDNa score. We plotted the mean difference of the adjusted SHR between each of the patterns of renal function and CKD (e.g., Normal minus CKD; AKI minus CKD; AKI on CKD minus CKD). This was completed at 2-point intervals from a final MELDNa score of 6 to 40. As highlighted by the confidence intervals, the AKI and AKI on CKD groups had statistically greater adjusted SHR, while the normal group had statistically lower adjusted SHR. Further, as the MELDNa increases the difference between each of the patterns of renal function (AKI, AKI on CKD and normal) and CKD increases (Figure 3).
Figure 3. Contrasts of Adjusted Mean Subhazard Ratio in Reference to CKD at Varying Final MELDNa Scores.
Error bars represent 95% Confidence Intervals
Logistic Regression Models of Renal Function Pattern and Final MELDNa For Waitlist Mortality
In bivariate logistic regression models of patients in our cohort, odds ratios for 6-month (at listing to 180d) waitlist mortality were calculated using MELDNa at listing with and without renal function pattern at day 90 (Table 3). In the univariate models, there was a statistically significant increase in the odds of waitlist mortality for MELDNa at listing (OR 1.14, 95CI 1.13 – 1.16), AKI compared to normal renal function (OR 7.01, 95CI 5.89 – 8.35) and AKI on CKD compared to normal renal function (OR 4.74, 95CI 3.38 – 6.67). In the bivariate model, these same variables remained significant: MELDNa at listing (OR 1.14, 95CI 1.12 – 1.16), AKI compared to normal renal function (OR 6.23, 95CI 5.19 – 7.45), and AKI on CKD compared to normal renal function (OR 3.65, 95CI 2.56 – 5.22). Patients with CKD had an odds of waitlist mortality that was similar to those with normal renal function (OR 0.82, 95CI 0.58 – 1.16). When we examined the ROC curves generated from the logistic regression models to predict 6-month waitlist mortality, the addition of the pattern of renal function to MELDNa at listing increased the accuracy of the model from an AUC of 0.71 to an AUC of 0.80 (p<0.001) (Table 3).
Table 3.
Odds of Waitlist Mortality at 6 Months Based on Pattern of Renal Function, Controlling for MELDNa
| Models and Variables | 6-Month Waitlist Mortality | AUC | ||
|---|---|---|---|---|
| OR | 95% CI | P | ||
| 1 | ||||
| MELDNa at Listing | 1.14 | 1.13 – 1.16 | <0.001 | 0.71* |
| 2 | ||||
| MELDNa at Listing | 1.14 | 1.12 – 1.16 | <0.001 | |
| Renal Group | ||||
| Normal | - | - | - | 0.80* |
| AKI | 6.23 | 5.19 – 7.48 | <0.001 | |
| CKD | 0.82 | 0.58 – 1.16 | 0.27 | |
| AKI on CKD | 3.65 | 2.56 – 5.22 | <0.001 | |
Indicates that AUCs are statistically different at the p<0.05 level
DISCUSSION
In this study evaluating objectively-defined patterns of renal function, we aimed to describe the impact of patterns of renal function on survival in patients with cirrhosis. Confirming prior reports (6), we observed that each pattern of renal function has a varying effect on survival. More specifically, we demonstrated that those with an acute worsening of their renal function (i.e. AKI or AKI on CKD) were at significantly higher risk of waitlist mortality than both those with stable CKD and normal renal function, even after controlling for final MELDNa score. Further, we demonstrated that this effect increased as MELDNa score increased. This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest. In fact, we observed that a model including the renal function pattern and MELDNa score was more accurate at predicting mortality at 6-months than the model including MELDNa score alone, improving the C-statistic from 0.71 to 0.80.
We hypothesize that the mechanism by which renal function patterns have a varying impact on survival in patients with decompensated cirrhosis is related to the etiology of the renal dysfunction. We suspect that those patients who experience AKI and AKI on CKD in our cohort, likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality. These events inherently carry more risk than stable non-liver related elevations in serum creatinine that are seen in patients with CKD. Given this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality. As we have shown in our models, a simple, objective categorization of renal function patterns that we developed in this study may serve as an additive marker in the prediction of waitlist mortality and future studies should focus on the best way to incorporate the pattern of renal function into the MELDNa score in an attempt to maximize its accuracy.
We acknowledge the following limitations to this study. First, there was no standardized protocol for measuring serum creatinine levels. As such, there may be certain patients who are misclassified by renal function pattern in our cohort because of either insufficient data or more frequent assessments. However, the use of UNOS/OPTN dataset provided the power and follow up (median 1.6 years) necessary to overcome this shortcoming and reflects the information that is currently available in clinical practice, enchancing the applicability of our findings. That being said, it was not possible to determine the specific precipitants or etiologies of AKI or CKD from this national data registry, supporting the need for prospective cohorts to inform the natural history of renal dysfunction in patients with cirrhosis. Second, as with any analysis of UNOS registry data, our results are limited by the accuracy of the registry inputs. We minimized any impact input errors may have had in our results by focusing on objective data, such as change in serum creatinine. Third, the definitions of renal function patterns chosen require that patients be followed for a minimum of 90 days. Statistically, the possibility of introducing bias, particularly “immortal time bias” was addressed by treating renal function pattern as a time-dependent covariate (19). However, clinically, it means that our findings are only applicable to those patients who have been followed at a minimum of 90 days and are decompensated enough to require liver transplantation.
Despite these limitations, this is one of the first studies to demonstrate that the pattern of renal function has a significant impact on survival in patients with cirrhosis. Our finding that patients with AKI and AKI on CKD have nearly twice the risk of waitlist mortality compared to those with CKD even after adjusting for MELDNa score has important implications for clinical practice. Specifically, it highlights an opportunity to enhance mortality prediction in patients with cirrhosis. Our data demonstrate that not only can the inclusion of the renal function pattern improve the accuracy of the MELDNa score, it appears that this effect will have a greater impact in patients who are at greatest risk of mortality. The mechanism of this impact and whether these patterns can be identified at the time of listing for liver transplant warrants further study in cohorts with more granular data. Nevertheless, our data, focusing on the impact of the pattern of renal function on mortality in patients with cirrhosis, demonstrate an opportunity to enhance prognostication of mortality and provide the framework for further study of renal dysfunction in all patients with cirrhosis.
What You Need to Know.
Background:
Renal dysfunction increases risk of death for patients with cirrhosis. We investigated whether the risk is the same for patients with acute kidney injury (AKI), chronic kidney disease (CKD), or both.
Findings:
In an analysis of adults on the waitlist for liver transplantation, we found that even after adjusting for confounders including final MELD-Na score, there is a significant association between type of renal dysfunction and mortality in patients with cirrhosis. AKI and CKD increased risk of death almost 3-fold, followed by AKI or CKD alone.
Implications for patient care:
Including information on type of renal dysfunction could improve risk analysis for patients with cirrhosis.
Acknowledgments
Financial Support: This study was funded by K23AG048337 (Paul B. Beeson Career Development Award in Aging Research; Lai) and by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK060414; Cullaro), both of which played no role in the analysis of the data or the preparation of this manuscript
Abbreviations:
- AKI
Acute Kidney Injury
- CKD
Chronic Kidney Disease
- AKI on CKD
Acute on Chronic Kidney Disease
- MELD
Model for End-Stage Liver Disease
- HCC
Hepatocellular Carcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors of this manuscript have conflicts of interest to disclose as described by Gastroenterology: Giuseppe Cullaro – nothing to disclose. Elizabeth C. Verna – Advisory Committees or Review Panels: Gilead; Grant/Research Support: Salix, Merck. Jennifer C. Lai – nothing to disclose.
References
- 1.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48(6):2064–77. [DOI] [PubMed] [Google Scholar]
- 2.Cullaro G, Park M, Lai JC. “Normal” Creatinine Levels Predict Persistent Kidney Injury and Waitlist Mortality in Outpatients with Cirrhosis. Hepatology [Internet]. 2018. April 26 [cited 2018 Jun 22]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29698588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet [Internet]. 2014. May 17 [cited 2018 Jun 8];383(9930):1749–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24480518 [DOI] [PubMed] [Google Scholar]
- 4.Ginès P, Schrier RW. Renal Failure in Cirrhosis. N Engl J Med [Internet]. 2009. September 24 [cited 2017 Nov 4];361(13):1279–90. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMra0809139 [DOI] [PubMed] [Google Scholar]
- 5.Fede G, D’amico G, Arvaniti V, Tsochatzis E, Germani G, Georgiadis D, et al. Renal failure and cirrhosis: A systematic review of mortality and prognosis. J Hepatol [Internet]. 2012. [cited 2018 Jun 21];56:810–8. Available from: https://ac.elscdn.com/S0168827811008531/1-s2.0-S0168827811008531-main.pdf?_tid=79384cad-2e26-4623-8b6cefff1d8b608d&acdnat=1529603672_b3a27d3ed6fafcde9bf3ad400cd4f4a8 [DOI] [PubMed] [Google Scholar]
- 6.Martín–Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, et al. Prognostic Importance of the Cause of Renal Failure in Patients With Cirrhosis. Gastroenterology [Internet]. 2011. February [cited 2018 Jun 8];140(2):488–496. e4 Available from: http://www.ncbi.nlm.nih.gov/pubmed/20682324 [DOI] [PubMed] [Google Scholar]
- 7.Wong F, O’Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, et al. Association of AKI With mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height Contributes to the Gender Difference in Wait-List Mortality Under the MELD-Based Liver Allocation System. Am J Transplant [Internet]. 2010. December 1 [cited 2017 Sep 19];10(12):2658–64. Available from: http://doi.wiley.com/10.1111/j.1600-6143.2010.03326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and Mortality among Patients on the Liver-Transplant Waiting List. N Engl J Med [Internet]. 2008. September 4 [cited 2017 Sep 27];359(10):1018–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18768945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64(4):1–8. [DOI] [PubMed] [Google Scholar]
- 12.Boyle G Simultaneous Liver Kidney ( SLK ) Allocation Policy Simultaneous Liver Kidney ( SLK ) Allocation Policy. Optn/Unos. 2016;1–92. [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med [Internet]. 2009. May 5 [cited 2017 Aug 29];150(9):604–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19414839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krones E, Fickert P, Zitta S, Neunherz S, Artinger K, Reibnegger G, et al. The chronic kidney disease epidemiology collaboration equation combining creatinine and cystatin C accurately assesses renal function in patients with cirrhosis. BMC Nephrol [Internet]. 2015. December 1 [cited 2017 Dec 13];16(1):196 Available from: http://www.biomedcentral.com/1471-2369/16/196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Souza V, Hadj-Aissa A, Dolomanova O, Rabilloud M, Rognant N, Lemoine S, et al. Creatinine-versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis. Hepatology [Internet]. 2014. April [cited 2017 Dec 13];59(4):1522–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24123197 [DOI] [PubMed] [Google Scholar]
- 16.Francoz C, Nadim MK, Baron A, Prié D, Antoine C, Belghiti J, et al. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: validation of current recommendations. Hepatology [Internet]. 2014. April [cited 2017 Dec 13];59(4):1514–21. Available from: http://doi.wiley.com/10.1002/hep.26704 [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. Proportional Hazards Model for the Subdistribution of a Competing Risk A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;1459(April 2013):37–41. [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics [Internet]. 1988. September [cited 2018 Jul 9];44(3):837–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3203132 [PubMed] [Google Scholar]
- 19.Suissa S Immortal Time Bias in Pharmacoepidemiology. Am J Epidemiol [Internet]. 2008. January 7 [cited 2018 Jul 13];167(4):492–9. Available from: https://academic.oup.com/aje/article-lookup/doi/10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]