Abstract
As maternal child health (MCH) programs expand in the setting of HIV, health systems are challenged to reach those most vulnerable and at the greatest need. Cross-sectional surveys of MCH clinics and recent mothers in the Siaya Health Demographic Surveillance System were conducted to assess correlates of accessing antenatal care and facility delivery. Of 376 recent mothers, 93.4% accessed antenatal care and 41.2% accessed facility delivery. Per-kilometer distance between maternal residence and the nearest facility offering delivery services was associated with 7% decreased probability of uptake of facility delivery. Compared with a reference of less than 1 km between home and clinic, a distance of more than 3 km to the nearest facility was associated with 25% decreased probability of uptake of facility delivery. Distance to care was a factor in accessing facility delivery services. Decentralization or transportation considerations may be useful to optimize MCH and HIV service impact in high-prevalence regions.
Keywords: antenatal care, distance, health systems, HIV, prevention of mother-to-child transmission
Interventions for the prevention of mother-to-child transmission (PMTCT) of HIV have become increasingly effective over the past decade, with the potential to decrease infant transmission rates to less than 2% (Shapiro et al., 2010). Although the scale up of PMTCT programs has led to increased delivery of antiretroviral (ARV) interventions, challenges persist in the capacity of health systems to reach those most vulnerable and at the greatest need (World Health Organization, 2007). As a result, the PEPFAR 3.0 guidance promotes a “datadriven approach to strategically target geographic areas and populations where we can achieve the most impact” (Office of the U.S. Global AIDS Coordinator, 2014, p. 7).
Population efficacy of PMTCT requires not only effective ARV regimens but also complete engagement at all steps of the PMTCT cascade of care—from accessing antenatal care (ANC) services, to HIV testing, to initiation of and adherence to PMTCT ARV interventions (Hamilton et al., 2017). Losses to care throughout the PMTCT cascade are associated with individual, community, and health systems factors (Kebaabetswe, 2007; Mandala et al., 2009; Mbonye, Hansen, Wamono, & Magnussen, 2010; Uwimana, Jackson, Hausler, & Zarowsky, 2012), including maternal failure to access ANC and facility deliveries (Kinuthia et al., 2015; Kohler, Okanda, et al., 2014), the venues at which PMTCT ARVs are delivered. A few studies have identified distance to these health services as a barrier to PMTCT uptake (Escamilla et al., 2015; Peltzer, Mosala, Shisana, Nqueko, & Mngqundaniso, 2007). In the most recent Kenya AIDS Indicator Survey, the most common response to why women did not attend antenatal care was that the clinic distance was too far (32.5%; National AIDS and STI Control Programme, 2014). Conversely, it has been suggested that HIV-related stigma, which has also been associated with decreased uptake of PMTCT services in a number of settings (Kohler, Ondenge, et al., 2014; Turan & Nyblade, 2013), might encourage women to seek care further from their homes in fear of being seen by fellow community members or lack of confidentiality by providers (Chung, Kohler, Attwa, Thiga, & John-Stewart, 2010; Munyaneza et al., 2018; Nakigozi et al., 2013; Theilgaard et al., 2011).
Geographical information system (GIS) mapping is a tool to identify health service gaps or areas with limited access to health care services and allows a quantitative exploration of the effect of geographic distance on the delivery of health care services (Ackers et al., 2014; Akullian, Mukose, Levine, & Babigumira, 2016; Chamla et al., 2007; Feikin et al., 2009; Fulcher & Kaukinen, 2005; Kaukinen & Fulcher, 2006; Papo, Bauni, Sanders, Brocklehurst, & Jaffe, 2011). GIS has not yet been widely implemented for assessing access to PMTCT services, although emerging evidence suggests that it can be used to identify critical distance cutoffs where women become less likely to access care and can inform policies such as decentralization of health services (Akullian et al., 2016; Escamilla et al., 2015).
The aim of our study was to map the service provision landscape for maternal child health (MCH) and PMTCT services in rural Western Kenya. The mapping and description of PMTCT service provision, linked together with maternal household survey data, enabled the assessment of distance to the nearest health facility on utilization and uptake of maternal health services for antenatal and delivery care.
Methods
Study Design
Our study was a secondary analysis of a community- based study that aimed to assess access to maternal HIV prevention services in Kenya (Kohler, Okanda, et al., 2014). A cross-sectional survey of MCH facilities was performed within the Health Demographic Surveillance System (HDSS) area in Siaya County, Kenya. Concurrently, a community-based survey was conducted among women in the region who had delivered an infant within the past year to assess which health facility women attended at last pregnancy, if any, as well as rates and correlates of uptake of services.
Study Location
The Siaya HDSS covers 383 rural villages with a population of approximately 220,000 in Nyanza Province, Kenya. Three regions make up this area: Karemo, Gem, and Asembo. Launched in September 2001 by the U.S. Centers for Disease Control and Prevention (CDC) in collaboration with the Kenya Medical Research Institute, the HDSS serves as a community-based platform for monitoring health outcomes. The HDSS provides demographic and health information as well as disease- and intervention-specific information (Adazu et al., 2005).
Study Population and Sample
All health facilities offering private, public, or mission-based practices, including ANC, labor and delivery, or PMTCT services, in the HDSS area were included. The facility manager or nurse providing the majority of antenatal HIV services was recruited to provide information about the facility. Eligible community-based participants were women, ages 14 years and older (the age of emancipation for mothers in Kenya), who resided in the HDSS area, and had delivered an infant between January and December, 2010 (n = 405). A comprehensive list of all public and private facilities in the HDSS region was generated. The study team accessed a number of sources to identify all providers of maternal health services in the HDSS region. These included meetings with District AIDS and Sexually Transmitted Infection Coordinators and District Medical Officers, area program managers of nongovernmental organizations supporting local facilities in the implementation of PMTCT, the eHealth-Kenya database Web site (Ministry of Health, 2011), and HIV research study and HDSS regional and community mobilization coordinators. For maternal surveys, samples were generated by the HDSS database and stratified by region: a random sample in Gem HDSS and a comprehensive sample of women living with HIV (WLWH) from Karemo and Asembo, where home-based counseling and testing had been implemented.
Data Collection
Geospatial mapping and surveys of the health facilities and women in the community were conducted simultaneously in 2011. Health facility surveys included information about services offered, PMTCT policies, and estimated numbers of clients. Maternal surveys included information about the health facility accessed for ANC and delivery care at last pregnancy, validated stigma indicators (Nyblade et al., 2005), method of transportation to care, and outcomes of uptake of care along the PMTCT cascade. Trained fieldworkers administered surveys and recorded GPS locations on handheld mobile devices using electronic forms (Pendragon Software Corporation, Buffalo Grove, IL). GPS coordinates of facilities that women reported attending outside the survey area were obtained from the Kenya Master Health Facility Web site and Google Maps.
Data Analysis
Each woman reported the health facility she attended at last pregnancy, and this information was used to link facility-level data to maternal outcomes. Fieldworkers were blinded to HIV status of community participants, even when status was known to the sampling team, thus self-report of HIV status was used for all analyses. Statistical analyses were restricted to the random sample. Descriptive measures included frequency, proportions, and medians with interquartile ranges. Chi-square tests were used to test univariate associations. Multivariate relative risk regression with log binomial link functions using STATA (STATACorp., College Station, TX) were used to test for associations between distance in kilometers from maternal residence to the nearest ANC or delivery services and uptake of MCH services. Analyses were adjusted for hypothesized confounding variables: age, education, stigma, and HIV status. Visualization of spatial data and calculation of geographic measurements (distance to the nearest health facility and distance to the health facility attended) were completed using ArcGIS software (Esri, Redlands, CA).
Ethical Considerations
Before study started, community engagement activities were held targeting the area senior medical officers, the local community advisory board, village reporters, chiefs, and assistant chiefs. Letters of support were obtained from area district and province medical officers, and the study was approved by the University of Washington Institutional Review Board (#36022) and the Kenya Medical Research Institute Ethical Review Committee (#1714). Written informed consent was obtained from all participants, and women in the community consented both to participate in the study and also to have data from the surveys linked to their HDSS records.
Results
Services and Characteristics of Maternal Health Facilities
Overall, 41 public, private, and mission health facilities were identified. One service provider declined to participate, resulting in a sample of 40 providers (Table 1). Most facilities were public government facilities (80%), with lowest tier dispensaries being the most prevalent (70%). All facilities offered general health care services, including ANC. Other services included family planning (95%), sexually transmitted infection services (93%), and tuberculosis care (75%); 38 facilities (95%) offered HIV testing. The two that did not offer HIV testing were private clinics. Delivery services were offered at 85% of facilities; 95% of delivery facilities offered HIV testing for women with no prior test, and 80% regularly offered ARVs for PMTCT to women delivering at the facility. Thirtythree facilities (83%) reported offering some PMTCT services for pregnant women, and 26 facilities (65%) reported offering ARVs for mothers and infants through the breastfeeding period. Eighty-five percent (n = 34) of the facilities reported that ARVs for PMTCT were available for infants.
Table 1.
Maternal Child Health Services in the Kenya Health Demographic Surveillance System, 2011
| Facility Information | N = 40, n (%) or median (IQR) |
|---|---|
| Type of facility | |
| District hospital | 2 (5.0) |
| Subdistrict hospital | 3 (7.5) |
| Health center | 1 (2.5) |
| Dispensary | 28 (70.0) |
| Mission hospital | 2 (5.0) |
| Private clinic | 2 (5.0) |
| Private hospital | 2 (5.0) |
| Managing authority | |
| Government | 32 (80.0) |
| Mission/Church | 4 (10.0) |
| NGO | 1 (2.5) |
| Private for-profit | 2 (5.0) |
| Other: government-NGO collaboration | 1 (2.5) |
| Services offered | |
| General health care | 40 (100.0) |
| Pediatric care | 39 (97.5) |
| ANC | 40 (100.0) |
| Family planning | 38 (95.0) |
| STI services | 37 (92.5) |
| TB care | 30 (75.0) |
| Estimated number of women seen per month for ANC | 40 (19–69) |
Note. ANC = antenatal care; IQR = interquartile range; NGO = nongovernmental organization; STI = sexually transmitted infection; TB = tuberculosis.
Uptake and Distance to Antenatal Care and Facility Delivery Services
In geospatial statistical analyses, we included women from the random sample with available GPS information (n = 376, 93%). Of these women, 351 (93%) accessed ANC care (Table 2). Median travel distance for ANC care was 2.5 km (interquartile range [IQR] = 1.6–3.7), and did not differ by HIV status (p = .39). Of the 337 women reporting travel time to a facility, the median travel time was 1 hr (IQR = 0.5–1.5). Most (87%) reported walking to the facility to receive ANC. Fewer than half of the women in the sample, 155 (41%), accessed a health facility for delivery services. Median travel distance for delivery was 2.7 km (95% confidence interval [CI], 1.5–5.2 km), which also did not differ by HIV status (p = .61). Reported mean travel time for facility delivery was 0.9 hr (IQR = 0.3–1.5), longer for WLWH (1.5 hr; IQR = 0.75–2) than uninfected women (0.5 hr; IQR = 0.3–1.5; p = .009). More WLWH (61%) walked to delivery services than uninfected women (50%), although differences in the mode of transportation were not statistically significant.
Table 2.
Uptake and Distance to Services by HIV Status for Recent Mothers in Kenya, 2011
| All Mothers,a
N = 376 |
Mothers Living With HIV, n = 41 |
Uninfected Mothers, n = 296 |
p Value | |
|---|---|---|---|---|
| N (%) or Median (IQR) | n (%) or Median (IQR) | n (%) or Median (IQR) | ||
| Mothers accessing ANC | 351 (93.4) | 41 (100.0) | 280 (94.6) | .13 |
| Kilometers traveled to ANC | 2.5 (1.6–3.7) | 2.0 (1.5–3.4) | 2.5 (1.6–3.7) | .39 |
| Travel time to ANC (in hrs) | 1.0 (0.5–1.5) | 1.0 (0.7–1.5) | 1.0 (0.5–1.5) | .40 |
| Walked to ANC | 305 (86.9) | 37 (90.2) | 240 (85.7) | .63 |
| Accessed delivery facility | 155 (41.2) | 28 (68.3) | 115 (38.9) | <.001 |
| Kilometers traveled to delivery facility | 2.7 (1.5–5.2) | 3.0 (1.7–13.6) | 2.7 (1.6–5.0) | .61 |
| Travel time to delivery facility (in hrs) | 0.9 (0.3–1.5) | 1.5 (0.9–2) | 0.5 (0.3–1.5) | .009 |
| Walked to delivery facility | 85 (55.2) | 17 (60.7) | 58 (50.0) | .40 |
Includes 39 women with missing HIV status.
Note. ANC = antenatal care.
Correlates of Antenatal Care Uptake and Facility Delivery
In the analysis adjusted for residential distance to the nearest facility providing ANC services, mothers who reported having HIV were 7% more likely to have sought ANC care during their pregnancies than those reporting negative status (adjusted risk ratio [aRR] = 1.07; 95% CI, 1.03–1.11; p ≤ .001; Table 3). Mothers with primary education were also more likely to access ANC (aRR = 1.06; 95% CI, 1.01–1.11; p = .01). Distance to the nearest clinic from home was not significantly associated with the uptake of ANC services. Stigma measures of shame and blame were not significantly associated with distance to ANC.
Table 3.
Factors Associated With Uptake of Care in Recent Mothers in Kenya, 2011
| Adjusted Relative Risk aRR (95% CI) | p Value | |
|---|---|---|
| Uptake of ANC | ||
| HIV status | 1.07 (1.03–1.11) | .001 |
| Maternal age (yrs) | 1.00 (0.99–1.00) | .326 |
| Primary education | 1.06 (1.01–1.11) | .014 |
| Stigma indicator: shame | 0.98 (0.93–1.03) | .341 |
| Kilometer distance to nearest facility | ||
| <1 km | Ref | |
| 1–2 km | 0.99 (0.94–1.05) | .843 |
| 2–3 km | 0.98 (0.92–1.05) | .590 |
| 3+ km | 0.97 (0.87–1.08) | .552 |
| Facility delivery | ||
| HIV status | 1.25 (1.14–1.37) | <.001 |
| Maternal age (yrs) | 1.00 (0.99–1.00) | .391 |
| Primary education | 1.09 (1.02–1.17) | .014 |
| Stigma indicator: shame | 1.01 (0.94–1.08) | .819 |
| Kilometer distance to nearest facility | ||
| < 1 km | Ref | |
| 1–2 km | 0.85 (0.76–0.94) | .001 |
| 2–3 km | 0.83 (0.74–0.92) | <.001 |
| 3+ km | 0.75 (0.66–0.85) | <.001 |
Note. ANC = antenatal care.
In the analysis adjusted for residential distance to the nearest delivery facility, those reporting HIV infection were 25% more likely to deliver in a facility compared with those reporting negative HIV status (aRR = 1.25; 95% CI, 1.14–1.37; p < .001). Residential distance to the nearest facility providing delivery services was associated with the likelihood of a women delivering at a facility; for every additional 1 km in residential distance from the nearest delivery facility, the probability of delivery in a facility declined by 7% (aRR = 0.93; 95% CI, 0.89–0.97; p ≤ .001). Compared with a reference of less than 1 km distance between home and clinic, 1–2 km residential distance was associated with 15% decreased probability of uptake of facility delivery (aRR = 0.85; 95% CI, 0.76–0.94; p = .001), 2–3 km distance between home and nearest clinic was associated with 17% decreased probability of uptake of facility delivery (aRR = 0.83; 95% CI, 0.74–0.92; p < .001), and more than 3 km between home and the nearest facility was associated with 25% decreased probability of uptake of facility delivery (aRR = 0.75; 95% CI, 0.66–0.85; p < .001). Stigma was also not associated with distance to delivery care.
Facility Characteristics and Maternal Reports of Access to Care
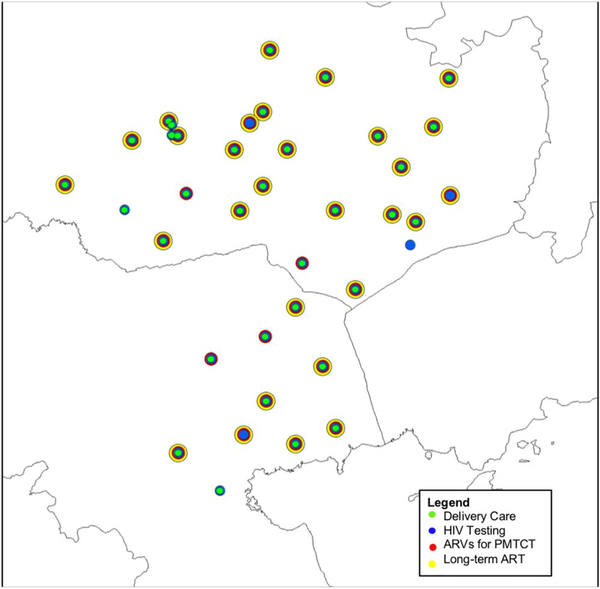
Service availability by site is shown in Figure 1. Twelve of the 40 facilities were not attended by any women who participated in the survey. Public facilities (78%) were more likely to be attended by surveyed women versus 38% of private facilities (p = .03). Facilities offering CD4+ T-cell and polymerase chain reaction testing were more commonly accessed (79% and 83%, respectively) than those that did not (21% and 17%; p = .06 and .01). No WLWH in our survey reported attending private clinics. WLWH who attended the mission-based hospital facilities in the region for ANC reported significantly higher uptake of maternal ARVs (97%) than those who attended government facilities (80%; p = .03); the same was true for the uptake of infant ARVs, with 100% of those attending mission facilities reporting administering infant ARVs compared with 77% of those attending government facilities (p < .001).
Figure 1.
Map of surveyed health facilities by services offered. Note. ARV = antiretroviral; PMTCT = prevention of mother-to-child transmission.
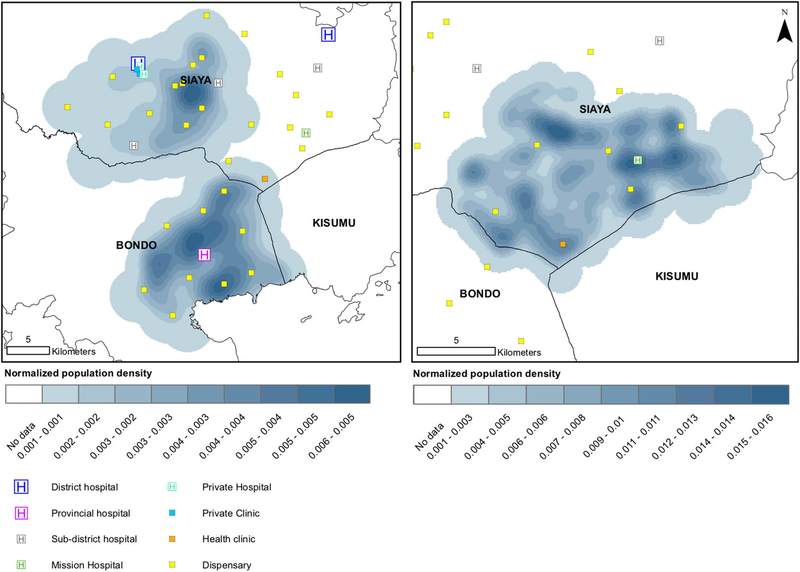
Population Density
Population density in the random sample of women relative to the location of health facilities, by facility level, is shown in Figure 2 (random sample, left; WLWH oversample, right). Most health facilities appeared close to population centers, although there are some areas of population density greater than 5 km from any care facilities, and the closest care was usually a dispensary.
Figure 2.
Map of providers relative to population density in the random sample (left) and oversample of people living with HIV (right).
Discussion
Our 2011 study comprehensively mapped and assessed all facilities offering PMTCT, including public, private, and mission facilities in the Siaya HDSS and linked this information to a community-based survey of women who had delivered an infant in the previous year. Generally, we found high availability (>80%) of HIV testing and ARVs for PMTCT. Most women did not deliver at a facility, and WLWH delivered in a facility more often than uninfected women. Although the distance traveled by WLWH and uninfected women was similar, WLWH had a longer travel time to delivery facilities, possibly reflecting more limited transportation resources. Health services generally mapped to population-dense centers, and we identified some areas with high population density, in which higher tier comprehensive services were limited.
Although some WLWH and uninfected women bypassed the closest facility, self-reported HIV stigma was not associated with the distance to care facility accessed for WLWH. Facilities that were not attended by women offered fewer services, suggesting that women sought a higher level of care and were not necessarily avoiding clinics where they may have been known in the community. This was supported by a Ugandan study showing that people living with HIV were only likely to bypass the nearest facility if that facility lacked ARV services (Akullian et al., 2016) and an HDSS study that found that more severe illness was associated with traveling further for pediatric care (Feikin et al., 2009). However, one study in Rwanda noted an increased likelihood of missed visits by adults initiating HIV care who lived less than 1 km from the health facility (Munyaneza et al., 2018).
As prevention and treatment programs try to reach more with fewer resources, it is unclear whether a centralized hub with comprehensive care or a decentralized approach, where services are more widely available, is the most effective and appropriate method to deliver services (Menzies et al., 2011; Siapka et al., 2014). Our data suggest that WLWH were likely to perceive the health benefits of attending HIV centers of excellence and may have been willing to travel some distance to reach it; however, there also appeared to be a critical cutoff point where distance became prohibitive. WLWH accessed facility delivery more frequently than uninfected women, perhaps recognizing the need for care. Importantly, the 25% reduction in the likelihood of accessing facility delivery services at 3 km distance has implications not only for HIV services but also for maternal and neonatal mortality. Our findings were consistent with a study from Zambia, in which every kilometer increase in distance resulted in a 10% reduction in the odds of receiving PMTCT services (Escamilla et al., 2015). This suggests marked losses in the uptake of services where services were far from the population in need. Scale-up of innovations, such as birthing centers, transport vouchers, clinic- or nurse-led text messaging, or ambulance services, may have dual HIV/MCH benefit for women who live far from health care services.
Our study was novel in its approach to use GIS mapping to assess PMTCT service availability in a rural community in Kenya and the ability to link data between the majority of women in the study area with the facilities they accessed. Limitations included the cross-sectional nature of the surveys, limited data on other social factors that may have influenced service uptake, and limited information on facilities that women attended outside the HDSS. The analysis also took place during a period of rapid transition of PMTCT policies prior to implementation of Option B+ and thus may not reflect current practice.
Conclusions
Geographical Information System mapping can provide an important tool for HIV service delivery planning. Although targeted centers of excellence may attract motivated clients, residential distance to health facilities appeared to significantly reduce uptake of services. Stigma was not noted to be a factor in this analysis; however, future research should explore the roles of stigma and distance in seeking care. Further decentralization or transport considerations may be useful to optimize MCH and HIV prevention service impact in HIV high-prevalence areas.
Key Considerations.
Geospatial mapping and analyses can be important tools for HIV service delivery planning.
The farther women live from a service facility, the lesser the uptake of facility-based delivery services.
Decentralization or transport considerations may be useful to optimize MCH and HIV prevention impact in HIV high-prevalence areas.
Acknowledgments
The study was funded through the A Kenya Free of AIDS grant (R24HD056799, PI: Morris/John-Stewart) from the National Institutes of Health and received assistance from the University of Washington Center for AIDS Research (P30 AI027757, PI: Holmes) and Pediatric HIV-1 in Africa (K24HD054314, PI: John-Stewart). The authors gratefully acknowledge the support and advice of Dr. Martina Morris, Dr. Barbra Richardson, Dr. Nancy Woods, and Dr. Lisa Manhart; the UW Center for Integrated Health of Women, Adolescents, and Children (Global WACh); the Director of the Kenya Medical Research Institute; and the Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System team.
Footnotes
Disclosures
This article was published with the approval of the Director, Kenya Medical Research Institute. All authors report no financial interests or potential conflicts of interest.
Contributor Information
Pamela K. Kohler, Associate Professor, Departments of Global Health and Psychosocial & Community Health, University of Washington, Seattle, Washington, USA..
Adam Akullian, Affiliate Assistant Professor, Department of Global Health, University of Washington, Seattle, Washington, USA..
John Okanda, Research Officer, KEMRI & CDC Research and Public Health Collaboration, Kisumu, Kenya..
George Otieno, Senior Data Analyst, KEMRI & CDC Research and Public Health Collaboration, Kisumu, Kenya..
John Kinuthia, Head of Research and Programs, Kenyatta National Hospital, Nairobi, Kenya..
Joachim Voss, Professor, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA..
Brenda Zierler, Professor, Department of Biobehavioral Nursing and Health Informatics, University of Washington, Seattle, Washington, USA..
Grace John-Stewart, Professor, Departments of Global Health, Medicine, Pediatrics, and Epidemiology, University of Washington, Seattle, Washington, USA..
References
- Ackers ML, Hightower A, Obor D, Ofware P, Ngere L, Kubaje A, & Laserson KF (2014). Health care utilization and access to human immunodeficiency virus (HIV) testing and care and treatment services in a rural area with high HIV prevalence, Nyanza Province, Kenya, 2007. The American Journal of Tropical Medicine and Hygiene, 90, 224–233. doi: 10.4269/ajtmh.13-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adazu K, Lindblade KA, Rosen DH, Odhiambo F, Ofware P, Kwach J,Slutsker L (2005).Health and demographic surveillance in rural western Kenya: A platform for evaluating interventions to reduce morbidity and mortality from infectious diseases. The American Journal of Tropical Medicine and Hygiene, 73, 1151–1158. doi: 10.4269/ajtmh.2005.73.1151 [DOI] [PubMed] [Google Scholar]
- Akullian AN, Mukose A, Levine GA, & Babigumira JB (2016). People living with HIV travel farther to access healthcare: A population-based geographic analysis from rural Uganda. Journal of the International AIDS Society, 19, 20171. doi: 10.7448/ias.19.1.20171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamla DD, Olu O, Wanyana J, Natseri N, Mukooyo E, Okware S,George M(2007).Geographical information system and access to HIV testing, treatment and prevention of mother-to-child transmission in conflict affected Northern Uganda. Conflict and Health, 1, 12. doi: 10.1186/1752-1505-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MH, Kohler P, Attwa M, Thiga J, & John-Stewart GC (2010). Comparing clinic retention between residents and nonresidents of Kibera, Kenya. Journal of Acquired Immune Deficiency Syndromes, 53, 422–424. doi: 10.1097/QAI.0b013e3181b32bd6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla V, Chibwesha CJ, Gartland M, Chintu N, Mubiana-Mbewe M, Musokotwane K, Chi BH (2015). Distance from household to clinic and its association with the uptake of prevention of mother-to-child HIV transmission regimens in rural Zambia. Journal of Acquired Immune Deficiency Syndromes, 70, e94–e101. doi: 10.1097/qai.0000000000000739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feikin DR, Nguyen LM, Adazu K, Ombok M, Audi A, Slutsker L,& Lindblade KA (2009).The impact of distance of residence from a peripheral health facility on pediatric health utilisation in rural western Kenya. Tropical Medicine and lnternational Health, 14,54–61. doi: 10.1111/j.1365-3156.2008.02193.x [DOI] [PubMed] [Google Scholar]
- Fulcher C, & Kaukinen C (2005). Mapping and visualizing the location of HIV service providers: An exploratory spatial analysis of Toronto neighborhoods. AIDS Care, 17, 386–396. doi: 10.1080/09540120512331314312 [DOI] [PubMed] [Google Scholar]
- Hamilton E, Bossiky B, Ditekemena J, Esiru G, Fwamba F, Goga AE, Guay L (2017).Using the PMTCT cascade to accelerate achievement of the global plan goals. Journal of Acquired Immune Deficiency Syndromes, 75, S27–S35. doi: 10.1097/QAI.0000000000001325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukinen C,& Fulcher C (2006).Mapping the social demography and location of HIV services across Toronto neighbourhoods. Health and Social Care in the Community, 14, 37–48. doi: 10.1111/j.1365-2524.2005.00595.x [DOI] [PubMed] [Google Scholar]
- Kebaabetswe PM (2007). Barriers to participation in the prevention of mother-to-child HIV transmission program in Gaborone, Botswana a qualitative approach. AIDS Care, 19, 355–360. doi: 10.1080/09540120600942407 [DOI] [PubMed] [Google Scholar]
- Kinuthia J, Kohler P, Okanda J, Otieno G, Odhiambo F, & John-Stewart G (2015). A community-based assessment of correlates of facility delivery among HIV-infected women in western Kenya. BMC Pregnancy and Childbirth, 15, 46. doi: 10.1186/s12884-015-0467-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler PK, Okanda J, Kinuthia J, Mills LA, Olilo G, Odhiambo F, ... John-Stewart G (2014). Community-based evaluation of PMTCT uptake in Nyanza Province, Kenya. PLoS One, 9, e110110. doi: 10.1371/journal.pone.0110110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler PK, Ondenge K, Mills LA, Okanda J, Kinuthia J, Olilo G, ...John-Stewart G (2014). Shame, guilt, and stress: Community perceptions of barriers to engaging in prevention of mother to child transmission (PMTCT) programs in western Kenya. AIDS Patient Care and STDS, 28, 643–651. doi: 10.1089/apc.2014.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala J,Torpey K,Kasonde P,Kabaso M, Dirks R, Suzuki C, Mukadi YD (2009). Prevention of mother-to-child transmission of HIV in Zambia: Implementing efficacious ARV regimens in primary health centers. BMC PublicHealth, 9, 314. doi: 10.1186/1471-2458-9-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonye AK, Hansen KS,Wamono F, & Magnussen P (2010). Barriers to prevention of mother-to-child transmission of HIV services in Uganda. Journal of Biosocial Science, 42, 271–283. doi: 10.1017/S002193200999040X [DOI] [PubMed] [Google Scholar]
- Menzies NA, Berruti AA, Berzon R, Filler S, Ferris R, Ellerbrock TV, & Blandford JM (2011). The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS, 25,1753–1760. doi: 10.1097/QAD.0b013e3283463eec [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health. (2011). e-Health Kenya. Retrieved from www.ehealth.or.ke/facilities
- Munyaneza F, Ntaganira J, Nyirazinyoye L, Birru E, Nisingizwe MP, Gupta N,…Hedt-Gauthier BL (2018). Community-based accompaniment and the impact of distance for HIV patients newly initiated on antiretroviral therapy: Early outcomes and clinic visit adherence in rural Rwanda. AIDS and Behavior, 22, 77–85. doi: 10.1007/s10461-016-1658-5 [DOI] [PubMed] [Google Scholar]
- Nakigozi G, Atuyambe L, Kamya M, Makumbi FE, Chang LW, Nakyanjo N, ... Gray R (2013). A qualitative study of barriers to enrollment into free HIV care: Perspectives of never- in-care HIV-positive patients and providers in Rakai, Uganda. Biomed Research International, 470245, 1–7. doi: 10.1155/2013/470245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National AIDS and STI Control Programme. (2014). Kenya AIDS Indicator Survey 2012. Retrieved from http://statistics.knbs.or.ke/nada/index.php/catalog/82
- Nyblade L, MacQuarrie K, Phillip F, Kwesigabo G, Mbwambo J, Ndega J, Stangl A (2005). Working report measuring HIV stigma: Results of a field test in Tanzania. Retrieved from https://www.icrw.org/wp-content/uploads/2016/10/Working-Report-Measuring-HIV-Stigma-Results-of-a-Field-Test-in-Tanzania.pdf
- Office of the U.S. Global AIDS Coordinator. (2014). PEPFAR 3.0 Controlling the epidemic: Delivering on the promise of an AIDS-free generation. Retrieved from http://www.pepfar.gov/documents/organization/234744.pdf
- Papo JK, Bauni EK, Sanders EJ, Brocklehurst P, & Jaffe HW (2011). Exploring the condom gap: Is supply or demand the limiting factor - condom access and use in an urban and a rural setting in Kilifi district, Kenya. AIDS, 25,247–255. doi: 10.1097/QAD.0b013e328341b9b8 [DOI] [PubMed] [Google Scholar]
- Peltzer K, Mosala T, Shisana O, Nqueko A, & Mngqundaniso N (2007). Barriers to prevention of HIV transmission from mother to child (PMTCT) in a resource poor setting in the Eastern Cape, South Africa. African Journal of Reproductive Health, 11, 57–66. doi: 10.2307/30032488 [DOI] [PubMed] [Google Scholar]
- Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C,... Essex M (2010). Antiretroviral regimens in pregnancy and breast-feeding in Botswana. The New England Journal of Medicine, 362, 2282–2294. doi: 10.1056/NEJMoa0907736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapka M, Remme M, Obure CD, Maier CB, Dehne KL, & Vassall A (2014). Is there scope for cost savings and efficiency gains in HIV services? A systematic review of the evidence from low- and middle-income countries. Bulletin of the World Health Organization, 92, 499–511AD. doi: 10.2471/blt.13.127639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilgaard ZP, Katzenstein TL, Chiduo MG,Pahl C,Bygbjerg IC, Gerstoft J, Tersbøl BP (2011). Addressing the fear and consequences of stigmatization - a necessary step towards making HAART accessible to women in Tanzania: A qualitative study. AIDS Research and Therapy, August 2;8:28. doi: 10.1186/1742-6405-8-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan JM, & Nyblade L (2013). HIV-related stigma as a barrier to achievement of global PMTCT and maternal health goals: A review of the evidence. AIDS and Behavior, 17, 2528–2539. doi: 10.1007/s10461-013-0446-8 [DOI] [PubMed] [Google Scholar]
- Uwimana J, Jackson D, Hausler H, & Zarowsky C (2012). Health system barriers to implementation of collaborative TB and HIV activities including prevention of mother to child transmission in South Africa. Tropical Medicine and International Health, 17, 658–665. doi: 10.1111/j.1365-3156.2012.02956.x [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2007). Everybody’s business: Strengthening health systems to improve health outcomes: WHO’s Framework for Action. Retrieved from http://www.who.int/healthsystems/strategy/everybodys_business.pdf