Abstract
In cystic fibrosis (CF), ductal plugging and acinar loss result in rapid decline of exocrine pancreatic function. This destructive process results in remodeled islets, with only a modest reduction in insulin producing β cells. However, β-cell function is profoundly impaired, with decreased insulin release and abnormal glucose tolerance being present even in infants with CF. Ultimately, roughly half of CF subjects develop diabetes (termed CF-related diabetes, CFRD). Importantly, CFRD increases CF morbidity and mortality via worsening catabolism and pulmonary disease. Current accepted treatment options for CFRD are aimed at insulin replacement, thereby improving glycemia as well as preventing nutritional losses and lung decline. CFRD is a unique form of diabetes with a distinct pathophysiology that is as yet incompletely understood. Recent studies highlight emerging areas of interest. First, islet inflammation and lymphocyte infiltration are common even in young children with CF and may contribute to β-cell failure. Second, controversy exists in the literature regarding the presence/importance of β-cell intrinsic functions of CFTR and its direct role in modulating insulin release. Third, loss of the CF transmembrane conductance regulator (CFTR) from pancreatic ductal epithelium, the predominant site of its synthesis, results in paracrine effects that impair insulin release. Finally, the degree of β-cell loss in CFRD does not appear sufficient to explain the deficit in insulin release. Thus, it may be possible to enhance the function of the remaining β cells using strategies such as targeting islet inflammation or ductal CFTR deficiency to effectively treat or even prevent CFRD.
Keywords: cystic fibrosis, diabetes, insulin secretion, beta-cell mass, inflammation
Overview
Cystic fibrosis (CF) is the most common markedly life-shortening autosomal recessive genetic condition in people of Northern European descent, affecting approximately 0.05% of live births. It is caused by loss-of-function mutations in the CF transmembrane conductance regulator (CFTR, Table 1), resulting in disruption of anion transport across epithelial cells predominantly in lung, gut and pancreas. This results in a severe multisystem disease, with premature death usually occurring secondary to the resultant pulmonary disease. Significant improvements in the pulmonary care of patients with cystic fibrosis (CF) have therefore resulted in substantially greater survival.
Table 1:
CFTR Mutations
| Mutation Class | Effect on CFTR | Example | Disease | Pancreatic insufficiency risk (Ahmed, et al. 2003) |
Diabetes risk (Adler, et al. 2008) |
|---|---|---|---|---|---|
| I | failed synthesis | G542X | Severe | High | High |
| II | failed protein processing | F508del | Severe | High | High |
| IV | reduced channel function | R117H | Less-severe | Low | Low |
| V | reduced synthesis or processing | A455E | Less-severe | Low | Low |
The genetics of CF are recessive, and the severity of CF disease is governed by the least severe CFTR mutation a patient carries. In most Caucasian populations, F508del is the most common mutation by far, and most CF patients have severe disease.
The Pancreas in Cystic Fibrosis
CFTR is essential to the function of the exocrine pancreas. Approximately 85% of CF patients develop severe exocrine pancreatic disease marked by exocrine pancreatic insufficiency (Wilschanski and Novak 2013). In these patients, pancreatic enzyme replacement is essential to normal growth and weight gain. The risk of pancreatic insufficiency is related to CFTR mutation severity (Table 1). CFTR is highly expressed in the epithelial cells of small pancreatic ducts that drain the pancreatic acini (Marino, et al. 1991) where it functions to drive secretion of chloride, water, and bicarbonate into the ducts. This serves to dilute and increase the pH of the viscous digestive enzyme secretions from pancreatic acinar cells and thereby (i) facilitates their movement through the ductal system for delivery into the intestinal lumen and (ii) helps prevent premature activation of these proteases. When CFTR function is absent, these small ducts become dilated and plugged with the thick secretions representing the earliest pathology observed in CF pancreatic disease. Widespread acinar destruction ensues, accompanied by inflammatory cell infiltration, fatty replacement, appearance of extensive fibrosis, and cystic dilation of larger ducts (Figure 1). This can begin in utero, and is extensive by 1–4 years of age such that very little acinar tissue remains (Andersen 1958). In many CF patients, the non-endocrine portion of the pancreas ultimately becomes comprised largely of adipose tissue. Importantly, the endocrine pancreas generally survives this process, albeit in a remodeled state, and with islets persisting within this highly adverse environment (Figure 1).
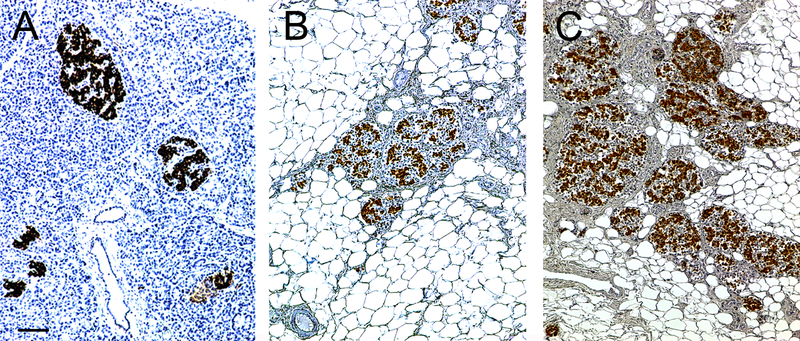
Figure 1.
Pancreas morphology (haematoxylin staining) in a non-CF control (A; 17 years old), CF-no diabetes (B; 18 years) and CFRD (C; 46 years) autopsy human pancreas specimen. Extensive fatty replacement and fibrosis are evident in the CF and CFRD cases. Islets, visualized by insulin immunohistochemistry (brown), appear remodeled but clearly present in CF and CFRD even when compared to non-CF control. Scale bar = 100 μm (Scale bar in A applies to all panels).
CF-Related Diabetes
Despite the relative sparing of islets within the pancreas, a continuum of abnormalities in glucose metabolism are extremely common in subjects with CF, encompassing impaired glucose tolerance (IGT; see Table 2 for diagnostic criteria) and CF related diabetes (CFRD). Also, many people with CF have abnormally high glucose levels at intermediate time points of an oral glucose tolerance test (OGTT; i.e. 15–90 minutes), despite meeting conventional criteria for normal glucose tolerance [NGT (Dobson, et al. 2004)]. Various specific criteria to define such OGTT profiles have been forwarded, termed indeterminate glycemia (INDET) or early glucose intolerance (EGI). In this review INDET/EGI, along with IGT, are collectively referred to as abnormal glucose tolerance (AGT).
Table 2.
Glucose tolerance categories based on OGTT
| Category | Fasting glucose mg/dl (mmol/L) | Intermediate time points mg/dl (mmol/L) |
120 minute time point mg/dl (mmol/L) |
|---|---|---|---|
| Normal glucose tolerance (NGT) | <100 (5.6) | <200 (11.1) | <140 (7.8) |
| Impaired fasting glucose (IFG) | >100 (5.6) <126 (7.0) |
<200 (11.1) | <140 (7.8) |
| Impaired glucose tolerance (IGT) | <126 (7.0) | <200 (11.1) | >140 (7.8) <200 (11.1) |
| Indeterminate glycemia (INDET) | <126 (7.0) | >200 (11.1) | <140 (7.8) |
| Early glucose intolerance (EGI) | <126 (7.0) | ≥155 (8.6) [for 1 hour timepoint] |
<140 (7.8) |
| Cystic Fibrosis Related Diabetes Mellitus without fasting hyperglycemia (CFRD) | <126 (7.0) | N/A | >200 (11.1) |
| Cystic Fibrosis Related Diabetes Mellitus with fasting hyperglycemia (CFRD) | ≥ 126 (7.0) | N/A | >200 (11.1) |
N/A –not applicable
Diabetes is one of the most common comorbidities in people with CF, with a prevalence of 2% in children, 19% in adolescents and 40%−50% of adults (Moran, et al. 2009a). Although CFRD is rare in childhood, it has been described in children of all ages including infants. Diabetes is more likely to develop in those with severe CFTR mutations (Table 1), increasing age, worse pulmonary function, under-nutrition, liver dysfunction and pancreatic insufficiency (Marshall, et al. 2005). Several genetic polymorphisms outside the CFTR locus also contribute to diabetes risk among CF patients, many of which are shared with type 2 diabetes genetic risk (Blackman, et al. 2013).
A significant increase in morbidity and mortality is seen in patients with CFRD compared to CF patients without diabetes, occurring secondary to worsening lung disease and respiratory failure rather than the vascular complications commonly seen in type 1 and type 2 diabetes. Age-adjusted mortality rates per 100 person-years reported in 2010 were 4.2 (3.4–5.1) in individuals with CFRD vs. 1.5 (1.3–1.7) in those with CF without diabetes (Blackman et al. 2013). CFRD related mortality has improved significantly over time, most likely due to annual diabetes screening and early institution of insulin treatment (Moran et al. 2009a). However, of concern, mortality rates among people with CFRD above the age of 30 have not declined in the last decade. This highlights the need for better understanding of CFRD pathophysiology and the development of improved treatments.
Compared to CF without diabetes, CFRD is associated with poorer lung function, decreased nutritional status (lower BMI), increased catabolism and higher rate of pulmonary exacerbations (Marshall et al. 2005). Even patients who have not developed CFRD but have AGT exhibit worse survival and higher lung transplant rates compared to CF patients without glucose abnormalities (Bismuth, et al. 2008). People with CF with IGT at baseline have a faster rate of decline in lung function compared with those with NGT (Milla, et al. 2000); and the degree of insulin deficiency is associated with higher rate of decline. More concerning, the decline in BMI and lung function starts 4 to 6 years before CFRD diagnosis (Lanng, et al. 1992).
Current guidelines recommend yearly OGTT screening starting at the age of 10 years (Moran, et al. 2010). Diagnosis is based on the American Diabetes Association (ADA) criteria for type 1 and type 2 diabetes, even though the morbidities associated with CFRD differ from those seen in type 1 and type 2 diabetes (Moran et al. 2009a). Further, these guidelines recommend monitoring only fasting and 120 min OGTT glucose levels, yet CF patients with elevated 60 min OGTT glucose levels are at high risk for worsening pulmonary disease (Brodsky, et al. 2011). Additionally, continuous glucose monitoring has shown that even CF patients with NGT commonly have intermittent, asymptomatic hyperglycemia (Moreau, et al. 2008). Together, these data suggest that current diagnostic criteria fail to capture all CF patients at risk for clinical decline due to impairments in glucose metabolism.
Abnormal Glucose Tolerance is An Early-Life Feature of Cystic Fibrosis
As outlined above, in cystic fibrosis (CF), even mild hyperglycemia is associated with significant decline in lung function (Milla et al. 2000). However, even though CF lung disease begins in the first years of life, glucose levels in very young children had not been characterized until very recently. This decade, data from CF animal models demonstrated glucose abnormalities starting at birth (Olivier, et al. 2012; Uc, et al. 2015; Yi, et al. 2016b). Additionally, data from humans showed that AGT in 6–10 year old children with CF predicts early progression to CFRD (Ode, et al. 2010). Although there are no systematic outcome studies of the long-term implications of early-life glycemic abnormalities in humans with CF, anecdotal evidence comes from a case report which describes an infant with hyperglycemia at 3 months, who developed waxing and waning glucose intolerance through childhood, finally culminating in CFRD with insulin requirement at 13 years (Fattorusso, et al. 2017), much earlier than the usual onset of CFRD, which has a median age of onset typically in the third decade of life.
Exocrine pancreatic disease in CF begins prior to birth, and is progressive thereafter, with 23% of children <1 year of age having severe exocrine pancreatic loss, compared to 75% of 1–4 year olds (Andersen 1958; Bogdani, et al. 2017). This early exocrine pancreatic pathology may have an important bearing on later CFRD risk. In fact, a greater degree of exocrine pancreatic disease at birth, as reflected by lower circulating immunoreactive trypsinogen levels, predicts increased risk for CFRD in later life (Soave, et al. 2014). Early life exocrine pancreatic disease may also drive endocrine pancreas pathology, as young children with CF already exhibit loss of islet β cells and islet inflammation (Bogdani et al. 2017; Hull, et al. 2018). Such early pathological changes in the endocrine pancreas may be functionally important, since cross-sectional data from a cohort of children aged 3 months to 5 years showed the greatest frequency of AGT at 2–4 years of age followed by a lower AGT frequency at 5–6 years (Yi, et al. 2016a). Together, these findings support an overarching relationship between exocrine pancreatic pathology, islet pathology/dysfunction, and AGT in CF.
Impaired Insulin Secretion is a Key Determinant of Hyperglycemia in Cystic Fibrosis
In CF subjects, β-cell function is substantially reduced compared to non-CF controls - even in patients with NGT (Merjaneh, et al. 2015; Sheikh, et al. 2017). This is clearly demonstrated in CF patients by measuring the disposition index (DI). DI is a robust measure of β-cell function quantified as the insulin response during an oral GTT, normalized for the prevailing insulin sensitivity, and is a strong predictor of future type 1 and type 2 diabetes in non-CF populations. DI is low in the general CF population, demonstrating an insulin secretory defect that exists even during stable pulmonary disease and adequate nutritional status (Merjaneh et al. 2015; Sheikh et al. 2017). First phase insulin secretion is particularly impaired, and represents an early abnormality in the development of AGT among those with CF (Mohan, et al. 2009; Sheikh et al. 2017). Furthermore, relative proinsulin secretion is elevated in CF patients who have AGT (Nyirjesy, et al. 2018; Sheikh et al. 2017), providing additional evidence for early β-cell dysfunction. These studies confirm that, in CF, β-cell dysfunction is present before deterioration of glucose tolerance is apparent, and long before the diagnosis of diabetes.
While impaired insulin secretion worsens with age, likely in all pancreatic insufficient CF subjects regardless of glycemic status (Moran et al. 2009a; Ode et al. 2010; Yi et al. 2016a), abnormal insulin secretion is already detectable in young people with CF, and is potentially present from birth. Evidence for the latter comes in part from CF animal models which develop spontaneous hyperglycemia; these exhibit abnormal insulin responses from birth (Olivier et al. 2012; Uc et al. 2015). Furthermore, very young humans with CF (3 months to 5 years of age) fail to exhibit the normal increase in insulin secretion seen in non-CF subjects within the same age range (Yi et al. 2016a). In addition, young CF subjects with AGT show an inability to increase insulin secretion even in the face of hyperglycemia (Yi et al. 2016a). Six to 10 year old CF children also have a delayed insulin response, which is more pronounced in those with AGT (Ode et al. 2010).
Overall, the literature suggests that even CF subjects with NGT have low β-cell reserve, which places them at risk for the development of hyperglycemia. Further, the clinical implications of a low β-cell reserve are likely more important in CF than in non-CF subjects given their catabolic state and the detrimental effects of insulin deficiency on weight gain and lung function in CF patients.
Islet Pathology in Cystic Fibrosis
As mentioned above, exocrine pancreas pathology is a major feature of CF, occurring due to pancreatic ductal plugging and extensive involution of acini (Andersen 1958). In line with this, pancreatic volume, estimated by MRI, is decreased in CF compared to healthy controls and to patients with type 1 diabetes [note, however, pancreas could only be visualized in a small subset of CF subjects (Sequeiros, et al. 2010)]. While destruction of the exocrine pancreatic acini is near total in CF, many ducts survive, especially larger ones, and islets are still present within the fibrotic/fatty pancreas parenchyma (Figure 1).
In subjects with CF without a diabetes diagnosis, compared to non-CF controls, most studies report a decrease in β-cell area (Abdul-Karim, et al. 1986; Couce, et al. 1996; Hart, et al. 2018; Lohr, et al. 1989) ranging from 11–52%. β-cell loss appears to be particularly severe in younger subjects (Bogdani et al. 2017), but does not differ between subjects that exhibit fatty vs. fibrotic exocrine pancreas pathology (Lohr et al. 1989). The manner in which β-cell measures are expressed affects interpretation of the data - β-cell area, when expressed relative to islet area, is reduced in most studies (Abdul-Karim et al. 1986; Bogdani et al. 2017; Couce et al. 1996; Lohr et al. 1989). β-cell normalized to pancreatic area was found to be decreased in one study (Hart et al. 2018), but unchanged in others (Bogdani et al. 2017; Hull et al. 2018). Such differences may be due to difficulties in distinguishing pancreas-intrinsic fat (i.e. that which is present due to fatty replacement) from fat which is located close to the pancreas.
In CF subjects with diabetes, studies demonstrating β-cell loss (relative to islet area) are relatively small (n≤8 in at least one group) but consistent, reporting a decrease of ~25–30% (Bogdani et al. 2017; Hull et al. 2018; Iannucci, et al. 1984; Soejima and Landing 1986). Interestingly, two larger studies (n=12–18 subjects per group) failed to find significant loss in β-cell area when quantified per islet area (Couce et al. 1996; Hull et al. 2018)or per pancreas area (Hull et al. 2018). Taken together, the literature is somewhat mixed but shows that loss of β cells may be a feature of CF both with and without diabetes.
An increase in glucagon-positive α-cells has been described in CF subjects both with and without diabetes (Bogdani et al. 2017; Hart et al. 2018; Hull et al. 2018; Lohr et al. 1989), with only one study reporting no difference (Abdul-Karim et al. 1986). Similarly, the fraction of somatostatin positive δ-cells is increased in humans with CF (Abdul-Karim et al. 1986; Bogdani et al. 2017; Lohr et al. 1989; Soejima and Landing 1986), with two other studies showing the same trend (Hart et al. 2018; Hull et al. 2018). PP cell area has also been shown to be increased in subjects with CF vs. non-CF controls (Hull et al. 2018; Lohr et al. 1989). A significant decrease in PP cells between CF subjects with and without diabetes was reported in one study (Hull et al. 2018) with no difference being observed in another (Iannucci et al. 1984). The relative areas of ghrelin-positive ϵ cells do not appear to have been determined in CF pancreas.
Abnormalities in non-endocrine islet components, particularly nerve fibers and/or islet capillaries, may also contribute to the islet lesion in CF. This has not been well studied, although data from a pig model of CF suggests that pancreatic/islet vascularization appears normal while nerve fiber density (determined by PGP9.5 immunoreactivity) may be reduced (Uc et al. 2015).
In summary, it is clear that the extent of β-cell loss seen in (human) CF is not as severe as the loss of pancreatic acini, nor as the loss of β-cell mass seen in type 1 diabetes. Even if 50% or more of β-cell mass was lost in CFRD, a functional β-cell defect must also exist to explain the magnitude of observed insulin secretion impairment. This raises the possibility that the function of remaining β cells could be targeted for therapy to improve insulin release in CF. From the literature, it is clear that non-β islet endocrine cells (i.e. α, δ and PP cells) are increased in CF/CFRD. This alteration in islet composition suggests that paracrine interactions among islet endocrine cells are likely to be disturbed, which could contribute to β-cell dysfunction and impaired glucose metabolism in CF subjects.
Release of Other Islet Hormones is Also Abnormal in Cystic Fibrosis
Inappropriately elevated glucagon levels are postulated to contribute to hyperglycemia and hepatic insulin resistance in type 2 diabetes, while an inappropriately low counter-regulatory glucagon response is an important contributor to hypoglycemia in diabetes. An insufficient glucagon response to arginine has been reported in CF subjects with impaired insulin release (Uc et al. 2015). Additionally, glucagon responses to hypoglycemia are impaired in CF subjects with pancreatic insufficiency, but are intact in exocrine sufficient CF subjects (Moran, et al. 1991). Glucagon suppression during glucose loading has been found to be both impaired [i.e. poor suppression, especially in subjects with AGT (Lanng, et al. 1993b)], and normal, albeit decreasing from a reduced baseline level (Sheikh et al. 2017). Despite these inconsistencies, the literature supports that dysregulated glucagon release may be a feature of CF.
Somatostatin is secreted by islet δ cells and potently inhibits insulin and glucagon secretion through paracrine action. In CFRD patients compared to normal controls, plasma somatostatin is increased following arginine stimulation (Meacham, et al. 1993), though it is not known if this reflects somatostatin secreted from islets or other locations with somatostatin secreting cells (such as the gut). Nonetheless, this data suggests that somatostatin tone is increased in CF and that increased somatostatin may therefore contribute to poor insulin secretion in CF.
Pancreatic polypeptide (PP) is released by PP-cells of the islet in response to feeding. Circulating PP levels fail to increase in response to feeding in CF patients across a range of ages (Allen, et al. 1983). The PP response to hypoglycemia is also decreased, more so in patients with exocrine insufficiency (Moran et al. 1991). PP release during an OGTT is likewise severely impaired (Lanng et al. 1993b).
While the data suggest that levels of non-β cell islet hormones are differentially altered in CF (i.e. glucagon and PP levels are largely decreased, while somatostatin levels may be increased), the morphological data show that α, δ and PP cell areas are all increased. Thus, there exists a disconnect between the frequency of these non-β endocrine cells and release of their hormone contents, further supporting the concept that functional, rather than morphological abnormalities represent the key pathogenic defect in the islet in CF.
Animal Models of CFRD
Until recently, experimental study of CFRD has been hampered due to a lack of suitable animal models. Mouse models of cystic fibrosis, including CFTR knockout and CFTR-ΔF508 mice, exhibit severe gastrointestinal disease and impaired growth but do not develop substantive pancreatic or lung disease. Blood glucose in CF mice is normal (Lanng et al. 1993b) or even reduced (Fontes, et al. 2015) during glucose tolerance testing. Mice with acute- or chronic β-cell specific CFTR deletion demonstrate normal glucose tolerance and do not exhibit altered insulin release in response to glucose or IBMX (Hart et al. 2018). Likewise, CFTR KO rats have a normal pancreas, although their glycemic phenotype is yet to be detailed. By contrast, CFTR knockout zebrafish undergo exocrine pancreatic destruction, though their glycemic phenotype also has yet to be described. CFTR knockout pigs and ferrets develop lung disease and severe gastrointestinal disease, requiring specialized care similar to humans with CF. Importantly, they also develop exocrine pancreatic disease which phenocopies human pancreatic CF including ductal plugging, acinar destruction, ductal dilation, inflammation, fibrosis, fatty replacement, and relative sparing of endocrine mass (Sun, et al. 2014; Uc et al. 2015; Yi et al. 2016b). Both models develop hyperglycemia with impaired insulin secretion (Uc et al. 2015; Yi et al. 2016b). As in humans, these abnormalities are detected in early life. Newborn CFTR knockout (KO) pigs have abnormal glucose tolerance, extensive exocrine pancreatic disease, and impaired insulin secretion (Uc et al. 2015). Similarly, newborn CFTR KO ferret kits have decreased first phase insulin secretion and abnormal glucose tolerance, but only mild exocrine pancreatic disease consisting of ductal plugging, pancreatic stellate cell activation, with no inflammatory infiltrate or cell apoptosis/necrosis (Olivier et al. 2012). Interestingly, CF ferrets experience an early-life hyperglycemic phase (Yi et al. 2016b), similar to that observed in humans with CF (Yi et al. 2016a), and which correlate with histopathologic changes in the pancreas (Yi et al. 2016a). In the first several weeks after birth, exocrine pancreatic disease accelerates in CF ferrets, culminating at 4–8 weeks in severe pancreas inflammation, inflammatory cell infiltration, extensive loss of insulin-positivity within islets and spontaneous diabetes. Strikingly, insulin-positivity and islet hormone mRNA expression reappear thereafter and blood glucose levels spontaneously normalize. This occurs as loss of acinar tissue becomes complete, inflammation recedes, and a fibrotic to adipogenic transition initiates fatty replacement of the exocrine pancreas. The islets are remodeled, with altered shape, size, and cellular composition (Rotti, et al. 2018). This euglycemic reprieve is temporary and is followed by a persistent decline in glucose tolerance, with this final phase being very similar to the clinical picture of CFRD in teenage and adult humans with CF (Yi et al. 2016a). Unfortunately, widespread utility of the CF pig and ferret models has been limited by the required technical expertise to rear these fragile animals, and the related expense. Two approaches, currently being undertaken, will hopefully mitigate technical and expense barriers, allowing widespread study of clinically-relevant CFRD models. Firstly, recent gene editing advances should enable re-engineering of the ferret and/or pig CF models to ameliorate their fragile state. Secondly, creation of murine model(s) of spontaneous CFRD, for example through humanization of pancreatic biology as has been done in other forms of diabetes, could also greatly propel CFRD research.
Potential Mechanisms Underlying Loss of β-Cell Function in CF
Islet Inflammation
Several studies have investigated the possibility that islet-intrinsic mechanisms may underlie the decline in β-cell function that characterizes CF. The concept that pro-inflammatory cytokines can elicit β-cell cytotoxicity is well established in the literature. However, whether β-cell dysfunction result from localized islet inflammation is somewhat controversial, with the production of cytokines, especially IL-1β, from β cells themselves being a particularly contentious issue. Surprisingly, immunoreactivity for IL-1β, which appears to localize to endocrine cells, is a robust and common feature of CF islet pathology (Hull et al. 2018). Two other studies recently described immune infiltration in islets from CF human autopsy pancreas sections, including in children younger than 4 years of age (Bogdani et al. 2017; Hart et al. 2018). This leukocyte infiltration is chiefly comprised of T-cells (CD8+ and CD4+), but not macrophages (Bogdani et al. 2017; Hart et al. 2018; Hull et al. 2018). It is clear that the islet pathology in CF differs from the profound autoimmune-mediated β-cell destruction seen in type 1 diabetes. However, these new data demonstrating the presence of T- lymphocytes in CF islets warrants additional study, as localized inflammation and presence of T-cells in the islet appear to be common features of CF. These findings, together with data from other forms of diabetes and from CF animal models, indicate that β cells and/or other cells in the islet are likely to also express other proinflammatory cytokines (aside from IL-1β) and chemokines, further contributing to islet inflammation. It is possible that this islet inflammation, especially if it occurs acutely, is a survival mechanism activating recovery and repair mechanisms. However, chronic inflammation is likely to be detrimental to β-cell function and survival, and may therefore represent a therapeutic target for CFRD.
Islet Amyloid Deposition
Islet amyloid formation is a long-recognized pathological hallmark of type 2 diabetes which is toxic to β-cells and is associated with decreased insulin release in animal models (Hull, et al. 2004). Islet amyloid deposition has been documented in CF (Couce et al. 1996; Hart et al. 2018; Hull et al. 2018; Iannucci et al. 1984), most commonly in CFRD (~70% of subjects), with 17% of those with CF and “borderline diabetes” also being affected (Couce et al. 1996). Islet amyloid is rarer in adult CF subjects without diabetes and has not been observed in children with CF (Bogdani et al. 2017; Hull et al. 2018). It is striking, though, that the age at which amyloid is frequently seen in CFRD (on average around 28 years) is decades earlier than that typically described in type 2 diabetes (Hull et al. 2004), suggesting that this cytotoxic process may be accelerated in CF. Interestingly, despite well-documented proinflammatory effects of islet amyloid (Masters, et al. 2010; Westwell-Roper, et al. 2011), IL-1β immunoreactivity in CF is not always coincident with islet amyloid deposition. In fact, the presence of IL-1β reactivity is much more widespread than amyloid, being present in adults with and without diabetes, and in children (Hull et al. 2018).
Islet Endocrine Cell Autonomous Actions of CFTR
There has been considerable interest in the possibility that CFTR might have direct actions in β cells and/or other islet endocrine cells. This interest stems in part from CFTR’s primary function as a chloride channel. It has long been postulated that chloride channels should contribute to β-cell electrophysiology (Di Fulvio, et al. 2014), and there are several molecules that could fulfill or contribute to this function. CFTR would be one such candidate; it is closely related phylogenetically to the sulfonylurea receptor, both are members of the ABCC subfamily of ABC (ATP binding cassette) transport proteins and the latter is known to be critical for β-cell function. Some studies provide data in support of CFTR activity in the β cell; evidence for this has been previously reviewed in detail (Koivula, et al. 2016). However, conflicting data also exist and as such this issue remains highly controversial.
In 2007, CFTR mRNA and protein expression was first reported in primary rat islet β cells and RIN-5mF insulinoma cells, at levels lower than in “non-β cells” (Boom, et al. 2007). Subsequent studies confirmed that RIN-5mF and MIN6 cells both express detectable CFTR (Guo, et al. 2014; Ntimbane, et al. 2016). Isolated primary human β cells were reported to have immunoreactivity for CFTR although mRNA data were not reported (Guo et al. 2014). Newer data from the same group (Edlund, et al. 2017) show the presence of scattered CFTR immunoreactive cells in a representative human islet, but the proportion of CFTR-positive cells which are also insulin-positive was not determined. In contrast, other groups did not detect CFTR in β cells, by immunohistochemistry (Boom et al. 2007; Hart et al. 2018) or in situ hybridization (Sun, et al. 2017), from rat, ferret and human pancreas, or based on data from the Human Protein Atlas (Hart et al. 2018; Uhlen, et al. 2015). Further, analysis of data from two single-cell RNASeq transcript datasets, representing over 12,000 single cells dispersed from isolated human islets (Baron, et al. 2016; Segerstolpe, et al. 2016) demonstrated an average CFTR expression per β cell of 0.14±0.47 reads per kilobase million (RPKM) or 1.05±1.02 transcripts per million (TPM) respectively. In these two studies, pancreatic β- and ductal cells comprised 12–29% and 13–17% of islet cell types, respectively. By comparison, these same analyses demonstrated an average CFTR expression per ductal cell of 308±250 RPMK or 207±827 TPM, respectively. Additional analyses using one of these same datasets (Segerstolpe et al. 2016) along with bulk RNA-Seq datasets from mouse and human β cells (Blodgett, et al. 2015; Bramswig, et al. 2013) also found CFTR mRNA is detectable only at low levels (< 6 RPKM), in a small proportion (~5%) of β cells (Hart et al. 2018). In sum, the available data suggest that if CFTR is produced in the β cell, its expression is low and/or occurs only in a minority of cells.
The extent to which β cells express CFTR, or not, is of critical importance because CFTR is expected to exert effects on β cell electrical activity and thus impact insulin secretion. The presence of CFTR in a minority of β cells could still have functional consequences if those cells were highly electrically active [such as “hub” β cells (Johnston, et al. 2016)]; however the presence of CFTR in such cells has not been demonstrated. CFTR-knockdown and/or pharmacological inhibition of CFTR activity in immortalized β-cell lines results in reduced glucose stimulated membrane depolarization (Guo et al. 2014) and reduced glucose-stimulated insulin secretion (Ntimbane et al. 2016). The presence of an cAMP (forskolin)-induced chloride whole cell current has been documented in isolated mouse and human β cells; this can be partially blocked with small molecule CFTR inhibitors, and is absent in β cells from mice with global expression of the ΔF508 CFTR mutation (Edlund, et al. 2014; Guo et al. 2014; Ntimbane et al. 2016). Furthermore, murine β cells from ΔF508 mice or with pharmacological inactivation of CFTR exhibited membrane hyperpolarization and slower glucose stimulated membrane depolarization, reduced generation of action potentials and smaller rises in intracellular calcium levels (Guo et al. 2014). Isolated human and mouse β cells treated with small molecule CFTR inhibitors exhibited no alteration of voltage-dependent calcium currents but showed blocked depolarization-evoked membrane capacitance (a measure of secretory granule exocytosis) (Edlund et al. 2014). In contrast to both of these studies, recent data from human β cells failed to detect any forskolin-activated chloride current (Hart et al. 2018), although the patch clamp conditions utilized differed from the previous publications, precluding direct comparisons of the data.
Some important caveats regarding specificity are important to bear in mind when interpreting the above studies. The two CFTR inhibitors used in the above studies, CFTR(inh)-172 and GlyH-101, are not specific for CFTR activity at the concentrations employed, 10 μM (Guo et al. 2014), and 10–40 and 40–50 μM respectively (Edlund et al. 2014). Both compounds inhibit mitochondrial function at 10 μM (Kelly, et al. 2010) and the activity of other chloride channels at 5 μM [(Friard, et al. 2017; Kelly et al. 2010; Melis, et al. 2014) and reviewed in (Di Fulvio et al. 2014)]. Furthermore, 20 μM CFTR(inh)-172 has been shown to reduce glucose-stimulated calcium currents and insulin secretion in CFTR-KO ferret islets (GlyH-101 not tested) (Sun et al. 2017), indicating that this compound, at the concentration used, likely has islet actions which are independent of CFTR. Secondly, sufficient data exist in the literature to warrant caution in the interpretation of CFTR immunoreactivity. Specifically, a variety of CFTR antibodies exhibit aberrant labeling, including non-specific labeling of cells which do not express CFTR (Hart et al. 2018; van Meegen, et al. 2013) and varying sensitivity for detection of low CFTR quantities (relevant for its detection in islet endocrine cells). Taken together, studies using inhibitors and antibodies directed against CFTR should be interpreted with these caveats in mind.
Data regarding insulin release in conditions of pharmacological or genetic inactivation of CFTR are also conflicting. Use of small molecule CFTR inhibitors, or islets from mice bearing the ΔF508 Cftr mutation resulted in reduced glucose-stimuated insulin release (GSIS) in one study (Guo et al. 2014). In contrast, data from mouse and human islets using the same CFTR inhibitors showed no effect on glucose-stimulated insulin release but reported inhibition of forskolin-augmented insulin release (Edlund et al. 2014). Similar to both studies, studies of ferret islets showed both glucose stimulated insulin secretion and forskolin amplified insulin secretion were impaired in perifused islets isolated from CFTR-KO versus control ferrets (Sun et al. 2017). Additionally, a CFTR inhibitor reduced insulin secretion from islets isolated from normal ferret and human islets, but also reduced insulin secretion in islets from CFTR-KO ferrets indicating non-specific inhibitor effects (Sun et al. 2017). Further contrasting data from mouse islets with acute or chronic β-cell selective CFTR deletion show no differences in glucose- or IBMX-mediated insulin release (Hart et al. 2018). This same study also showed no effect of CFTR activators to modulate insulin release in response to glucose or forskolin in human islets (Hart et al. 2018), and, importantly, also found no difference in insulin release from perifused islets derived from human donors with-or-without CF (Hart et al. 2018).
There has also been considerable interest in whether CFTR may influence α-cell function. CFTR immunoreactivity has been reported in human, rat and mouse α cells (Boom et al. 2007; Edlund et al. 2017). This is in contrast to studies showing no CFTR immunoreactivity in α cells from human islets and no CFTR RNA in α cells of ferret pancreas or in dispersed islet cells from ferrets and humans (Hart et al. 2018; Sun et al. 2017). As an aside, all four of these studies agree that CFTR immunoreactivity is absent from islet δ cells (Boom et al. 2007; Edlund et al. 2017; Hart et al. 2018; Sun et al. 2017). A CFTR inhibitor enhanced glucagon secretion from human and mouse islets in response to low glucose + forskolin (Edlund et al. 2017). The effect of this inhibitor was abrogated in human islets by siRNA-mediated CFTR knockdown, although CFTR knockdown did not itself influence glucagon release.
In summary, there has been great interest in whether there may be islet β and α cell-autonomous actions for CFTR. Conflicting data exist regarding whether endogenous CFTR expression and electrical activity occur in these cells and could thereby influence insulin secretion. Even if these effects do exist, pancreatic ductal cells remain the major site of CFTR expression in the pancreas, raising the possibility that CFTR expression in these cells may explain some or all of the effects of CFTR on insulin secretion (as detailed in the next section).
Potential Paracrine Actions of CFTR on Beta-cell Function
In the pancreas, CFTR is expressed predominantly, if not almost exclusively, in the ductal epithelium [(Marino et al. 1991; Wilschanski and Novak 2013) and Figure 2]. CFTR expression is highest in those duct cells nearest acini, especially centroacinar and intercalated duct cells (Marino et al. 1991; Wilschanski and Novak 2013). Islets share a physically close association with pancreatic ducts, and it is postulated that ductal cells influence islet function via paracrine mechanisms (Bertelli and Bendayan 2005). Based on the essential requirement of CFTR for proper ductal function (Wilschanski and Novak 2013) and because ducts are the major site of CFTR expression in the pancreas, it has been postulated that loss of CFTR from ductal epithelium contributes to islet dysfunction in CF via paracrine mechanisms.
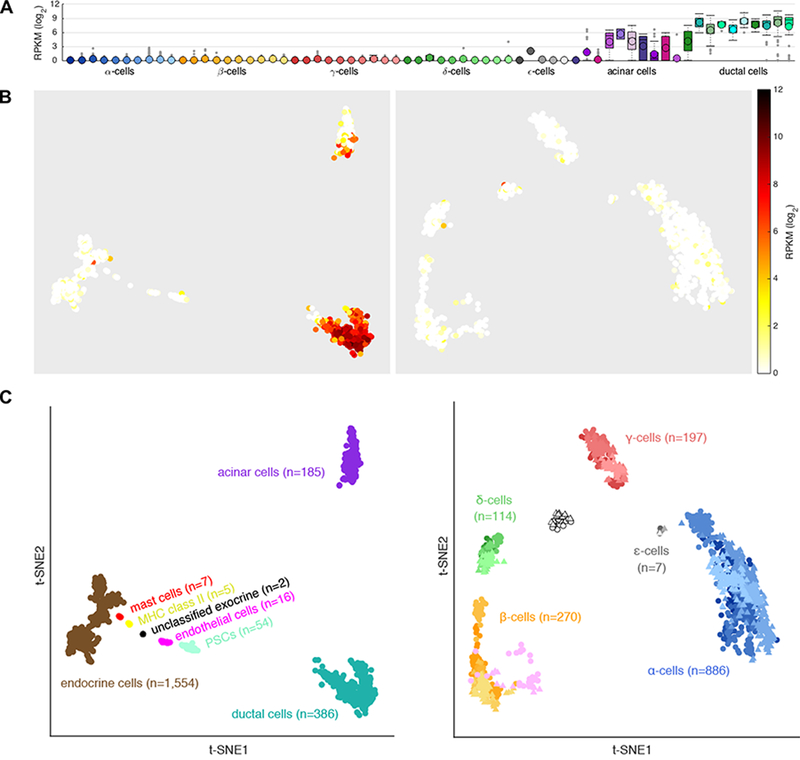
Figure 2.
CFTR expression in isolated human islet cells. Data captured from [(Segerstolpe, et al. 2016) as visualized at http://sandberg.cmb.ki.se/pancreas/]. (A) Single cell RNAseq results for CFTR expression in isolated islets collected from 10 individuals. Boxplots summarize CFTR expression in Reads Per Kilobase of transcript per Million reads (RPKM) on a log2 scale across the 7 major cell types for each donor. The first 6 boxes for each cell-type correspond to healthy individuals and the last 4 to individuals with type 2 diabetes. (B) t-SNE plot of cells grouped by transcriptome similarity and colored according to CFTR expression according to the indicated log2 RPKM scale. (C) Legend indicating cell types. (B, C) Left panels show all cell types (n=2,209) and right panels show endocrine cell types only (n=1,554).
The concept that islet function can be regulated by paracrine factors (along with multiple other inputs: autocrine, endocrine, neural, etc.) is established in the literature, providing support for such a hypothesis. In fact, persistence of pancreatic ductal-derived cells in preparations of isolated islets, even following several days of culture or after transplantation, has been well-documented in the literature [(Ichii, et al. 2008; Keymeulen, et al. 2006; Segerstolpe et al. 2016; Sun et al. 2017; Warnock, et al. 2005) and Figure 3]. Further, cultured islet preparations from humans and ferrets contain ductal cells which express CFTR at much higher levels than endocrine cells [as illustrated in Figure 2 and (Baron et al. 2016; Hart et al. 2018; Segerstolpe et al. 2016; Sun et al. 2017)].
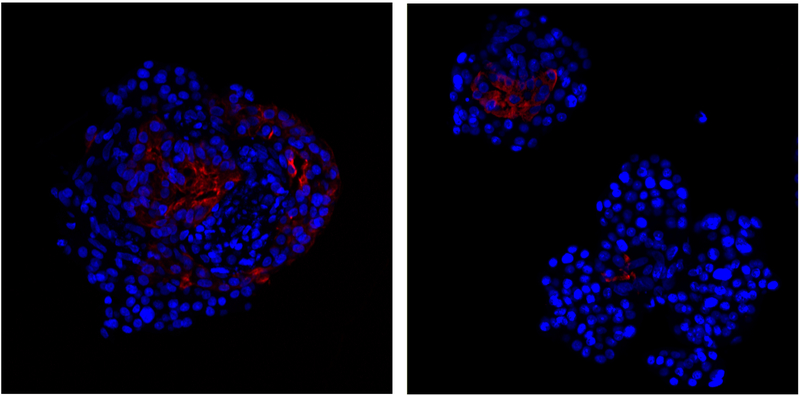
Figure 3.
Presence of cytokeratin 7-positive duct cells (red) in isolated human islets, immediately upon receipt from the IIDP. Cytokeratin 7 immunoreactivity was detected using the anti-Cytokeratin 7 antibody EPR1619Y from AbCam (catalogue no. ab68459) based on the approach described in (Sun et al. 2017).
Given these data, it is possible that the persistence of CFTR-expressing ductal cells in isolated islet preparation could exert effects on insulin release and explain/confound some of the existing studies in the literature. Indeed, we utilized this property of isolated islets to test the hypothesis that CFTR expressed in ductal cells may influence insulin secretion. Acute knockdown of CFTR in human and ferret islets does indeed result in decreased glucose-stimulated insulin secretion in static islet cultures [Figure 4; see Supplemental Methods]. Since ductal cells were the only cell type in these studies to express CFTR mRNA (Sun et al. 2017), these results suggest that CFTR-dependent paracrine actions from islet-associated ductal cells are crucial for insulin secretion and provides an additional or alternative mechanism to the role of intrinsic CFTR action in β (or α) cells. The mechanism underlying this effect remains to be determined, and whether this effect occurs in vivo is also currently unknown. If this observation does translate to the in vivo situation, it suggests that restoration of ductal CFTR action may be an effective strategy to improve insulin secretion and therefore treat CFRD.
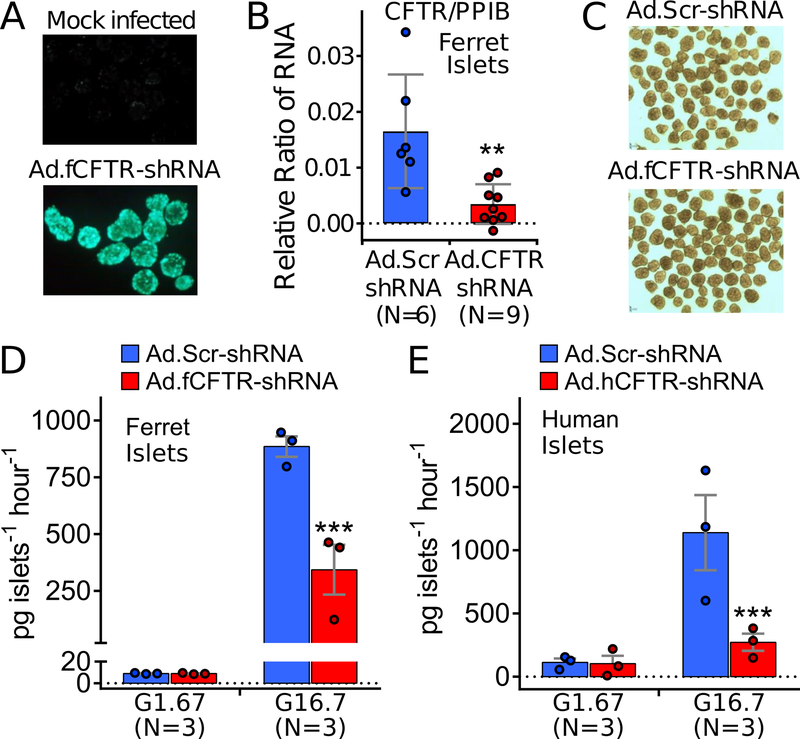
Figure 4.
CFTR-knockdown in wild-type adult ferret and human islets reduces glucose stimulated insulin secretion. (A) Infection of adult ferret islets with a fCFTR-shRNA expressing recombinant adenovirus (Ad.fCFTR-shRNA) that contains a CMV-driven EGFP transgene in the E3 region of the virus. Fluorescent photomicrographs of mock and Ad.fCFTR-shRNA infected islets are shown. (B) Evaluation of fCFTR and housekeeping (PPIB) RNA levels in CF and WT adult ferret islets 48 hrs following Ad.fCFTR-shRNA or Ad.Scr-shRNA (scrambled control) infection. Plots represent mean±SEM for N experiments; ** P<0.01, two-tailed student’s t-test. (C) Photomicrograph of adult ferret islets 48 hours following infection with Ad.fCFTR-shRNA and Ad.Scr-shRNA. (D, E) Insulin secretion in response to 1.67 mmol/l glucose (G1.67) or 16.7 mmol/l glucose (G16.7) by adult WT ferret islets (D) and human islets (E) 48 hrs following infection with Ad.fCFTR-shRNA, Ad.hCFTR-shRNA, or Ad.Scr-shRNA as indicated. (D,E) Graphs show the mean±SEM for the N independent batches, with dots showing the mean per batch performed on 1–4 replicates. Statistical analysis comparing Scr-shRNA versus CFTR-shRNA within each glucose category was performed by mixed effects modeling treating batch replicates as repeated measures using the R package nlme; *** P≤0.0001.
Influence of Extra-Pancreatic Factors on Islets in CF
Gastrointestinal disease is prominent in CF and levels of several gut hormones that impact islet function have been shown to be deranged in CF patients. Active glucagon-like peptide-1 (GLP1) responses during mixed meal testing vary between studies, being normal or low (Hillman, et al. 2012; Sheikh et al. 2017). By contrast, gastric inhibitory polypeptide (GIP) levels during mixed meal testing are more consistently decreased in CF patients, especially those who are pancreatic insufficient (Sheikh et al. 2017). Fasting plasma acyl-ghrelin levels in CF patients are elevated (Cohen, et al. 2008), but the potential connection of ghrelin to CFRD has not been explored. Although low GLP-1 and GIP and elevated ghrelin would be expected to potentially contribute to diminished β-cell function, evidence suggest there is no epidemiological linkage of relative GLP-1 and GIP deficiency to declining glucose tolerance in CF (Lanng, et al. 1993a; Nyirjesy et al. 2018). A very recent small study evaluating a GLP-1 receptor agonist did show improved glucose excursion in CF patients with impaired glucose tolerance, however, this appeared to be primarily secondary to slowing of gastric emptying, indicating that abnormalities in incretin function, have a complex relationship to the pathophysiology of CFRD (Geyer, et al. 2018). Relatedly, available literature supports the hypothesis that meticulous attention to pancreatic enzyme supplementation decreases postprandial hyperglycemia in CF, by improving incretin secretion and by slowing gastric emptying (Perano, et al. 2014).
Insulin Resistance and Cystic Fibrosis
Although insulin resistance is not a prominent clinical feature of patients with CF at baseline health, studies employing sensitive techniques have repeatedly demonstrated insulin resistance in CF patients [as reviewed in (Yi et al. 2016b)]. Exocrine pancreatic status predicts insulin sensitivity in CF patients. Those CF patients who are exocrine pancreatic sufficient exhibit comparable insulin sensitivity to non-CF subjects. However, CF patients with exocrine pancreatic insufficiency demonstrate primarily hepatic insulin resistance, the mechanistic origins of which are not well understood. Additionally, transient insulin resistance is common in CF patients owing to systemic inflammation during pulmonary infections and also due to systemic corticosteroid treatment when used. It is therefore likely that insulin resistance in CF increases stress on the β cells and thus contributes to their dysfunction.
Specific Considerations for the Treatment of Diabetes in Cystic Fibrosis
Unique to CFRD, compared to other forms of diabetes mellitus, the primary goal of treatment is preservation of lung function. Weight and BMI maintenance is also essential to survival in CF, as CFRD induces a catabolic state that contributes to clinical decline in CF patients. Insulin is the only currently recommended medical treatment for CFRD (Moran et al. 2010), as it is the only agent to date that has been shown to prevent BMI decline in CFRD patients (Moran, et al. 2009b). The advent of aggressive insulin treatment for CFRD has narrowed the survival gap between CF patients with and without CFRD. However, insulin treatment is labor intensive for patients and so it would significantly improve quality of life if other less complex treatments were effective for CFRD. Unfortunately, there is a paucity of adequate data to support the use of other treatments at this time. Several smaller clinical studies have examined the efficacy of various oral type 2 diabetes medications to treat CFRD, though none have yielded sufficient evidence to produce consensus recommending their use (Moran et al. 2010). Although sulfonylurea and meglinide secretagogues stimulate insulin secretion in CF patients, two clinical trials using repaglinide have failed to produce durable improvements in BMI among CFRD patients (Ballmann, et al. 2018; Moran et al. 2009b). Comprehensive review of the clinical literature on the remaining pharmaceutical classes currently used to treat type 2 diabetes is beyond this scope of this article. However, several conceptual considerations are informative. Many of these agents (which include biguanides, alpha-glucosidase inhibitors, incretin mimetics, DPP-4 inhibitors, amylin, and glycosurics) promote some degree of catabolism through weight loss, appetite reduction, macronutrient malabsorptiont, and/or glucose wasting. Thus, use or investigation of any of these agents for CFRD treatment must cautiously track and assess their effect on catabolism and body mass. Although thiazolidinediones have been shown to increase weight in type 2 diabetes, their side-effect of reducing bone mineral density could be very problematic for CF patients, who are at markedly increased risk for osteoporosis at baseline.
There has been considerable recent progress in understanding the pathophysiology of CFRD, potentially unveiling new possible treatment or preventative approaches. Given the prominent role of exocrine pancreatic inflammation, which is especially extreme in the young pancreas but persists even after resolution of the inflammatory infiltrate, it is tempting to wonder whether anti-inflammatory agents might treat CFRD. Focusing on the endocrine pancreas, there is precedent - which comes from the connection of islet inflammation, amyloidosis, and type 2 diabetes (Marchetti 2016; Masters et al. 2010; Westwell-Roper et al. 2011). Clinical trials using anti-inflammatories in type 2 diabetes have met with mixed results. Anakinra, an IL-1 receptor antagonist, demonstrated improvements in insulin secretion, fasting glucose, and glycated hemoglobin in a phase 2 study of 70 adults (Larsen, et al. 2007). However, in a larger study using canakinumab, a monoclonal antibody against IL-1β, non-sustained reductions in hemoglobin A1c were observed in 10,061 patients, suggesting limitations to anti-inflammatory therapy in type 2 diabetes (Everett, et al. 2018). Despite these results, the presence of islet inflammation/infiltration and the degree of islet amyloidosis in relatively young CF patients could indicate that responses to anti-inflammatory medications may be more effective than in other forms of diabetes (Bogdani et al. 2017; Couce et al. 1996; Hart et al. 2018; Hull et al. 2018). Of course, given that CF patients are extremely susceptible to fatal lung infection, agents that dampen the immune system must be approached with extreme caution unless delivered selectively to the pancreas.
The over-abundance of δ cells in the CF islet suggests that there may be increased paracrine inhibition of insulin release by somatostatin. In such a case, it is conceivable that a selective somatostatin receptor antagonist might improve β-cell function. Indeed such agents have been considered for treatment of type 2 diabetes. Small molecule inhibition of the glucagon receptor has also been tested in the context of type 2 diabetes. Despite favorable effects on lowering glucose levels, concerns of hepatic transaminitis may limit this approach in patients with cystic fibrosis. GLP-1 based therapies have several desirable properties that might be beneficial in CFRD, including augmentation of insulin secretion and inhibition of glucagon release. The potential efficacy of GLP-1-based therapy in cystic fibrosis subjects is currently under study.
Recent advances in gene editing technologies have reignited interest and enthusiasm for gene and cell-based therapies of CF affected organs. While the current focus has primarily been on the lung, whether correction of CFTR mutations by gene editing in the CF pancreas would reverse the course of disease is an interesting prospect. The question as to which pancreatic cell type(s) are responsible for CFTR actions that ultimately support insulin secretion is paramount to determining which therapeutic strategy should be employed, and underscores the critical nature of future work to resolve the marked discrepancies in the current literature.
Looking Forward
Remarkable progress has been made in pulmonary care for patients with CF, leading to significant improvements in longevity. However, there have been no concomitant advances that reduce the burden of diabetes among CF patients. A new era of CF care is dawning, as small molecule drugs that restore CFTR function and dramatically improve lung function have recently come into clinical use. It remains to be determined how these agents will impact long term diabetes risk. Because of the CF pancreatic defects incurred early in life, the utility of CFTR restoring drugs for treating CFRD is not yet clear. Early-life treatment may be required in order to prevent pancreatic pathology. On the other hand, these drugs might help treat or prevent CFRD by recovering CFTR dependent islet-associated ductal function, by improving gut incretin function, and/or by reducing the inflammation and insulin resistance of systemic illness. Given these uncertainties, it is crucial to understand all of the pathophysiologic drivers of CFRD, so that we can design preventative strategies to ameliorate the morbidity and mortality of this disease.
Recent advances in both animal models and translational human studies have shed light on the pathogenesis of CFRD but more work on the fundamental mechanisms of disease is needed. Future work should focus on understanding the precise role of islet inflammation in CFRD, including the role, if any, of amyloid deposition, better understanding the cellular and molecular details underlying impaired insulin secretion, and defining the mechanisms underlying CFTR dependent pancreatic duct/endocrine cross talk. Because β-cell mass is relatively well retained in CFRD, with greater knowledge it may be possible to enhance the function of the remaining β cells and thus effectively prevent and treat CFRD.
Supplementary Material
ACKNOWLEDGMENTS
Many excellent articles are unfortunately not cited here due to limitations on the number of references allowed, but these were considered and discussed during the writing of this article. We apologize to those whose work is not directly acknowledged.
FUNDING
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases awards R01DK115791, R24DK096518 and P30DK089507, UK Cystic Fibrosis Trust (SRC007), Cystic Fibrosis Foundation awards R565CR11, SINGH15R0, VERKMA15R0, MERJAN17AB and HULL16I0, and a faculty scholar award from the Fraternal Order of Eagles Diabetes Research Center.
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Rebecca Hull is a Senior Editor of the Journal of Endocrinology. Rebecca Hull was not involved in the review or editorial process for this paper, on which she is listed as an author.
REFERENCES
- Abdul-Karim FW, Dahms BB, Velasco ME & Rodman HM 1986. Islets of Langerhans in adolescents and adults with cystic fibrosis. A quantitative study. Arch Pathol Lab Med 110 602–606. [PubMed] [Google Scholar]
- Allen JM, Penketh AR, Adrian TE, Lee YC, Sarson DL, Hodson ME, Batten JC & Bloom SR 1983. Adult cystic fibrosis: postprandial response of gut regulatory peptides. Gastroenterology 85 1379–1383. [PubMed] [Google Scholar]
- Andersen DH 1958. Cystic fibrosis of the pancreas. J Chronic Dis 7 58–90. [DOI] [PubMed] [Google Scholar]
- Ballmann M, Hubert D, Assael BM, Staab D, Hebestreit A, Naehrlich L, Nickolay T, Prinz N, Holl RW & Group CS 2018. Repaglinide versus insulin for newly diagnosed diabetes in patients with cystic fibrosis: a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 6 114–121. [DOI] [PubMed] [Google Scholar]
- Baron M, Veres A, Wolock SL, Faust AL, Gaujoux R, Vetere A, Ryu JH, Wagner BK, Shen-Orr SS, Klein AM, et al. 2016. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst 3 346–360 e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelli E & Bendayan M 2005. Association between endocrine pancreas and ductal system. More than an epiphenomenon of endocrine differentiation and development? J Histochem Cytochem 53 1071–1086. [DOI] [PubMed] [Google Scholar]
- Bismuth E, Laborde K, Taupin P, Velho G, Ribault V, Jennane F, Grasset E, Sermet I, de BJ, Lenoir G, et al. 2008. Glucose tolerance and insulin secretion, morbidity, and death in patients with cystic fibrosis. J Pediatr 152 540–545, 545. [DOI] [PubMed] [Google Scholar]
- Blackman SM, Commander CW, Watson C, Arcara KM, Strug LJ, Stonebraker JR, Wright FA, Rommens JM, Sun L, Pace RG, et al. 2013. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes 62 3627–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett DM, Nowosielska A, Afik S, Pechhold S, Cura AJ, Kennedy NJ, Kim S, Kucukural A, Davis RJ, Kent SC, et al. 2015. Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 64 3172–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdani M, Blackman SM, Ridaura C, Bellocq JP, Powers AC & Aguilar-Bryan L 2017. Structural abnormalities in islets from very young children with cystic fibrosis may contribute to cystic fibrosis-related diabetes. Sci Rep 7 17231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom A, Lybaert P, Pollet JF, Jacobs P, Jijakli H, Golstein PE, Sener A, Malaisse WJ & Beauwens R 2007. Expression and localization of cystic fibrosis transmembrane conductance regulator in the rat endocrine pancreas. Endocrine 32 197–205. [DOI] [PubMed] [Google Scholar]
- Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M & Kaestner KH 2013. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J Clin Invest 123 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J, Dougherty S, Makani R, Rubenstein RC & Kelly A 2011. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care 34 292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RI, Tsang D, Koenig S, Wilson D, McCloskey T & Chandra S 2008. Plasma ghrelin and leptin in adult cystic fibrosis patients. J Cyst Fibros 7 398–402. [DOI] [PubMed] [Google Scholar]
- Couce M, O’Brien TD, Moran A, Roche PC & Butler PC 1996. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab 81 1267–1272. [DOI] [PubMed] [Google Scholar]
- Di Fulvio M, Brown PD & Aguilar-Bryan L 2014. Chloride channels and transporters in β-cell physiology In The Islets of Langerhans, pp 401–451. Ed Islam MS. New York, NY, USA: Springer-Verlag. [Google Scholar]
- Dobson L, Sheldon CD & Hattersley AT 2004. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabet Med 21 691–696. [DOI] [PubMed] [Google Scholar]
- Edlund A, Esguerra JL, Wendt A, Flodstrom-Tullberg M & Eliasson L 2014. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med 12 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund A, Pedersen MG, Lindqvist A, Wierup N, Flodstrom-Tullberg M & Eliasson L 2017. CFTR is involved in the regulation of glucagon secretion in human and rodent alpha cells. Sci Rep 7 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, Glynn RJ, Libby P & Ridker PM 2018. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol 71 2392–2401. [DOI] [PubMed] [Google Scholar]
- Fattorusso V, Casale A, Raia V, Mozzillo E & Franzese A 2017. Long-term follow-up in a girl with cystic fibrosis and diabetes since the first year of life. Diabetes Ther 8 1187–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes G, Ghislain J, Benterki I, Zarrouki B, Trudel D, Berthiaume Y & Poitout V 2015. The deltaF508 mutation in the cystic fibrosis transmembrane conductance regulator is associated with progressive insulin resistance and decreased functional beta-cell mass in mice. Diabetes 64 4112–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friard J, Tauc M, Cougnon M, Compan V, Duranton C & Rubera I 2017. Comparative effects of chloride channel inhibitors on LRRC8/VRAC-mediated chloride conductance. Front Pharmacol 8 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MC, Sullivan T, Tai A, Morton JM, Edwards S, Martin AJ, Perano SJ, Gagliardi L, Rayner CK, Horowitz M, et al. 2018. Exenatide corrects postprandial hyperglycaemia in young people with cystic fibrosis and impaired glucose tolerance: A randomized crossover trial. Diabetes Obes Metab. [DOI] [PubMed] [Google Scholar]
- Guo JH, Chen H, Ruan YC, Zhang XL, Zhang XH, Fok KL, Tsang LL, Yu MK, Huang WQ, Sun X, et al. 2014. Glucose-induced electrical activities and insulin secretion in pancreatic islet beta-cells are modulated by CFTR. Nat Commun 5 4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart NJ, Aramandla R, Poffenberger G, Fayolle C, Thames AH, Bautista A, Spigelman AF, Babon JAB, DeNicola ME, Dadi PK, et al. 2018. Cystic fibrosis-related diabetes is caused by islet loss and inflammation. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman M, Eriksson L, Mared L, Helgesson K & Landin-Olsson M 2012. Reduced levels of active GLP-1 in patients with cystic fibrosis with and without diabetes mellitus. J Cyst Fibros 11 144–149. [DOI] [PubMed] [Google Scholar]
- Hull RL, Gibson RL, McNamara S, Deutsch GH, Fligner CL, Frevert CW, Ramsey BW & Sanda S 2018. Islet interleukin-1beta immunoreactivity is an early feature of cystic fibrosis that may contribute to beta-cell failure. Diabetes Care 41 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull RL, Westermark GT, Westermark P & Kahn SE 2004. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab 89 3629–3643. [DOI] [PubMed] [Google Scholar]
- Iannucci A, Mukai K, Johnson D & Burke B 1984. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol 15 278–284. [DOI] [PubMed] [Google Scholar]
- Ichii H, Miki A, Yamamoto T, Molano RD, Barker S, Mita A, Rodriguez-Diaz R, Klein D, Pastori R, Alejandro R, et al. 2008. Characterization of pancreatic ductal cells in human islet preparations. Lab Invest 88 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, Piemonti L, Marchetti P, Bugliani M, Bosco D, et al. 2016. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab 24 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, Trudel S, Brouillard F, Bouillaud F, Colas J, Nguyen-Khoa T, Ollero M, Edelman A & Fritsch J 2010. Cystic fibrosis transmembrane regulator inhibitors CFTR(inh)-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. J Pharmacol Exp Ther 333 60–69. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Gillard P, Mathieu C, Movahedi B, Maleux G, Delvaux G, Ysebaert D, Roep B, Vandemeulebroucke E, Marichal M, et al. 2006. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci U S A 103 17444–17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivula FNM, McClenaghan NH, Harper AGS & Kelly C 2016. Islet-intrinsic effects of CFTR mutation. Diabetologia 59 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanng S, Thorsteinsson B, Nerup J & Koch C 1992. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur J Pediatr 151 684–687. [DOI] [PubMed] [Google Scholar]
- Lanng S, Thorsteinsson B, Pociot F, Marshall MO, Madsen HO, Schwartz M, Nerup J & Koch C 1993a. Diabetes mellitus in cystic fibrosis: genetic and immunological markers. Acta Paediatr 82 150–154. [DOI] [PubMed] [Google Scholar]
- Lanng S, Thorsteinsson B, Roder ME, Orskov C, Holst JJ, Nerup J & Koch C 1993b. Pancreas and gut hormone responses to oral glucose and intravenous glucagon in cystic fibrosis patients with normal, impaired, and diabetic glucose tolerance. Acta Endocrinol (Copenh) 128 207–214. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T & Donath MY 2007. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356 1517–1526. [DOI] [PubMed] [Google Scholar]
- Lohr M, Goertchen P, Nizze H, Gould NS, Gould VE, Oberholzer M, Heitz PU & Kloppel G 1989. Cystic fibrosis associated islet changes may provide a basis for diabetes. An immunocytochemical and morphometrical study. Virchows Arch A Pathol Anat Histopathol 414 179–185. [DOI] [PubMed] [Google Scholar]
- Marchetti P 2016. Islet inflammation in type 2 diabetes. Diabetologia 59 668–672. [DOI] [PubMed] [Google Scholar]
- Marino CR, Matovcik LM, Gorelick FS & Cohn JA 1991. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest 88 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG & Morgan WJ 2005. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 146 681–687. [DOI] [PubMed] [Google Scholar]
- Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, et al. 2010. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol 11 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham LR, Caplan DB, McKean LP, Buchanan CN, Parks JS & Culler FL 1993. Preservation of somatostatin secretion in cystic fibrosis patients with diabetes. Arch Dis Child 68 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis N, Tauc M, Cougnon M, Bendahhou S, Giuliano S, Rubera I & Duranton C 2014. Revisiting CFTR inhibition: a comparative study of CFTRinh −172 and GlyH-101 inhibitors. Br J Pharmacol 171 3716–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merjaneh L, He Q, Long Q, Phillips LS & Stecenko AA 2015. Disposition index identifies defective beta-cell function in cystic fibrosis subjects with normal glucose tolerance. J Cyst Fibros 14 135–141. [DOI] [PubMed] [Google Scholar]
- Milla CE, Warwick WJ & Moran A 2000. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 162 891–895. [DOI] [PubMed] [Google Scholar]
- Mohan K, Miller H, Dyce P, Grainger R, Hughes R, Vora J, Ledson M & Walshaw M 2009. Mechanisms of glucose intolerance in cystic fibrosis. Diabet Med 26 582–588. [DOI] [PubMed] [Google Scholar]
- Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, Robinson KA, Sabadosa KA, Stecenko A & Slovis B 2010. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 33 2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Diem P, Klein DJ, Levitt MD & Robertson RP 1991. Pancreatic endocrine function in cystic fibrosis. J Pediatr 118 715–723. [DOI] [PubMed] [Google Scholar]
- Moran A, Dunitz J, Nathan B, Saeed A, Holme B & Thomas W 2009a. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 32 1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, Tullis E, Liou TG, Allen H & Cystic Fibrosis Related Diabetes Therapy Study G 2009b. Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the cystic fibrosis related diabetes therapy trial. Diabetes Care 32 1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau F, Weiller MA, Rosner V, Weiss L, Hasselmann M, Pinget M, Kessler R & Kessler L 2008. Continuous glucose monitoring in cystic fibrosis patients according to the glucose tolerance. Horm Metab Res 40 502–506. [DOI] [PubMed] [Google Scholar]
- Ntimbane T, Mailhot G, Spahis S, Rabasa-Lhoret R, Kleme ML, Melloul D, Brochiero E, Berthiaume Y & Levy E 2016. CFTR silencing in pancreatic beta-cells reveals a functional impact on glucose-stimulated insulin secretion and oxidative stress response. Am J Physiol Endocrinol Metab 310 E200–212. [DOI] [PubMed] [Google Scholar]
- Nyirjesy SC, Sheikh S, Hadjiliadis D, De Leon DD, Peleckis AJ, Eiel JN, Kubrak C, Stefanovski D, Rubenstein RC, Rickels MR, et al. 2018. Beta-cell secretory defects are present in pancreatic insufficient cystic fibrosis with 1-hour oral glucose tolerance test glucose >/=155 mg/dL. Pediatr Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode KL, Frohnert B, Laguna T, Phillips J, Holme B, Regelmann W, Thomas W & Moran A 2010. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes 11 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, et al. 2012. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 122 3755–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perano SJ, Couper JJ, Horowitz M, Martin AJ, Kritas S, Sullivan T & Rayner CK 2014. Pancreatic enzyme supplementation improves the incretin hormone response and attenuates postprandial glycemia in adolescents with cystic fibrosis: a randomized crossover trial. J Clin Endocrinol Metab 99 2486–2493. [DOI] [PubMed] [Google Scholar]
- Rotti PG, Xie W, Poudel A, Yi Y, Sun X, Tyler SR, Uc A, Norris AW, Hara M, Engelhardt JF, et al. 2018. Pancreatic and islet remodeling in cystic fibrosis transmembrane conductance regulator (CFTR) knockout ferrets. Am J Pathol 188 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstolpe A, Palasantza A, Eliasson P, Andersson EM, Andreasson AC, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK, et al. 2016. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 24 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeiros IM, Hester K, Callaway M, Williams A, Garland Z, Powell T, Wong FS, Jarad NA & Bristol Cystic Fibrosis Diabetes G 2010. MRI appearance of the pancreas in patients with cystic fibrosis: a comparison of pancreas volume in diabetic and non-diabetic patients. Br J Radiol 83 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh S, Gudipaty L, De Leon DD, Hadjiliadis D, Kubrak C, Rosenfeld NK, Nyirjesy SC, Peleckis AJ, Malik S, Stefanovski D, et al. 2017. Reduced beta-cell secretory capacity in pancreatic-insufficient, but not pancreatic-sufficient, cystic fibrosis despite normal glucose tolerance. Diabetes 66 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soave D, Miller MR, Keenan K, Li W, Gong J, Ip W, Accurso F, Sun L, Rommens JM, Sontag M, et al. 2014. Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: a Mendelian randomization study. Diabetes 63 2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima K & Landing BH 1986. Pancreatic islets in older patients with cystic fibrosis with and without diabetes mellitus: morphometric and immunocytologic studies. Pediatr Pathol 6 25–46. [DOI] [PubMed] [Google Scholar]
- Sun X, Olivier AK, Yi Y, Pope CE, Hayden HS, Liang B, Sui H, Zhou W, Hager KR, Zhang Y, et al. 2014. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am J Pathol 184 1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Yi Y, Xie W, Liang B, Winter MC, He N, Liu X, Luo M, Yang Y, Ode KL, et al. 2017. CFTR influences beta cell function and insulin secretion through non-cell autonomous exocrine-derived factors. Endocrinology 158 3325–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uc A, Olivier AK, Griffin MA, Meyerholz DK, Yao J, Abu-El-Haija M, Buchanan KM, Vanegas Calderon OG, Abu-El-Haija M, Pezzulo AA, et al. 2015. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 128 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, et al. 2015. Proteomics. Tissue-based map of the human proteome. Science 347 1260419. [DOI] [PubMed] [Google Scholar]
- van Meegen MA, Terheggen SW, Koymans KJ, Vijftigschild LA, Dekkers JF, van der Ent CK & Beekman JM 2013. CFTR-mutation specific applications of CFTR-directed monoclonal antibodies. J Cyst Fibros 12 487–496. [DOI] [PubMed] [Google Scholar]
- Warnock GL, Meloche RM, Thompson D, Shapiro RJ, Fung M, Ao Z, Ho S, He Z, Dai LJ, Young L, et al. 2005. Improved human pancreatic islet isolation for a prospective cohort study of islet transplantation vs best medical therapy in type 1 diabetes mellitus. Arch Surg 140 735–744. [DOI] [PubMed] [Google Scholar]
- Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van RN, Ehses JA & Verchere CB 2011. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol 187 2755–2765. [DOI] [PubMed] [Google Scholar]
- Wilschanski M & Novak I 2013. The cystic fibrosis of exocrine pancreas. Cold Spring Harb Perspect Med 3 a009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, Norris AW, Wang K, Sun X, Uc A, Moran A, Engelhardt JF & Ode KL 2016a. Abnormal glucose tolerance in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 194 974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y, Sun X, Gibson-Corley K, Xie W, Liang B, He N, Tyler SR, Uc A, Philipson LH, Wang K, et al. 2016b. A transient metabolic recovery from early life glucose intolerance in cystic fibrosis ferrets occurs during pancreatic remodeling. Endocrinology 157 1852–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.