Abstract
A health care learning community engages providers and families in a collaborative environment to improve outcomes. Vermont Oxford Network (VON), a voluntary organization dedicated to improving the quality, safety and value of care through a coordinated program of data-driven quality improvement, education, and research, is a worldwide learning community in newborn medicine. Through collection of pragmatic structured data items and benchmarking reports, quality improvement collaboratives, pragmatic trials, and observational research, VON facilitates quality improvement by multidisciplinary teams and families in neonatal intensive care units (NICU) in low, middle, and high resource countries. By bringing health professionals and families together across disciplines and geographies to enable shared learning and knowledge dissemination, VON empowers individuals, organizations, and systems to meet the shared vision that every infant around the world can and should achieve their full potential.
Keywords: Neonatology, global health, quality improvement, evidence-based practice
Introduction
A health care learning community engages providers and families in a collaborative environment to improve outcomes (1). The community connects people across disciplines and geographies to enable shared learning and knowledge creation and dissemination, and to set common goals and measure collective progress (2). Such communities require data to facilitate learning, a culture of continuous improvement, and individuals, organizations, and systems with a shared vision (3). Vermont Oxford Network (VON) is a voluntary worldwide multidisciplinary learning community dedicated to improving the quality, safety and value of care through a coordinated program of data-driven quality improvement, education, and research in newborn medicine (4).
Data to facilitate learning
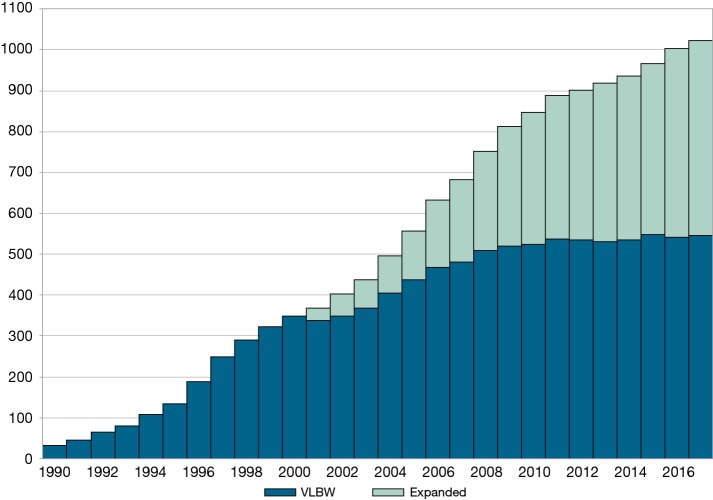
Currently, over 1,100 hospitals submit standardized, structured data to VON (Figure 1). The data have pragmatic definitions designed to help hospitals improve the quality of care for newborns and their families. In 2018, 532 hospitals finalized data on very low birth weight or very preterm infants, defined as infants 401 to 1,500 grams or 22 to 29 weeks at birth, and 491 hospitals finalized data on all infants admitted to neonatal intensive care units (NICU), including the very low birth weight and very preterm population (5).
Figure 1.
Hospital participation in databases over time by database type (EXP or VLBW). EXP, expanded; VLBW, very-low-birth-weight.
Reports based on these data give hospitals information for quality improvement in their local context. Process and outcome measures, collected over time and compared to peers, help hospitals identify potential areas for improvement. Hospitals can benchmark performance against the entire network, the type of NICU based on services provided, special groups such as hospital systems, or the country or state in which a hospital is located. VON reports the hospital rates at the 25th and 75th percentiles for all unadjusted measures so that hospitals can benchmark against the best and worst quartiles of performance. VON also reports risk- and volume-adjusted observed-to-expected measures to help hospitals understand if performance is better, worse, or as expected. Evaluation of year-by-year performance helps hospitals observe the effect of quality improvement programs. Hospitals can obtain monthly, quarterly, or semi-annual run charts by discharge date to ascertain the effect of changes on a more granular time scale. Evidence from peer-reviewed publications (6-14) and abstracts presented at national meetings suggests that multidisciplinary teams actively use VON data to fuel improvement.
Annual survey
All hospitals complete an annual survey on resources and capabilities, and to classify hospitals into VON NICU types. Using responses to whether the center was required by state regulation to transfer infants to another center for assisted ventilation based on the infant characteristics or duration of ventilation required, or whether one of eight surgeries was performed at the center (omphalocele repair, ventriculoperitoneal shunt, tracheoesophageal fistula/esophageal atresia repair, bowel resection/renanastomosis, meningomyelocele repair, cardiac catheterization, cardiac surgery requiring bypass), centers are divided into three groups: ventilation restrictions or no surgery; surgery except cardiac requiring bypass; all surgery (Table 1). NICUs with ventilation restrictions correspond to Level II under the current American Academy of Pediatrics levels of neonatal care. NICUs that do not perform surgery on site and NICUs that perform all surgery except cardiac requiring bypass correspond to Level III. NICUs that perform all surgery correspond to Level IV (15). These data contribute vital information to the learning community about hospitals’ resources and are used in reporting and in research (16-18).
Table 1. Vermont Oxford Network hospitals participating in the very-low-birth-weight or expanded databases by VON NICU type, 2017.
| Types | n [%] |
|---|---|
| Type A: restrictions on ventilation or no surgery | 427 [42] |
| Type B: no ventilation restrictions and surgery* except cardiac requiring bypass | 429 [42] |
| Type C: no ventilation restrictions and surgery* including cardiac requiring bypass | 161 [16] |
*, omphalocele repair, ventriculoperitoneal shunt, tracheoesophageal fistula/esophageal atresia repair, bowel resection/renanastomosis, meningomyelocele repair, or cardiac catheterization. NICU, neonatal intensive care units.
Extremely-low-birth-weight (ELBW) follow-up
For hospitals interested in benchmarking care of extremely low birth weight and low gestational age infants (401–1,000 grams or 22–27 weeks’ gestational age), VON offers data collection on health and developmental outcomes at 18–24 months. Reports based on these data are used to benchmark differences in infant procedures and outcomes among participating hospitals and in research (19-21). Recently, follow-up teams at hospitals in Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont formed the New England Follow-Up Network. Using the VON follow-up data collection and reporting, these teams identified areas for improvement that they will work on collectively including the follow-up rate, collection of data on social determinants of health, and growth at 2-year follow-up.
Global health
VON also offers a unique database for hospitals in low-resource settings to help teams focus on improving the processes and outcomes most important in these environments. The neonatal period represents the most vulnerable time for a child’s survival globally. In 2017, 2.5 million deaths, or roughly 47% of all deaths under the age of 5 years, occurred during the first 28 days after birth; 98% of these deaths occurred in low- and middle-income countries, translating to 7,000 newborn deaths every day (22). The most common causes of neonatal death worldwide are known preterm birth (35%), intrapartum-related causes (24%), and sepsis (14%) (23). The United Nations Sustainable Development Goal is to reduce neonatal mortality to 12 per 1,000 live births globally by 2030 (24). Quality improvement methods are not yet part of standard educational training or routine health care delivery in most low-income countries, and timely, actionable, facility-level data has not been available for improvement purposes (25).
As an innovator in neonatal care, VON partners with colleagues in low-income countries to change the landscape of neonatal care, and develop tools, mentorship, and a community to help meet the United Nations Sustainable Development Goal. In 2018, twenty Ethiopian public and academic hospitals joined VON, utilizing the Global Health Database. In partnership with the Ethiopian Pediatrics Society and the Federal Ministry of Health, VON facilitated the formation of the Ethiopian Neonatal Network, utilizing this standardized data platform among the teams in this community. Approximately 10,000 infants were registered in the database in the first year. Using newly available, real-time, local facility-level reports, teams identified gaps in the quality of care (26), created specific, measurable, achievable, relevant, and time-bound (SMART) aims, and embarked on their first mentored quality improvement project. Teams at these hospitals are working on increasing the percentage of deliveries attended by providers trained in neonatal resuscitation or Helping Babies Breathe, reducing admission hypothermia in the neonatal unit, and increasing the percentage of low-birth weight infants that receive kangaroo mother care. Given the success in developing a learning community in Ethiopia, the Global Database’s favorable ratio of data burden to data value, and the ability of some teams to achieve their SMART aims within the first year of database participation, we plan for expansion to additional low-income countries in Africa by 2022.
Quality measurement
As part of its commitment to the newborn learning community, VON submits its measures to the National Quality Forum, a not-for-profit, nonpartisan organization that leads collaboration in the United States to improve health and healthcare quality through measurement. “Proportion of Infants 22 to 29 Weeks Gestation Screened for Retinopathy of Prematurity” and “Late-Onset Sepsis or Meningitis in Neonates (Risk-Adjusted)” were both endorsed in 2008 (27), 2011 (28), and 2016 (29) as part of a suite of perinatal measures that hospitals can use to evaluate the quality and efficiency of newborn health care. The Leapfrog Group includes two VON measures in its annual hospital survey, antenatal steroid treatment and a risk-adjusted composite death or morbidity measure. VON also worked closely with the Centers for Disease Control and Prevention on a new neonatal late onset sepsis/meningitis measure and the neonatal standardized antimicrobial administration rates for the National Healthcare Safety Network (30).
A culture of continuous improvement
For more than 25 years, VON has organized quality improvement collaboratives of multidisciplinary teams working collectively to improve newborn outcomes. These activities serve the heart of the organization’s mission to improve the quality, safety, and value of care for newborn infants and their families.
Over 700 neonatal care teams have participated in one or more of VON’s quality improvement collaboratives. Some hospitals join VON solely to participate in a collaborative. In these collaboratives, multidisciplinary teams of health professionals work together under the guidance of clinical and quality improvement experts to identify, test, and implement “potentially better” practices designed to achieve their stated improvement aims. “Potentially better” practices, rather than “better” or “best” practices, indicate that a practice is not better or best until it is adapted, tested, and shown to be effective in each hospital’s unique context (31). Teams learn the four key habits for improvement: the habit for change; the habit for evidence-based practice; the habit of systems thinking; and the habit for collaborative learning (32,33). Teams apply the Model for Improvement to achieve their goals, setting SMART aims, identify measures to track improvement, adapt and test the potentially better practices provided in collaborative toolkits through a series of Plan Do Study Act (PDSA) cycles (34). This context-dependent aspect of improvement is an underappreciated strength of the VON learning community. As multiple teams test and implement potentially better practices in unique ways suited to their local conditions and share what they have learned, everyone’s understanding of these practices is enhanced as the community searches the clinical fitness landscape for the highest peaks of performance (35).
Neonatal Improvement Collaborative for Quality (NICQ)
Teams in the NICQ self-select into “homerooms” of 6 to 10 hospital teams. A dedicated faculty including clinical experts, a quality improvement coach and a NICU parent supports each homeroom. Each team is expected to have a NICU parent as a working member of the improvement team. Teams participate in faculty-facilitated visits to other units during which both host and visitor contribute to deep immersion learning and exploration of the clinical landscape. Since 1995, teams in the NICQ improvement collaboratives have addressed a wide range of improvement topics including hospital acquired infection, chronic lung disease and respiratory care, necrotizing enterocolitis and nutrition, brain injury, obstetrical care, nurse staffing and turnover, micropremature infants, family centered care, and costs, among others (32,33,36-41).
Internet Newborn Improvement Collaborative for Quality (iNICQ)
Teams in the iNICQ collaborative focus on a single improvement topic over a 1 to 3-year timeframe. Recent improvement topics have included neonatal abstinence syndrome (42), alarm safety (43), and antibiotic stewardship (44). The current iNICQ collaborative, Critical Transitions: The Ins and Outs of Newborn Care involves over 120 neonatal teams from five countries focused on improving the quality and safety of the multiple transitions experienced by newborn infants and their families before during and after their initial hospitalization (45).
VON Day Audits
Often, teams participating in the NICQ collaborative use reporting from the VON databases to measure improvements. The iNICQ collaboratives have supplemented measures collected with VON Day Audits, short, focused surveys of unit policies, practices, or guidelines about the program topic and a patient audit related to the topic. The surveys are meant to be completed on a single day in a 2-week audit window. The audits allow teams to identify gaps in performance, select potentially better practices to close those gaps.
The VON Day audits are used by individual teams to track their improvement progress and support assessment of the overall collaboratives. In a 2-year period of the neonatal abstinence syndrome iNICQ program, the mean number of NAS-focused policies increased while the median length of pharmacological treatment and length of stay decreased, as did the proportion of infants discharged on medication for NAS (42). Teams participating in a one-year iNICQ program on alarm safety showed significant progress toward implementation of Joint Commission Alarm Safety goals for oximeter monitoring (43). VON Day Audits were also used to measure delivery room management (46) and antibiotic stewardship (44). In the iNICQ collaborative on antibiotic stewardship conducted in partnership with the Centers for Disease Control and Prevention, participating teams increased adherence to the CDC core stewardship practices while reducing antibiotic use rates in their units.
Inclusion of families
A hallmark of NICQ and iNICQ participation is inclusion of family (47), who are full members of the improvement teams and serve as paid faculty in all VON collaboratives. Each NICQ homeroom includes a paid parent faculty member. There is also a paid parent faculty member on the overall faculty for NICQ and iNICQ, and all NICU teams are encouraged to have paid family advisors. VON is now working closely with the Point of Care Foundation to integrate Experience Based Co-Design into both the NICQ and iNICQ collaboratives (48). Using EBCD, staff and families work closely together to deeply understand both the staff and family experience of care using stories, narratives, observation, and filming. This knowledge is then applied to redesign the key processes of care.
Generating new knowledge
A learning community has the power to generate new knowledge. VON has a long history of conducting pragmatic randomized clinical trials and observational research contributing knowledge to the newborn learning community.
Pragmatic trials
Pragmatic trials measure the benefit of a treatment produced in routine clinical practice. Such trials conducted by VON include a comparison of two surfactants for the treatment of respiratory distress syndrome; the trial had a design similar to a large National Institutes of Child Health and Development (NICHD) trial but with more precise estimates of the effects of the surfactant preparations (49). A trial on early postnatal dexamethasone for chronic lung disease identified serious side effects of early steroid treatment (50,51), and a trial of prophylactic emollient ointment and its effects on infection and skin integrity provided evidence that went against evolving guidelines and recommendations (52). A trial of different types of initial respiratory management supported a less invasive approach to delivery room stabilization of preterm infants (53), and a trial of occlusive wrap for heat loss prevention improved knowledge on early thermal stability (54). VON also conducted a unique pragmatic cluster randomized trial at the hospital level with 114 NICUs participating in a quality improvement intervention designed to foster evidence-based practices regarding surfactant administration (55).
Observational research
Observational research using the very low birth weight and all NICU admission databases has informed the learning community on trends in outcomes and practices (56-59). Nearly 90% of the very-low-birth-weight infants born in the United States are reported to VON, making results representative of the full population and therefore generalizable. The large number of cases in the databases support research on outcomes in specific populations, such as infants with necrotizing enterocolitis (17,18,60-63), gastroschisis (64,65), and hypoplastic left heart syndrome (66). Topics in health services research and newborn care include neonatal care utilization in the United States (16,67) and internationally (68), nurse staffing (69-72), and disparities in care (73-76).
The large number of cases in the VON databases can contribute new evidence to widely accepted practices. Despite the abundant evidence on the efficacy of antenatal steroids in improving neonatal outcomes, gaps in the evidence still exist. Trials do not address the effect of antenatal steroids in less mature infants. Current guidelines recommend giving antenatal corticosteroids to infants at or above 24 weeks’ gestation and recommend “consideration” of antenatal steroid treatment in infants 23 weeks’ gestation. For infants at 22 weeks’ gestation, corticosteroids are not recommended (77). However, there is evidence from observational studies that antenatal steroids may benefit even these least mature infants (78). In a multicenter observational cohort study of 29,932 liveborn infants who received postnatal life support at 431 VON hospitals in the United States, survival to hospital discharge was higher for infants with ANS exposure (72.3%) compared with infants without ANS exposure (51.9%). The adjusted risk ratio for survival of infants at 22 weeks was 2.11 (95% CI, 1.68 to 2.65) and at 23 weeks was 1.54 (95% CI, 1.40 to 1.70). It is doubtful (though not unethical) that future trials will address the efficacy of steroids in this extremely immature population despite the real need to further understand the downstream consequences on the development of these particularly fragile infants, but large databases with standardized definitions may help provide new information for decision making.
Knowledge from a learning community can raise the bar for future improvement work. In a study of 408,164 very low birth weight infants at 756 NICUs in the United States, 99.1% of units achieved the 2005 shrunken adjusted rate of the best quartile for death prior to discharge, while 98% for late onset infection, 88% for severe intraventricular hemorrhage, 82% for severe retinopathy of prematurity, 76% for necrotizing enterocolitis, and 41% for chronic lung disease. It took 3 years before 75% of units achieved the 2005 shrunken adjusted rate from the best quartile for death prior to discharge, 5 years for late onset infection, 6 years for severe retinopathy of prematurity and severe intraventricular hemorrhage, and 8 years for necrotizing enterocolitis (59). These findings set a new standard for NICUs working to reduce mortality and morbidities.
Knowledge from a learning community can also set the bar for quality improvement among neonatal units in low-income countries. In a study of 5,703 infants at 20 NICUs in Ethiopia using the Vermont Oxford Network Global Health Database, Essential Newborn Care practices were recorded for all infants admitted to the NICU and stratified by inborn and outborn status to inform referral feedback to community sites. Among all NICU admissions, 80% had a delivery provider trained in neonatal resuscitation, 85% were dried at birth, 53% were placed skin-to-skin, 42% received delayed cord clamping, and 41% had early initiation of breastfeeding (26). These quality gaps were paired with the hospital-level annual survey, which provided additional data for advocacy including NICU physician and nurse staffing, physical space, and equipment (79). As specialized neonatal care and neonatal intensive care in low-income countries emerges in response to the demand to meet the Sustainable Development Goals, the optimal levels of care provided, practices and staffing are not well understood. As additional countries join the Vermont Oxford Network Global Neonatal Database, the opportunities for understanding and improving neonatal intensive care will be greatly enhanced and catalyzed by leaders within the growing neonatal improvement community in low-income countries.
NICU by the Numbers
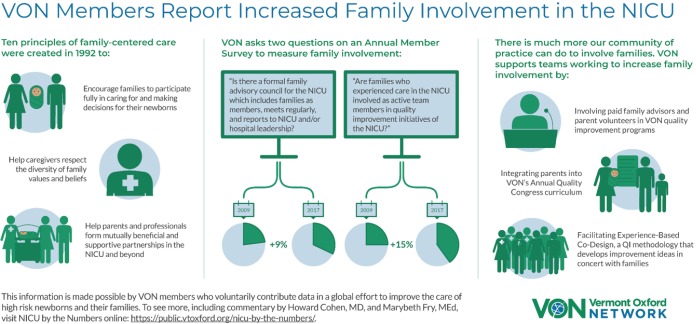
Recently, VON started a series of two-page data briefs called “NICU by the Numbers.” The topics of these short reports, such as hypothermia on admission, the gap in antenatal steroid receipt for outborn infants, international variation in human milk at discharge, and family involvement in quality improvement (Figure 2) are meant to highlight important and potentially actionable issues and are free and publicly available (80).
Figure 2.
Family involvement in the NICU, published in NICU by the Numbers. NICU, neonatal intensive care units.
Cochrane Neonatal
As the host organization for Cochrane Neonatal, VON provides financial support and resources for the creation and dissemination of systematic reviews of evidence-based medicine for newborns. These resources help the learning community provide the best possible evidence-based care for infants and families around the world. Cochrane Neonatal has created over 350 systematic reviews of neonatal interventions with the engagement of over 1,000 researchers and authors worldwide. Over 100 of these Cochrane Neonatal reviews have informed national and international guidelines, changing practice in neonatal care and significantly improving neonatal outcomes. Cochrane Neonatal is one of over 50 review groups affiliated with Cochrane, an international not-for-profit organization dedicated to providing up-to-date, accurate information about the effects of healthcare.
Challenges for the future
Each year at the Vermont Oxford Network Annual Quality Congress in the State of the Network address, we set challenges for the VON community.
Our first challenge is to dramatically reduce, if not eliminate entirely, the major morbidities of neonatal intensive care. We first introduced this challenge regarding late onset infection over a decade ago. At the time, there was great skepticism that this could be achieved. Infection was considered unavoidable. However, since that challenge was first posed, infection rates for very low birth weight infants wer reduced from 21.9% in 2005 to 10.1% in 2014 (59). We have now extended that challenge to all the major morbidities. There is skepticism, but we fully expect that in the next decade we will see dramatic improvements in all the major morbidities of neonatal care.
Our second challenge is to engage neonatal providers from low- and middle-income countries as full participants in our learning community. VON sponsored neonatology fellowship training for four promising pediatric leaders from low-income countries in Africa. Recognizing that these neonatologists will provide clinical care and, more importantly, serve as neonatal leaders at a national and international level, we are proud to be involved and support their continued professional development. This track record in capacity building is one we intend to continue. In Ethiopia, the neonatologists helped develop the national neonatal guidelines and started the first advanced practice neonatal nursing master’s program in the country. We are learning from their passion, resourcefulness, and progress while striving to sustainably enrich our worldwide community of practice with additional low-income country teams.
The final challenge for the VON learning community is to accept that our responsibility to the infants and families we serve extends beyond the hospital walls. In The Color of Law, Richard Rothstein documents how, from Reconstruction to the present local, state, and federal policies, regulations and laws have been used to segregate Americans by race resulting in dramatic inequalities in social, economic, and educational opportunities (81). Black and other minority Americans live in poorer neighborhoods (82,83), attend lower quality schools (84,85), and receive care at lower quality hospitals (75,76,86-90). We must expand the old paradigm of “follow-up” to include broader responsibility for “follow through.” Caring for high risk infants and families is more than a technical exercise. It is one that encompasses the social determinants that will ultimately drive the health and well-being of infants and families. Again, there is skepticism. Clinicians question how they can affect social factors such as poverty, housing, food insecurity, and racism, segregation, and inequality. VON has a long history of identifying racial and ethnic disparities in care that can inform and motivate the search for practical interventions (73-76). We will work closely with teams to identify practical interventions that individual health professionals, hospitals, and health systems can implement to help remedy these inequalities including advocacy at the local state and national levels.
Conclusions
Since 1988, the VON has grown from 34 neonatal units in the United States to a worldwide learning community including teams from over 1,500 neonatal units in 42 countries. Outcomes for infants and families have improved substantially (59,91,92). However, our work is only beginning. By continuing to bring health professionals and families together across disciplines and geographies to enable shared learning and knowledge creation and dissemination with the common goal of insuring that every infant around the world must achieve their full potential, the size, strength, and efficacy of our learning community will continue to thrive.
Acknowledgments
We are indebted to our colleagues who submit data to Vermont Oxford Network on behalf of infants and their families. Participating hospitals are listed in http://fp.amegroups.cn/cms/tp.2019.07.01-1.pdf.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci 2015;10:21. 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center on the Developing Child at Harvard University. Learning Communities 2017. Available online: https://developingchild.harvard.edu/collective-change/key-concepts/learning-communities/
- 3.Center on the Developing Child at Harvard University. Distributed Leadership 2017. Available online: https://developingchild.harvard.edu/collective-change/key-concepts/distributed-leadership/
- 4.Wenger E. Communities of Practice. New York: Cambridge University Press, 1998. [Google Scholar]
- 5.Vermont Oxford Network. Manual of Operations: Part 2. Data Definitions and Infant Data Forms. Burlington, VT: Vermont Oxford Network, 2018. [Google Scholar]
- 6.Owens JD, Soltau T, McCaughn D, et al. Multi-hospital community NICU quality improvement improves survival of ELBW infants. J Miss State Med Assoc 2015;56:237-42. [PubMed] [Google Scholar]
- 7.Wallingford B, Rubarth L, Abbott A, et al. Implementation and evaluation of "golden hour" practices in infants younger than 33 weeks‘ gestation. Newborn Infant Nurs Rev 2012;12:86-96. 10.1053/j.nainr.2012.03.008 [DOI] [Google Scholar]
- 8.Harer MW, Vergales B, Cady T, et al. Implementation of a multidisciplinary guideline improves preterm infant admission temperatures. J Perinatol 2017;37:1242-7. 10.1038/jp.2017.112 [DOI] [PubMed] [Google Scholar]
- 9.Burhouse A, Lea C, Ray S, et al. Preventing cerebral palsy in preterm labour: a multiorganisational quality improvement approach to the adoption and spread of magnesium sulphate for neuroprotection. BMJ Open Qual 2017;6:e000189. 10.1136/bmjoq-2017-000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbs L, Tooke L, Harrison MC. Short-term outcomes of inborn v. outborn very-low-birth-weight neonates (<1 500 g) in the neonatal nursery at Groote Schuur Hospital, Cape Town, South Africa. S Afr Med J 2017;107:900-3. 10.7196/SAMJ.2017.v107i10.12463 [DOI] [PubMed] [Google Scholar]
- 11.Chee YY, Wong MS, Wong RM, et al. Neonatal outcomes of preterm or very-low-birth-weight infants over a decade from Queen Mary Hospital, Hong Kong: comparison with the Vermont Oxford Network. Hong Kong Med J 2017;23:381-6. [DOI] [PubMed] [Google Scholar]
- 12.Talavera MM, Bixler G, Cozzi C, et al. Quality improvement initiative to reduce the necrotizing enterocolitis rate in premature infants. Pediatrics 2016. doi: . 10.1542/peds.2015-1119 [DOI] [PubMed] [Google Scholar]
- 13.Birenbaum HJ, Pfoh ER, Helou S, et al. Chronic lung disease in very low birth weight infants: Persistence and improvement of a quality improvement process in a tertiary level neonatal intensive care unit. J Neonatal Perinatal Med 2016;9:187-94. 10.3233/NPM-16915098 [DOI] [PubMed] [Google Scholar]
- 14.Pinheiro JM, Boynton S, Furdon SA, et al. Use of chemical warming packs during delivery room resuscitation is associated with decreased rates of hypothermia in very low-birth-weight neonates. Adv Neonatal Care 2011;11:357-62. 10.1097/ANC.0b013e318229aa8f [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics Committee on Fetus And Newborn Levels of neonatal care. Pediatrics 2012;130:587-97. 10.1542/peds.2012-1999 [DOI] [PubMed] [Google Scholar]
- 16.Edwards EM, Horbar JD. Variation in use by NICU types in the United States. Pediatrics 2018. doi: . 10.1542/peds.2018-0457 [DOI] [PubMed] [Google Scholar]
- 17.Sparks EA, Gutierrez IM, Fisher JG, et al. Patterns of surgical practice in very low birth weight neonates born in the United States: a Vermont Oxford Network analysis. J Pediatr Surg 2014;49:1821-4.e8. 10.1016/j.jpedsurg.2014.09.032 [DOI] [PubMed] [Google Scholar]
- 18.Fullerton BS, Sparks EA, Morrow KA, et al. Hospital transfers and patterns of mortality in very low birth weight neonates with surgical necrotizing enterocolitis. J Pediatr Surg 2016;51:932-5. 10.1016/j.jpedsurg.2016.02.051 [DOI] [PubMed] [Google Scholar]
- 19.Mercier CE, Dunn MS, Ferrelli KR, et al. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Neonatology 2010;97:329-38. 10.1159/000260136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fullerton BS, Hong CR, Velazco CS, et al. Severe neurodevelopmental disability and healthcare needs among survivors of medical and surgical necrotizing enterocolitis: A prospective cohort study. J Pediatr Surg 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Hong CR, Fullerton BS, Mercier CE, et al. Growth morbidity in extremely low birth weight survivors of necrotizing enterocolitis at discharge and two-year follow-up. J Pediatr Surg 2018;53:1197-202. 10.1016/j.jpedsurg.2018.02.085 [DOI] [PubMed] [Google Scholar]
- 22.United Nations Inter-agency Group for Child Mortality Estimation. Levels & Trends in Child Mortality: Report 2018. New York, NY: United Nations Children’s Fund, 2018. [Google Scholar]
- 23.United Nations Children‘s Fund. Neonatal mortality March 2018. Available online: https://data.unicef.org/topic/child-survival/neonatal-mortality/
- 24.United Nations. Sustainable Development Goal 3: Ensure healthy lives and promote well-being for all at all ages. Available online: https://sustainabledevelopment.un.org/sdg3
- 25.Ehret DY, Patterson JK, Bose CL. Improving neonatal care: a global perspective. Clin Perinatol 2017;44:567-82. 10.1016/j.clp.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 26.Ehret DEY, Edwards EM, Tariku A, et al. The Ethiopian Neonatal Network-Establishing quality improvement as a strategy to achieve the Sustainable Development Goal of Neonatal Mortality Reduction. Pediatric Academic Societies Meeting, April 27, 2019, Baltimore, MD. [Google Scholar]
- 27.National Quality Forum. National Voluntary Consensus Standards for Perinatal Care 2008: A Consensus Report. Washington, D.C.: NQF, 2009. [Google Scholar]
- 28.National Quality Forum. Perinatal and Reproductive Health Endorsement Maintenance: Technical Report. Washington, D.C.: NQF, 2012. [Google Scholar]
- 29.National Quality Forum. Perinatal and Reproductive Health, 2015-2016: Final Report. Washington, D.C.: NQF, 2016.
- 30.O‘Leary EN, van Santen KL, Edwards EM, et al. Using NHSN‘s antimicrobial use option to monitor and improve antibiotic stewardship in neonates. Hosp Pediatr 2019;9:340-7. [DOI] [PMC free article] [PubMed]
- 31.Plsek PE. Quality improvement methods in clinical medicine. Pediatrics 1999;103:203-14. [PubMed] [Google Scholar]
- 32.Horbar JD, Plsek PE, Leahy K. NIC/Q 2000: establishing habits for improvement in neonatal intensive care units. Pediatrics 2003;111:e397-410. [PubMed] [Google Scholar]
- 33.Horbar JD, Rogowski J, Plsek PE, et al. Collaborative quality improvement for neonatal intensive care. NIC/Q Project Investigators of the Vermont Oxford Network. Pediatrics 2001;107:14-22. 10.1542/peds.107.1.14 [DOI] [PubMed] [Google Scholar]
- 34.Associates in Process Improvement. Model for Improvement. Available online: http://www.apiweb.org/
- 35.Eppstein MJ, Horbar JD, Buzas JS, et al. Searching the clinical fitness landscape. PLoS One 2012;7:e49901. 10.1371/journal.pone.0049901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horbar JD, Gould JB. Evidence-based quality improvement in neonatal and perinatal medicine. Pediatrics 1999;103:202-393. [Google Scholar]
- 37.Horbar JD, Plsek PE, Schriefer JA, et al. Evidence-based quality improvement in neonatal and perinatal medicine: the NIC/Q 2002 experience. Pediatrics 2006;118:S57-202. 10.1542/peds.2006-0913A [DOI] [Google Scholar]
- 38.Payne NR, Finkelstein MJ, Liu M, et al. NICU practices and outcomes associated with 9 years of quality improvement collaboratives. Pediatrics 2010;125:437-46. 10.1542/peds.2009-1272 [DOI] [PubMed] [Google Scholar]
- 39.Rogowski JA, Horbar JD, Plsek PE, et al. Economic implications of neonatal intensive care unit collaborative quality improvement. Pediatrics 2001;107:23-9. 10.1542/peds.107.1.23 [DOI] [PubMed] [Google Scholar]
- 40.Kaempf JW, Zupancic JA, Wang L, et al. A risk-adjusted, composite outcomes score and resource utilization metrics for very low-birth-weight infants. JAMA Pediatr 2015;169:459-65. 10.1001/jamapediatrics.2014.3566 [DOI] [PubMed] [Google Scholar]
- 41.Mola SJ, Annibale DJ, Wagner CL, et al. NICU bedside caregivers sustain process improvement and decrease incidence of bronchopulmonary dysplasia in infants < 30 weeks gestation. Respir Care 2015;60:309-20. 10.4187/respcare.03235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patrick SW, Schumacher RE, Horbar JD, et al. Improving care for neonatal abstinence syndrome. Pediatrics 2016;137:e20153835. 10.1542/peds.2015-3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagadorn JI, Sink DW, Buus-Frank ME, et al. Alarm safety and oxygen saturation targets in the Vermont Oxford Network iNICQ 2015 collaborative. J Perinatol 2017;37:270-6. 10.1038/jp.2016.219 [DOI] [PubMed] [Google Scholar]
- 44.Ho T, Buus-Frank ME, Edwards EM, et al. Adherence of newborn-specific antibiotic stewardship programs to CDC recommendations. Pediatrics 2018. doi: . 10.1542/peds.2017-4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermont Oxford Network. iNICQ 2019: The "Ins" and "Outs" of Newborn Care. Improving Critical Transitions for Every Newborn 2018. Available online: https://public.vtoxford.org/quality-education/the-ins-outs-of-neonatal-care-an-inicq-for-critical-transitions/
- 46.Edwards EM, Soll RF, Ferrelli K, et al. Identifying improvements for delivery room resuscitation management: results from a multicenter safety audit. Matern Health Neonatol Perinatol 2015;1:2. 10.1186/s40748-014-0006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celenza JF, Zayack D, Buus-Frank ME, et al. Family involvement in quality improvement: from bedside advocate to system advisor. Clin Perinatol 2017;44:553-66. 10.1016/j.clp.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 48.EBCD: Experience-based co-design toolkit The Point of Care Foundation. Available online: https://www.pointofcarefoundation.org.uk/resource/experience-based-co-design-ebcd-toolkit/introduction/experience-based-co-design/
- 49.Vermont-Oxford Neonatal Network A multicenter, randomized trial comparing synthetic surfactant with modified bovine surfactant extract in the treatment of neonatal respiratory distress syndrome. Pediatrics 1996;97:1-6. [PubMed] [Google Scholar]
- 50.Vermont Oxford Network Steroid Study Group Early postnatal dexamethasone therapy for the prevention of chronic lung disease. Pediatrics 2001;108:741-8. 10.1542/peds.108.3.741 [DOI] [PubMed] [Google Scholar]
- 51.American Academy of Pediatrics Committee on Fetus and Newborn Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002;109:330-8. 10.1542/peds.109.2.330 [DOI] [PubMed] [Google Scholar]
- 52.Edwards WH, Conner JM, Soll RF, et al. The effect of prophylactic ointment therapy on nosocomial sepsis rates and skin integrity in infants with birth weights of 501 to 1000 g. Pediatrics 2004;113:1195-203. 10.1542/peds.113.5.1195 [DOI] [PubMed] [Google Scholar]
- 53.Dunn MS, Kaempf J, de Klerk A, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128:e1069-76. 10.1542/peds.2010-3848 [DOI] [PubMed] [Google Scholar]
- 54.Reilly MC, Vohra S, Rac VE, et al. Randomized trial of occlusive wrap for heat loss prevention in preterm infants. J Pediatr 2015;166:262-8.e2. 10.1016/j.jpeds.2014.09.068 [DOI] [PubMed] [Google Scholar]
- 55.Horbar JD, Carpenter JH, Buzas J, et al. Collaborative quality improvement to promote evidence based surfactant for preterm infants: a cluster randomised trial. BMJ 2004;329:1004. 10.1136/bmj.329.7473.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics 2002;110:143-51. 10.1542/peds.110.1.143 [DOI] [PubMed] [Google Scholar]
- 57.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129:1019-26. 10.1542/peds.2011-3028 [DOI] [PubMed] [Google Scholar]
- 58.Soll RF, Edwards EM, Badger GJ, et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics 2013;132:222-8. 10.1542/peds.2013-0501 [DOI] [PubMed] [Google Scholar]
- 59.Horbar JD, Edwards EM, Greenberg LT, et al. Variation in performance of neonatal intensive care units in the United States. JAMA Pediatr 2017;171:e164396. 10.1001/jamapediatrics.2016.4396 [DOI] [PubMed] [Google Scholar]
- 60.Fitzgibbons SC, Ching Y, Yu D, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009;44:1072-5; discussion 1075-6. 10.1016/j.jpedsurg.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 61.Fisher JG, Jones BA, Gutierrez IM, et al. Mortality associated with laparotomy-confirmed neonatal spontaneous intestinal perforation: a prospective 5-year multicenter analysis. J Pediatr Surg 2014;49:1215-9. 10.1016/j.jpedsurg.2013.11.051 [DOI] [PubMed] [Google Scholar]
- 62.Hull MA, Fisher JG, Gutierrez IM, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J Am Coll Surg 2014;218:1148-55. 10.1016/j.jamcollsurg.2013.11.015 [DOI] [PubMed] [Google Scholar]
- 63.Velazco CS, Fullerton BS, Hong CR, et al. Morbidity and mortality among "big" babies who develop necrotizing enterocolitis: A prospective multicenter cohort analysis. J Pediatr Surg 2017;53:108-12. 10.1016/j.jpedsurg.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 64.Fullerton BS, Velazco CS, Sparks EA, et al. Contemporary outcomes of infants with gastroschisis in North America: a multicenter cohort study. J Pediatr 2017;188:192-7.e6. 10.1016/j.jpeds.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 65.Hong CR, Fullerton BS, Han SM, et al. Impact of disease-specific volume and hospital transfer on outcomes in gastroschisis. J Pediatr Surg 2019;54:65-9. 10.1016/j.jpedsurg.2018.10.034 [DOI] [PubMed] [Google Scholar]
- 66.Mechak JT, Edwards EM, Morrow KA, et al. Effects of gestational age on early survivability in neonates with hypoplastic left heart syndrome. Am J Cardiol 2018;122:1222-30. 10.1016/j.amjcard.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 67.Shrestha M, Scarpino SV, Edwards EM, et al. The interhospital transfer network for very low birth weight infants in the United States. EPJ Data Science 2018;7(27). [Google Scholar]
- 68.Adams M, Bassler D, Bucher HU, et al. Variability of very low birth weight infant outcome and practice in Swiss and US neonatal units. Pediatrics 2018;141:e20173436. 10.1542/peds.2017-3436 [DOI] [PubMed] [Google Scholar]
- 69.Rogowski JA, Staiger D, Patrick T, et al. Nurse staffing and NICU infection rates. JAMA Pediatr 2013;167:444-50. 10.1001/jamapediatrics.2013.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogowski JA, Staiger DO, Patrick TE, et al. Nurse staffing in neonatal intensive care units in the United States. Res Nurs Health 2015;38:333-41. 10.1002/nur.21674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hallowell SG, Rogowski JA, Spatz DL, et al. Factors associated with infant feeding of human milk at discharge from neonatal intensive care: Cross-sectional analysis of nurse survey and infant outcomes data. Int J Nurs Stud 2016;53:190-203. 10.1016/j.ijnurstu.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hallowell SG, Spatz DL, Hanlon AL, et al. Characteristics of the NICU work environment associated with breastfeeding support. Adv Neonatal Care 2014;14:290-300. 10.1097/ANC.0000000000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morales LS, Staiger D, Horbar JD, et al. Mortality among very low-birthweight infants in hospitals serving minority populations. Am J Public Health 2005;95:2206-12. 10.2105/AJPH.2004.046730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lake ET, Staiger D, Edwards EM, et al. Nursing care disparities in neonatal intensive care units. Health Serv Res 2018;53 Suppl 1:3007-26. 10.1111/1475-6773.12762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lake ET, Staiger D, Horbar J, et al. Disparities in perinatal quality outcomes for very low birth weight infants in neonatal intensive care. Health Serv Res 2015;50:374-97. 10.1111/1475-6773.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horbar JD, Edwards EM, Greenberg LT, et al. Racial segregation and inequality in NICU care for very low birth weight and very preterm infants. JAMA Pediatr. 2019 doi: 10.1001/jamapediatrics.2019.0241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.ACOG Committee Opinion No 475: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol 2011;117:422-4. 10.1097/AOG.0b013e31820eee00 [DOI] [PubMed] [Google Scholar]
- 78.Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks‘ gestation. JAMA Netw Open 2018;1:e183235. 10.1001/jamanetworkopen.2018.3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ehret DEY, Edwards EM, Tariku A, et al. Landscape of low-income country neonatal intensive care units: a survey of current practices and staffing in Ethiopia. Pediatric Academic Societies Meeting, April 27, 2019, Baltimore, MD. [Google Scholar]
- 80.Vermont Oxford Network Available online: https://public.vtoxford.org/nicu-by-the-numbers/
- 81.Rothstein R. The Color of Law. New York, NY: Liveright, 2017. [Google Scholar]
- 82.Firebaugh G, Acciai F. For blacks in America, the gap in neighborhood poverty has declined faster than segregation. Proc Natl Acad Sci USA 2016;113:13372-7. 10.1073/pnas.1607220113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Firebaugh G, Farrell CR. Still large, but narrowing: The sizable decline in racial neighborhood inequality in metropolitan America, 1980-2010. Demography 2016;53:139-64. 10.1007/s13524-015-0447-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rivkin S. Desegregation since the Coleman Report. Education Next 2016;16:28-37. [Google Scholar]
- 85.Kozol J. Savage Inequalities: Children in America‘s Schools. New York, NY: Broadway Paperbacks, 1991. [Google Scholar]
- 86.Cornely PB. Segregation and discrimination in medical care in the United States. Am J Public Health Nations Health 1956;46:1074-81. 10.2105/AJPH.46.9.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reynolds PP. Hospitals and civil rights, 1945-1963: The case of Simkins v Moses H. Cone Memorial Hospital. Ann Intern Med 1997;126:898-906. 10.7326/0003-4819-126-11-199706010-00009 [DOI] [PubMed] [Google Scholar]
- 88.Smith DB. The racial segregation of hospital care revisited: Medicare discharge patterns and their implications. Am J Public Health 1998;88:461-3. 10.2105/AJPH.88.3.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hebert PL, Chassin MR, Howell EA. The contribution of geography to black/white differences in the use of low neonatal mortality hospitals in New York City. Med Care 2011;49:200-6. 10.1097/MLR.0b013e3182019144 [DOI] [PubMed] [Google Scholar]
- 90.Jha AK, Orav EJ, Epstein AM. Low-quality, high-cost hospitals, mainly in South, care for sharply higher shares of elderly black, Hispanic, and medicaid patients. Health Aff (Millwood) 2011;30:1904-11. 10.1377/hlthaff.2011.0027 [DOI] [PubMed] [Google Scholar]
- 91.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314:1039-51. 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Helenius K, Sjors G, Shah PS, et al. Survival in very preterm infants: an international comparison of 10 national neonatal networks. Pediatrics 2017. doi: . 10.1542/peds.2017-1264 [DOI] [PubMed] [Google Scholar]