Abstract
Background and Objectives
Diffuse long coronary artery disease (DLCAD) still has unfavorable clinical outcomes after successful percutaneous coronary intervention (PCI). Therefore, we aimed to evaluate the effectiveness and safety of Resolute™ zotarolimus-eluting stent (R-ZES; Resolute™ Integrity) for patients with DLCAD.
Methods
From December 2011 to December 2014, 1,011 patients who underwent PCI using R-ZES for CAD with longer than 25 mm lesion were prospectively enrolled from 21 hospitals in Korea. We assessed the clinical outcome of major adverse cardiac events (MACE) defined as the composite of cardiac death, non-fatal myocardial infarction (MI), and clinically-driven target vessel revascularization at 12 months.
Results
Mean age was 63.8±10.8 years, 701 (69.3%) patients were male, 572 (87.0%) patients had hypertension, 339 (33.8%) patients had diabetes, 549 (54.3%) patients diagnosed with acute MI and 545 (53.9%) patients had multi-vessel disease (MVD). A total of 1,697 stents were implanted into a total of 1,472 lesions. The mean diameter was 3.07±0.38 mm and the length was 28.27±6.97 mm. Multiple overlapping stents were performed in 205 (13.8%) lesions. A 12-month clinical follow-up was available in 1,004 patients (99.3%). The incidences of MACE and definite stent thrombosis at 12-month were 3.0% and 0.3% respectively. On multivariate Cox-regression analysis, multiple overlapping stents implantation, previous congestive heart failure, MVD, and age ≥75 years were independent predictors of one-year MACE.
Conclusions
Our study shows that R-ZES has an excellent 1-year clinical outcome in Korean patients with DLCAD.
Keywords: Drug-eluting stents, Coronary artery disease, Treatment outcome
INTRODUCTION
With the gradual development of new drug-eluting stent (DES) and medical treatments in patients with coronary artery disease (CAD), there have been significant improvements in restenosis and clinical outcomes after stent implantation. However, high restenosis rate and poor prognosis are still reported in patients with diffuse long coronary artery disease (DLCAD) in the era of DES as well as in the era of bare metal stent (BMS).1),2),3)
The Resolute™ zotarolimus-eluting stent (R-ZES; Resolute™ Integrity; Medtronic Inc., Santa Rosa, CA, USA) is a zotarolimus-eluting system with Integrity™ cobalt alloy stent platform that further enhances the flexibility and deliverability of the stent in complex lesions by incorporation of a continuous sinusoidal design and with a new BioLinx™ polymer that allows a slower drug elution, therefore, might be expected to improve clinical outcomes compared to other DES. Some available data have shown the clinical efficacy and safety of R-ZES for treatment of CAD.4),5),6),7)
However, there have been a little data for the effectiveness and safety of R-ZES (Resolute™ Integrity) in patients with DLCAD. Therefore, the purpose of this study is to determine the effectiveness and safety of R-ZES in patients with DLCAD, a particularly long lesion ≥25 mm.
METHODS
Study design and patient population
We designed a multicenter, prospective, and observational study to assess the clinical outcomes in patients with DLCAD who underwent successful PCI using R-ZES (mainly Resolute™ Integrity) from 21 large-volume percutaneous coronary intervention (PCI) centers in Korea.
The selection and exclusion criteria of the subjects were as follows. The inclusion criteria for our study were at least 18 years old patients with CAD, those eligible for PCI with lesions suitable for R-ZES implantation, presence of one or more de novo stenosis ≥70% in a native coronary artery from 2.25 to 4.0 mm in diameter with more than 25mm length that can be covered with one or multiple stents with no limitation to the number of treated lesions or number of treated vessels. In principle, we initially implanted Resolute™ Integrity in all patients and allowed limited use of other stents only if Resolute™ Integrity was not available for additional stenting.
The exclusion criteria were patents with previous other type of DES or BMS implantation in target vessel, chronic renal failure (serum creatinine >2.0 mg/dL), severe hepatic impairment (serum alanine and aspartate aminotransferase ≥3 times the upper limit of normal), hypersensitivity or contraindication to antiplatelet agents, active bleeding or significant risk of bleeding or life expectancy <12 months. We had also excluded the patients with cardiogenic shock, left main disease (≥50% stenosis), and saphenous vein graft lesion.
The baseline data including clinical, procedural, and lesional characteristics and in-hospital medications were obtained by electronic medical record reviews. Patients were followed at 1, 6, and 12 months after index procedure. The clinical outcome data were collected by a specialized study coordinator at each center using web-based electronic case report form. The outcome data of the patients who had not been followed-up were confirmed by telephone interviews. The study protocol was approved by the local Institutional Review Board. The approval number was 2011-184 of Chonnam National University Hospital, and all patients provided written informed consent for participation before or after PCI.
Procedure and post-intervention medications
PCI with R-ZES was performed in a routine manner without limitation on the use of single or multiple overlapping stent technique. The appropriate length and diameter of the R-ZES ensuring complete coverage of the lesion was chosen by the operator's visual estimate. The vascular access, pre-dilatation or post-dilatation, use of intravascular ultrasound (IVUS) and peri-procedural anti-thrombotic therapies were freely determined based on the patient status according to the clinical decision of operators in each institutes. Anti-platelet agents were administered to all patients prior to the intervention, with aspirin 300 mg and clopidogrel 300–600 mg, prasugrel 60 mg or ticagrelor 180 mg. After the intervention, the patients received aspirin 100 mg once daily indefinitely and clopidogrel 75 mg once daily, prasugrel 5–10 mg once daily or ticagrelor 90 mg twice daily for at least 1 year. Other medical treatments were also administered based on the standard treatment regimen for patients with CAD in a non-restrictive manner.
Study end-points
The primary end-point was the occurrence of a major adverse cardiac events (MACE), defined as the composite of cardiac death, non-fatal myocardial infarction (MI), definite stent thrombosis (ST), and clinically-driven target vessel revascularization (TVR) at 12-month follow-up. The secondary endpoints included cerebrovascular accident (CVA), clinically-driven target lesion revascularization (TLR), the need for rehospitalization due to recurrent ischemia, and the Thrombolysis In Myocardial Infarction (TIMI) defined major or minor bleeding at 12-month follow-up. MI was defined as the third universal definition of myocardial infarction. A nonfatal myocardial infarction was defined as a MI event requiring hospitalization unrelated to death, however, periprocedural MI was excluded in this study. Clinically-driven TVR was defined as revascularization performed on the treated lesion or vessel of a patient who complained of clinical symptoms such as chest pain that had increased in frequency, duration, or intensity.
Statistical analyses
Categorical variables were expressed as frequencies and percentages, and continuous variables as mean±standard deviation. Cumulative incidence of clinical events was calculated using the Kaplan-Meier method. Cox regression analysis was used to identify the factors affecting the occurrence of MACE following the implantation of R-ZES. Only variables with a p value <0.1 in the univariate analysis were included in the multivariate model. Sub-group analyses were performed based on the several clinical statuses of patients. All statistical tests were 2-tailed, and a p value <0.05 was considered statistically significant. All the statistical analyses were performed using SPSS (Statistical Package for Social Science, SPSS Inc., Chicago, IL, USA) for Windows, version 24.0.
RESULTS
Baseline characteristics
From December 2011 to December 2014, we prospectively recruited 1,034 patients who underwent PCI using R-ZES for CAD with long lesion (≥25 mm) from 21 hospitals in Korea. Of these, a total of 1,011 patients were analyzed, except for 23 patients with inadequate registration or withdrawal of permission.
Baseline clinical, procedural, and lesional characteristics are presented in Table 1. The mean age was 63.8±10.8 years. Of them, 701 (69.3%) patients were male, 572 (56.6%) patients had hypertension, 339 (33.5%) patients had diabetes mellitus (DM), 10 (1.0%) patents had previous congestive heart failure, and 549 (54.3%) patients presented with acute myocardial infarction (AMI).
Table 1. Baseline clinical, angiographic, and procedural characteristics and in-hospital medications.
| Variables | Values (%) | |||
|---|---|---|---|---|
| Variables of patients | Patients (n=1,011) | |||
| Age (years) | 63.75±10.82 | |||
| Age ≥75 years | 169 (16.7) | |||
| Male sex | 701 (69.3) | |||
| Hypertension | 572 (56.6) | |||
| Diabetes mellitus/Insulin-dependent | 339 (33.5)/13 (1.3) | |||
| Dyslipidemia | 344 (34.6) | |||
| Current smoker | 284 (28.5) | |||
| Chronic renal failure | 9 (0.9) | |||
| Previous cerebrovascular accident | 60 (6.0) | |||
| Peripheral artery disease | 14 (1.4) | |||
| Family history of coronary artery disease | 30 (3.0) | |||
| Previous percutaneous coronary intervention | 51 (5.1) | |||
| Previous coronary artery bypass graft surgery | 4 (0.4) | |||
| Previous MI | 24 (2.4) | |||
| Previous congestive heart failure | 10 (1.0) | |||
| Left ventricular ejection fraction (%) | 58.74±11.35 | |||
| Body mass index (kg/m2) | 24.68±3.55 | |||
| Clinical presentation | ||||
| ST elevation MI | 142 (14.0) | |||
| Non-ST elevation MI | 407 (40.3) | |||
| Angina or silent ischemia | 462 (45.7) | |||
| Unfractionated heparin | 510 (50.4) | |||
| Low molecular weight heparin | 155 (15.3) | |||
| Glycoprotein IIb/IIIa inhibitor | 15 (1.5) | |||
| In-hospital medications | ||||
| Aspirin | 989 (99.3) | |||
| Clopiodgrel | 859 (86.7) | |||
| Prasugrel | 52 (5.1) | |||
| Ticagrelor | 47 (4.6) | |||
| Cilostazol | 137 (13.6) | |||
| Beta-blockers | 747 (75.7) | |||
| Angiotensin converting enzyme inhibitors | 372 (37.8) | |||
| Angiotensin II receptor blockers | 366 (37.1) | |||
| Calcium channel blockers | 206 (20.4) | |||
| Statin | 903 (91.4) | |||
| Oral hypoglycemic agents | 214 (21.7) | |||
| Target vessel | ||||
| Left anterior descending artery | 550 (54.4) | |||
| Left circumflex artery | 138 (13.6) | |||
| Right coronary artery | 320 (31.7) | |||
| Left main | 3 (0.3) | |||
| Involved vessel number | ||||
| Single vessel | 466 (46.1) | |||
| Two vessels | 336 (33.2) | |||
| Three vessels | 209 (20.7) | |||
| Vascular access | ||||
| Transradial approach | 620 (61.3) | |||
| Transfemoral approach | 391 (38.7) | |||
| Use of intravascular ultrasound | 378 (37.4) | |||
| Variables of lesions | Lesions (n=1,472) | |||
| Treated vessel | ||||
| Left anterior descending artery | 716 (48.6) | |||
| Left circumflex artery | 285 (19.4) | |||
| Right coronary artery | 464 (31.5) | |||
| Left main | 7 (0.5) | |||
| Lesion preparation before stenting | ||||
| Balloon dilatation | 1,248 (84.8) | |||
| Cutting balloon dilatation | 1 (0.1) | |||
| Thrombectomy | 44 (3.0) | |||
| Use of intravascular ultrasound | 522 (36.1) | |||
| Post-dilatation after stenting | 470 (31.9%) | |||
| Pre-PCI TIMI antegrade flow 0 to 2 | 708 (48.4) | |||
| Post-PCI TIMI antegrade flow 2 or 3 | 1,445 (98.2) | |||
| Type of stent | 1,697 (100.0) | |||
| Resolute™ Integrity | 1,542 (90.9) | |||
| Endeavor Resolute™ | 144 (8.5) | |||
| Xience™/Promus™ | 6 (0.4)/3 (0.2) | |||
| Biomatrix™ | 1 (0.06) | |||
| Bare metal stents | 1 (0.06) | |||
| Technique of stent implantation | ||||
| Single | 1,282 | |||
| Multiple overlapping | 205 | |||
| Two overlapping stents | 200 | |||
| Three overlapping stents | 5 | |||
| Number of stents per patient | 1.64±0.84 | |||
| Stent diameter per lesion, mm | 3.07±0.38 | |||
| Stent length per lesion, mm | 28.27±6.97 | |||
| Restenotic lesion | 3 (0.2) | |||
| Chronic total occlusion | 54 (3.7) | |||
| Moderate to severe tortuosity | 137 (9.3) | |||
| Moderate to severe calcification | 211 (14.4) | |||
| Bifurcation lesion | 133 (9.2) | |||
| ACC/AHA classification B2/C lesion | 1,303 (88.6) | |||
The data are presented as number (%) or mean±standard deviation.
ACC/AHA = American College of Cardiology/American Heart Association; MI = myocardial infarction; PCI = percutaneous coronary intervention; ST = stent thrombosis; TIMI = thrombolysis in myocardial infarction.
A total of 1,697 stents (Resolute™ Integrity: 90.9%) were implanted in 1,472 lesions and mean diameter of stent was 3.07±0.38 mm and mean stent length was 28.27±6.97 mm. The target lesion was common in the left anterior descending artery (54.4%) and patients with multi-vessel disease (MVD) accounted for 53.9%. A chronic total occlusion was present in 54 (3.7%) lesions and bifurcation lesion in 133 (9.2%) lesions. Post-dilatations were performed in 369 patients (470 lesions) and IVUS in 378 patients (522 lesions). A single long stent implantation was performed in most lesions, however, 2 overlapping stents were performed in 200 lesions and 3 overlapping stents in 5 lesions.
Clinical outcomes
A 12-month clinical follow-up was available in 1,004 patients (99.3%). The cumulative incidences of clinical events during follow-up are summarized in Table 2.
Table 2. Cumulative incidence of clinical events at 12-month.
| Clinical events | 12-month cumulative clinical events (n=1,004) | |
|---|---|---|
| Cardiac death | 5 (0.5) | |
| Non-fatal MI | 8 (0.8) | |
| Stent thrombosis, definite | 3 (0.3) | |
| Cerebrovascular accident | 9 (0.9) | |
| Repeat revascularization, clinically-driven | 36 (3.6) | |
| Target lesion revascularization | 17 (1.7) | |
| Target vessel revascularization | 19 (1.9) | |
| Non-target lesion revascularization | 19 (1.9) | |
| Bleeding complication | 12 (1.2) | |
| TIMI major | 5 (0.5) | |
| TIMI minor | 7 (0.7) | |
| Rehospitalization | 68 (6.8) | |
| MACE | 30 (3.0) | |
The data are presented as number (%).
MACE = major adverse cardiac events; MI = myocardial infarction; TIMI = thrombosis in myocardial infarction.
During the 12-month period, definite ST occurred in only 3 patients, including 1 with acute ST at 1 day and 2 with late ST at 70 and 322 days on dual antiplatelet treatments. The incidences of cumulative TVR and MACE at 12-month were 1.7% and 3.0% respectively.
On multivariate Cox-regression analysis, multiple overlapping stents implantation, previous congestive heart failure, MVD and patients with age ≥75 years were independent predictors of 1-year MACE (hazard ratio [HR], 3.879, 95% confidence interval [CI],1.779–8.459; HR, 8.071, 95% CI, 1.307–49.855; HR, 5.248, 95% CI, 1.557–17.689; and HR, 2.489, 95% CI,1.097–5.648, respectively) (Table 3).
Table 3. Independent predictors of 12-month major adverse cardiovascular events.
| Variable | Univariate analysis | Multivariate Cox regression analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Multiple overlapping vs. single stenting | 5.079 (2.311–11.163) | <0.001 | 3.879 (1.779 – 8.459) | 0.001 |
| Previous CHF | 9.990 (2.014–49.556) | 0.026 | 8.071 (1.307–49.855) | 0.003 |
| Multi-vessel disease | 6.800 (2.029–22.795) | <0.001 | 5.248 (1.557–17.689) | 0.007 |
| Age ≥75 years | 3.247 (1.447–7.285) | 0.003 | 2.489 (1.097–5.648) | 0.029 |
| Diabetes mellitus | 2.021 (0.927–4.410) | 0.072 | 1.570 (0.717–3.436) | 0.259 |
| Previous MI | 3.598 (0.800–16.178) | 0.075 | 1.398 (0.223–8.779) | 0.721 |
| Chronic total occlusion | 2.546 (0.739–8.778) | 0.125 | - | - |
| Statin | 1.921 (0.717–5.142) | 0.187 | - | - |
| TFI vs. TRI | 1.606 (0.737–3.501) | 0.230 | - | - |
| Potent P2Y12 inhibitors | 1.646 (0.556–4.873) | 0.364 | - | - |
| Diagnosed with MI | 0.715 (0.327–1.562) | 0.398 | - | - |
| Hypertension | 0.748 (0.343–1.630) | 0.463 | - | - |
| LVEF <50% | 1.416 (0.547–3.667) | 0.472 | - | - |
| Current smoker | 1.432 (0.591–3.046) | 0.481 | - | - |
| Dyslipidemia | 0.730 (0.302–1.766) | 0.484 | - | - |
| Beta-blocker | 1.222 (0.549–2.722) | 0.623 | - | - |
| Male sex | 1.203 (0.530–2.729) | 0.658 | - | - |
| Previous CVA | 1.306 (0.301–5.662) | 0.720 | - | - |
| Calcium channel blocker | 0.865 (0.322–2.323) | 0.774 | - | - |
| ACEi or ARB | 0.949 (0.431–2.088) | 0.896 | - | - |
ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CHF = congestive heart failure; CI = confidence interval; CVA = cerebrovascular accident; HR = hazard ratio; LVEF = left ventricular ejection fraction; MI = myocardial infarction; TFI = trans-femoral intervention; TRI = trans-radial intervention.
Subgroup analysis
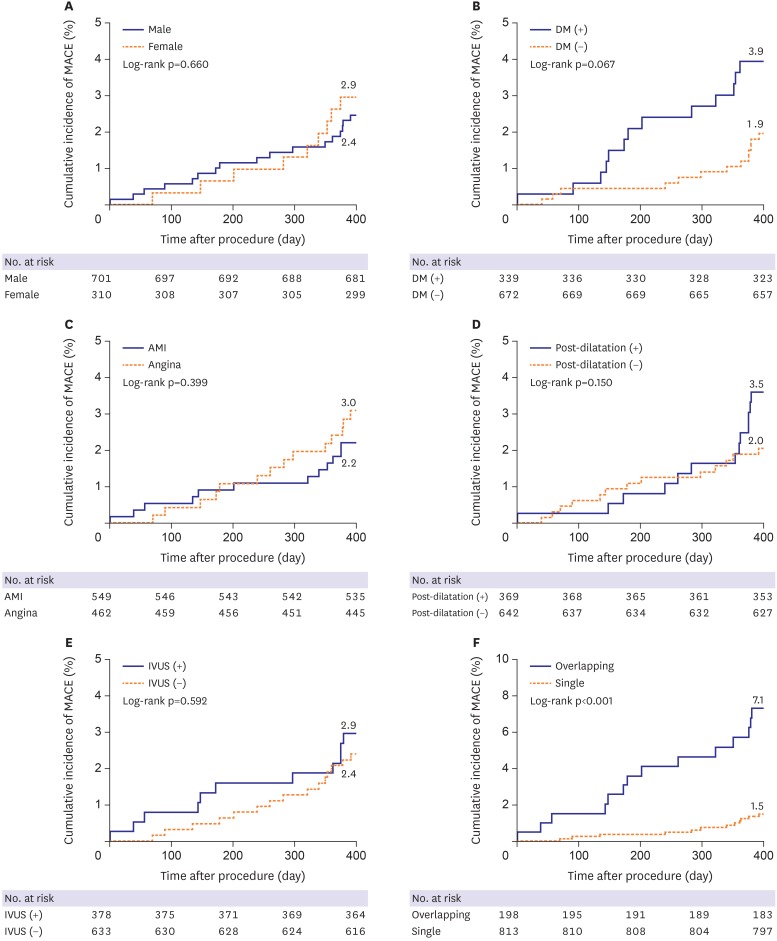
Subgroup analyses were performed according to parameters of gender, DM, presentation with AMI, post-dilatation after stenting or not, use of IVUS, and multiple overlapping stents implantation. Kaplan-Meier cumulative event curves showed no statistically significant differences in the incidence of MACE between males and females (2.4% vs. 2.9%, p=0.660), between patients with and without DM (3.9% vs. 1.9%, p=0.067), between those who presented with AMI and angina (2.2% vs. 3.0%, p=0.399), between patients with and without post-dilatation (3.5% vs. 2.0%, p=0.150), and between patients with IVUS and no IVUS (2.9% vs. 2.4%, p=0.592), however, significantly different between single stent and multiple overlapping stents implantation (1.5% vs. 7.1%, p<0.001) (Figure 1).
Figure 1. Kaplan-Meier survival curves for MACE among subgroups. Cumulative incidences of MACE according to (A) sex, (B) the presence of DM, (C) presentation with AMI, (D) performance of post-dilatation, (E) use of IVUS, and (F) the presence of multiple overlapping stents implantation.
AMI = acute myocardial infarction; DM = diabetes mellitus; IVUS = intravascular ultrasound MACE = major adverse cardiac events.
DISCUSSION
This is a first study to assess 1-year clinical outcomes in patients with DLCAD receiving R-ZES (mainly Resolute™ Integrity) from a large-scale, prospective, and real world-registry in Korea. A total of 1,011 patients with 1,472 lesions were enrolled and analyzed. In our results, the incidence of TVR and MACE were relatively low and that of definite ST was rare at 12-month follow up. Therefore, our study demonstrated that R-ZES has an excellent 1-year clinical outcome in Korean patients with DLCAD.
The R-ZES has been reported to have excellent long-term efficacy and safety outcomes in various studies. The RESOLUTE Global Clinical Trial Program included 7,618 patients treated with R-ZES reported that the 5-year cumulative incidence of target lesion failure (TLF defined as a composite of cardiac death, MI or clinically-driven TLR) was 13.4% and definite or probable ST was 1.2%.8)
These excellent results are comparable to those of other second-generation DES. The data of RESOLUTE-Korea registry showed that the TLF of R-ZES was 2.9% at l-year follow-up and similar with that of everolimus-eluting stent (EES).9) R-ZES had lower incidences of TLR and ST, and showed comparable one-year safety and efficacy compared with EES. A report from the RESOLUTE All-Comer Trial demonstrated similar safety and efficacy between R-ZES and EES throughout 4 years. The rates of TLF (15.2% vs. 14.6%, p=0.68), clinically-indicated TLR (7.0% vs. 6.5%, p=0.62), and definite/probable ST (2.3% vs. 1.6%, p=0.23) were similar with the R-ZES and EES at 4 year.10)
Compared with the biodegradable polymer biolimus-eluting stent (BES), the durable-polymer R-ZES also had similar in 3-year safety and efficacy clinical outcomes, including ST. MACE occurred in 8.6% assigned to the durable-polymer R-ZES and in 9.6% assigned to the biodegradable-polymer BES (p=0.36). TLR (5.4% vs. 5.5%) and definite very late ST (0.4% vs. 0.7%) did not differ significantly between the 2 groups.11)
Recently, novel biodegradable polymer stents with thinner strut, flexible designs and refined coatings have been available compared to the early biodegradable polymer stent. The thin strut durable polymer R-ZES were also non-inferior to very thin strut biodegradable polymer everolimus-eluting (Synergy™, Boston Scientific, Natick, MA, USA) or sirolimus-eluting stents (SES; Orsiro, Biotronik, Bulach, Switzerland) in treating complex all-comers with a high proportion of patients with acute coronary syndromes. The incidence of target vessel failure (TVF) was about 5% in all 3 groups at 12-month follow-up.12)
The length of coronary artery lesion or stent has been well-known as predictors of restenosis and poor clinical outcomes after PCI in the first-generation DES era as well as in the BMS era.2),13),14) However, some data have reported that the second-generation DES, especially such as R-ZES, is relatively safe and effective for diffuse long lesions.
In the RESOLUTE 38-mm sub-study, the 38-mm length of the R-ZES had a low rate of TLF (5.4%) and ST (0.9%).15) Prospective study to evaluate safety and efficacy of Zotarolimus Eluting Stent (PSEZES) trial reported that the incidence of TLF was 6.36% and binary restenosis was 7.5% in R-ZES for patients with long coronary artery lesions (>20 mm).16)
LONG-DES IV trial showed that R-ZES implantation had similar angiographic and clinical outcomes as compared with the first-generation SES implantation for patients with de novo long CAD.17) However, other study presented that R-ZES had a superior long-term safety than SES, with a similar clinical efficacy for treating long coronary lesions at 3-year follow-up. The cumulative TLR rate was similar between 2 groups (4.6% vs. 4.6%, p=0.911), however, the occurrence of target lesion-related definite ST was significantly lower in R-ZES than in SES (0.0% vs. 2.0%, p=0.028).18)
A study comparing between R-ZES (Resolute™ Integrity) and X-EES (Xience Xpedition™) used single stent length of more than 30 mm for long CAD showed no significant between-group differences in the rate of adverse clinical events. The incidence of TLF was 5% in R-ZES and 4% in X-EES groups (HR, 1.25, 95% CI, 0.86–5.6; p=0.19).19)
While most studies on R-ZES for DLCAD were small-scale studies, our study seems to be more meaningful because it is a relatively large-scale and prospective study. Our study reported that the incidence of MACE was 3.0% and that of definite ST was 0.3% at 12-month follow-up. In our study, the incidence of MACE appeared to be comparable or somewhat lower compared with that in other studies for DLCAD. We have not performed routine follow-up angiography and any stress test, but have evaluated only the clinically-driven TVR during follow-up periods. That is why we could not actually evaluate the binary stenosis or a silent ischemia. Therefore, it is possible that our results were assessed lower than actual. However, most of all (99.3%) have clearly completed a 12-month clinical follow-up in our study. In conclusion, as with other studies, R-ZES was actually safe and effective in our study. The good clinical outcomes of R-ZES did not differ according to gender, the presence of DM, and the diagnosis of AMI.
Also, post-dilatation or use of IVUS could not affect the clinical outcomes of patients with DLCAD in our study. These procedures were performed by the choice of the operator and were more likely to be used in more complex lesions, so selection bias could not be ruled out. Therefore, a large randomized trial should be needed to assess the impact of post-dilatation or IVUS for patients with DLCAD.
However, multiple overlapping stents implantation was different from single stent implantation in clinical outcomes and was still a predictor of MACE in patients with DLCAD. DES overlap has been well-known to be associated with impaired angiographic and long-term clinical outcome, including death or periprocedural MI.20),21),22) However, the second-generation DES overlap seems to be much better than the first-generation DES overlap. The porcine model study showed that BES appears to be reliable on the inflammatory response at overlapping segments as well as non-overlapping segments.23) One retrospective study reported that the stent overlap with EES versus the first-generation DES was associated with lower rates of MACE and ST.24) The study for full metal jacket with ZES for DLCAD showed that the incidence of MACE and clinically-driven TLR were 11.7% and 5.0% at 3 year follow-up.25) Despite improvements in the second-generation DES era, DES overlap was still associated with high incidence of TVF and poor clinical outcomes. Therefore, we should make efforts to improve the clinical outcomes in patients with multiple overlapping DES for DLCAD.
Our study has several limitations. First, our study was not a randomized, comparative trial but based on a prospective, observational registry, even though our study enrolled a relatively large number of patients. Therefore, we could not evaluate the effect of R-ZES compared with that of other second-generation DES in patients with DLCAD. In addition, selection bias was hardly avoidable in the post hoc sub-group analysis of our study. Second, we could not evaluate quantitative angiographic outcomes, but only evaluated clinical outcomes. Therefore, we could not evaluate the angiographic pattern of in-stent restenosis between overlapping and non-overlapping segments of R-ZES. Third, Resolute™ integrity was used over 90% in our study. However, with additional stenting, other DES or BMS was inevitably used at around 0.6%. Nevertheless, other DES or BMS had nothing to do with the clinical outcomes.
In conclusion, our study shows that R-ZES implantation was safe and effective at 1-year follow-up in Korean patients with DLCAD. These effectiveness and safety of R-ZES were consistent for patients with DLCAD, regardless of gender, the presence of DM, and presented with AMI. However, it still seems to be necessary to improve the clinical outcome of multiple overlapping R-ZES in patients with DLCAD.
ACKNOWLEDGEMENTS
This study was funded by Medtronic. We thank Eo Jin Lee (Department of Cardiology, Chonnam National University Hospital) for technical assistance.
Footnotes
Funding: This study was supported by Medtronic.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Hong YJ.
- Data curation: Hwang JY, Kim DH, Rhew JY, Ryu JK, Park JS, Park TH, Yang TH, Oh SK, Lee BR, Lee SU, Lee SG, Chun KJ, Cho JH, Cha KS, Chae JK, Hur SH, Hwang SH, Park HS, Kim DI.
- Formal analysis: Koh YY, Ki YJ, Kim SS, Kim HK, Choi DH.
- Writing - original draft: Park KH.
- Writing - review & editing: Ahn Y.
References
- 1.Kastrati A, Schömig A, Elezi S, et al. Predictive factors of restenosis after coronary stent placement. J Am Coll Cardiol. 1997;30:1428–1436. doi: 10.1016/s0735-1097(97)00334-3. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi Y, De Gregorio J, Kobayashi N, et al. Stented segment length as an independent predictor of restenosis. J Am Coll Cardiol. 1999;34:651–659. doi: 10.1016/s0735-1097(99)00303-4. [DOI] [PubMed] [Google Scholar]
- 3.Claessen BE, Smits PC, Kereiakes DJ, et al. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv. 2011;4:1209–1215. doi: 10.1016/j.jcin.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S. The resolute™ integrity zotarolimus-eluting stent in coronary artery disease: a review. Cardiol Ther. 2013;2:17–25. doi: 10.1007/s40119-012-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung AC, Leon MB, Jain A, et al. Clinical evaluation of the Resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J Am Coll Cardiol. 2011;57:1778–1783. doi: 10.1016/j.jacc.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Qiao S, Chen L, Chen S, Wang W, Zhu G. One-year outcomes from an all-comers chinese population of patients implanted with the resolute zotarolimus-eluting stent. Am J Cardiol. 2014;113:613–620. doi: 10.1016/j.amjcard.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 7.Richardt G, Leschke M, Abdel-Wahab M, et al. Clinical outcomes of the Resolute zotarolimus-eluting stent in patients with in-stent restenosis: 2-year results from a pooled analysis. JACC Cardiovasc Interv. 2013;6:905–913. doi: 10.1016/j.jcin.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Yeh RW, Silber S, Chen L, et al. 5-year safety and efficacy of resolute zotarolimus-eluting stent: the RESOLUTE Global Clinical Trial Program. JACC Cardiovasc Interv. 2017;10:247–254. doi: 10.1016/j.jcin.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Park KW, Lee JM, Kang SH, et al. Safety and efficacy of second-generation everolimus-eluting Xience V stents versus zotarolimus-eluting resolute stents in real-world practice: patient-related and stent-related outcomes from the multicenter prospective EXCELLENT and RESOLUTE-Korea registries. J Am Coll Cardiol. 2013;61:536–544. doi: 10.1016/j.jacc.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Taniwaki M, Stefanini GG, Silber S, et al. 4-year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the RESOLUTE All-Comers trial (A Randomized Comparison of a Zotarolimus-Eluting Stent With an Everolimus-Eluting Stent for Percutaneous Coronary Intervention) J Am Coll Cardiol. 2014;63:1617–1625. doi: 10.1016/j.jacc.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Raungaard B, Christiansen EH, Bøtker HE, et al. Comparison of durable-polymer zotarolimus-eluting and biodegradable-polymer biolimus-eluting coronary stents in patients with coronary artery disease: 3-year clinical outcomes in the Randomized SORT OUT VI Trial. JACC Cardiovasc Interv. 2017;10:255–264. doi: 10.1016/j.jcin.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 12.von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016;388:2607–2617. doi: 10.1016/S0140-6736(16)31920-1. [DOI] [PubMed] [Google Scholar]
- 13.Kastrati A, Elezi S, Dirschinger J, Hadamitzky M, Neumann FJ, Schömig A. Influence of lesion length on restenosis after coronary stent placement. Am J Cardiol. 1999;83:1617–1622. doi: 10.1016/s0002-9149(99)00165-4. [DOI] [PubMed] [Google Scholar]
- 14.Choi IJ, Koh YS, Lim S, et al. Impact of the stent length on long-term clinical outcomes following newer-generation drug-eluting stent implantation. Am J Cardiol. 2014;113:457–464. doi: 10.1016/j.amjcard.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Hiremath S, Zambahari R, et al. One-year outcomes of percutaneous coronary intervention with the 38-mm Resolute zotarolimus-eluting stent. Am J Cardiol. 2013;112:1335–1341. doi: 10.1016/j.amjcard.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Bahuleyan CG, Krishna Kumar VV, Babu S. Prospective study to evaluate safety and efficacy of Zotarolimus Eluting Stent (PSEZES) in patients with long coronary artery lesions. Indian Heart J. 2015;67:233–238. doi: 10.1016/j.ihj.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn JM, Park DW, Kim YH, et al. Comparison of resolute zotarolimus-eluting stents and sirolimus-eluting stents in patients with de novo long coronary artery lesions: a randomized LONG-DES IV trial. Circ Cardiovasc Interv. 2012;5:633–640. doi: 10.1161/CIRCINTERVENTIONS.111.965673. [DOI] [PubMed] [Google Scholar]
- 18.Im E, Kim BK, Ko YG, et al. Comparison of 3-year clinical outcomes between Resolute™ zotarolimus- and sirolimus-eluting stents for long coronary artery stenosis. J Interv Cardiol. 2013;26:378–383. doi: 10.1111/joic.12047. [DOI] [PubMed] [Google Scholar]
- 19.Patra S, Chakraborty RN, Pande A, et al. Zotarolimus-eluting Resolute Integrity versus everolimus-eluting Xience Xpedition stents in the management of very long (>30mm) de novo coronary artery stenosis. Cardiovasc Revasc Med. 2017;18:160–164. doi: 10.1016/j.carrev.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Kereiakes DJ, Wang H, Popma JJ, et al. Periprocedural and late consequences of overlapping Cypher sirolimus-eluting stents: pooled analysis of five clinical trials. J Am Coll Cardiol. 2006;48:21–31. doi: 10.1016/j.jacc.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 21.Dawkins KD, Grube E, Guagliumi G, et al. Safety and efficacy of multiple, overlapping polymer-based paclitaxel-eluting stents. EuroIntervention. 2007;3:213–221. doi: 10.4244/eijv3i2a37. [DOI] [PubMed] [Google Scholar]
- 22.Räber L, Jüni P, Löffel L, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55:1178–1188. doi: 10.1016/j.jacc.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 23.Park KH, Jeong MH, Kim JM, et al. The impact of triple anti-platelet therapy for endothelialization and inflammatory response at overlapping bioabsorbable polymer coated drug-eluting stents in a porcine coronary model. Int J Cardiol. 2013;168:1853–1858. doi: 10.1016/j.ijcard.2012.12.070. [DOI] [PubMed] [Google Scholar]
- 24.Kitabata H, Loh JP, Pendyala LK, et al. Safety and efficacy outcomes of overlapping second-generation everolimus-eluting stents versus first-generation drug-eluting stents. Am J Cardiol. 2013;112:1093–1098. doi: 10.1016/j.amjcard.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 25.Durante A, Foglia Manzillo G, Burzotta F, et al. Long term follow-up of “full metal jacket” of de novo coronary lesions with new generation zotarolimus-eluting stents. Int J Cardiol. 2016;221:1008–1012. doi: 10.1016/j.ijcard.2016.07.050. [DOI] [PubMed] [Google Scholar]