Abstract
In adult congenital heart disease (ACHD), residua and sequellae after initial repair develop late complications such as cardiac failure, arrhythmias, thrombosis, aortopathy, pulmonary hypertension and others. Acquired lesions with aging such as hypertension, diabetes mellitus, obesity can be negative influence on original cardiovascular disease (CVD). Also, atherosclerosis may pose an additional health problem to ACHD when they grow older and reach the age at which atherosclerosis becomes clinically relevant. In spite of the theoretical risk of atherosclerosis in ACHD due to above mentioned factors, cyanotic ACHDs even after repair are noted to have minimal incidence of coronary artery disease (CAD). Acyanotic ACHD has similar prevalence of CAD as the general population. However, even in cyanotic ACHD, CAD can develop when they have several risk factors for CAD. The prevalence of risk factor is similar between ACHD and the general population. Risk of premature atherosclerotic CVD in ACHD is based, 3 principal mechanisms: lesions with coronary artery abnormalities, obstructive lesions of left ventricle and aorta such as coarctation of the aorta and aortopathy. Coronary artery abnormalities are directly affected or altered surgically, such as arterial switch in transposition patients, may confer greater risk for premature atherosclerotic CAD. Metabolic syndrome is more common among ACHD than in the general population, and possibly increases the incidence of atherosclerotic CAD even in ACHD in future. Thus, ACHD should be screened for metabolic syndrome and eliminating risk factors for atherosclerotic CAD.
Keywords: Adult congenital heart disease, Metabolic syndrome, Cardiovascular diseases, Coronary artery disease, Cyanotic congenital heart disease
INTRODUCTION
Improvements in quality and level of care in the fields of pediatric cardiology and cardiovascular surgery have resulted in the survival and increased life expectancy of congenital heart disease patients to adulthood. At present, 85–90% of those born with congenital heart disease (CHD) grow up into adults. Consequently, in Japan the number of adults with CHD became even comparing with children with CHD in 1997, and now in 2019, there are more adults with CHD (500,000) than children with CHD, and the number is expected to grow at an estimated rate of 5% per year.1)
Surgical treatment made survival to adulthood possible for CHD patients; however, these operations are seldom curative. Postoperative residua and sequelae develop new-onset late complications such as cardiac failure, arrhythmias, thrombosis, aortopathy and pulmonary hypertension and others vary in severity among different CHDs. Patients with moderate to complex lesions, both unoperated and postoperative, require lifelong surveillance.
Acquired lesions with aging such as hypertension, diabetes mellitus, obesity can be negative impact on original cardiovascular disease (CVD). Also, atherosclerosis may pose an additional health problem to adult congenital heart disease (ACHD) patients when they grow older and reach the age at which atherosclerosis becomes clinically relevant.2),3),4),5),6),7)
Modifiable and non-modifiable risk factors acquired later in life such as smoking, development of hypertension and diabetes, and dyslipidemia also pose significant additional health risk in these patients. Ironically, obesity is of great concern in ACHD nowadays. In spite of the theoretical risk of atherosclerosis in ACHD patients due to above mentioned factors, ACHDs, most especially the cyanotic types even after repair, were noted to have minimal incidence of coronary artery disease (CHD).2),8),9),10),11),12)
Acyanotic ACHD has similar prevalence of CAD as general population.11) However, even in cyanotic ACHD patients, CAD can develop when they have risk factors for CAD such as obesity, dyslipidemia, hypertension, diabetes mellitus, smoking habit and/or positive family history of CVD, and lead a life of limited exercise levels. The prevalence of having risk factors for CVD is similar between ACHD and the general population.
Metabolic syndrome increases the risk for atherosclerotic CAD, and its prevalence increases with increasing age and body mass index. ACHD is now living longer and accruing CAD risk factors. ACHD may be at increased risk for premature CAD for several reasons: abnormal coronary artery anatomy, inflammation and scarring of the coronary arteries from surgical manipulation or the underlying CHD, reperfusion injuries during surgery, turbulent blood flow and postsurgical residua and sequallae from underlying anatomic abnormalities, associated genetic syndromes, ventricular hypertrophy or dilation, systemic hypertension and aortopathy. CHD includes a heterogeneous group of conditions and there are few data to identify those at highest risk for CAD. Atherosclerosis may pose an additional health problem to ACHD patients.2),3),4),5),6) Development of metabolic syndrome, hypertension, diabetes, dyslipidemia and obesity also pose significant additional risk in ACHD.2),7)
PREVALENCE OF CORONARY ARTERY DISEASE IN ADULT CONGENITAL HEART DISEASE
In spite of the theoretical risk of atherosclerosis in patients with ACHD, most of the cyanotic ACHDs were noted to have minimal incidence of CAD.1),7),8),9),10) In 2004, a series by Perloff2) found out that there were minimal or absent signs of atherosclerosis on coronary angiography in 25 cyanotic women, mean age 43±4 years and 24 cyanotic men, mean age 41±6 years. More recent series in 2009 describing 250 patients who underwent selective coronary angiography for reasons other than suspected CAD in the United Kingdom revealed that the prevalence of significant CAD in a hospital adult CHD cohort (9.2%) was similar to that in the general population. No patient younger than 40 years old had significant CAD. Systemic arterial hypertension and hyperlipidemia were strong predictors of CAD. However, none of the cyanotic patients included had significant CAD.3)
In total, prevalence of significant CAD in ACHD was similar to the general population. Traditional cardiovascular risk factors for CAD also applied to ACHD, in whom primary prevention of CAD was as important as in the general population.
Yalonetsky et al.4) studied 12,124 ACHD on CAD and reported that 1% (141/12,124) had both CHD and obstructive CAD (age, 56±13 years). In those 141 ACHD, especially atrial septal defect (35%), bicuspid aortic valve (18%), tetralogy of Fallot (9%) followed by coarctation of the aorta (7%) and Eisenmneger syndrome (6%) revealed higher prevalence of obstructive CAD. Traditional risk factors were commonly observed in these patients (82%). Especially, all Eisenmenger syndrome patients revealed several risk factors of CAD. They concluded that CAD was observed in ACHD even in cyanotic ACHD when they had traditional risk factors. Systemic arterial hypertension and hyperlipidemia were strong predictors of CAD.
CYANOTIC CONGENITAL HEART DISEASE AND CORONARY ARTERY DISEASE
Coronary circulation in cyanotic adult congenital heart disease
The coronary arteries were tortuous and markedly dilated in 15% inherently cyanotic ACHD patients by coronary arteriography.2),11),12) Mild to moderate dilatation of the extramural coronary arteries in cyanotic ACHD is in response to endothelial vasodilator nitric oxide and prostaglandins, of which the collaboration is provoked by increased endothelial shear stress of the various erythrocytotic perfusae2),13),14) as well as the systemic vascular bed. Striking dilatation is due to medial structural degeneration that attenuates the coronary artery walls and act in concert with vasodilatation by endothelial nitric oxide synthase.10),13),15) At the same time, angiogenesis in the lung field can be developed from coronary artery, ascending aorta, subclavian artery to pulmonary arteries, and other collateral arteries such as pulmonary arterial to pulmonary vein, venous-venous collaterals and other multiple collaterals due to vascular endothelial growth factor and other growth factors developed in cyanotic ACHD.16)
Factors related to lower incidence of coronary atherosclerosis in cyanotic adult congenital heart disease
Numerous physiologic consequences particularly in cyanotic CHD may be responsible for the low incidence of atherosclerosis in this population. Hypoxemia, hypocholesterolemia, up regulated levels of nitric oxide, hyperbilirubinemia, and low platelet counts are the factors those are “protective” of atherosclerosis in cyanotic ACHD.9),10)
Hypocholesterolemia
Hypoxemic erythrocytotic residents of high altitudes lack coronary atherosclerosis and have low cholesterol levels.2),17) Cyanotic CHD is one of the causes of hypocholesterolemia, reduced levels of total cholesterol and low-density lipoprotein (LDL) cholesterol (Figure 1 and Table 1).11) Decreased total cholesterol is related to low intima-media thickness as has been demonstrated in the general population.18) The etiology of which includes cyanosis (systemic arterial hypoxemia), erythrocytosis, and genetic factors.11) Persistence of hypocholestrolemia after surgical elimination of cyanosis, hypoxemia and erythrocytosis implies induction or suppression of genes that possibly induce hypocholesterolemia.
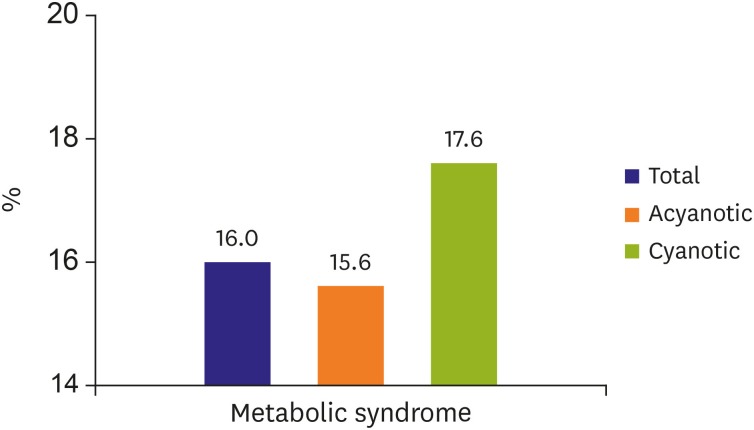
Figure 1. Prevalence of metabolic syndrome.
Metabolic syndrome was observed in 16% adult congenital disease patients, on the contrary, metabolic syndrome in the general population in Japan was 5.5%. When any 3 of these are observed, metabolic syndrome is defined:
1) Waist circumference: >85 cm
2) Triglycerides: ≥150 mg/dL (1.7 mmol/L)*
3) High-density cholesterol: ≤40 mg/dL (1.0 mmol/L) in males and ≤50 mg/dL (<1.3 mmol/L) in females*
4) Systolic blood pressure ≥130 and/or diastolic blood pressure >85 mmHg*
5) Fasting blood sugar: ≥100 mg/dL*
*Drug treatment for the specific condition is an alternate indicator.
Table 1. Plasma lipid levels in ACHD.
| Types of ACHD | Total cholesterol | HDL cholesterol | LDL cholesterol | Triglyceride |
|---|---|---|---|---|
| Group A (n=143, 18–69 years) | 162±40 (87–313)* | 37±10 (16–67)† | 104±38 (47–191)‡ | 122±95 (34–544)‡ |
| Group B (n=47, 22–69 years) | 166±37 (90–260)* | 41±10 (28–54)† | 108±21 (78–135)‡ | 135±113 (44–489) |
| Group C (n=41, 22–75 years) | 210±46 (119–324) | 54±16 (33–79) | 133±26 (106–177) | 166±171 (50–812) |
| Group D (n=48, 21–70 years) | 195±39 (137–285) | 48±12 (27–66) | 122±25 (64–183) | 146±86 (44–335) |
Group A, cyanotic adult congenital heart disease (ACHD), group B, repaired cyanotic ACHD, group C, acyanotic ACHD, and group D, repaired acyanotic ACHD. Data shown are mean±SD (mg/dL) and (range).
ACHD = adult congenital heart disease; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
*p<0.01 (group A or B vs. group C or D), †p<0.01 (group A or B vs. group C or D), ‡p<0.05 (group A or B vs. group C or D).
However, recently study on the prevalence of subclinical atherosclerosis in a larger population of patients with cyanotic CHD was published.19) They reported that there were no differences between cyanotic CHD and controls in: coronary artery calcification score, carotid plaques, carotid plaque thickness max or carotid intima media thickness. And further no significant differences in lipoprotein concentrations. The reason of this difference is not known at this point of time.
Low to normal levels of high-density lipoprotein (HDL) was also noted in cyanotic ACHDs.11) The cyanotic state and the use of β-blockers were found to be independent predictors for reduced HDL.2) Another possible hypothesis is the existence of hypoalphalipoproteinemia in patients with ACHD, because apo A-I plays an important role in HDL function.2) Furthermore, a number of rare genetic disorders are associated with low HDL cholesterol levels. Also limited exercise levels of cyanotic ACHD will be another cause of hypo HDL level. The genes that account for the variation in serum HDL cholesterol in the general population, however, have not been identified yet.
Hypoxemia
Hypoxemia is associated with a decrease in atherogenic oxidized plasma LDL and a decrease in intimal oxidized LDL. Larger LDL particles are relatively resistant to oxidation, and the lack of small dense oxidation-sensitive LDL in cyanotic CHD may act in a similar fashion.2)11) A relationship or lack between oxygen saturation and atherosclerosis in various vascular beds is relevant. In the normal pulmonary circulation, atherosclerosis is common especially above age 40 years, despite hypoxemia. When oxygen saturation in the pulmonary bed is increased by a left-to-right shunt, the prevalence of atherosclerosis remains the same or increases slightly comparing with those without a left-to-right shunt.2) Except in the presence of hypercholesterolemia, there is no relationship between atherosclerosis in the hypoxemic normal pulmonary circulation and in the normally oxygenated systemic circulation.
Nitric oxide
Nitric oxide is an antiatherogenic property because the paracrine molecule opposes platelet adherence and aggregation, stimulates disaggregation of preformed platelet aggregates, inhibits monocyte adherence and infiltration, turns off transcription of the molecule intercellular adhesion molecule that governs adhesion of monocytes to endothelium, and inhibits smooth muscle proliferation.13) Nitric oxide, a ubiquitous signaling molecule synthesized from L arginine and oxygen, and bioavailability of nitric oxide is increased in cyanotic CHD because increased endothelial shear stress provoked by the viscous erythrocytotic perfusate which is a major factor in inducing elaboration of nitric oxide and endothelial nitric oxide synthase gene expression.2),13),20) Nitric oxide is an anti-atherogenic molecule because it counters platelet aggregation, stimulates disaggregation of preformed platelet aggregates, inhibits monocyte adherence and infiltration, and turns off transcription of intercellular adhesion molecule-1 which governs the endothelial adhesion of monocytes and inhibits smooth muscle proliferation.10),20) In addition, red blood cells are nitric oxide reservoirs, and red cell mass is increased in cyanotic CHD.21)
Hyperbilirubinemia
Hyperbilirubinemia is frequently seen in cyanotic patients due to secondary erythrocytosis. Hyperbilirubinemia is a feature of cyanotic ACHD because bilirubin is formed from the breakdown of heme, a process made excessive by the increased red cell mass.2),22) Unconjugated and conjugated bilirubins are endogenous antioxidants that inhibit LDL oxidation and reduce atherosclerotic risk.20),23) Gilbert's disease, a benign hereditary disorder of hepatic bilirubin metabolism, is accompanied by elevated levels of unconjugated bilirubin and followed by immunity from coronary atherosclerosis.23)
Low platelet counts and secondary erythrocytosis
Low platelet counts are antiatherogenic,10),24) and platelet counts are typically low or thrombocytopenic in cyanotic CHD because whole megakaryocytes are shunted from the systemic venous circulation into the systemic arterial circulation and cannot shed platelets by cytoplasmic fragmentation in the pulmonary vascular bed. Platelet counts correlate negatively with hematocrit levels and with the magnitude of the right-to-left shunt. Hematologic abnormalities in cyanotic ACHDs, in particular: thrombocytopenia and secondary erythrocytosis may cause either bleeding or thrombosis, respectively. Although not actual risk factors for atherosclerosis, thrombocytopenia and secondary erythrocytosis are frequently seen in severely cyanotic patients. Low platelet levels were negatively associated with the severity of pulmonary-to-systemic shunting and therefore with the level of cyanosis. Contrary to the increased bleeding tendency caused by thrombocytopenia, hyperviscosity, which is a frequent complication in cyanotic ACHD patients, increases thrombotic risk.22) We recognized this increased hyperviscosity by demonstrating significantly elevated hemoglobin, hematocrit and erythrocyte levels in cyanotic ACHD patients. The literature is ambiguous, however, on the thrombotic risk of hyperviscosity, indicating the necessity of further investigation.2)
CARDIOVASCULAR RISK FACTORS FOR CARDIOVASCULAR DISEASE IN VARIOUS ADULT CONGENITAL HEART DISEASES
Risk of premature atherosclerotic CVD in ACHD is mainly based on 2 background mechanisms: lesions with coronary artery abnormalities such as origin of the left main coronary artery from the right sinus of Valsalva and obstructive lesions of the left ventricle and aorta such as coarctation of the aorta. In addition, surgical repair of CHDs may result in abnormalities of the coronary arteries. Cardiovascular risk may also vary by type of heart defect. Specific conditions in which the coronary arteries are directly affected or altered surgically may confer greater risk for premature atherosclerotic CAD. Coronary artery re-implantation at the time of arterial switch repair in complete transposition of the great arteries has been shown to result in abnormal coronary flow reserve, and intracoronary ultrasound reveals that some patients develop intimal proliferation, a precursor to atherosclerosis.25) Left-sided obstructive lesions may also be associated with accentuated cardiovascular risk. Coarctation of the aorta, even after repair, is commonly associated with systemic hypertension; aortic stenosis can be associated with left ventricular hypertrophy and diastolic dysfunction, known risk factors for adult-onset cardiovascular morbidity and mortality.7) Further, aortopathy (aortic root dilatation and stiffness of the aorta) with aortic pathophysiological abnormalities in ACHD can have negative influence on coronary artery flow and that may induce CAD in advanced age.26) Aortopathy is observed in Marfan syndrome, bicuspid aortic valve, tetralogy of Fallot, complete transposition of the great arteries and hypoplastic left heart syndrome and other CHDs.27),28)
Lesions with coronary artery abnormalities
Complete transposition of the great arteries after arterial switch operation, Ross procedure, anomalous origin of left coronary artery from the pulmonary artery (Bland-White-Garland syndrome)
Coronary manipulation is necessary in arterial switch operation for complete transposition of the great arteries and anomalous origin of left coronary artery from the pulmonary artery. Coronary ostial stenosis or coronary artery kinking could progress over time, and there may be increased risk of associated atherosclerosis.29) By intravascular ultrasound study, coronary intimal thickening/early coronary atherosclerosis was found in translocated coronary arteries in patients with complete transposition of the great arteries post arterial switch.30) Epicardial coronary dilatation in response to nitroglycerin was significantly less in the patients with complete transposition of the great arteries 5 years after arterial switch. Coronary arterial flow reserve was reduced after acetylcholine administration.31) Nearly one-third of patients with arterial switch operation and Ross procedure were obese or overweight. Obesity and associated comorbidities may pose additional CAD in patients with coronary artery implantation surgery.32)
Coronary artery anomalies and atherosclerosis
Origin of the left main coronary artery from the right sinus of Valsalva, passing between the aorta and pulmonary artery, has been associated with sudden death, particularly during or immediately after exercise.33) In these patients, autopsies may reveal subendocardial scars and occasionally, large myocardial infarction. Atherosclerosis in a segment of the abnormal artery has been demonstrated even in young individuals with this disorder.34) Six of 8 patients with both right coronary artery & left coronary artery originated from one aortic sinus, showed significant narrowing of coronary artery due to atherosclerotic plaque.34) Also, symptomatic patients with single coronary artery and ischemia have nearly always associated with atherosclerosis. Right coronary artery is reported to have a high incidence of coronary atheroma.34)
Obstructive lesions of the left ventricle and aorta
CHDs that have been shown to be associated with increased risk of CVD in adulthood is obstructive lesions of the left side of the heart.
Coarctation of the aorta
Coarctation of the aorta even after completely repair commonly associated with systemic hypertension or exercise induced hypertension, which relates to pathophysiology for acquired CVD such as early onset atherosclerosis.35),36) Arterial abnormalities may persist after coarctation of the aorta repair and result in long-term systemic hypertension and increased risk for CVD. Hypertension is related to constriction of the aorta at the site of repair, but coarctation of the aorta may be associated with abnormal vascular reactivity, arterial wall abnormality (aortic medial degeneration: aortopathy) or baroreceptor function.37),38),39) The prevalence of hypertension at rest after repair of coarctation is at least 10%.40) Exercise-induced systolic hypertension may also occur in patients after repair of coarctation of the aorta, even when the blood pressure is normal at rest.41) To evaluate for this, patients with coarctation should have routine exercise/blood pressure evaluation. Beyond hypertension, coarctation of the aorta is associated with other important sequallae that lead to morbidity and mortality. Cerebrovascular accidents occur in association with systemic hypertension especially in patients with cerebral arterial aneurysm in which around 10% of patients with coarctation of the aorta accompanied.42) Aortic dissection in the ascending aorta or near the repair site in coarctation of the aorta may occur.28) Persistent hypertension, association with bicuspid aortic valve, aortic atherosclerosis, and dilated aorta (aortopathy), all of these predispose coarctation of the aorta patients to the serious cardiovascular risk.35),36) Aortic dissection in the ascending aorta or near the repair site may occur whether or not an aneurysm forms in the aorta at the site of the repair. Persistent hypertension, older age at repair, association with bicuspid aortic valve, aortic atherosclerosis, and dilation of the aorta proximal to the repair site all predispose coarctation patients to this serious risk.41)
Aortic stenosis
Aortic stenosis occurs most often at the level of aortic valve, but can happens at supravalvular and subvalvular regions and those can result in myocardial damage and CVD. Aortic stenosis associated with left ventricular hypertrophy and diastolic dysfunction, known as risk factors for adult onset CVD mortality and morbidity. Valvular aortic stenosis occurs in 3% to 6% of patients with CHDs.43) Left ventricular hypertrophy is known to be an independent risk factor for CVD.44) Myocardial blood flow may be compromised in patients with aortic stenosis, despite normal coronary artery patency. Increased myocardial work results in increased demand for oxygen, exceeding the capacity of the coronary supply (abnormal coronary flow reserve). Aortic stenosis during childhood can progress and may therefore be associated with increased left ventricular mass and increased risk for CVD in adults. Increase in the left ventricular outflow tract gradient is enhanced by progressive calcification of the aortic valve.43)
Supravalvular aortic stenosis (most commonly associated with Williams syndrome45)) is associated with coronary ostial stenosis and hypertension in the ascending aorta and subsequent coronary arterial stenosis, and that may result in myocardial ischemia and exercise induced syncope. Often associated renal arterial stenosis also induces systemic hypertension.35),46)
Aortopathy and coronary artery disease
Aorta in some types of CHDs such as Marfan syndrome, bicuspid aortic valve, coarctation of the aorta, tetralogy of Fallot and the other various CHDs will become dilate, aneurysmal and rupture.15),28) In these ACHD patients, reduced aortic elasticity and increased stiffness with dilated aorta can induce aortic regurgitation, reduced coronary artery flow and negative impact on left ventricular systolic and diastolic function (left ventricular hypertrophy and failure). Therefore, aortopathy in ACHD can have unfavorable influence on coronary artery flow and that may induce CAD in advanced age.28) Senzaki et al.47) reported that there is an abnormal ventriculoarterial coupling (increased aortic stiffness) in repaired tetralogy of Fallot. They found that tetralogy of Fallot patients comparing with controls (38 repaired tetralogy of Fallot vs. 55 controls) revealed higher impedance, pulse wave velocity, arterial wave reflection and lower total peripheral arterial compliance, and they found increased aortic wall stiffness and increased aortic root diameter. They concluded central and peripheral arterial wall stiffness are increased even after tetralogy of Fallot repair. Abnormal arterial elastic properties in repaired tetralogy of Fallot induce increased left ventricular afterload, decreased coronary artery flow and aortic dilatation, and these may develop systolic and diastolic dysfunction of left ventricle.
CAUSES OF DEATH IN ADULT CONGENITAL HEART DISEASE
Significant advances over the last 5 decades have allowed most patients with CHD to survive into adulthood. Mortality of CHD has been declining year by year and cause of death is changing. Among adults with cyanotic lesions such as tetralogy of Fallot and complete transposition of the great arteries after repair, the primary contributing cause of death was arrhythmia followed by heart failure.48) However, for adults with noncyanotic lesions, the major contributing cause before 1990 was arrhythmia; after 1990, myocardial infarction became the leading contributing cause of death in US. Myocardial infarction is now the leading contributing cause for adults with noncyanotic CHD consistent with late survival and an increasing impact of acquired heart disease. However, arrhythmia remains the primary contributing cause of death for those with cyanotic lesions. In near future, whether the leading cause of death will change or not in cyanotic ACHD remains unknown because of their antiatherogenic properties.
RISK FACTORS FOR CORONARY ARTERY DISEASE AND METABOLIC SYNDROME IN ADULT CONGENITAL HEART DISEASE
We conducted the retrospective multicenter study on CVD risk factors and metabolic syndrome in ACHD patients,49) and attempted to shed light on: i) whether traditional cardiovascular risk factors for atherosclerosis also apply to ACHD patients or not, and ii) whether primary prevention of CAD in ACHD is as important as in the general population or not.
Metabolic syndrome
Metabolic syndrome (Figures 1 and 2) is a constellation of risk factors for CVD, including obesity, dyslipidemia, insulin resistance, and hypertension.50) These risk factors are associated with excess acquired CVD, type 2 diabetes mellitus and mortality.51)
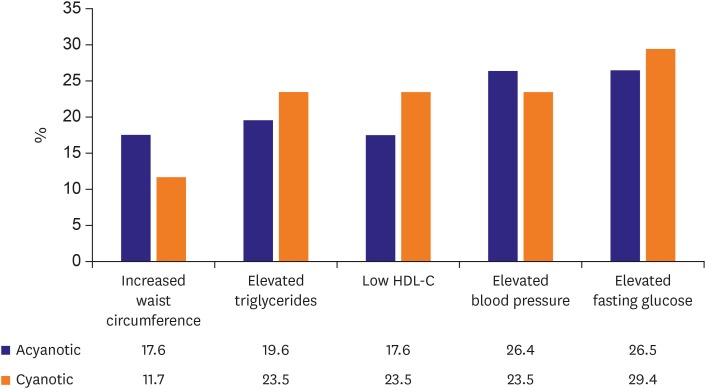
Figure 2. Metabolic syndrome components.
Waist circumference and blood pressure are higher in acyanotic ACHD comparing cyanotic ACHD. Triglycerides and fasting blood glucose are higher and HDL cholesterol is lower in cyanotic ACHD. Total number of patients, cyanotic ACHD, and acyanotic ACHD were 224 (age, 37.2±15.7; males, 45%; females, 55%), 105 (47%), and 117(53%).
Definition of abnormal component is as follows; Increased waist circumference: >85 cm in male, >90 cm in female, elevated triglycerides: ≥150 mg/dL, low HDL cholesterol:<40 mg/dL, elevated blood pressure: >140/90 mmHg, elevated fasting glucose: 100 mg/dL.
ACHD = adult conge natal heart disease; HDL, high-density lipoprotein.
From our study on CVD risk in ACHD patients,49) metabolic syndrome was observed in 16% ACHD patients, on the contrary, metabolic syndrome in the general population in Japan was 5.5% (National Cholesterol Education Program Adult Treatment Panel III).52) From recent study of metabolic syndrome in ACHD in US,50) metabolic syndrome was also more common in ACHD patients than in controls (15.0% vs. 7.4%). Thus, patients with CHD should be screened for metabolic syndrome and risk factors mitigated where possible to prevent atherosclerotic CAD. A sedentary lifestyle may be an independent risk factor for accelerated atherosclerosis and obesity. Therefore, preventive cardiology should be included during routine ACHD care and follow-up. Preventive cardiology including healthy lifestyle counseling, blood pressure monitoring, and close screening for lipid abnormalities and insulin resistance, should be performed for ACHD patients. Cardiac rehabilitation has been shown to improve the exercise performance of children with CHD even those with known residual cardiac dysfunction.53) Some ACHD may have limitation in their ability to perform physical activity. Exercise capacity should be evaluated and appropriate aerobic activities should be encouraged in most ACHD patients. Additionally, because atherosclerosis is known to start during childhood, pediatric cardiologists have a role in preventative cardiology counseling in children with CHD during transition process.
Overweight/obesity
We analyzed prevalence of obesity±overweight in ACHD (n=224, 101 males [45%], 123 females [55%]. Prevalence of obesity (BMI ≥30 kg/m2) is higher in our ACHD patients49) compared to general Japanese population (ACHD patients vs. male, female; 10.1% vs. 2.9%, 3.4%).54) However, prevalence of obesity/overweight (BMI ≥25 kg/m2) in our ACHD patients is similar to general Japanese population, age 30–40 years, (ACHD patients vs. male, female; 21.9% vs. 28.6%, 14.3%). In a previous report, prevalence of obesity and overweight in ACHD patients was similar to national data among those with and without heart disease.55) In one published study, 26% of 1,532 patients in a large pediatric cardiology outpatient clinic were overweight or obese.7) In a study from Belgium, 1,976 individuals with CHD were more often obese and hypertensive compared with the general population.6) Obesity was present in 30% of adults with moderate and complex CHD requiring additional surgery.5) Nearly one-third of patients with a history of complete transposition of the great arteries after arterial switch operation are obese.25) While some studies have demonstrated lower rates of obesity in patients with single ventricles, obesity was present in 11% of pediatric and 17% of adult Fontan patients.55),56),57) Several recent studies have shown that patients post Fontan procedure have an increased prevalence of obesity.58),59),60) Association of obesity such as diastolic dysfunction and ventricular hypertrophy may complicate the management of ACHD patients who are already at risk for ventricular dysfunction, arrhythmias, and heart failure.61),62) Obesity also has been shown to be independently associated with hypertension and endothelial dysfunction. Contributing factors for obesity in ACHD patients especially moderate to severe ACHD including post Fontan procedure are suspected to be due to physical inactivity (self-limitation and anxiety in physical activities). ACHD patients frequently self-restrict exercise because of perceived risks of underlying CHD or because of limited capacity for exercise.63),64) There is a report that showed in ACHD only 7 hours/week spent in physical activities vs. 28 hours/week of sedentary activities.7) Also, there is a desire to gain weight early in life when weight gain was poor, so they love high caloric supplemental diet. ACHD patients possess unique risk factors for developing obesity, including exercise restriction and differing nutritional strategies in infancy and early childhood.6),55),65),66) Exercise restriction is common in ACHD patients and has been shown to promote obesity in patients with CHD. Of course, some restrictions on competitive sports are recommended in certain high-risk populations. Most patients with repaired CHD may exercise safely, but medical providers, as well as parents, often impose additional exercise restrictions.67) Aside from predisposing to obesity and hyperlipidemia, the lack of aerobic exercise is associated with an increased risk of hospitalization and death in ACHD patients. Enhanced physical activity and aerobic exercise play an important role in decreasing cardiovascular risk. However, questions still remain regarding this effect in ACHD patients. Patients with severe forms of CHD often exhibit failure to thrive early in life and require increased caloric supplementation to achieve appropriate weight gain in infancy, but they experience a rapid growth once cardiac surgery is performed.68) Similar to the general population, obesity creates the major risk for development of metabolic syndrome in ACHD patients. Little is known about the long-term effects of obesity on ACHD patients. Possibly genetic predisposition also can be related, but it remains unknown. Similar growth patterns seen in infants without CHD are associated with obesity and a greater risk of adult CVD.69) In addition, although most children have normal nutritional requirements after surgical palliation, medical providers and parents may continue to stress weight gain as a goal.70) Lerman et al.71) recently reported that compared with controls, ACHD had similar prevalence of overweight and obesity but lower prevalence of morbid obesity. These relationships were not attenuated by adjustment for CHD severity. In spite of higher incidence of obese, some ACHD patients especially complex ACHD or cyanotic ACHD revealed sarcopenia. Following recent study on sarcopenia in ACHD,72) ACHD patients have higher calorie, protein, and fat intake than those in a national survey despite decreased skeletal muscle mass. Amino acid intake plus resistance training positively improved body fat percentage, skeletal muscle mass, and edema in ACHD.
Hypertension
In a cohort of ACHD patients, systolic blood pressure was higher in the overweight and obese patients.7),54) Increased afterload by hypertension can inducer left ventricular hypertrophy, then increased left ventricular mass, and those factors adversely affect the delicate physiology of complex ACHD (e.g. Fontan or systemic right ventricular physiology) and on the underlying CHD with valvular regurgitation and ventricular dysfunction. Also, systemic hypertension is another important cardiovascular risk factor that can lead to premature CAD, stroke, arrhythmia, and CVD.
The prevalence of hypertension was lower in our ACHD patients49) compared to the general Japanese population (15.1 vs. 26.5% in males). Regarding recent paper on blood pressure in 100 ACHD patients (age, 37.0±15.0 years),73) average systolic blood pressure was 117.7±20.1 mmHg and diastolic blood pressure was 69.7±14.3 mmHg with central systolic blood pressure of 119.9±21.5 mmHg, respectively. Hypertension was observed in 16 (16%). The determinants of high systolic blood pressure were age, body mass index, hemoglobin A1c level and total cholesterol level. They concluded that the high systolic blood pressure is common in ACHD, therefore, monitoring and the strict blood pressure control are mandatory. In the Belgium study, 13% of ACHD were hypertensive.6) In an older population of ACHD patients (age >65 years), 47% were found to be hypertensive,74) however, many of them were simple CHD. Generally, hypertension in this population is “essential” hypertension. There are patients with moderate or complex CHD who are at increased risk of hypertension including coarctation of the aorta. While hypertension was seen in 4% of the CHD group as a whole, it was as high as 46% in patients after coarctation of the aorta repair.75) Hypertension may increase the risk of aortic dilation in prior coarctation of the aorta repair, bicuspid aortic valve, tetralogy of Fallot, and complete transposition of the great arteries after arterial switch operation. The long-term effects of longstanding hypertension on the ACHD remain unknown
Dyslipidemia
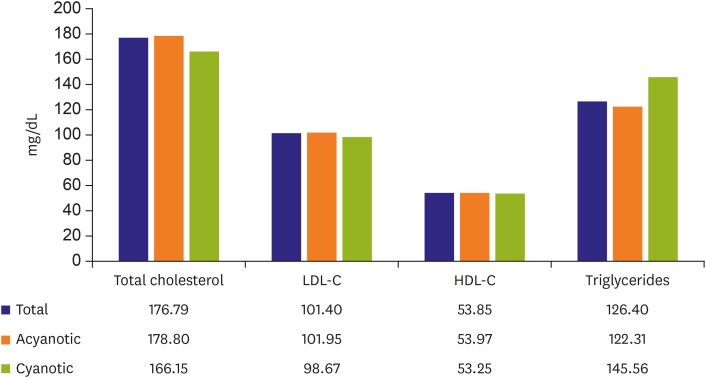
In our ACHD study (49), lipid profiles such as total cholesterol (mean, 177 mg/dL), LDL cholesterol (mean, 101 mg/dL), HDL cholesterol (mean, 54 mg/dL), and triglycerides (mean, 126 mg/dL) are within normal limits, and there are no differences between cyanotic and acyanotic ACHD (Figure 3). In other report on CHD patients and serum cholesterol levels,76) children with cyanotic CHD revealed lower cholesterol than acyanotic CHD and arrhythmias patients, and that tendency increases with age. However, the tendency is not significant. There is no evidence to suggest that rates of dyslipidemia are higher in ACHD as compared to the general population.4),77) One UK single-center study of 250 ACHD patients (mean age, 51 years) found the prevalence of dyslipidemia was 19.1%, with dyslipidemia being a strong risk factor for CAD.3) Prevalence of dyslipidemia, however, decreased with increasing cardiac lesion complexity. Data from the Quebec Congenital Heart Disease Database found a prevalence of dyslipidemia in 27% of patients over the age of 65, which is lower than estimates for the general population.78) Cyanotic patients have a lower total cholesterol than acyanotic patients.10),11) This effect persists even in patients who have been surgically corrected to an acyanotic circulation.11) Martínez-Quintana and Rodríguez-González79) reported that ACHD patients showed lower serum total cholesterol and LDL cholesterol levels than no-congenital patients although no significant differences were seen in lipoprotein (a) concentrations between both groups.80)
Figure 3. Lipid profile.
Lipid profile is not significantly different between cyanotic ACHD and acyanotic ACHD. Total number of patients, cyanotic ACHD, and acyanotic ACHD were 224 (age, 37.2±15.7; males, 45%; females, 55%), 105 (47%), and 117(53%).
ACHD = adult conge natal heart disease; LDL = low-density lipoprotein; HDL = high-density lipoprotein.
What we do know is that cyanotic ACHD patients have significantly lower total and LDL cholesterol levels than non-congenital patients presenting, therefore, possibly a lower risk of developing atherosclerosis.8),10)
Type 2 diabetes mellitus and impaired fasting glucose levels
Ohuchi et al.81) reported lower fasting but higher postprandial blood glucose and glycated hemoglobin levels in cyanotic ACHD without reparative surgery or Fontan patients compared with healthy controls. Dellborg et al.82) reported CHD and secondary risk factors for CVD frequently coexist and the development of type 2 diabetes mellitus in the ACHD population is not also uncommon with an estimated prevalence of between 4% and 8%. In our ACHD study, prevalence of type 2 diabetes mellitus and impaired fasting glucose is 9.2% and 5.9%, respectively, and there is no difference in prevalence between cyanotic and acyanotic ACHD. Martínez-Quintana et al.80) identified elevated risk of metabolic derangements in ACHD patients, consistent with decreased insulin sensitivity. These study results conform to previously published findings of abnormal glucose tolerance and low HDL levels in ACHD patients.80),81) This pathophysiology should be considered in managing long-term survivors of complex ACHD.81) Those ACHD patients show activated neurohormonal activities, abnormal cardiac autonomic nervous activity, and inflammatory cytokines as demonstrated in non-ACHD patients with heart failure and some of those prognostic values are emerging.83) Treatment of conventional cardiovascular risk factors in ACHD could be considered secondary prevention given the relatively high morbidity and high risk for mortality observed in patients with the combination of ACHD and type 2 diabetes mellitus. However, a prognostic value of abnormal glucose regulation still remains unclear, especially the prognostic power for the mortality in ACHD.
Summary on coronary artery disease risk factors and metabolic syndrome in adult congenital heart disease
Former studies showed that the presence of cardiovascular risk factors such as obesity and metabolic syndrome are higher in ACHD patients compared to the general population. Still, preventive and therapeutic measures for primary and secondary prevention of cardiovascular events should be accorded to this special group of patients when necessary.
RECOMMENDATIONS FOR ADULT CONGENITAL HEART DISEASE FOR PREVENTION OF DEVELOPING METABOLIC SYNDROME, ATHEROSCLEROSIS AND CARDIOVASCULAR DISEASE
All the known risk factors for inducing atherosclerosis will possibly occur in ACHD patients at the similar prevalence as seen in the general population. In the setting of repaired ACHD may represent higher tendency for premature atherosclerosis. Many children with repaired CHD may have limitation for physical activity during school days. A life style of avoiding exercise or walking is an independent risk for atherosclerosis. Also, these ACHD patients are more prone to be obese. Cardiac rehabilitation has been shown to improve the exercise performance of ACHD patients, even those with known residual cardiac dysfunction. Because ACHD patients have other abnormalities that may make the heart more vulnerable to both the development of atherosclerosis and the adverse sequelae of a cardiovascular event, it seems prudent to be aggressive about the evaluation of their CVD risk status. This is particularly true in ACHD patients that have risk factors for developing CVD. Specific ACHDs are at risk for premature CVD. Even in cyanotic ACHD regardless of repair, eliminating risk factors of CVD is mandatory.
CONCLUSIONS
Cyanotic ACHD even after repair has low incidence of CAD. Acyanotic ACHD has similar prevalence of CAD as general population. However, even in cyanotic ACHD patients, CAD can develop when they have risk factors for CAD such as obesity, dyslipidemia, hypertension, diabetes mellitus, smoking habit and/or positive family history of CVD and lead a life of limited exercise levels. The prevalence of having risk factors for CVD is similar between ACHD and the general population. Because ACHD patients have other abnormalities that may make the heart more vulnerable to both the development atherosclerosis and adverse sequalae of a cardiovascular event, it seems reasonable to regularly evaluate their CVD risk status. This is especially true for ACHD patients with specific CHD at risk, therefore, all ACHD patients should be cared for not having risk factors for CAD.
Points are follows:
(1) ACHD patients have similar prevalence of risk factors for CVD as general population.
(2) Cyanotic ACHD has lower incidence of CAD.
(3) Acyanotic ACHD has similar prevalence of CAD as general population.
(4) Increased risk of coronary atherosclerosis is observed in congenital coronary artery anomalies, complete transposition of the great arteries post-arterial switch operation and coarctation of the aorta.
(5) Aortopathy may be an additional risk factor for CVD.
(6) Primary prevention for CVD is even more important in young adults with CHD.
Footnotes
Conflict of Interest: The author has no financial conflicts of interest.
References
- 1.Niwa K. Adults with congenital heart disease transition. Curr Opin Pediatr. 2015;27:576–580. doi: 10.1097/MOP.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 2.Perloff JK. The coronary circulation in cyanotic congenital heart disease. Int J Cardiol. 2004;97(Suppl 1):79–86. doi: 10.1016/j.ijcard.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Giannakoulas G, Dimopoulos K, Engel R, et al. Burden of coronary artery disease in adults with congenital heart disease and its relation to congenital and traditional heart risk factors. Am J Cardiol. 2009;103:1445–1450. doi: 10.1016/j.amjcard.2009.01.353. [DOI] [PubMed] [Google Scholar]
- 4.Yalonetsky S, Horlick EM, Osten MD, Benson LN, Oechslin EN, Silversides CK. Clinical characteristics of coronary artery disease in adults with congenital heart defects. Int J Cardiol. 2013;164:217–220. doi: 10.1016/j.ijcard.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Zaidi AN, Bauer JA, Michalsky MP, et al. The impact of obesity on early postoperative outcomes in adults with congenital heart disease. Congenit Heart Dis. 2011;6:241–246. doi: 10.1111/j.1747-0803.2011.00522.x. [DOI] [PubMed] [Google Scholar]
- 6.Moons P, Van Deyk K, Dedroog D, Troost E, Budts W. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612–616. doi: 10.1097/01.hjr.0000197472.81694.2b. [DOI] [PubMed] [Google Scholar]
- 7.Pemberton VL, McCrindle BW, Barkin S, et al. Report of the National Heart, Lung, and Blood Institute's Working Group on obesity and other cardiovascular risk factors in congenital heart disease. Circulation. 2010;121:1153–1159. doi: 10.1161/CIRCULATIONAHA.109.921544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffels MG, Mulder KM, Trip MD, et al. Atherosclerosis in patients with cyanotic congenital heart disease. Circ J. 2010;74:1436–1441. doi: 10.1253/circj.cj-09-0858. [DOI] [PubMed] [Google Scholar]
- 9.Niwa K. The coronary circulation in adults with congenital heart disease. Intern Med. 2006;45:1199–1200. doi: 10.2169/internalmedicine.45.0169. [DOI] [PubMed] [Google Scholar]
- 10.Perloff JK. Cyanotic congenital heart disease the coronary arterial circulation. Curr Cardiol Rev. 2012;8:1–5. doi: 10.2174/157340312801215836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fyfe A, Perloff JK, Niwa K, Child JS, Miner PD. Cyanotic congenital heart disease and coronary artery atherogenesis. Am J Cardiol. 2005;96:283–290. doi: 10.1016/j.amjcard.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 12.Chugh R, Perloff JK, Fishbein M, Child JS. Extramural coronary arteries in adults with cyanotic congenital heart disease. Am J Cardiol. 2004;94:1355–1357. doi: 10.1016/j.amjcard.2004.07.135. [DOI] [PubMed] [Google Scholar]
- 13.Malinski T. Normal and pathological distribution of nitric oxide in the cardiovascular system. Pol J Pharmacol. 1998;50:387–391. [PubMed] [Google Scholar]
- 14.Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res. 1993;72:1276–1284. doi: 10.1161/01.res.72.6.1276. [DOI] [PubMed] [Google Scholar]
- 15.Niwa K, Perloff JK, Bhuta SM, et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393–400. doi: 10.1161/01.cir.103.3.393. [DOI] [PubMed] [Google Scholar]
- 16.Hamada H, Ebata R, Higashi K, et al. Serum vascular endothelial growth factor in cyanotic congenital heart disease functionally contributes to endothelial cell kinetics in vitro. Int J Cardiol. 2007;120:66–71. doi: 10.1016/j.ijcard.2006.08.106. [DOI] [PubMed] [Google Scholar]
- 17.Arias-Stella J, Topilsky M. Anatomy of the coronary artery circulation at high altitude in high altitude physiology. Edinburgh: Churchill Livingstone Publishers; 1971. pp. 149–157. [Google Scholar]
- 18.European Association for Cardiovascular Prevention & Rehabilitation. Reiner Z, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 19.Tarp JB, Sørgaard MH, Christoffersen C, et al. Subclinical atherosclerosis in patients with cyanotic congenital heart disease. Int J Cardiol. 2019;277:97–103. doi: 10.1016/j.ijcard.2018.08.104. [DOI] [PubMed] [Google Scholar]
- 20.Niwa K. Coronary artery anomalies and coronary artery disease in adults with congenital cardiac disease. Asia Pac Cardiol. 2007;1:70–72. [Google Scholar]
- 21.Gross SS, Lane P. Physiological reactions of nitric oxide and hemoglobin: a radical rethink. Proc Natl Acad Sci U S A. 1999;96:9967–9969. doi: 10.1073/pnas.96.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwa K, Perloff JK, Kaplan S, Child JS, Miner PD. Eisenmenger syndrome in adults: ventricular septal defect, truncus arteriosus, univentricular heart. J Am Coll Cardiol. 1999;34:223–232. doi: 10.1016/s0735-1097(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 23.Madhavan M, Wattigney WA, Srinivasan SR, Berenson GS. Serum bilirubin distribution and its relation to cardiovascular risk in children and young adults. Atherosclerosis. 1997;131:107–113. doi: 10.1016/s0021-9150(97)06088-7. [DOI] [PubMed] [Google Scholar]
- 24.Lill MC, Perloff JK, Child JS. Pathogenesis of thrombocytopenia in cyanotic congenital heart disease. Am J Cardiol. 2006;98:254–258. doi: 10.1016/j.amjcard.2006.01.083. [DOI] [PubMed] [Google Scholar]
- 25.Pasquali SK, Marino BS, Powell DJ, et al. Following the arterial switch operation, obese children have risk factors for early cardiovascular disease. Congenit Heart Dis. 2010;5:16–24. doi: 10.1111/j.1747-0803.2009.00359.x. [DOI] [PubMed] [Google Scholar]
- 26.Niwa K. Pathological background. In: Niwa K, Kaemmerer H, editors. Aortopathy. Tokyo: Springer; 2017. pp. 15–30. [Google Scholar]
- 27.Niwa K. Landmark lecture: Perloff lecture: Tribute to Professor Joseph Kayle Perloff and lessons learned from him: aortopathy in adults with CHD. Cardiol Young. 2017;27:1959–1965. doi: 10.1017/S1047951117002116. [DOI] [PubMed] [Google Scholar]
- 28.Niwa K. Aortopathy in congenital heart disease in adults: aortic dilatation with decreased aortic elasticity that impacts negatively on left ventricular function. Korean Circ J. 2013;43:215–220. doi: 10.4070/kcj.2013.43.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Click RL, Holmes DR, Jr, Vlietstra RE, Kosinski AS, Kronmal RA. Anomalous coronary arteries: location, degree of atherosclerosis and effect on survival--a report from the Coronary Artery Surgery Study. J Am Coll Cardiol. 1989;13:531–537. doi: 10.1016/0735-1097(89)90588-3. [DOI] [PubMed] [Google Scholar]
- 30.Pedra SR, Pedra CA, Abizaid AA, et al. Intracoronary ultrasound assessment late after the arterial switch operation for transposition of the great arteries. J Am Coll Cardiol. 2005;45:2061–2068. doi: 10.1016/j.jacc.2005.02.076. [DOI] [PubMed] [Google Scholar]
- 31.Gagliardi MG, Adorisio R, Crea F, Versacci P, Di Donato R, Sanders SP. Abnormal vasomotor function of the epicardial coronary arteries in children five to eight years after arterial switch operation: an angiographic and intracoronary Doppler flow wire study. J Am Coll Cardiol. 2005;46:1565–1572. doi: 10.1016/j.jacc.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 32.Pasquali SK, Marino BS, Pudusseri A, et al. Risk factors and comorbidities associated with obesity in children and adolescents after the arterial switch operation and Ross procedure. Am Heart J. 2009;158:473–479. doi: 10.1016/j.ahj.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 34.Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. Am Heart J. 1986;111:941–963. doi: 10.1016/0002-8703(86)90646-0. [DOI] [PubMed] [Google Scholar]
- 35.Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 36.Vriend JW, van Montfrans GA, Romkes HH, et al. Relation between exercise-induced hypertension and sustained hypertension in adult patients after successful repair of aortic coarctation. J Hypertens. 2004;22:501–509. doi: 10.1097/00004872-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Gidding SS, Rocchini AP, Moorehead C, Schork MA, Rosenthal A. Increased forearm vascular reactivity in patients with hypertension after repair of coarctation. Circulation. 1985;71:495–499. doi: 10.1161/01.cir.71.3.495. [DOI] [PubMed] [Google Scholar]
- 38.Beekman RH, Katz BP, Moorehead-Steffens C, Rocchini AP. Altered baroreceptor function in children with systolic hypertension after coarctation repair. Am J Cardiol. 1983;52:112–117. doi: 10.1016/0002-9149(83)90080-2. [DOI] [PubMed] [Google Scholar]
- 39.Strafford MA, Griffiths SP, Gersony WM. Coarctation of the aorta: a study in delayed detection. Pediatrics. 1982;69:159–163. [PubMed] [Google Scholar]
- 40.Brouwer RM, Erasmus ME, Ebels T, Eijgelaar A. Influence of age on survival, late hypertension, and recoarctation in elective aortic coarctation repair. Including long-term results after elective aortic coarctation repair with a follow-up from 25 to 44 years. J Thorac Cardiovasc Surg. 1994;108:525–531. [PubMed] [Google Scholar]
- 41.Freed MD, Rocchini A, Rosenthal A, Nadas AS, Castaneda AR. Exercise-induced hypertension after surgical repair of coarctation of the aorta. Am J Cardiol. 1979;43:253–258. doi: 10.1016/s0002-9149(79)80012-0. [DOI] [PubMed] [Google Scholar]
- 42.Connolly HM, Huston J, 3rd, Brown RD, Jr, Warnes CA, Ammash NM, Tajik AJ. Intracranial aneurysms in patients with coarctation of the aorta: a prospective magnetic resonance angiographic study of 100 patients. Mayo Clin Proc. 2003;78:1491–1499. doi: 10.4065/78.12.1491. [DOI] [PubMed] [Google Scholar]
- 43.Roberts WC. Anatomically isolated aortic valvular disease. The case against its being of rheumatic etiology. Am J Med. 1970;49:151–159. doi: 10.1016/s0002-9343(70)80070-5. [DOI] [PubMed] [Google Scholar]
- 44.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 45.Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]
- 46.Daniels SR, Loggie JM, Schwartz DC, Strife JL, Kaplan S. Systemic hypertension secondary to peripheral vascular anomalies in patients with Williams syndrome. J Pediatr. 1985;106:249–251. doi: 10.1016/s0022-3476(85)80297-3. [DOI] [PubMed] [Google Scholar]
- 47.Senzaki H, Iwamoto Y, Ishido H, et al. Arterial haemodynamics in patients after repair of tetralogy of Fallot: influence on left ventricular after load and aortic dilatation. Heart. 2008;94:70–74. doi: 10.1136/hrt.2006.114306. [DOI] [PubMed] [Google Scholar]
- 48.Pillutla P, Shetty KD, Foster E. Mortality associated with adult congenital heart disease: trends in the US population from 1979 to 2005. Am Heart J. 2009;158:874–879. doi: 10.1016/j.ahj.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Javier AD, Niwa K. Cardiovascular risk factors among adults with congenital heart disease. Nihon Shoni Junkanki Gakkai Zasshi. 2013;29:S137. [Google Scholar]
- 50.Deen JF, Krieger EV, Slee AE, et al. Metabolic syndrome in adults with congenital heart disease. J Am Heart Assoc. 2016;5:e001132. doi: 10.1161/JAHA.114.001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 52.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 53.Rhodes J, Curran TJ, Camil L, et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339–1345. doi: 10.1542/peds.2004-2697. [DOI] [PubMed] [Google Scholar]
- 54.Ministry of Health, Labour and Welfare (JP) Results of National health and nutrition survey by the Japanese Ministry of Health, Labour and Welfare [Internet] Tokyo: Ministry of Health, Labour and Welfare; Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/h29-houkoku.html. [Google Scholar]
- 55.Shustak RJ, McGuire SB, October TW, Phoon CK, Chun AJ. Prevalence of obesity among patients with congenital and acquired heart disease. Pediatr Cardiol. 2012;33:8–14. doi: 10.1007/s00246-011-0049-y. [DOI] [PubMed] [Google Scholar]
- 56.Martinez SC, Byku M, Novak EL, et al. Increased body mass index is associated with congestive heart failure and mortality in adult Fontan patients. Congenit Heart Dis. 2016;11:71–79. doi: 10.1111/chd.12296. [DOI] [PubMed] [Google Scholar]
- 57.Vogt KN, Manlhiot C, Van Arsdell G, Russell JL, Mital S, McCrindle BW. Somatic growth in children with single ventricle physiology impact of physiologic state. J Am Coll Cardiol. 2007;50:1876–1883. doi: 10.1016/j.jacc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 58.Freud LR, Webster G, Costello JM, et al. Growth and obesity among older single ventricle patients presenting for Fontan conversion. World J Pediatr Congenit Heart Surg. 2015;6:514–520. doi: 10.1177/2150135115598212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung ST, Hong B, Patterson L, Petit CJ, Ham JN. High overweight and obesity in Fontan patients: a 20-year history. Pediatr Cardiol. 2016;37:192–200. doi: 10.1007/s00246-015-1265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wellnitz K, Harris IS, Sapru A, Fineman JR, Radman M. Longitudinal development of obesity in the post-Fontan population. Eur J Clin Nutr. 2015;69:1105–1108. doi: 10.1038/ejcn.2015.68. [DOI] [PubMed] [Google Scholar]
- 61.Daniels SR, Kimball TR, Morrison JA, Khoury P, Witt S, Meyer RA. Effect of lean body mass, fat mass, blood pressure, and sexual maturation on left ventricular mass in children and adolescents. Statistical, biological, and clinical significance. Circulation. 1995;92:3249–3254. doi: 10.1161/01.cir.92.11.3249. [DOI] [PubMed] [Google Scholar]
- 62.Maser RE, Lenhard MJ. An overview of the effect of weight loss on cardiovascular autonomic function. Curr Diabetes Rev. 2007;3:204–211. doi: 10.2174/157339907781368931. [DOI] [PubMed] [Google Scholar]
- 63.Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 64.Reybrouck T, Mertens L. Physical performance and physical activity in grown-up congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:498–502. doi: 10.1097/01.hjr.0000176510.84165.eb. [DOI] [PubMed] [Google Scholar]
- 65.Cohen MS. Clinical practice: the effect of obesity in children with congenital heart disease. Eur J Pediatr. 2012;171:1145–1150. doi: 10.1007/s00431-012-1736-2. [DOI] [PubMed] [Google Scholar]
- 66.Stefan MA, Hopman WM, Smythe JF. Effect of activity restriction owing to heart disease on obesity. Arch Pediatr Adolesc Med. 2005;159:477–481. doi: 10.1001/archpedi.159.5.477. [DOI] [PubMed] [Google Scholar]
- 67.Longmuir PE, Brothers JA, de Ferranti SD, et al. Promotion of physical activity for children and adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013;127:2147–2159. doi: 10.1161/CIR.0b013e318293688f. [DOI] [PubMed] [Google Scholar]
- 68.Owens JL, Musa N. Nutrition support after neonatal cardiac surgery. Nutr Clin Pract. 2009;24:242–249. doi: 10.1177/0884533609332086. [DOI] [PubMed] [Google Scholar]
- 69.Barker DJ, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 70.Vieira TC, Trigo M, Alonso RR, et al. Assessment of food intake in infants between 0 and 24 months with congenital heart disease. Arq Bras Cardiol. 2007;89:219–224. doi: 10.1590/s0066-782x2007001600002. [DOI] [PubMed] [Google Scholar]
- 71.Lerman JB, Parness IA, Shenoy RU. Body weights in adults with congenital heart disease and the obesity frequency. Am J Cardiol. 2017;119:638–642. doi: 10.1016/j.amjcard.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 72.Shiina Y, Matsumoto N, Okamura D, et al. Sarcopenia in adults with congenital heart disease: nutritional status, dietary intake, and resistance training. J Cardiol. 2019;74:84–89. doi: 10.1016/j.jjcc.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Murakami T, Shiraishi M, Shiina Y, Tateno S, Kawasoe Y, Niwa K. B1-2. High blood pressure in adult patients with congenital heart disease. J Adult Congenit Heart Dis. 2013;5:99. [Google Scholar]
- 74.Afilalo J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Marelli AJ. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509–1515. doi: 10.1016/j.jacc.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 75.Engelfriet P, Boersma E, Oechslin E, et al. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The Euro Heart Survey on adult congenital heart disease. Eur Heart J. 2005;26:2325–2333. doi: 10.1093/eurheartj/ehi396. [DOI] [PubMed] [Google Scholar]
- 76.Higashi K, Tateno S, Niwa K, et al. Hypocholesterolemia in cyanotic congenital heart disease in children. Nihon Shoni Junkanki Gakkai Zasshi. 2006;22:432. [Google Scholar]
- 77.Lui GK, Fernandes S, McElhinney DB. Management of cardiovascular risk factors in adults with congenital heart disease. J Am Heart Assoc. 2014;3:e001076. doi: 10.1161/JAHA.114.001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joffres M, Shields M, Tremblay MS, Connor Gorber S. Dyslipidemia prevalence, treatment, control, and awareness in the Canadian Health Measures Survey. Can J Public Health. 2013;104:e252–e257. doi: 10.17269/cjph.104.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martínez-Quintana E, Rodríguez-González F. Lipoprotein(a) concentrations in adult congenital heart disease patients. Congenit Heart Dis. 2014;9:63–68. doi: 10.1111/chd.12093. [DOI] [PubMed] [Google Scholar]
- 80.Martínez-Quintana E, Rodríguez-González F, Nieto-Lago V, Nóvoa FJ, López-Rios L, Riaño-Ruiz M. Serum glucose and lipid levels in adult congenital heart disease patients. Metabolism. 2010;59:1642–1648. doi: 10.1016/j.metabol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 81.Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30–39. doi: 10.1016/j.ahj.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 82.Dellborg M, Björk A, Pirouzi Fard MN, et al. High mortality and morbidity among adults with congenital heart disease and type 2 diabetes. Scand Cardiovasc J. 2015;49:344–350. doi: 10.3109/14017431.2015.1085595. [DOI] [PubMed] [Google Scholar]
- 83.Ohuchi H, Yasuda K, Ono S, et al. Low fasting plasma glucose level predicts morbidity and mortality in symptomatic adults with congenital heart disease. Int J Cardiol. 2014;174:306–312. doi: 10.1016/j.ijcard.2014.04.070. [DOI] [PubMed] [Google Scholar]