Abstract
Most patients with gastric mucosa‐associated lymphoid tissue (MALT) lymphoma are infected with Helicobacter pylori, and eradication therapy is the first‐line treatment for localized disease with H pylori infection. However, there were several reports showing effectiveness of eradication therapy in even H pylori negative cases. Gastric MALT lymphomas are endoscopically classified into three common types: superficial, ulcerative, and elevated types. For the past 20 years, we have encountered 200 cases of localized gastric MALT lymphoma. Among them, only 4 cases (2%) showed similar macroscopic findings to those of nodular gastritis (gastric MALT lymphoma with nodular gastritis‐like appearance; M‐NGA). Here, we compared clinicopathological characteristics and prevalence of non‐H pylori Helicobacter (NHPH) infection between M‐NGA and other common types of gastric MALT lymphoma. To examine the prevalence of NHPH infection, DNA was extracted from formalin‐fixed paraffin‐embedded biopsy tissues from four cases of M‐NGA, 20 cases of common endoscopic types of gastric MALT lymphoma, and 10 cases of nodular gastritis. We used a highly sensitive polymerase chain reaction assay to detect the presence of five species of NHPH (Helicobacter suis, H felis, H bizzozeronii, H salomonis, and H heilmannii). H suis infection was detected in 4, 2, and 0 of the 4, 20, and 10 cases of M‐NGA, other types of gastric MALT lymphoma, and nodular gastritis, respectively. Other NHPH species were not detected in any cases. Complete response rate by eradication therapy was 4/4 in M‐NGA cases. Therefore, nodular gastritis‐like MALT lymphoma, which shows a very rare phenotype, is closely associated with NHPH infection, and eradication therapy may be the first‐choice treatment.
Keywords: eradication therapy, Helicobacter suis, mucosa‐associated lymphoid tissue, nodular gastritis, non‐H pylori Helicobacter (NHPH)
1. INTRODUCTION
Helicobacter pylori (H pylori) is a major cause of atrophic gastritis and is also involved in the pathogenesis of several gastrointestinal diseases, including gastric ulcer, gastric adenocarcinoma, and gastric mucosa‐associated lymphoid tissue (MALT) lymphoma.1 Recently, non‐H pylori Helicobacters (NHPHs; also referred to as H heilmannii‐like organisms or H heilmannii sensu lato) have been found to be associated with a range of gastric disorders, especially MALT lymphoma.2, 3 Five NHPH species (H suis, H felis, H bizzozeronii, H salomonis, and H heilmannii) are known to infect humans and cause gastritis.3 While NHPH infection is very common in dogs, cats, pigs, and nonhuman primates,4, 5 its prevalence in humans is much lower than that of H pylori, ranging from 0.1% to 6%.6, 7, 8, 9, 10, 11, 12, 13
Nodular gastritis, a form of chronic gastritis referred to as “gooseflesh‐like gastritis,” is frequently associated with follicular lymphoid hyperplasia with intraepithelial lymphocytosis.14 The term “nodular” is not included in the Sydney classification because it is not yet widely accepted by pathologists worldwide. Endoscopically, gastric MALT lymphomas are usually classified into three types: superficial, ulcerative, or elevated types. Gastric MALT lymphoma with nodular gastritis‐like appearance (M‐NGA) is very rare.15 In the present study, we examined the prevalence of NHPH infection and its association with macroscopic features of gastric MALT lymphoma.
2. PATIENTS AND METHODS
2.1. Patients
From 1996 to 2015, 200 patients (79 men and 121 women; median age: 62 years) were diagnosed with gastric MALT lymphoma and followed up at the Hiroshima University Hospital after treatment. We macroscopically classified gastric MALT lymphoma into three types: superficial, elevated, and ulceration types (collectively referred to as the common type MALT lymphoma in this study). Of the 200 cases, 196 were common type MALT lymphoma, and the remaining four cases exhibited a distinctive appearance with a nodular elevation diffusely extending from the antrum to the corpus. This appearance is very similar to that of nodular gastritis. Therefore, the four cases were classified as M‐NGA. In this study, we randomly selected 20 cases from the 196 cases of common type MALT lymphoma and then compared clinicopathological data and status of NHPH infection between common type MALT lymphoma and M‐NGA. The cases were examined for H pylori infection and the presence of the API2‐MALT1 chimeric transcript. H pylori infection was evaluated serologically by anti‐H pylori IgG antibody and by the urea breath test. In the more recent cases, polymerase chain reaction (PCR) was performed to assess IGH gene rearrangements using Invivoscribe IGH chain clonality assay (Invivoscribe Technologies, San Diego, CA) according to the manufacturer's instructions. The presence of gastric mucosal atrophy was determined by endoscopic examination based on the criteria of Kimura and Takemoto as previously described.16 All patients were endoscopically followed every 6 months. MALT lymphoma was determined to exhibit complete response (CR) when the absence of lymphoma was confirmed both macroscopically and pathologically. The characteristics, treatments, and outcomes of the patients were analyzed retrospectively.
To eradicate Helicobacter species, patients with gastric MALT lymphoma received a 1‐week course of orally administered lansoprazole (30 mg/day), amoxicillin (750 mg/day), and clarithromycin (400 mg/day). A second endoscopy was performed at 3‐12 months after completion of the antimicrobial treatment. These studies were conducted in accordance with the Declaration of Helsinki and were approved by the Institutional Review Board of the Hiroshima University Hospital. Informed consent was obtained from all patients.
2.2. DNA extraction and gastric Helicobacter species‐specific PCR assay
Paraffin block sections (10 × 10 μm) were collected in microtubes, and DNA was extracted using the Gene Read DNA FFPE Kit (Qiagen Japan, Tokyo, Japan) following the manufacturer's instructions. PCR amplification of the urease gene from NHPH species (H suis, H bizzozeronii, H felis, H salomonis, and H heilmannii ss) was performed using species‐specific primers according to a previous report (Table 1).17 PCR reactions were conducted using the buffer and DNA polymerase from KOD FX Neo (TOYOBO, Osaka, Japan) according to the manufacturer's instructions. The PCR amplification of H suis, H bizzozeronii, H felis, and H heilmannii was performed under the following conditions: 5 minutes of preincubation at 95°C, followed by 40 cycles of 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C. A final extension was performed for 7 minutes at 72°C H salomonis amplification was performed with 5 minutes of preincubation at 95°C, followed by 40 cycles of 30 seconds at 94°C, 30 seconds at 62°C, and 30 seconds at 72°C. A final extension was performed for 7 minutes at 72°C as previously reported.17 To confirm the specificity of NHPH species‐specific PCR assays, approximately 30% of the positive PCR products was randomly selected for sequencing using both forward and reverse primers. Nucleotide sequences were analyzed using the BLAST tool on the website of the DNA Data Bank of Japan (http://www.ddbj.nig.ac.jp/index-j.html).
Table 1.
Primers for detection of non‐H pylori Helicobacter
| Target gene | Target species | Primer sequences (F: forward, R: reverse) | Annealing temperature (℃) | Cycle number | Fragment size (bp) |
|---|---|---|---|---|---|
| ureA | H suis | F: CACCACCCCGGGGAAGTGATCTTG | 60 | 40 | 253 |
| R: CTACATCAATCAAATGCACGGTTTTTTCTTCG | |||||
| ureA | H bizzozeronii | F: CGCTTTGAACCCGGTGAGAAAA | 60 | 40 | 172 |
| R: TATCGCAACCGCAATTCACAACA | |||||
| ureB | H felis | F: TCCCACTACCGGGGATCGTG | 60 | 40 | 350 |
| R: CAGCGGTTACAATCAAGCCCTCA | |||||
| ureAB | H salomonis | F: CTTTGGGTCTGTGCCTGCCTG | 62 | 40 | 219 |
| R: CATCGCGGATAGTCTTACCGCCT | |||||
| ureA | H heilmannii ss | F: CTTTCTCCTGGTGAAGTGATTCTC | 60 | 40 | 368 |
| R: CAGTTGATGGTGCCAAAG |
2.3. Statistical analysis
Between‐group differences were evaluated using Student's t‐test for quantitative data and chi‐squared test for categorical data. Yates' correction or Fisher's exact test was used as required. All tests were two‐sided, and a P value < 0.05 was considered statistically significant. For multiple comparisons, one‐way analysis of variance (one‐way ANOVA) and the Bonferroni post hoc test were used appropriately. All analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan).18
3. RESULTS
3.1. Case reports of M‐NGA
Clinicopathological characteristics of the four cases of M‐NGA are shown in Table 2 (case A1‐A4). All four patients were males, with an average age of 40 years. Representative endoscopic and pathological images and PCR assay results for case A1 are shown in Figure 1. Endoscopically, the gastric mucosa exhibited a coarse, granular appearance in the antrum, angles, and lower corpus (Figure 1A,B). Indigo carmine was used to enhance and image the nodules (Figure 1C,D). Pathological findings compatible with MALT lymphoma were detected in biopsy tissues taken from areas indicated with yellow arrows in Figure 1C,D. Nodular size was larger and more heterogeneous as compared to that of typical nodular gastritis. Histologically, a diffuse distribution of small lymphocytes with slightly irregular nuclei was observed (Figure 1I). Lymphoid cells proliferated diffusely and formed nodules of various sizes with follicular hyperplasia (Figure 1I). The atypical lymphoid cells had invaded the epithelium, resulting in the destruction of mucosal glands and formation of lymphoepithelial lesions (Figure 1J). Neoplastic cells were positive for CD20 (Figure 1K), negative for CD3 (Figure 1L), and positive for CD79a (Figure 1M). H suis infection was confirmed by PCR (Figure 1N). The endoscopic and pathological images of the other three cases (A2, A3, and A4) are shown in Figures 2, 3, 4. Endoscopic and pathological features were similar in all four cases of M‐NGA (Figures 1A‐D,I‐M; 2A‐D,I‐M; 3A‐D,I‐M; and 4A‐D,H‐L). IGH rearrangement was evaluated in case A4, and monoclonal IgH rearrangement was confirmed (Figure 4G). Serum anti‐H pylori IgG antibody and urea breath tests were only positive in case A3. The other three cases were negative for H pylori infection. Although H suis‐specific PCR band was detected in all cases, pathological diagnosis was very difficult. An NHPH with a straight appearance, corkscrew‐shaped spirals, and large size was pathologically observed in proper gastric gland regions with hematoxylin and eosin staining only in case 2. Three months after eradication therapy with lansoprazole, amoxicillin, and clarithromycin, we confirmed that H suis infection had disappeared by PCR. Moreover, at 3 months after eradication therapy, gastroscopy showed that the coarse, granular appearance and enlarged mucosal folds were no longer present, and multiple heterogeneous whitish spots were observed in all four cases (Figures 1E‐H, 2E‐G, 3E‐G, and 4E,F). CR was confirmed pathologically.
Table 2.
Cases of gastric MALT lymphoma with nodular gastritis‐like appearance
| No. | Age (y) | Sex | HP | API2‐MALT1 | Treatment | Eradication regimen | Outcome | Gastric mucosa atrophy | Bacterial species (PCR) | NHPH diagnosis (histological) | NHPH after eradication | Authors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 36 | M | N | N | Eradication | LAC | CR | (‐) | H suis | N | N | Current study |
| A2 | 37 | M | N | N | Eradication | LAC | CR | (‐) | H suis | P | N | |
| A3 | 42 | M | P | N | Eradication | LAC | CR | C‐2 | H suis | N | N | |
| A4 | 45 | M | N | N | Eradication | LAC | CR | (‐) | H suis | N | N | |
| B1 | ND | ND | N | ND | Eradication | LAC | CR | ND | ND | P | N | Okiyama et al10 |
| B2 | ND | ND | N | ND | Eradication | LAC | CR | ND | ND | P | N | Okiyama et al10 |
Abbreviations: CR, complete response; F, female; HP, H pylori infection; LAC, lansoprazole, amoxicillin, clarithromycin; M, male; MALT, mucosa‐associated lymphoid tissue; N, negative; ND, not described; NHPH, non‐H pylori Helicobacter; P, positive.
Figure 1.
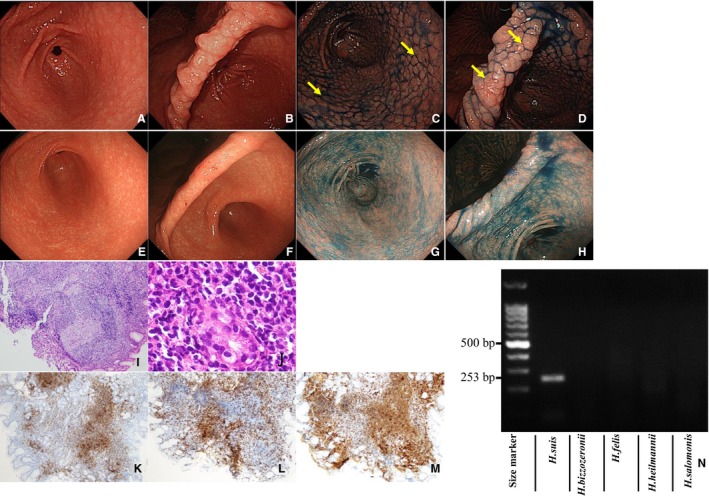
Characterization of case A1. Endoscopic examination (before eradication): Multiple heterogeneous nodules similar to nodular gastritis in antrum (A) and anglus (B). Indigo carmine dye makes the nodules more obvious in the antrum (C) and anglus (D). Endoscopic examination (after eradication): no nodules in the antrum (E) or anglus (F), even with indigo carmine dye in the antrum (G) and anglus (H). Pathological examination (before eradication): Lymphoma cells proliferate diffusely and form heterogeneous nodules with lymphoid hyperplasia. HE, ×100 (I). Atypical lymphoid cells invading the epithelium, resulting in the destruction of mucosal glands and formation of lymphoepithelial lesions. HE, ×400 (J). Immunohistochemistry (before eradication): Neoplastic cells are positive for CD20 (K), negative for CD3 (L), and positive for CD79a (M). PCR assay (before eradication): Positive for Helicobacter suis (253 bp); negative for other non‐H pylori Helicobacter (N). Biopsies taken from areas indicated with yellow arrows showed pathological findings compatible with mucosa‐associated lymphoid tissue lymphoma. Abbreviation: HE, hematoxylin and eosin
Figure 2.
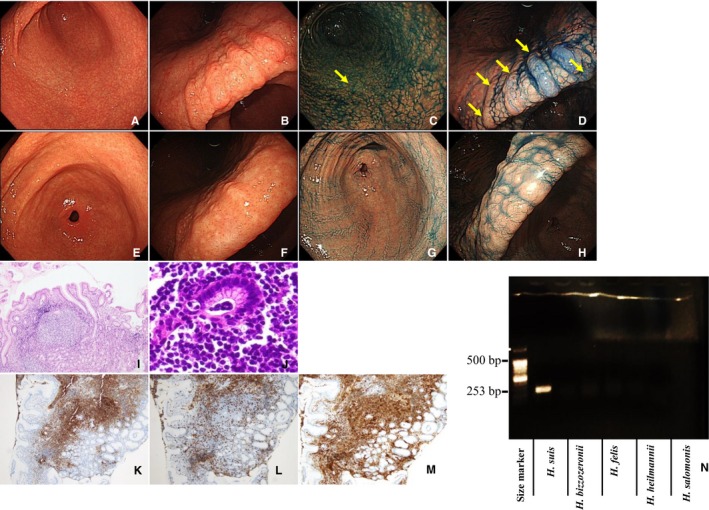
Characterization of case A2. Endoscopic examination (before eradication): Multiple heterogeneous nodules similar to nodular gastritis in antrum (A) and anglus (B). Indigo carmine dye makes the nodules more obvious in the antrum (C) and anglus (D). Endoscopic examination (after eradication): no nodules in the antrum (E) or anglus (F), even with indigo carmine dye in the antrum (G) and anglus (H). Pathological examination (before eradication): lymphoma cells proliferate diffusely and form heterogeneous nodules with lymphoid hyperplasia. HE, ×100 (I). Atypical lymphoid cells invading the epithelium, resulting in the destruction of mucosal glands and formation of lymphoepithelial lesions. HE, ×400 (J). Immunohistochemistry (before eradication): Neoplastic cells are positive for CD20 (K), negative for CD3 (L), and positive for CD79a (M). PCR assay (before eradication): Positive for Helicobacter suis (253 bp); negative for other non‐H pylori Helicobacter (N). Biopsies taken from areas indicated with yellow arrows showed pathological findings compatible with mucosa‐associated lymphoid tissue lymphoma. Abbreviation: HE, hematoxylin and eosin
Figure 3.
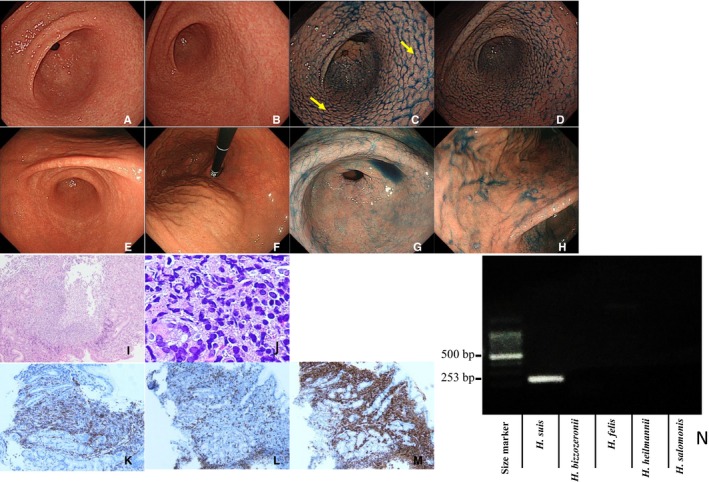
Characterization of case A3. Endoscopic examination (before eradication): Multiple heterogeneous nodules similar to nodular gastritis in antrum (A) and anglus (B). Indigo carmine dye makes the nodules more obvious in the antrum (C) and anglus (D). Endoscopic examination (after eradication): no nodules in the antrum (E) or anglus (F), even with indigo carmine dye in the antrum (G) and anglus (H). Pathological examination (before eradication): lymphoma cells proliferate diffusely and form heterogeneous nodules with lymphoid hyperplasia. HE, ×100 (I). Atypical lymphoid cells invading the epithelium, resulting in the destruction of mucosal glands and formation of lymphoepithelial lesions. HE, ×400 (J). Immunohistochemistry (before eradication): Neoplastic cells are positive for CD20 (K), negative for CD3 (L), and positive for CD79a (M). PCR assay (before eradication): Positive for Helicobacter suis (253 bp); negative for other non‐H pylori Helicobacter (N). Biopsies taken from areas indicated with yellow arrows showed pathological findings compatible with mucosa‐associated lymphoid tissue lymphoma. Abbreviation: HE, hematoxylin and eosin
Figure 4.
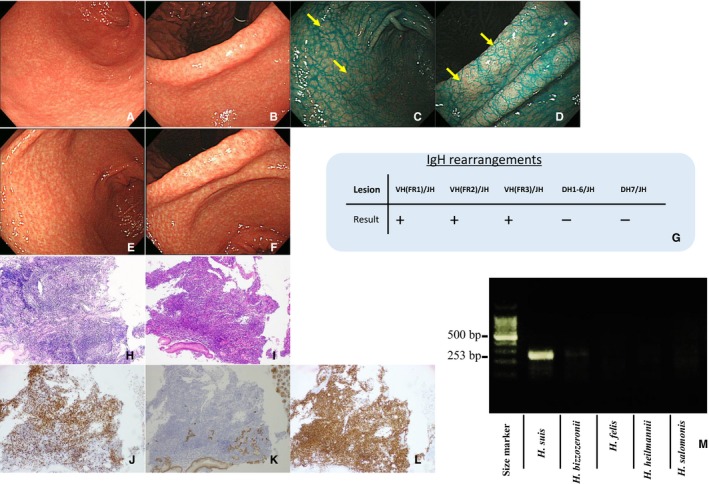
Characterization of case A4. Endoscopic examination (before eradication): Multiple heterogeneous nodules similar to nodular gastritis in antrum (A) and anglus (B). Indigo carmine dye makes the nodules more obvious in the antrum (C) and anglus (D). Endoscopic examination (after eradication): No nodules in the antrum (E) or anglus (F). Status of monoclonal IgH rearrangement (G). Pathological examination (before eradication): Lymphoma cells proliferate diffusely and form heterogeneous nodules with lymphoid hyperplasia. HE, ×100 (H). Atypical lymphoid cells invading the epithelium, resulting in the destruction of mucosal glands and formation of lymphoepithelial lesions. HE, ×400 (I). Immunohistochemistry (before eradication): Neoplastic cells are positive for CD20 (J), negative for CD3 (K), and positive for CD79a (L). PCR assay (before eradication): Positive for Helicobacter suis (253 bp); negative for other non‐H pylori Helicobacter (M). Biopsies taken from areas indicated with yellow arrows showed pathological findings compatible with mucosa‐associated lymphoid tissue lymphoma. Abbreviation: HE, hematoxylin and eosin
3.2. Prevalence of NHPH infection in M‐NGA
To compare the prevalence of NHPH infection, we utilized 20 cases of common type gastric MALT lymphoma for which the clinical course had been followed for more than 2 years. Notably, all four cases of M‐NGA were infected with H suis, which is one of the NHPH; however, H pylori infection was detected in only one case (case A3). In common type MALT lymphoma, the prevalence of H pylori infection was 75% and that of NHPH was 10%, indicating that the prevalence rate of NHPH infection was significantly higher in M‐NGA than in common type MALT lymphoma (Table 3). In two cases of common type MALT lymphoma, H suis infection was detected, both of which had superficial type MALT lymphoma. No characteristic findings such as nodules were found in the areas of MALT lymphoma in either case (Figure 5E‐H). However, in case A6, an endoscopic finding similar to nodular gastritis was observed in the antrum area (Figure 5E,5). MALT lymphoma was pathologically detected only in superficial lesions observed in the gastric body, not in this antrum lesion.
Table 3.
Clinicopathological features of M‐NGA, common type gastric MALT lymphoma, and nodular gastritis
| M‐NGA (n = 4) |
Common type (n = 20) |
Nodular gastritis (n = 10) |
P‐value |
|
|---|---|---|---|---|
| Age: average ± SD | 40 ± 3.6 | 64 ± 12.6 | 38 ± 13 |
0.0012*
0.00011* 0.7427 |
| Sex: male/female | 4/0 (100%) | 10/10 (50%) | 7/3 (70%) |
0.064 0.2974 0.22 |
| API2‐MALT1 chimeric transcript: positive/negative | 0/4 (0%) | 1/19 (5%) | ND |
0.6478 ND ND |
| HP infection: positive/negative | 1/3 (25%) | 15/5 (75%) | 10/0 (100%) |
0.053 0.083 0.002* |
| NHPH infection: positive/negative | 4/0 (100%) | 2/18 (10%) | 0/10 (10%) |
0.0001*
0.30 0.0002* |
| Effect of eradication therapy: CR/NC | 4/0 (100%) | 13/7 (65%) | 10/0 (100%) |
0.16 0.033 1 |
Abbreviations: CR, complete response; HP, Helicobacter pylori; M‐NGA, gastric MALT lymphoma with nodular gastritis‐like appearance; MALT, mucosa‐associated lymphoid tissue; NC, no change; ND, no data; NHPH, non‐H pylori Helicobacter.
Statistically significant (significance level = 0.05/3).
Figure 5.
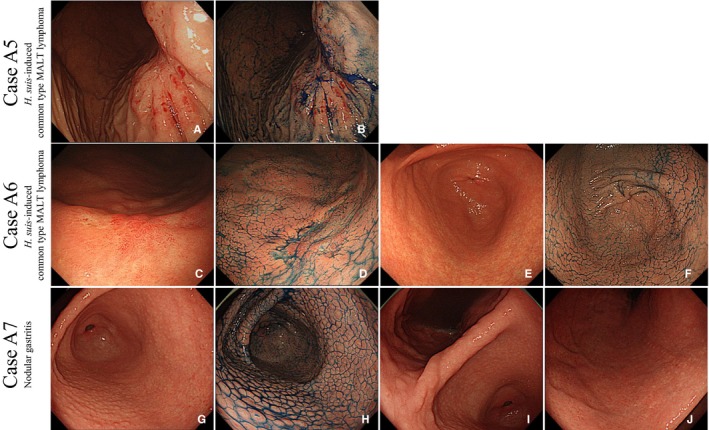
Characterization of cases A5, A6, and A7. Cases A5 and A6: Endoscopic pictures of common type MALT lymphoma with Helicobacter suis infection. In case A5, the superficial phenotype of gastric MALT lymphoma was observed in the gastric body under white light (A) and chromoendoscopy (B). In case A6, the superficial phenotype was observed in the gastric body under white light (C) and chromoendoscopy (D). Mild nodular gastritis without MALT lymphoma was observed in the gastric antrum under white light (E) and chromoendoscopy (F). Case A7: Representative endoscopic pictures of nodular gastritis. Endoscopic examination (before eradication): Multiple homogeneous nodules are seen in the antrum. Whitish spots exist on the top of each nodule (G). No nodule is observed in the gastric anglus (H). Mild atrophy is observed in the gastric body (I). Indigo carmine dye makes the nodules more obvious in the antrum (J). Abbreviation: MALT, mucosa‐associated lymphoid tissue
Ten cases of nodular gastritis were also examined, all of which were positive for H pylori infection but negative for NHPH infection. Among these three groups, sex and efficacy of eradication therapy were not significantly different. Average age was significantly lower in M‐NGA and nodular gastritis groups than in the common type MALT lymphoma group. H pylori infection rate was significantly higher in the nodular gastritis group than in the M‐NGA group. Remarkably, infection rate of NHPH was significantly higher in M‐NGA group than in the other two groups.
4. DISCUSSION
In this paper, we reported four cases of M‐NGA, focusing on the relationship between M‐NGA and NHPH infection. According to previous reports, NHPH species are more likely to induce MALT lymphoma than other Helicobacter species.19, 20, 21 There are several methods to detect NHPHs, including culture, immunohistochemistry, rapid urease test, urea breath test, serum antibody, and stool antigen; however, their low sensitivity limits their application for diagnosis.22, 23, 24 Genetic diagnosis using PCR is the most effective method for diagnosing NHPH infections.25 In all of our M‐NGA cases, infection of H suis, one of the NHPH species, was detected by PCR analysis. Among them, one case exhibited double infection with H pylori and H suis, while the other three cases were negative for H pylori infection.
It has been reported that H suis can result in benign phenotypes, such as gastritis or gastric ulcers, and that it causes relatively mild changes compared with those of H pylori infection.25, 26, 27, 28 In terms of the general histological features of H suis, it has been reported that H suis infection causes lymphoid hyperplasia, epithelial hyperplasia, and parietal cell necrosis.25, 26, 27, 28 H suis infection has been reported to induce lymphoid hyperplasia in animal experiments; this lymphoid hyperplasia occurs in the surface mucosa but not the deep mucosa, and these changes are known to be observed mainly in the L region of the stomach.28, 29 H suis is also reported to cause epithelial cell hyperproliferation.30 Furthermore, NHPHs have induced mucosal hypertrophy/nodular hyperplasia in experimental models.31 In general, the more inflammation present in the mucosa, the stronger the mononuclear cell infiltration and neutrophil infiltration observed. We speculate that these phenomena could cause an endoscopic nodular gastritis‐like appearance and that hyperplastic stimulation could subsequently cause malignant lymphoma. Unlike H pylori, the duration of H suis infection could be transient.32 Thus, NHPHs may induce endoscopic changes in a relatively short period of time. In contrast, the prevalence of NHPH infection in the common type gastric MALT lymphoma was significantly lower than that in M‐NGA (4/4, 100% vs 2/18, 16%) (Table 3). We observed two cases (cases A5 and A6) of H suis‐induced common type MALT lymphoma. In both cases, the phenotype of MALT lymphoma was the superficial type (Figure 5A‐D), and while nodular gastritis was observed in the antrum of one case (Figure 5E,5), MALT lymphoma was not detected in the lesion. From these cases, it can be inferred that H suis infection does not necessarily exhibit a nodular gastritis‐like appearance. In nodular gastritis, all cases were infected with H pylori, but not NHPH.
We also summarized the clinicopathological characteristics of six cases of M‐NGA (Table 2), which include three cases reported previously (B1‐B2)10 and our four identified cases (A1‐A4). The median age of all patients was 40 years. All six cases had NHPH infection, but only one patient was co‐infected with H pylori. In all six cases, NHPH infection was eradicated, and MALT lymphoma cells completely disappeared following eradication therapy comprising PPI + clarithromycin + amoxicillin for 1 week. Atrophic gastritis was observed in only one case with H pylori and H suis infections (case A3). In the other M‐NGA lymphoma cases, endoscopic and pathologic examinations did not reveal any atrophic change in the gastric mucosa. This is consistent with previous reports that showed that NHPH is less likely to cause atrophic gastritis.25, 32, 33
We also compared endoscopic findings between nodular gastritis and M‐NGA. Endoscopically, nodular gastritis shows diffuse and homogenous miliary pattern that is localized to the antrum. Typical endoscopic pictures are shown in Figure 5A‐D (case A5). Homogeneous nodules having whitish spots on the top were observed in the gastric antrum (Figure 5A,B), but not in the gastric anglus (Figure 5C). Mild atrophy was observed in the gastric body (Figure 5D) different from the observation in the M‐NGA cases. In contrast, M‐NGA nodules were larger and more heterogeneous and extended to the corpus of the stomach. Atrophic change in the gastric mucosa was detected in nodular gastritis but not in M‐NGA. It is of considerable interest to know the lateral spread of MALT lymphoma in the endoscopic images, as it is possible that nodular gastritis is the precursor of MALT lymphoma. In all four cases of M‐NGA, biopsies were taken from the area where nodules were observed (Figure 1C,D, 2C,D, 3C, and 4C,D). All tissues taken from the areas showed pathological findings compatible with MALT lymphoma.
In conclusion, we assessed four cases of M‐NGA, a distinct type of gastric MALT lymphoma. M‐NGA is closely associated with NHPH infection. Both NHPH infection and MALT lymphoma cells were completely eradicated by eradication therapy. Therefore, NHPH infection may be one of the causes of the pathogenesis of M‐NGA.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
ACKNOWLEDGMENTS
This work was carried out with the kind cooperation of the Analysis Center of Life Science, Natural Science Center for Basic Research and Development, Hiroshima University.
Takigawa H, Masaki S, Naito T, et al. Helicobacter suis infection is associated with nodular gastritis‐like appearance of gastric mucosa‐associated lymphoid tissue lymphoma. Cancer Med. 2019;8:4370–4379. 10.1002/cam4.2314
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Santos IS, Boccio J, Santos AS, et al. Prevalence of Helicobacter pylori infection and associated factors among adults in Southern Brazil: a population‐based cross‐sectional study. BMC Public Health. 2005;5:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baele M, Pasmans F, Flahou B, Chiers K, Ducatelle R, Haesebrouck F. Non‐Helicobacter pylori Helicobacters detected in the stomach of humans comprise several naturally occurring Helicobacter species in animals. FEMS Immunol Med Microbiol. 2009;55:306‐313. [DOI] [PubMed] [Google Scholar]
- 3. Haesebrouck F, Pasmans F, Flahou B, et al. Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin Microbiol Rev. 2009;22:202‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neiger R, Dieterich C, Burnens A, et al. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Queiroz DM, Rocha GA, Mendes EN, De Moura SB, De Oliveira AM, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996;111:19‐27. [DOI] [PubMed] [Google Scholar]
- 6. Heilmann KL, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991;32:137‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hilzenrat N, Lamoureux E, Weintrub I, Alpert E, Lichter M, Alpert L. Helicobacter heilmannii‐like spiral bacteria in gastric mucosal biopsies. Prevalence and clinical significance. Arch Pathol Lab Med. 1995;119:1149‐1153. [PubMed] [Google Scholar]
- 8. Ierardi E, Monno RA, Gentile A, et al. Helicobacter heilmannii gastritis: a histological and immunohistochemical trait. J Clin Pathol. 2001;54:774‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jhala D, Jhala N, Lechago J, Haber M. Helicobacter heilmannii gastritis: association with acid peptic diseases and comparison with Helicobacter pylori gastritis. Mod Pathol. 1999;12:534‐538. [PubMed] [Google Scholar]
- 10. Okiyama Y, Matsuzawa K, Hidaka E, Sano K, Akamatsu T, Ota H. Helicobacter heilmannii infection: clinical, endoscopic and histopathological features in Japanese patients. Pathol Int. 2005;55:398‐404. [DOI] [PubMed] [Google Scholar]
- 11. Debongnie JC, Donnay M, Mairesse J. Helicobacter pylori: 200 patients with a follow‐up of 5 years. An observation endoscopic study. Acta Gastroenterol Belg. 1995;58:35‐42. [PubMed] [Google Scholar]
- 12. Chen Y, Zhou D, Wang J. Biological diagnostic and therapeutic study on the infection of Helicobacter heilmannii . Zhonghua Yi Xue Za Zhi. 1998;78:490‐493. [PubMed] [Google Scholar]
- 13. Yali Z, Yamada N, Wen M, Matsuhisa T, Miki M. Gastrospirillum hominis and Helicobacter pylori infection in Thai individuals: comparison of histopathological changes of gastric mucosa. Pathol Int. 1998;48:507‐511. [DOI] [PubMed] [Google Scholar]
- 14. Miyamoto M, Haruma K, Yoshihara M, et al. Nodular gastritis in adults is caused by Helicobacter pylori infection. Dig Dis Sci. 2003;48:968‐975. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura S, Sugiyama T, Matsumoto T, et al. Long‐term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow‐up study of 420 patients in Japan. Gut. 2012;61:507‐513. [DOI] [PubMed] [Google Scholar]
- 16. Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87‐97. [Google Scholar]
- 17. Liu J, He L, Haesebrouck F, et al. Prevalence of coinfection with gastric non‐Helicobacter pylori Helicobacter (NHPH) species in Helicobacter pylori‐infected patients suffering from gastric disease in Beijing, China. Helicobacter. 2015;20:284‐290. [DOI] [PubMed] [Google Scholar]
- 18. Kanda Y. Investigation of the freely available easy‐to‐use software “EZR” for medical statistics. Bone Marrow Transpl. 2012;48:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joo M, Kwak JE, Chang SH, et al. Helicobacter heilmannii‐associated gastritis: clinicopathologic findings and comparison with Helicobacter pylori‐associated gastritis. J Korean Med Sci. 2007;22:63‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morgner A, Lehn N, Andersen LP, et al. Helicobacter heilmannii‐associated primary gastric low‐grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821‐828. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura M, Murayama SY, Serizawa H, et al. "Candidatus Helicobacter heilmannii" from a cynomolgus monkey induces gastric mucosa‐associated lymphoid tissue lymphomas in C57BL/6 mice. Infect Immun. 2007;75:1214‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wuppenhorst N, von Loewenich F, Hobmaier B, Vetter‐Knoll M, Mohadjer S, Kist M. Culture of a Gastric non‐Helicobacter pylori Helicobacter from the stomach of a 14‐year‐old girl. Helicobacter. 2013;1:4370‐5. [DOI] [PubMed] [Google Scholar]
- 23. Andersen LP, Norgaard A, Holck S, Blom J, Elsborg L. Isolation of a "Helicobacter heilmanii"‐like organism from the human stomach. Eur J Clin Microbiol. 1996;15:95‐96. [DOI] [PubMed] [Google Scholar]
- 24. Kivisto R, Linros J, Rossi M, Rautelin H, Hanninen ML. Characterization of multiple Helicobacter bizzozeronii isolates from a finnish patient with severe dyspeptic symptoms and chronic active gastritis. Helicobacter. 2010;15:58‐66. [DOI] [PubMed] [Google Scholar]
- 25. Goji S, Tamura Y, Sasaki M, et al. Helicobacter suis‐infected nodular gastritis and a review of diagnostic sensitivity for Helicobacter heilmannii‐like organisms. Case Rep Gastroenterol. 2015;9:179‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hellemans A, Chiers K, Decostere A, De Bock M, Haesebrouck F, Ducatelle R. Experimental infection of pigs with “Candidatus Helicobacter suis.” Vet Res Commun. 2007;31:385‐395. [DOI] [PubMed] [Google Scholar]
- 27. De Witte C, Devriendt B, Flahou B, et al. Helicobacter suis induces changes in gastric inflammation and acid secretion markers in pigs of different ages. Vet Res. 2017;48:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Bruyne E, Flahou B, Chiers K, et al. An experimental Helicobacter suis infection causes gastritis and reduced daily weight gain in pigs. Vet Microbiol. 2012;160:449‐454. [DOI] [PubMed] [Google Scholar]
- 29. Simpson KW, Strauss‐Ayali D, Scanziani E, et al. Helicobacter felis infection is associated with lymphoid follicular hyperplasia and mild gastritis but normal gastric secretory function in cats. Infect Immun. 2000;68:779‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flahou B, Haesebrouck F, Pasmans F, et al. Helicobacter suis causes severe gastric pathology in mouse and mongolian gerbil models of human gastric disease. PLoS ONE. 2010;5:e14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterson RA, Danon SJ, Eaton KA. Comparison of gastritis and gastric epithelial proliferation in Helicobacter heilmannii‐infected nude and BALB/c mice. Vet Pathol. 2001;38:173‐183. [DOI] [PubMed] [Google Scholar]
- 32. Debongnie JC, Donnay M, Mairesse J. Gastrospirillum hominis ("Helicobacter heilmanii"): a cause of gastritis, sometimes transient, better diagnosed by touch cytology? Am J Gastroenterol. 1995;90:411‐416. [PubMed] [Google Scholar]
- 33. Baele M, Decostere A, Vandamme P, et al. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol. 2008;58(Pt 6):1350‐1358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


