Abstract
Aims
This study aimed to evaluate the prognostic effect of neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) for patients with breast cancer (BC).
Methods
A literature search was performed by searching medical databases. Basic characteristics and prognostic data were extracted from included studies. Primary outcomes, such as overall survival (OS) and disease‐free survival (DFS), were synthesized and compared. Subgroup analyses were performed according to pathology, geographical region, cut‐off value, and tumor progression.
Results
A total of 39 studies comprising 17079 BC patients were included in this meta‐analysis. Among them, 28 studies with 142 64 BC patients investigated predicting role of NLR for OS, showing elevated NLR were associated poor prognosis (hazard ratio [HR]: 1.78, 95% confidence interval [CI]: 1.49‐2.13, P < 0.001). Twenty‐seven studies containing 115 04 patients explored the role of NLR in predicting DFS, showing elevated NLR was associated with poor DFS with HR of 1.60 (95% CI: 1.42‐1.96, P < 0.001). Twelve studies explored the role of PLR in predicting OS, showing patients with higher PLR were associated with a significantly worse prognosis with a pooled HR of 1.32 (95% CI: 1.11‐1.57, P = 0.002). Eleven studies with 5013 patients shown patients with elevated PLR were associated shorter DFS (HR: 1.43, 95% CI: 1.09‐1.86, P = 0.009). Subgroup analyses shown a greater magnitude of association between NLR and OS in triple‐negative BC patients than in HER2‐positive ones.
Conclusions
Our study suggested that elevated NLR and PLR were associated with poor OS as well as high risk of recurrence for BC patients. Subgroup analyses confirmed the prognostic effect of NLR and PLR in HER2‐positive BC patients. As easily accessible parameters, NLR and PLR should be identified as useful biomarkers in the management of BC.
Keywords: breast cancer, meta‐analysis, neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio, prognosis
1. INTRODUCTION
Breast cancer (BC) is the most frequently diagnosed malignance among women and the one of the most common causes of cancer‐related death.1, 2 Clinical therapeutic strategies and prognosis of BC are based on tumor characteristics, patient factors and response to treatment. However, its high heterogeneity results in a broad range of clinical outcomes even among BC patients with similar clinical staging and pathologic grading.3 Tumor microenvironment, inflammation, and immune response have been reported to play important roles in tumor progression and prognosis.4, 5
Recently, substantial evidence shown that inflammation‐based models, such as the systemic immune‐inflammation index, neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR) and inflammation‐based index, were useful indicators for predicting the prognosis of various solid cancers.6, 7 NLR and PLR, two of the most frequently applied indicators, have been widely investigated for their value in predicting prognosis of BC patients. A majority of researches demonstrated elevated peripheral NLR and PLR were recognized as poor prognostic factors.8, 9 Nevertheless, there were still others revealed patients with elevated PLR were associated with better survival outcomes.10
Therefore, this study was conducted to evaluate the prognostic value of NLR and PLR on overall survival (OS) and disease‐free survival (DFS) for BC patients. Moreover, by performing subgroup analyses, we quantified the effect of NLR and PLR in different subgroups.
2. METHODS
2.1. Search strategy
A comprehensive literature search of relevant studies was performed through the online medical databases PubMed, Embase, Web of Science, the Cochrane Library and Scopus. Studies focused on the correlation of BC and NLR as well as PLR were taken into retrievement. Search terms were confined to the following main words and Medical Subject Headings terms: “neutrophil”, “platelet”, “lymphocyte”, “neutrophil‐to‐lymphocyte”, “platelet‐to‐lymphocyte”, “breast cancer”, and “breast carcinoma”. Moreover, by using cross‐references from the references of primary selected studies and relevant studies, a backward search was also performed to ensure a comprehensive search. Literatures were restricted to those written in English. There was no restriction on geographical region. Two reviewers (Wanying Guo and Xin Lu) completed the electronic search independently.
2.2. Study inclusion and exclusion criteria
Reviewers screened the eligible studies based upon inclusion and exclusion criteria which were prespecified. The final decision on inclusion and exclusion criteria were approved by all the authors. Any disagreements between the two reviewers were solved and made final decision by the senior reviewer (Miao Deng).
The criteria for inclusion were as following:
Articles analyzed BC patients.
Articles evaluated prognostic value of NLR or PLR.
Articles assessed the OS or DFS of BC patients.
The criteria for exclusion were as following:
Articles not focused on the prognosis of BC patients.
Articles concerned on neither pretreatment NLR nor PLR.
Reviews or editorials.
Case reports or conference abstracts.
Articles without data of interest (OS or DFS).
Articles with duplicate sample set, such as those published by same authors or departments, were picked out for further screening. In this case, only the articles with largest sample size or those published most recently were finally included in the present meta‐analysis. However, for those studies analyzed two or more independent sample sets such as training and validation cohorts, the cohorts were enrolled in this study and analyzed independently.
2.3. Data management and statistical analysis
The software of Endnote (version X8) was used for preliminary screening and sorting. Data were extracted from the enrolled literatures by two authors (Wanying Guo and Xin Lu) after reading full text intensively. The baseline information included full list of authors as well as affiliations, year of publication, geographical region, research centers, models of use, sample size, cut‐off values for NLR and PLR, mean or median ages, proportion of triple negative patients, indications for surgical treatment, follow‐up time, and treatment strategies. The hazard ratios (HRs) with 95% confidential intervals (CIs) were directly extracted from the tables or texts. However, in several studies, the HRs and 95% CIs were not shown directly. In such cases, the software of Engauge Digitizer (version 4.1) was used to extract HRs and 95% CIs by computing the Kaplan‐Meier graph.11, 12 The primary outcomes were pooled using the Cochrane Collaboration's Review Manager (version 5.3, Cochrane Collaboration, Oxford, UK).13 Random effect model was applied routinely only if no obvious heterogeneity was observed among the included literatures (I 2 < 40%). Heterogeneity within studies was explored by using the chi‐square test with a P value of 0.10 for significance. Moreover, the heterogeneities were quantified using the I 2 statistics. Sensitivity analyses of main outcomes were conducted by using the software of Stata (version 12.0).14 The publication bias was investigated using funnel plots. Moreover, the symmetry properties of funnel plot was examined by using Begg and Egger tests.15
2.4. Risk of bias assessment
All the included studies were critically assessed for methodological quality by 2 researchers independently (Lu X and Guo WY) by using the Quality In Prognosis Studies tool. Each study was graded for the following domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. The risk of bias for each domain is graded as low (−), moderate (±), or high (+).
2.5. Subgroup analysis
To identify the sources of heterogeneity, subgroup analyses were conducted according to etiologies, geographical region, cut‐off values, and tumor stage. Subgroup of triple‐negative included BC patients with negatively expressed estrogen receptor, progesterone receptor, and HER2, while subgroup of HER2‐positve enrolled BC patients with positive HER2 expression. Patients from different continents were classified into Asia, America, and Europe subgroups respectively. Patients with early stage and metastatic tumors were analyzed in different subgroups. It was difficult to subgroup analysis which based on therapeutic strategies because of most of BC patients received surgical treatment as well as other treatments like adjuvant chemotherapy, endocrine therapy or trastuzumab therapy.
3. RESULTS
3.1. Characteristics of included studies
Literature research identified 1215 records: 115 from PubMed, 296 from Embase, 313 from Web of Science, 15 from the Cochrane Library, and 436 from Scopus. Meanwhile, 4 studies were identified through references. As shown in Figure 1, after screening titles and abstracts, 362 duplications and an additional 781 studies were excluded. Full text of the remaining 76 studies were read rigorously. Thirty‐seven more were excluded: 7 were published by same centers, 10 enrolled duplicate patients, 6 were conference abstracts and 14 were without survival data. Finally, a total of 39 studies with 17079 patients were included in the present meta‐analysis.8, 9, 10, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Among them, 35 studies with 159 39 BC patients analyzed the effectiveness of NLR and 15 studies with 7949 patients analyzed PLR. The characteristics of the 39 included studies were summarized in Table 1.
Figure 1.
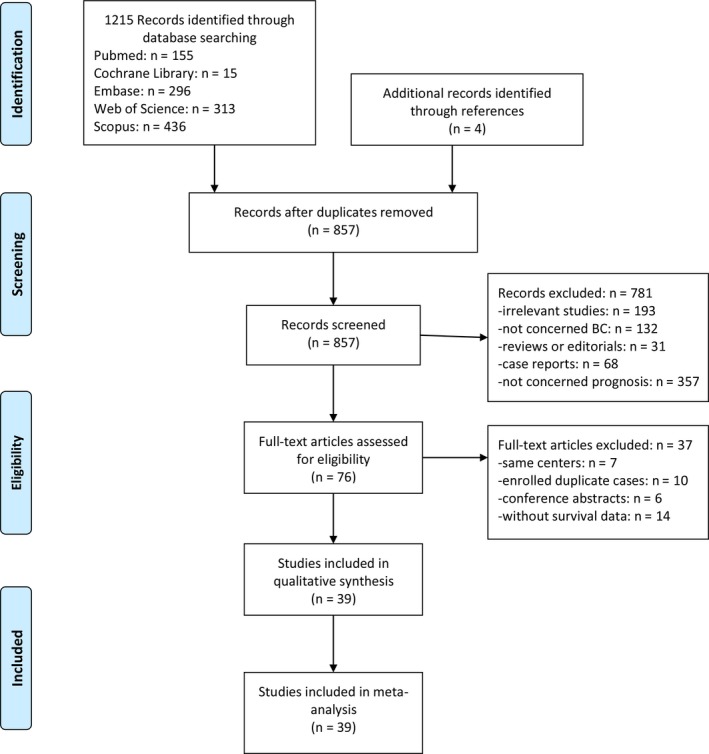
Flow diagram showing study retrieval and selection process.
Table 1.
Characteristics of included studies
| Study | Year | Country | Design, center | Models | Endpoint | HRs | Sample size | Cut‐off | Ages (Years) | Triple negative tumors (%) | Diagnosis | Follow‐up (months) | Therapies |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allan et al | 2016 | Costa Rica | Retrospective, single center | NLR & PLR | OS & DFS | Reported | 172 | NLR: 3; PLR: 250 | 54.2 ± 12.7 | 34 (19.6) | Nonmetastatic breast cancer | 79.3 (2‐90) | Adjuvant chemotherapy |
| Asano et al | 2015 | Japan | Retrospective, single center | NLR | OS & DFS | Reported | 177 | NLR: 3 | ≤56:87;>56:90 | 61 (34.5) | Early stage breast cancer | 40.8 (7.2‐72) | Neoadjuvant chemotherapy |
| Asano et al | 2016 | Japan | Retrospective, single center | PLR | OS & DFS | Reported | 177 | PLR: 150 | ≤56:87;>56:90 | 61 (34.5) | Early stage breast cancer | 37.2 (1.2‐72) | Neoadjuvant chemotherapy + surgery |
| Azab et al | 2013 | USA | Retrospective, single center | NLR & PLR | OS | Reported | 437 | NLR: 3.3; PLR: 185 | 63.6 ± 0.7 | NA | Breast cancer with all stages | Mean: 60 | Surgery or chemotherapy or radiotherapy |
| Blanchette et al | 2018 | Canada | Retrospective, single center | PLR | OS & FFS | Reported | 154 | PLR: 185 | 56 (47‐63) | 0 (0) | HER2‐positive metastatic breast cancer | 34 (6‐124) | Trastuzumab therapy |
| Bozkurt et al | 2015 | Turkey | Retrospective, single center | NLR | OS & DFS | Reported | 85 | NLR: 2 | ≤50:57;>50:28 | 85 (100) | Early stage breast cancer | NA | Surgery + adjuvant chemotherapy |
| Chae et al | 2018 | Korea | Retrospective, single center | NLR | DFS | Reported | 87 | NLR: 1.7 | 45.8 ± 11.2 | 87 (100) | Early stage breast cancer | Median: 57 | Neoadjuvant chemotherapy + surgery |
| Chen et al | 2016 | China | Retrospective, single center | NLR | DFS & CSS | Reported | 215 | NLR: 2.1 | 46.41 ± 9.82 | 18 (8.4) | TNM stage II‐III | 57.6 ± 27.1 | Neoadjuvant chemotherapy + surgery |
| Cho et al | 2018 | Korea | Retrospective, single center | NLR & PLR | DFS & CSS | Reported | 661 | NLR: 1.34; PLR: 185.5 | 52.7 ± 11.5 | 117 (17.7) | All TNM stages | 72 (1‐189) | Surgery |
| Cihan et al | 2014 | Turkey | Retrospective, single center | NLR & PLR | OS & DFS | Reported | 350 | NLR: 3; PLR: 160 | 55.3 ± 0.3 | NA | All TNM stages | 0.3‐112 | Surgery + adjuvant therapies |
| Dirican et al | 2015 | Turkey | Retrospective, single center | NLR | OS & DFS | Reported | 1527 | NLR: 4 | ≤35:92;36‐50:619;>50:816 | 214 (14) | All TNM stages | 30 (2‐75) | Surgery |
| Ferroni et al | 2018 | Italy | Retrospective, Database | NLR | OS & DFS | Reported | 475 | NLR: 2 | 57 ± 13 | 57 (12) | All TNM stages | 45.6 (3.12‐139.2) | Surgery |
| Forget et al | 2014 | Belgium | Retrospective, Database | NLR | OS & DFS | Reported | 425 | NLR: 3.3 | 25‐89 | NA | Resectable breast cancer | 69.8 (53.5‐89.9) | Surgery |
| Gunduz et al | 2015 | Turkey | Retrospective, single center | PLR | DFS | Reported | 62 | PLR: 200 | 52 (24‐73) | NA | Advanced breast cancer | Median: 48.4 | Surgery + adjuvant chemotherapy |
| Hernandez et al | 2017 | Spain | Retrospective, single center | NLR | OS & DFS | Reported | 150 | NLR: 3.3 | 49.8 (28‐77) | 38 (25.3) | TNM stage I‐III | 24 (1‐144) | Neoadjuvant chemotherapy + surgery |
| Hong et al | 2016 | China | Retrospective, single center | NLR | OS & DFS | Reported | 487 | NLR: 1.93 | 55 (28‐89) | 94 (19.2) | Primary invasive breast cancer | Median: 55 | Surgery |
| Iwase et al | 2017 | Japan | Retrospective, single center | NLR | OS | Reported | 89 | NLR: 3 | 50.9 ± 11.3 | 24 (27) | Recurrent breast cancer | NA | Chemotherapy |
| Jia et al | 2015 | China | Retrospective, single center | NLR | OS & DFS | Reported | 1570 | NLR: 2 | 48.9 ± 11.8 | 225 (14.3) | Operable breast cancer | 79 (4‐172) | Surgery |
| Koh et al | 2014 | Korea | Retrospective, single center | NLR | OS & DFS | Reported | 157 | NLR: 2.25 | 44 (24‐71) | 0 (0) | ER/PR(+) and HER2(−) | 21 (1‐108) | Neoadjuvant chemotherapy + surgery |
| Koh et al | 2015 | Malaysia | Retrospective, single center | NLR & PLR | OS& DFS | Reported | 1435 | NLR: 4; PLR: 185 | Median: 52 | 208 (14.5) | All TNM stages | NA | Surgery or chemotherapy or radiotherapy |
| Krenn‐Pilko et al | 2014 | Austria | Retrospective, single center | PLR | OS & DFS | Reported | 747 | PLR: 292 | 57.9 ± 12.2 | NA | Nonmetastatic breast cancer | 98 ± 29.2 | Surgery |
| Krenn‐Pilko et al | 2016 | Austria | Retrospective, single center | NLR | OS & DFS | Reported | 747 | NLR: 3 | 58.1 ± 12.2 | NA | Nonmetastatic breast cancer | Median: 106 | Surgery |
| Lee et al | 2018 | Korea | Retrospective, single center | NLR | OS & DFS | Reported | 358 | NLR: 3.16 | Median: 51 | 358 (100) | Advanced triple‐negative breast cancer | NA | Surgery |
| Limori et al | 2018 | Japan | Retrospective, single center | NLR | OS | Reported | 34 | NLR: 3 | 63 (44‐88) | NA | Stage IV breast cancer | NA | Endocrine therapy |
| Liu et al | 2016 | China | Retrospective, single center | NLR & PLR | OS & DFS | Reported | 318 | NLR: 3; PLR: 147 | 45 (19‐71) | 161 (50.6) | HR(−) nonmetastatic breast cancer | 58.1 (5.9‐136.1) | Surgery |
| Mando et al | 2018 | Argentina | Retrospective, single center | NLR | DFS | Reported | 85 | NLR: 2 | 56 (44‐66) | 5 (5.9) | Early stage breast cancer | 38.6 (29.4‐60.1) | Surgery |
| Miyagawa et al | 2018 | Japan | Retrospective, single center | NLR | OS | Reported | 59 | NLR: 3 | 34‐83 | 12 (21.4) | Metastatic breast cancer | NA | Chemotherapy |
| Nakano et al | 2014 | Japan | Retrospective, single center | NLR | DFS & CSS | Reported | 167 | NLR: 2.5 | 57.9 ± 10.9 | NA | Operable breast cancer with stage I‐III | 85.8 (19.8‐148.9) | Neoadjuvant chemotherapy or surgery |
| Orditura et al | 2016 | Italy | Retrospective, single center | NLR | DFS | Reported | 300 | NLR: 1.97 | ≤35:9;>35:291 | 30 (10) | Early stage breast cancer | Median: 84 | Surgery |
| Pistelli et al | 2014 | Italy | Retrospective, single center | NLR | OS & DFS | Reported | 90 | NLR: 3 | 53 (28‐79) | 90 (100) | Early stage breast cancer | 53.8 (13.1‐95.2) | Surgery |
| Qiu et al | 2018 | China | Retrospective, single center | NLR | OS & DFS | Reported | 406 | NLR: 2.85 | 44 (21‐75) | 406 (100) | Early stage breast cancer | 54.3 (7.8‐126.5) | Surgery + chemotherapy |
| Takeuchi et al | 2017 | Japan | Retrospective, single center | NLR & PLR | DFS | Reported | 296 | NLR: 2.06; PLR: 162.28 | <50:61;≥50:235 | NA | Localized breast cancer | Median: 41 | Surgery |
| Takuwa et al | 2018 | Japan | Retrospective, single center | NLR | OS | Reported | 171 | NLR: 1.9 | 59 (31‐89) | NA | Metastatic breast cancer | 44 (0‐217) | Multidisciplinary therapy |
| Templeton et al | 2018 | Spain | Prospective, 65 GEICAM institutions | NLR | OS & DFS | Reported | 1243 | NLR: 1.35 | 50 (23‐76) | 107 (8.6) | Early stage breast cancer | Median: 120 | Surgery + chemotherapy |
| Ulas et al | 2015 | Turkey | Retrospective, two centers | NLR & PLR | OS & DFS | Reported | 51 | NLR: 2.38; PLR: 161.28 | 51.4 ± 10.4 | NA | HER2‐positive early breast cancer | 26 (6‐84) | Surgery + adjuvant chemotherapy |
| Vernieri et al | 2018 | Italy | Retrospective, single center | NLR & PLR | OS | Reported | 57 | NLR: 2.5; PLR: 200 | 56 (33.7‐78.9) | 57 (100) | Metastatic breast cancer | NA | Chemotherapy |
| Wariss et al | 2017 | Brazil | Retrospective, one center | NLR & PLR | OS | Reported | 2288 | NLR: 4; PLR: 150 | 55 (18‐98) | 301 (12.7) | All TNM stages | NA | All kinds of therapies |
| Yao et al | 2014 | China | Retrospective, one center | NLR & PLR | OS & DFS | Reported | 608 | NLR: 2.56; PLR: 107.64 | 52.4 ± 10.8 | 98 (17.2) | Operable breast cancer | 42 (8‐62) | Surgery |
| Zhang et al | 2016 | China | Retrospective, one center | NLR | OS & DFS | Reported | 162 | NLR: 1.81 | 50.8 ± 10.6 | NA | TNM stage I‐III | NA | Surgery |
CSS, cancer‐specific survival; DFS, disease‐free survival; FFS, failure‐free survival; HRs, hazard ratios; N/A, not available; NLR, neutrophil‐to‐lymphocyte ratio; OS, overall survival; PLR, platelet‐to‐lymphocyte ratio; TNM, Tumor, node, metastases; GEICAM, Spanish Group for the Investigation of Breast Cancer Ages and Follow‐up periods were expressed as mean ± SD or median (range).
The bolds represent summary of subgroup analyses for OS and DFS.
3.2. Overall and subgroup analysis for NLR
Twenty‐eight studies comprising 142 64 BC patients investigated predicting role of NLR for OS. The pooled results shown that patients with elevated NLR prior treatment were associated worse prognosis compared to those with lower NLR (HR: 1.78; 95% CI: 1.49‐2.13; P < 0.001). Twenty‐seven cohort studies containing 115 04 patients explored the prognostic role of NLR in predicting DFS. The results demonstrated that patients with elevated NLR were associated with poor DFS, with a HR of 1.60 (95% CI: 1.42‐1.96; P < 0.001) (Figure 2).
Figure 2.
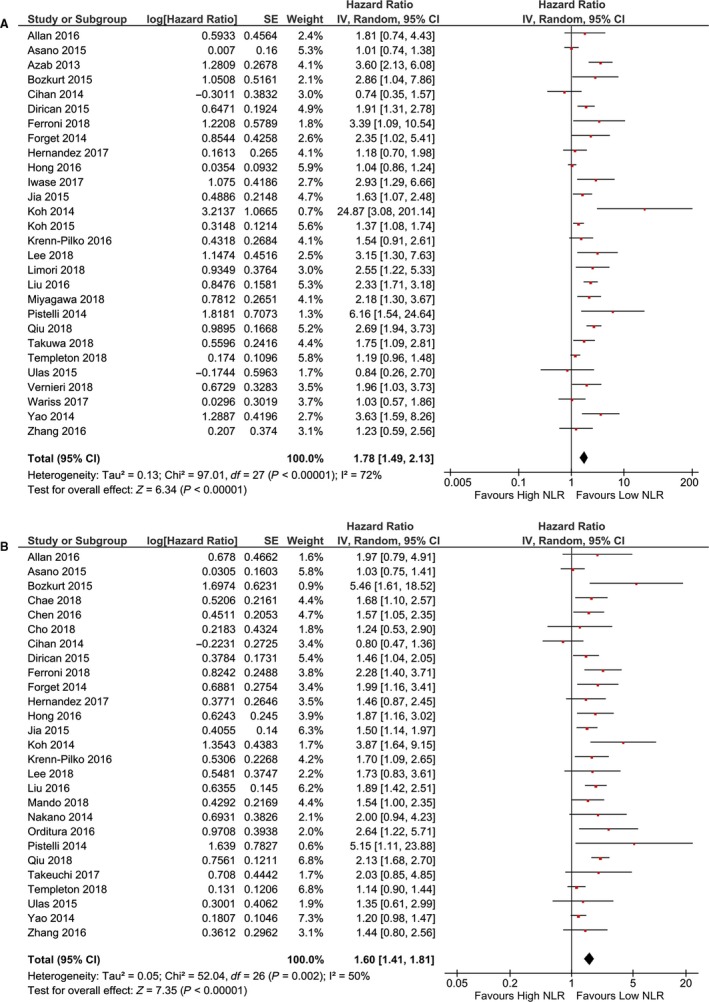
Forest plot of NLR in predicting OS (a) and DFS (b) of BC patients.
Results for subgroup analyses were shown in Table 2. The results of other subgroups displayed similar outcomes to overall result except subgroup of HER2‐positive and America. Six studies analyzed patients with HER2‐positively expressed showed that NLR was not significantly associated with OS (HR: 1.27; 95% CI: 0.94‐1.72; P = 0.12). In subgroup of America, the pooled result also showed no significant correlation between NLR with OS (HR: 1.91; 95% CI: 0.83‐4.37; P = 0.13). The results of subgroups for predicting DFS were similar to overall result. However, subgroup analyses based on tumor progression was unable to conduct due to insufficient data.
Table 2.
Subgroup analyses of NLR for OS and DFS
| Subgroups | Independent cohorts | Sample size | HR* (95% CI) (H/L*) | P value | Study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| χ 2 | df* | I 2, % | P value | |||||
| Overall survival | 28 | 142 64 | 1.78 (1.49‐2.13) | <0.0001 | 97.01 | 27 | 72 | <0.0001 |
| Pathology | ||||||||
| Triple‐negative | 12 | 2157 | 2.18 (1.75‐2.73) | <0.0001 | 15.70 | 11 | 30 | 0.15 |
| Her2‐positive | 6 | 1094 | 1.27 (0.94‐1.72) | 0.12 | 8.19 | 5 | 39 | 0.15 |
| Cut‐off value | ||||||||
| <3 | 12 | 5608 | 1.79 (1.33‐2.41) | 0.0001 | 47.85 | 11 | 77 | <0.0001 |
| ≥3 | 16 | 8656 | 1.79 (1.42‐2.24) | <0.0001 | 44.85 | 15 | 67 | <0.0001 |
| Tumor progression | ||||||||
| Early‐stage | 16 | 6984 | 1.70 (1.33‐2.18) | <0.0001 | 65.10 | 15 | 77 | <0.0001 |
| Metastatic | 6 | 768 | 2.17 (1.68‐2.81) | <0.0001 | 2.27 | 5 | 0 | 0.81 |
| Geographical region | ||||||||
| Asia | 18 | 8180 | 1.79 (1.42‐2.25) | <0.0001 | 71.50 | 17 | 76 | <0.0001 |
| America | 3 | 2897 | 1.91 (0.83‐4.37) | 0.13 | 9.69 | 2 | 79 | 0.008 |
| Europe | 7 | 3187 | 1.65 (1.20‐2.27) | 0.002 | 11.94 | 6 | 50 | 0.06 |
| Disease‐free survival | 27 | 115 04 | 1.60 (1.42‐1.96) | <0.0001 | ||||
| Pathology | ||||||||
| Triple‐negative | 10 | 1674 | 1.77 (1.44‐2.18) | <0.0001 | 14.40 | 9 | 38 | 0.11 |
| Her2‐positive | 5 | 881 | 1.41 (1.05‐1.89) | 0.02 | 8.66 | 4 | 54 | 0.07 |
| Cut‐off value | ||||||||
| <3 | 17 | 7190 | 1.67 (1.42‐1.96) | <0.0001 | 34.16 | 16 | 53 | 0.005 |
| ≥3 | 10 | 4313 | 1.50 (1.21‐1.86) | 0.0003 | 17.63 | 9 | 49 | 0.04 |
| Geographical region | ||||||||
| Asia | 18 | 7817 | 1.57 (1.34‐1.83) | <0.0001 | 37.75 | 17 | 55 | 0.003 |
| America | 2 | 257 | 1.61 (1.09‐2.85) | 0.02 | 0.23 | 1 | 0 | 0.63 |
| Europe | 7 | 3430 | 1.75 (1.30‐2.35) | 0.0002 | 13.92 | 6 | 57 | 0.03 |
Abbreviations: CI*, confidence interval; df*, degrees of freedom; HR, Hazard Ratio; H, High group, L, Low group.
The bolds represent summary of subgroup analyses for OS and DFS.
3.3. Overall and subgroup analyses for PLR
As shown in Table 3, 12 studies with 6930 patients explored the prognostic role of PLR in predicting OS of patients with BC. The pooled outcome suggested that patients with higher PLR were associated with a significantly poor prognosis (HR: 1.32; 95% CI: 1.11‐1.57; P = 0.002). Eleven studies with 5013 patients investigated predicting role of PLR for DFS shown that patients with elevated PLR were associated with shorter DFS (HR: 1.43; 95% CI: 1.09‐1.86; P = 0.009) (Figure 3).
Table 3.
Subgroup analyses of PLR for OS and DFS
| Subgroups | Independent cohorts | Sample size | HR* (95% CI) (H/L*) | P value | Study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| χ 2 | df* | I 2, % | P value | |||||
| Overall survival | 12 | 6930 | 1.32 (1.11‐1.57) | 0.002 | ||||
| Pathology | ||||||||
| Triple‐negative | 4 | 727 | 1.54 (1.03‐2.33) | 0.04 | 9.65 | 3 | 69 | 0.02 |
| Her2‐positive | 5 | 894 | 1.18 (0.83‐1.70) | 0.36 | 9.70 | 4 | 59 | 0.05 |
| Cut‐off value | ||||||||
| <185 | 6 | 3928 | 1.09 (0.97‐1.22) | 0.15 | 3.94 | 5 | 0 | 0.56 |
| ≥185 | 6 | 3002 | 1.81 (1.33‐2.46) | 0.0001 | 14.04 | 5 | 64 | 0.02 |
| Tumor progression | ||||||||
| Early‐stage | 6 | 2209 | 1.26 (0.97‐1.63) | 0.08 | 11.83 | 5 | 58 | 0.04 |
| Metastatic | 2 | 211 | 1.69 (1.27‐2.27) | 0.0004 | 0.14 | 1 | 0 | 0.71 |
| Geographical region | ||||||||
| Asia | 6 | 3075 | 1.14 (1.02‐1.28) | 0.02 | 5.23 | 5 | 4 | 0.39 |
| America | 4 | 3051 | 1.89 (1.13‐3.14) | 0.01 | 17.88 | 3 | 83 | 0.0005 |
| Europe | 2 | 804 | 1.70 (1.10‐2.62) | 0.02 | 0.25 | 1 | 0 | 0.62 |
| Disease‐free survival | 11 | 5013 | 1.43 (1.09‐1.86) | 0.009 | 44.24 | 10 | 77 | <0.0001 |
| Pathology | ||||||||
| Triple‐negative | 1 | 161 | 1.40 (0.97‐2.00) | 0.07 | NA | NA | NA | NA |
| Her2‐positive | 3 | 406 | 0.74 (0.42‐1.31) | 0.30 | 5.75 | 2 | 65 | 0.06 |
| Cut‐off value | ||||||||
| <185 | 6 | 1936 | 1.28 (0.99‐1.66) | 0.06 | 10.03 | 5 | 50 | 0.07 |
| ≥185 | 5 | 3077 | 1.63 (0.86‐3.07) | 0.13 | 32.74 | 4 | 88 | <0.0001 |
| Geographical region | ||||||||
| Asia | 9 | 4094 | 1.28 (0.98‐1.68) | 0.07 | 34.19 | 8 | 77 | <0.0001 |
| America | 1 | 172 | 4.13 (1.60‐10.66) | 0.003 | N/A | N/A | N/A | N/A |
| Europe | 1 | 747 | 2.02 (1.18‐3.46) | 0.01 | N/A | N/A | N/A | N/A |
Abbreviations: CI*, confidence interval; df*, degrees of freedom; HR, Hazard Ratio; H, High group, L, Low group; N/A, not available.
The bolds represent summary of subgroup analyses for OS and DFS.
Figure 3.
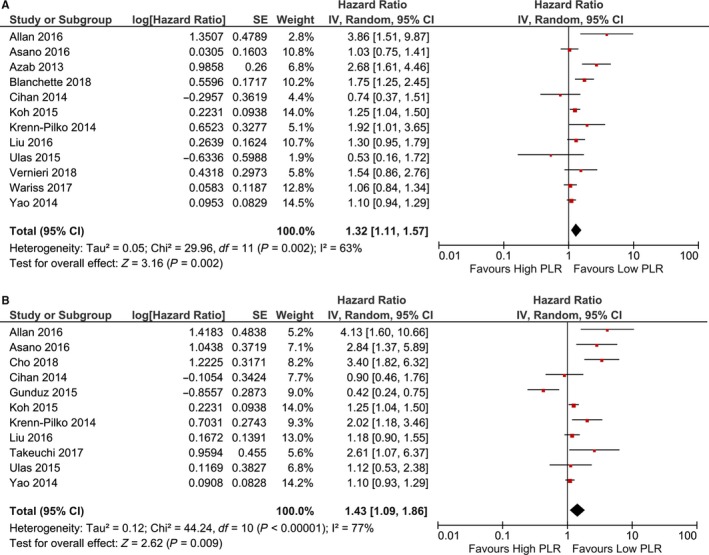
Forest plot of PLR in predicting OS (a) and DFS (b) of BC patients.
Subgroup analyses shown there were no statistically significant correlation between PLR and OS in patients with HER2‐positive expression or patients with early stage tumor. Subgroup analysis based on cut‐off value suggested a value that more than 185 was better for PLR in predicting prognosis of BC patients (HR: 1.81; 95% CI: 1.33‐2.46; P < 0.001). In subgroup analyses for PLR in predicting DFS, no significant association was found (Table 3).
3.4. Sensitivity analyses and publication bias
The sensitivity analyses was conducted to investigate the stabilities of the pooled HRs of OS and DFS by omitting enrolled studies in turn. The results showed that the pooled HRs did not alter significantly after eliminating the included studies in sequence which suggested the steady of our findings (Figure 4).
Figure 4.
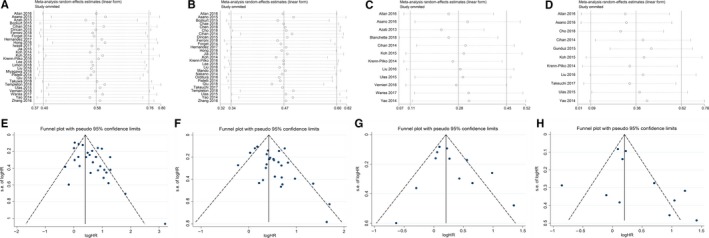
Sensitivity analyses and funnel plots of OS and DFS for NLR (a, b and e, f) and PLR (c, d and g, h).
The funnel plots showed no obvious publication bias among the enrolled studies (studies distributed around the center line symmetrically). Moreover, 2 kinds of statistical tests further validated the dissymmetry of the funnel plot by using Egger's test and Begg's test (Table 4).
Table 4.
Assessment for publication bias
| Number of estimate | Z value | P for Begg's test | t | P for Egger's test | |
|---|---|---|---|---|---|
| NLR for OS | 28 | 1.10 | 0.272 | 1.74 | 0.096 |
| NLR for DFS | 27 | 1.66 | 0.097 | 1.20 | 0.241 |
| PLR for OS | 12 | 1.03 | 0.304 | 1.31 | 0.220 |
| PLR for DFS | 11 | 1.40 | 0.161 | 1.38 | 0.202 |
Abbreviations: DFS, disease‐free survival; NLR, neutrophil‐to‐lymphocyte ratio; OS, overall survival; PLR, platelet‐to‐lymphocyte ratio.
4. DISCUSSION
Recently, more and more studies focused on correlation between inflammation and solid malignancies revealed that tumor initiation, progression, and metastasis were affected by host systemic inflammatory response as well as tumor microenvironment.4, 51, 52 Hence, we performed a meta‐analysis, including 39 studies comprising 17079 BC patients, to evaluate the prognostic role of NLR and PLR in predicting OS and DFS. A significant prognostic effect for NLR and PLR was found on OS and DFS after pooling results. High NLR level was associated with both poor OS as well as DFS for BC patients. Meanwhile, patients with elevated PLR were associated worse OS as well as higher risk of recurrence compared to those with decreased PLR level.
Although the underlying molecular mechanisms have not been adequately illuminated. Neutrophil was considered to be related to cancer‐associated inflammation with the potential mechanism of responding to the ectopic interleukin‐8 released in tumor proliferation, progression and metastasis.53 Moreover, cancer‐associated cytokines like tumor necrosis factor‐α and interleukin‐6 contribute to neutrophilia in solid cancers.54, 55 Neutrophilia inhibits the cytotoxic activity of immune cells like lymphocytes, natural killer cells and T cells which would counteract the anti‐tumor immune response.56, 57 A high platelet counts was considered to be related to metastasis of BC cells with the mechanism of contribution to lysophosphatidic acid‐dependent metastasis.58 Platelets could also promote tumor angiogenesis and stroma formation by secreting vascular endothelial growth factor and facilitating migration of inflammatory cells.59, 60 Loi et al found elevated lymphocytic infiltration in BC was associated with favorable prognosis, especially in those node‐positive and HER2‐negative BC.61 The lymphocytes played an important role in cell‐mediated anti‐tumor immune responses and tumor immunological surveillance.62, 63, 64
In view of the heterogeneity of BC, subgroup analyses according to different subtypes like triple‐negative and HER2‐positive. The results shown a greater magnitude of association between NLR and OS in triple‐negative BC patients than in HER2‐positive ones. And negative prognostic effect was found for NLR and PLR in HER2‐positive BC patients. A previous meta‐analysis performed by Zhang et al included 11 studies and 1 conference abstract to evaluate the prognostic value of PLR in BC.65 Their process of extracting data were not rigorous enough that several HRs with 95% were not consistent with original researches.10, 47 The pooled results of our study were more credible and stable because of more rigorous in data extraction and subgroup analyses. Nonetheless, future prospective studies with large sample size were in need to confirm our outcomes especially the prognostic effect of NLR and PLR on HER2‐positive BC patients. Our subgroup analyses also suggested that a cut‐off value no less than 185 for PLR in predicting OS was more preferable.
Individualized therapy based on tumor‐associated biological characteristics and host circumstance was advocated in treatment strategies for BC. Several treatments such as surgical resection combined with adjuvant chemotherapy or neoadjuvant chemotherapy, endocrine therapy, and targeted therapy were optional for BC patients with different tumor stages. This study performed subgroup analysis based on tumor stage suggested comparative effect of NLR and PLR in predicting OS. The data were insufficient to conduct subgroup analysis based on treatment strategies.
To our knowledge, this was the most comprehensive meta‐analysis with largest sample size to estimate the prognostic role of PLR as well as NLR for BC. However, there were still several limitations should be taken into consideration when interpreting our findings. First, although there was no obvious publication bias, all the included studies were retrospectively designed. High proportion of retrospective individual studies would give rise to inherent bias inevitably. It would be preferable for future studies to design and collect data prospectively. Second, in the subgroup analysis of pathology, only triple‐negative and HER2‐positive BC patients were enrolled. Furthermore, all the included studies were written in English which would result in potential publication bias. Finally, future international multi‐center studies with larger sample size were in need to confirm our results.
Nevertheless, the present meta‐analysis was performed at an appropriate time as an adequate number of studies with sufficient data in a large patient cohort investigating the prognostic effect of NLR and PLR for BC patients have been accumulated, allowing evaluation through meta‐analysis. A meta‐analysis is considered to be a statistical inspection of scientific studies which is associated with higher evidence level than the individual studies themselves.66 Subgroup analyses were performed to minimize heterogeneity stemming from different BC‐specific subtypes, optional cut‐off values, geographical regions, and tumor stages.
In conclusion, this study suggested that elevated NLR and PLR were associated with poor OS as well as high risk of recurrence for BC patients. Subgroup analyses confirmed negative prognostic value of NLR and PLR for HER2‐positive BC patients. As easily accessible parameters, NLR and PLR should be identified as useful biomarkers in the management of BC.
CONFLICT OF INTEREST
The authors declare that there are no conflict of interest.
AUTHOR CONTRIBUTIONS
Wanying Guo and Miao Deng contributed to the designation of this study. Wanying Guo and Xin Lu contributed to literature research. Qipeng Liu and Ting Zhang contributed to data extraction. Xin Lu, Peng Li and Weiqiang Qiao performed the statistical analysis. All the authors participated in drafting the manuscript.
Supporting information
ACKNOWLEDGMENTS
None.
Guo W, Lu X, Liu Q, et al. Prognostic value of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio for breast cancer patients: An updated meta‐analysis of 17079 individuals. Cancer Med. 2019;8:4135–4148. 10.1002/cam4.2281
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65(1):5‐29. [DOI] [PubMed] [Google Scholar]
- 2. Runowicz CD, Leach CR, Henry NL, et al. Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43‐73. [DOI] [PubMed] [Google Scholar]
- 3. Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil‐to‐lymphocyte ratio in breast cancer: a systematic review and meta‐analysis. Breast Cancer Res. 2017;19(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 5. Fridman WH, Zitvogel L, Sautes‐Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717‐734. [DOI] [PubMed] [Google Scholar]
- 6. Fu H, Zheng J, Cai J, et al. Systemic immune‐inflammation index (SII) is useful to predict survival outcomes in patients after liver transplantation for hepatocellular carcinoma within hangzhou criteria. Cell Physiol Biochem. 2018;47(1):293‐301. [DOI] [PubMed] [Google Scholar]
- 7. Pinato DJ, Karamanakos G, Arizumi T, et al. Dynamic changes of the inflammation‐based index predict mortality following chemoembolisation for hepatocellular carcinoma: a prospective study. Aliment Pharmacol Ther. 2014;40(11–12):1270‐1281. [DOI] [PubMed] [Google Scholar]
- 8. Krenn‐Pilko S, Langsenlehner U, Thurner E‐M, et al. The elevated preoperative platelet‐to‐lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524‐2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho U, Park HS, Im SY, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS ONE. 2018;13(7):e0200936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gündüz S, Göksu SS, Arslan D, et al. Factors affecting disease‐free survival in patients with human epidermal growth factor receptor 2‐positive breast cancer who receive adjuvant trastuzumab. Mol Clin Oncol. 2015;3(5):1109‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li H, Zhang J‐B, Chen X‐L, et al. Different techniques for harvesting grafts for living donor liver transplantation: a systematic review and meta‐analysis. World J Gastroenterol. 2017;23(20):3730‐3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li H, Zhu B, Huang J, Chen X, Wang J, Wu H. Liver hanging maneuver versus conventional approach for open hepatectomy: a meta‐analysis. HPB. 2018; pii: S1365-182X(18)34469-1. 10.1016/j.hpb.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 13. Lu X, Guo W, Xu W, et al. Prognostic value of the Glasgow prognostic score in colorectal cancer: a meta‐analysis of 9,839 patients. Cancer Manage Res. 2019;11:229‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li H, Zheng J, Cai JY, et al. Laparoscopic VS open hepatectomy for hepatolithiasis: an updated systematic review and meta‐analysis. World J Gastroenterol. 2017;23(43):7791‐7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucendo AJ, Arias A, Tenias JM. Systematic review: the association between eosinophilic oesophagitis and coeliac disease. Aliment Pharmacol Ther. 2014;40(5):422‐434. [DOI] [PubMed] [Google Scholar]
- 16. Zhang P, Zong Y, Liu M, Tai Y, Cao Y, Hu C. Prediction of outcome in breast cancer patients using test parameters from complete blood count. Mol Clin Oncol. 2016;4(6):918‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yao M, Liu Y, Jin H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. OncoTargets Therapy. 2014;7:1743‐1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vernieri C, Mennitto A, Prisciandaro M, et al. The neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios predict efficacy of platinum‐based chemotherapy in patients with metastatic triple negative breast cancer. Sci Rep. 2018;8(1):8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Templeton AJ, Rodriguez‐Lescure A, Ruiz A, et al. Prognostic role for the derived neutrophil‐to‐lymphocyte ratio in early breast cancer: a GEICAM/9906 substudy. Clin Transl Oncol. 2018;20(12):1548‐1556. [DOI] [PubMed] [Google Scholar]
- 20. Takeuchi H, Kawanaka H, Fukuyama S, Kubo N, Hiroshige S, Yano T. Comparison of the prognostic values of preoperative inflammation‐based parameters in patients with breast cancer. PLoS ONE. 2017;12(5):e0177137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu X, Song Y, Cui Y, Liu Y. Increased neutrophil‐lymphocyte ratio independently predicts poor survival in non‐metastatic triple‐negative breast cancer patients. IUBMB Life. 2018;70(6):529‐535. [DOI] [PubMed] [Google Scholar]
- 22. Pistelli M, De Lisa M, Ballatore Z, et al. Pre‐treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer. 2015;15:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orditura M, Galizia G, Diana A, et al. Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis‐free survival (DMFS) in early breast cancer: a propensity score‐matched analysis. ESMO Open. 2016;1(2):e000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakano K, Hosoda M, Yamamoto M, Yamashita H. Prognostic significance of pre‐treatment neutrophil: lymphocyte ratio in Japanese patients with breast cancer. Anticancer Res. 2014;34(7):3819‐3824. [PubMed] [Google Scholar]
- 25. Miyagawa Y, Araki K, Bun A, et al. Significant association between low baseline neutrophil‐to‐lymphocyte ratio and improved progression‐free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab‐paclitaxel. Clin Breast Cancer. 2018;18(5):400‐409. [DOI] [PubMed] [Google Scholar]
- 26. Mando P, Rizzo M, Roberti MP, et al. High neutrophil to lymphocyte ratio and decreased CD69(+)NK cells represent a phenotype of high risk in early‐stage breast cancer patients. OncoTargets Therapy. 2018;11:2901‐2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu C, Huang Z, Wang Q, et al. Usefulness of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in hormone‐receptor‐negative breast cancer. OncoTargets Therapy. 2016;9:4653‐4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iimori N, Kashiwagi S, Asano Y, et al. Clinical significance of the neutrophil‐to‐lymphocyte ratio in endocrine therapy for stage IV breast cancer. In Vivo. 2018;32(3):669‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee J, Kim DM, Lee A. Prognostic role and clinical association of tumor‐infiltrating lymphocyte, programmed death ligand‐1 expression with neutrophil‐lymphocyte ratio in locally advanced triple‐negative breast cancer. Cancer Res Treat. 2019;51(2):649‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krenn‐Pilko S, Langsenlehner U, Stojakovic T, et al. The elevated preoperative derived neutrophil‐to‐lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol. 2016;37(1):361‐368. [DOI] [PubMed] [Google Scholar]
- 31. Koh C‐H, Bhoo‐Pathy N, Ng K‐L, et al. Utility of pre‐treatment neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koh YW, Lee HJ, Ahn JH, Lee JW, Gong G. Prognostic significance of the ratio of absolute neutrophil to lymphocyte counts for breast cancer patients with ER/PR‐positivity and HER2‐negativity in neoadjuvant setting. Tumour Biol. 2014;35(10):9823‐9830. [DOI] [PubMed] [Google Scholar]
- 33. Jia W, Wu J, Jia H, et al. The peripheral blood neutrophil‐to‐lymphocyte ratio is superior to the lymphocyte‐to‐monocyte ratio for predicting the long‐term survival of triple‐negative breast cancer patients. PLoS ONE. 2015;10(11):e0143061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwase T, Sangai T, Sakakibara M, et al. An increased neutrophil‐to‐lymphocyte ratio predicts poorer survival following recurrence for patients with breast cancer. Mol Clin Oncol. 2017;6(2):266‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hong J, Mao Y, Chen X, et al. Elevated preoperative neutrophil‐to‐lymphocyte ratio predicts poor disease‐free survival in Chinese women with breast cancer. Tumour Biol. 2016;37(3):4135‐4142. [DOI] [PubMed] [Google Scholar]
- 36. Marín Hernández C, Piñero Madrona A, Gil Vázquez PJ, et al. Usefulness of lymphocyte‐to‐monocyte, neutrophil‐to‐monocyte and neutrophil‐to‐lymphocyte ratios as prognostic markers in breast cancer patients treated with neoadjuvant chemotherapy. Clin Transl Oncol. 2018;20(4):476‐483. [DOI] [PubMed] [Google Scholar]
- 37. Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease‐free survival and overall survival in conservative breast cancer surgery. Br J Anaesth. 2014;113(Suppl 1):i82‐i87. [DOI] [PubMed] [Google Scholar]
- 38. Ferroni P, Roselli M, Buonomo OC, et al. Prognostic significance of neutrophil–to–lymphocyte ratio in the framework of the 8th TNM edition for breast cancer. Anticancer Res. 2018;38(8):4705‐4712. [DOI] [PubMed] [Google Scholar]
- 39. Dirican A, Kucukzeybek BB, Alacacioglu A, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol. 2015;20(1):70‐81. [DOI] [PubMed] [Google Scholar]
- 40. Cihan YB, Arslan A, Cetindag MF, Mutlu H. Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev. 2014;15(10):4225‐4231. [DOI] [PubMed] [Google Scholar]
- 41. Chen YI, Chen K, Xiao X, et al. Pretreatment neutrophil‐to‐lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chae S, Kang KM, Kim HJ, et al. Neutrophil‐lymphocyte ratio predicts response to chemotherapy in triple‐negative breast cancer. Curr Oncol. 2018;25(2):e113‐e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bozkurt O, Karaca H, Berk V, et al. Predicting the role of the pretreatment neutrophil to lymphocyte ratio in the survival of early triple‐negative breast cancer patients. J BUON. 2015;20(6):1432‐1439. [PubMed] [Google Scholar]
- 44. Blanchette PS, Desautels DN, Pond GR, et al. Factors influencing survival among patients with HER2‐positive metastatic breast cancer treated with trastuzumab. Breast Cancer Res Treat. 2018;170(1):169‐177. [DOI] [PubMed] [Google Scholar]
- 45. Asano Y, Kashiwagi S, Onoda N, et al. Platelet‐lymphocyte ratio as a useful predictor of the therapeutic effect of neoadjuvant chemotherapy in breast cancer. PLoS ONE. 2016;11(7):e0153459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramos‐Esquivel A, Rodriguez‐Porras L, Porras J. Neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio as prognostic factors in non‐metastatic breast cancer patients from a Hispanic population. Breast Dis. 2017;37(1):4135‐6. [DOI] [PubMed] [Google Scholar]
- 47. Ulas A, Avci N, Kos T, et al. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2‐positive early breast cancer receiving adjuvant trastuzumab? J BUON. 2015;20(3):714‐722. [PubMed] [Google Scholar]
- 48. Asano Y, Kashiwagi S, Onoda N, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple‐negative breast cancer. Ann Surg Oncol. 2016;23(4):1104‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long‐term mortality in breast cancer patients. Med Oncol. 2013;30(1):432. [DOI] [PubMed] [Google Scholar]
- 50. Takuwa H, Tsuji W, Yamamoto Y, Shintaku M, Yotsumoto F. Low neutrophil‐lymphocyte ratio correlates with extended survival in patients with metastatic breast cancer who achieved clinically complete response following multidisciplinary therapy: a retrospective study. Oncol Lett. 2018;15(5):6681‐6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. DeNardo DG, Coussens LM. Inflammation and breast cancer. balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9(4):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin‐8. Clin Cancer Res. 2004;10(15):4895‐4900. [DOI] [PubMed] [Google Scholar]
- 54. Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non‐small‐cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00‐03. Eur J Cancer. 2009;45(11):1950‐1958. [DOI] [PubMed] [Google Scholar]
- 55. Turan N, Edwards MJ, Bates S, et al. IL‐6 pathway upregulation in subgroup of severe asthma is associated with neutrophilia and poor lung function. Clin Exp Allergy. 2018;48(4):475‐478. [DOI] [PubMed] [Google Scholar]
- 56. el‐Hag A, Clark RA Immunosuppression by activated human neutrophils. dependence on the myeloperoxidase system. J Immunol. 1987;139(7):2406‐2413. [PubMed] [Google Scholar]
- 57. Rotondo R, Bertolotto M, Barisione G, et al. Exocytosis of azurophil and arginase 1‐containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011;89(5):721‐727. [DOI] [PubMed] [Google Scholar]
- 58. Leblanc R, Lee SC, David M, et al. Interaction of platelet‐derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood. 2014;124(20):3141‐3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22(9):913‐922. [DOI] [PubMed] [Google Scholar]
- 60. Ghasemzadeh M, Hosseini E. Intravascular leukocyte migration through platelet thrombi: directing leukocytes to sites of vascular injury. Thromb Haemost. 2015;113(6):1224‐1235. [DOI] [PubMed] [Google Scholar]
- 61. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor‐infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node‐positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin‐based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860‐867. [DOI] [PubMed] [Google Scholar]
- 62. Hopewell EL, Zhao W, Fulp WJ, et al. Lung tumor NF‐kappaB signaling promotes T cell‐mediated immune surveillance. J Clin Invest. 2013;123(6):2509‐2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wick DA, Webb JR, Nielsen JS, et al. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin Cancer Res. 2014;20(5):1125‐1134. [DOI] [PubMed] [Google Scholar]
- 64. Wang Y, Liu T, Tang W, et al. Hepatocellular carcinoma cells induce regulatory T cells and lead to poor prognosis via production of transforming growth factor‐beta1. Cell Physiol Biochem. 2016;38(1):306‐318. [DOI] [PubMed] [Google Scholar]
- 65. Zhang M, Huang XZ, Song YX, Gao P, Sun JX, Wang ZN. High platelet‐to‐lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with breast cancer: a meta‐analysis. BioMed Res Int. 2017;2017:4135–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kok NF, Grunhagen DJ, Ayez N, Verhoef C. Influence of margins on overall survival after hepatic resection for colorectal metastasis: a meta‐analysis. Ann Surg. 2015;261(1):e15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


