Abstract
Background and Objectives
In a previous genome‐wide screening, we identified hypermethylated CpG islands around glutamate decarboxylase 1 (GAD1) in lung adenocarcinoma (LADC). In this study, we aimed to investigate the methylation and expression status of GAD1 and its prognostic value in patients with LADC.
Methods
GAD1 methylation and mRNA expression status were analyzed using 33 tumorous and paired non‐tumorous LADC samples and publicly available datasets. The prognostic value of GAD1 overexpression was investigated using publicly available datasets of mRNA levels and 162 cases of LADC by immunohistochemistry.
Results
The methylation and mRNA expression levels of GAD1, each having a positive correlation, were significantly higher in LADC tumors than in paired non‐tumorous tissues. LADC patients with higher GAD1 mRNA expression showed significantly poorer prognosis for overall survival in publicly available datasets. Higher immunoreactivity of GAD1 was significantly associated with the pathological stage, pleural invasion, lymph vessel invasion, and poorer prognosis for cancer‐specific and disease‐free survival. Multivariate analysis revealed that GAD1 protein overexpression is an independent prognosticator for disease‐free survival.
Conclusions
GAD1 mRNA and protein expression levels were significant prognostic factors in LADC, suggesting that they might be useful biomarkers to stratify patients with worse clinical outcomes after resection.
Keywords: DNA methylation, expression, GAD1, lung adenocarcinoma, prognosis
1. INTRODUCTION
Lung adenocarcinoma (LADC) is the predominant histological subtype of lung cancer and has the highest mortality rate worldwide.1, 2 Although progress in the treatment of LADC has improved short‐term survival, the impacts on long‐term survival remain modest.3 Therefore, a better understanding of the mechanisms of LADC tumor progression is needed and useful prognostic molecular markers for accurately predicting the clinical outcomes of LADC are of great clinical significance.
To identify genes in the tumor that are specifically methylated at an early‐stage of LADC, we had previously performed a genome‐wide screening of aberrantly methylated CpG islands (CGIs) using paired tumorous and non‐tumorous tissues of early‐stage LADC, and identified TRIM58 as a novel candidate tumor‐suppressor gene for this disease.4 Through this screening, the glutamate decarboxylase 1 gene (GAD1) was found to be nearby hypermethylated CGIs in LADC. Because paradoxical hypermethylation‐associated overexpression of GAD1 was reported recently in colorectal and liver cancers5 and GAD1 overexpression has been reported in various neoplastic tissues, such as oral, nasopharyngeal, colorectal, liver, and gastric cancers,5, 6, 7, 8, 9 we focused on GAD1 as a potential LADC‐related gene in the present study. Moreover, the methylation and expression status and clinicopathological significance of GAD1 in LADC tumorigenesis have also not been examined previously.
Therefore, in the present study, we investigated the DNA methylation and mRNA and protein expression status of GAD1 in resected LADC tumors. Moreover, we assessed the prognostic significance of GAD1 expression in LADC using our tumor panel and publicly available datasets.
2. MATERIALS AND METHODS
2.1. Selection of candidate CGI
Previously obtained Human Methylation 450K array‐based methylation screening data of 12 paired tumorous/non‐tumorous stage‐I LADC sample sets from patients (6 smokers and 6 never‐smokers) who underwent surgery at Tokushima University Hospital (Tokushima, Japan) between April 1999 and March 2015 were reevaluated (Table S1).4
2.2. Patients and tissue samples
We included tumors and non‐tumorous tissues of LADC that were surgically resected at Tokushima University Hospital between April 1999 and November 2013 for additional analyses. No patients had been administered preoperative radiation, chemotherapy, or immunotherapy. For pyrosequencing‐based methylation analysis and real‐time PCR‐based expression analysis, 33 LADC samples were used (Table S2). For immunohistochemical staining, 162 LADC samples were used (Table S3). The mean follow‐up duration for the 162 patients with LADC was 48 months (range, 0.6‐147 months), with 45 recurrences (27.8%) and 34 deaths (21.0%) among the patients. Tumor staging was determined based on the seventh tumor‐node‐metastasis (TNM) classification for lung cancer.10 The tumors were classified according to the predominant histological subtype, as proposed by the 2015 WHO classification.11
This study was performed in accordance with the principles outlined in the Declaration of Helsinki. The ethics committee of Tokushima University Hospital approved the study (approval number 3048), and formal written consent was obtained from all patients or their representatives.
2.3. DNA and RNA preparation and bisulfite conversion of genomic DNA
DNA and RNA were extracted using standard methods. Bisulfite conversion of DNA was conducted using the EpiTect Bisulfite Kit (QIAGEN GmbH, Hilden, Germany) following the manufacturer's instructions.
2.4. Bisulfite pyrosequencing
Bisulfite‐treated genomic DNA was amplified using a set of primers designed with PyroMark Assay Design Software version 2.0.01.15 (QIAGEN GmbH, Table S4). The target region for sequencing began 10 nucleotides (nt) before and ended 26 nt after cg15126544. PCR product pyrosequencing and methylation quantification were performed with sequencing primers using the PyroMark 24 Pyrosequencing System, version 2.0.6 (QIAGEN GmbH), according to the manufacturer's instructions.
2.5. Real‐time quantitative reverse‐transcription polymerase chain reaction (rqRT‐PCR)
Complementary DNA was generated from isolated total RNA using the PrimeScript II first strand cDNA Synthesis Kit (TaKaRa, Shiga, Japan). rqRT‐PCR was performed using KAPA PROBE FAST qPCR Kits (Kapa Biosystems, Wilmington, MA, USA) and TaqMan Gene Expression Assays (Thermo Fisher Scientific, Waltham, MA, USA; Table S4) according to the manufacturers’ instructions. GAPDH mRNA levels were used as internal controls for normalization. Relative expression of GAD1 mRNA was calculated using Human Lung Total RNA (TaKaRa) as a normal lung control.
2.6. Data mining in bioinformatics
Available RNA sequencing data (IlluminaHiSeq_RNASeqV2 Level 3) containing 488 tumor and 58 non‐tumor samples and Infinium Human Methylation 450K data (Level 3) containing 473 tumor and 32 nontumorous samples of LADC cases with clinical annotations were downloaded from The Cancer Genome Atlas (TCGA) Research Network (http://cancergenome.nih.gov). mRNA expression data and DNA methylation data were available for 36 and 29 paired tumorous/non‐tumorous sample sets, respectively; both types of data were available for 18 sets. Tumorous samples with mRNA expression data and survival data were available for 423 cases. Survival analyses were conducted on patients with normalized mRNA expression and overall survival (OS) profiles. Patients were divided into low‐ and high‐expression groups according to the median GAD1 mRNA expression value.
Kaplan‐Meier Plotter (KM plotter, http://kmplot.com/analysis/), a publicly available online database of published microarray datasets for primary tumors with clinical information,12 was also used to generate OS curves in 9 studies from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/, Table S5) by setting the auto‐selected best value of GAD1 mRNA expression as the cutoff. All other parameters were left at default settings.
2.7. Immunohistochemical staining
Paraffin sections (4‐µm thick) were subjected to immunohistochemical staining using the Envision system (ChemMate Envision kit; Dako, Glostrup, Denmark) according to the manufacturer's instructions. Antigen retrieval was performed by heating the dewaxed and dehydrated sections in Dako Real Target Retrieval Solution, pH 9 (Dako), using a 2100 retriever (Aptum Biologics, Ltd., Southampton, UK). A mouse anti‐GAD67 monoclonal antibody (Sigma‐Aldrich, St. Louis, MO, USA; G5419), diluted to 1:200 with antibody diluents (Dako), was used as the primary antibody. The proportion and intensity of GAD1 staining in the LADC samples were scored (Table S6A) independently by two different researchers.
2.8. Statistical analysis
Student's t test or Fischer's exact test was used for comparisons between two groups. The paired t test was used for comparisons between paired samples. The relationship between continuous variables was investigated by calculating the Spearman's correlation coefficient. For survival analysis, Kaplan‐Meier survival curves were constructed for groups based on univariate predictors, and differences among groups were tested with the log‐rank test. Univariate and multivariate survival analyses were performed using the likelihood ratio test of the stratified Cox proportional hazard regression analysis. Differences were assessed using two‐sided tests and were considered significant at a P < 0.05. Statistical analyses were performed using IBM SPSS version 24 (IBM Corporation, Armonk, NY) or the Survival package for R (https://cran.r-project.org).
3. RESULTS
3.1. Methylation status of CGIs and each CpG site within CGIs around GAD1
In a previous array‐based, genome‐wide methylation screening of 12 paired tumorous/non‐tumorous LADC sample sets,4 CGI‐3 around GAD1 was ranked 14th as a hypermethylated CGI with a high P‐value (Table S1). Because hypermethylation‐associated overexpression of GAD1 was reported in colorectal and liver cancers,5 we reevaluated the results of the array‐based methylation status of each CpG site within CGI‐1‐4 (Figure 1A) around GAD1 (Figure 1B). The methylation levels of all CpG sites determined by array‐based analysis within CGI‐3 and in tumors were significantly higher than those in paired non‐tumorous tissues. Although the methylation levels in tumors were higher in CpG sites within CGI‐3 than in those within CGI‐4, the average β‐value in non‐tumor tissues was extremely and specifically low at cg15126544 and showed the largest difference of average β‐value between tumor and non‐tumor tissues at this site (Figure 1B and Table S7), which is localized within the CCCTC‐binding factor (CTCF)‐binding site of GAD1. Similar results were observed in the Level 3 Infinium Human Methylation 450K data of 29 LADC tumors and paired non‐tumor tissues from TCGA dataset (Figure S1). Because hypermethylation around this CTCF‐binding site has been reported as a possible cause of GAD1 overexpression,5 we further assessed the methylation status of cg15126544 and GAD1 mRNA expression levels.
Figure 1.
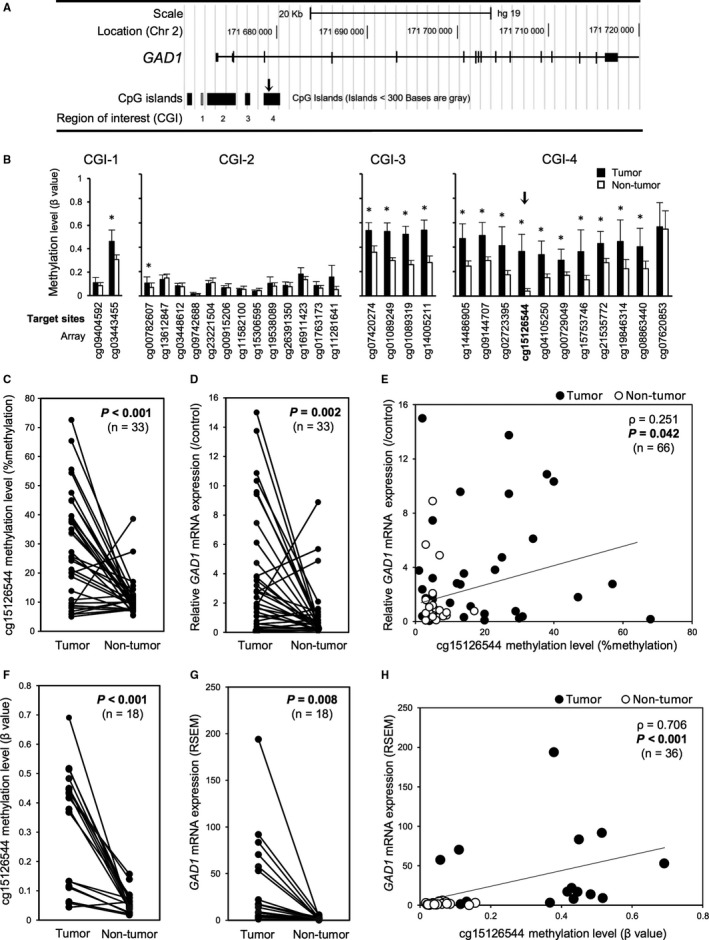
DNA methylation and mRNA expression status of GAD1 in patients with LADC. (A) A schematic diagram of the GAD1 gene structure with CGIs around GAD1. The arrow indicates the location of cg15126544. (B) The average β‐value (methylation level) of each CpG site targeted in the array‐based methylation experiment involving 12 LADC cases. *P < 0.05 vs. paired non‐tumorous tissues. (C) Linear plots of the average DNA methylation values (percentages) of cg15126544 in 33 LADC tumorous and paired non‐tumorous tissues, as determined by quantitative pyrosequencing. Samples from the same patient are linked with straight lines. (D) Linear plots of expression levels of GAD1 mRNA relative to those of the control normal human lung in 33 LADC tumorous and paired non‐tumorous tissues. Relative expression of GAD1 mRNA was calculated using Human Lung Total RNA as a normal control. (E) Correlation between the average methylation levels of cg15126544 (x‐axis) and relative GAD1 mRNA expression levels (y‐axis) in 33 LADC tumorous and paired non‐tumorous tissues. (F) Linear plots of the methylation levels (β‐values) of cg15126544 determined through an array‐based methylation experiment using HumanMethylation450K array in 18 paired LADC tumor and non‐tumorous tissue samples obtained from the TCGA dataset (http://cancergenome.nih.gov). (G) Linear plots of mRNA expression of GAD1 determined by RNA sequencing and quantified by RNA‐Seq by Expectation Maximization (RSEM) in 18 paired LADC tumor and non‐tumorous tissue samples obtained from the TCGA dataset. (H) Correlation between the methylation levels (β‐values) of cg15126544 (x‐axis) and GAD1 mRNA expression levels (y‐axis) in 18 paired LADC tumor and non‐tumorous tissue samples obtained from the TCGA dataset
3.2. Correlation between GAD1 expression and CGI methylation in LADC clinical cases
The DNA methylation status and mRNA expression status were investigated in our panel of LADC tumorous and paired non‐tumorous tissues (Table S2) using pyrosequence‐based methylation assays and rqRT‐PCR‐based expression analysis, respectively. Of the 33 sample sets, 26 (78.8%) demonstrated significantly higher methylation levels in tumor samples than in non‐tumorous tissues (Figure 1C). In the same cases, the mean GAD1 mRNA expression levels in the tumors were significantly higher than those in the paired non‐tumorous tissues (Figure 1D). There was a slightly positive (ρ = 0.251) but significant correlation between methylation levels at cg15126544 and GAD1 mRNA expression (Figure 1E). The LADC sample set containing 18‐paired samples obtained from TCGA demonstrated similar results both in methylation levels at cg15126544 and GAD1 mRNA expression (Figure 1F,G and Figure S1). A significant and highly positive correlation between them was also observed in TCGA dataset (ρ = 0.706, Figure 1H). Because the gene expression status of cancer cells directly affects their phenotypes, including malignant features, we focused on GAD1 expression in tumors to further assess its prognostic significance in patients with LADC.
3.3. Association of GAD1 mRNA expression levels with prognosis in LADC tumors
In our LADC cohort, a sufficient number of cases with high‐quality RNA suitable for expression analysis was not available for survival analysis. Therefore, to test the association between GAD1 mRNA expression levels in tumors and patients’ prognosis, we first performed survival analysis of 423 patients with LADC using data obtained from TCGA dataset. The OS rate of patients with LADC with higher GAD1 mRNA expression in tumors was significantly poorer than that of patients with lower GAD1 mRNA expression in tumors (Figure 2A). Univariate Cox regression analysis using data obtained from TCGA dataset confirmed that high GAD1 mRNA expression was associated with a worse prognostic significance for OS (Table 1). In multivariate Cox regression analysis, high GAD1 mRNA expression was also significantly associated with a poorer OS rate, suggesting that GAD1 mRNA expression is an independent prognostic factor for OS (P = 0.036, Table 1).
Figure 2.
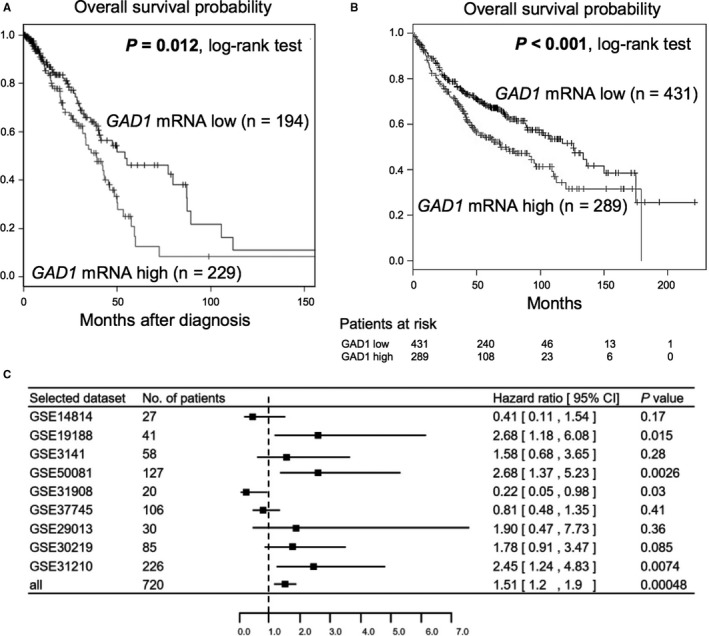
Publicly available datasets showing association between GAD1 mRNA expression status and prognosis in patients with LADC. (A) Kaplan‐Meier curve for OS rate of 423 LADC patients according to GAD1 mRNA expression levels using data obtained from the TCGA dataset. P‐values were calculated using the log‐rank test. Statistically significant P‐values are in boldface type. (B) Kaplan‐Meier curve for OS rate of 720 LADC patients in cohorts GSE14814, GSE19188, GSE3141, GSE50081, GSE31908, GSE37745, GSE29013, GSE30219, and GSE31210 according to GAD1 mRNA expression levels obtained from the online survival analysis software, Kaplan‐Meier plotter (KM plotter; http://www.kmplot.com). P‐values were calculated using the log‐rank test. Statistically significant P‐values are in boldface type. (C) Subgroup analysis of KM plotter databases for GAD1 mRNA expression in LADC. Hazard ratios (HR, center of the box) and 95% confidence intervals (CI, horizontal line) were calculated with Cox's regression models
Table 1.
Cox proportional hazard regression analysis of overall survival in 400 patients with LADC in TCGA dataset
| Factor (number) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | P‐value | Hazard ratio | 95% confidence interval | P‐value | |
|
Sex Male (n = 184) vs. Female (n = 216) |
1.048 | 0.704‐1.560 | 0.818 | 1.087 | 0.705‐1.675 | 0.706 |
|
Age (years) >67 (n = 210) vs. ≤67 (n = 190) |
1.348 | 0.897‐2.025 | 0.151 | 1.639 | 1.079‐2.490 | 0.021 |
|
Smoking history Positive (n = 339) vs. Negative (n = 61) |
1.069 | 0.569‐2.006 | 0.836 | 1.521 | 0.766‐3.020 | 0.230 |
|
Pathological stage II, III, IV (n = 184) vs. I (n = 216) |
2.620 | 1.725‐3.979 | 6.21E‐6 | — | — | — |
|
Tumor size pT2‐4 (n = 272) vs. pT1 (n = 128) |
1.631 | 0.978‐2.720 | 0.0609 | 1.565 | 0.922‐2.658 | 0.097 |
|
N stage (pN) pN1‐3 (n = 136) vs. pN0 (n = 264) |
2.475 | 1.662‐3.688 | 8.32E‐6 | 2.487 | 1.649‐3.750 | 1.38E‐5 |
|
M stage (pM) pM1 (n 19) vs. pM0 (n = 381) |
1.539 | 0.773‐3.066 | 0.220 | 1.528 | 0.752‐ 3.103 | 0.241 |
|
GAD1 mRNA expression High (n = 217) vs. Low (n = 183) |
1.749 | 1.165‐2.626 | 6.97E‐3 | 1.573 | 1.029‐2.404 | 0.036 |
Statistically significant values are in boldface type.
The analysis was performed in 400 patients with complete clinical information in the TCGA dataset.
The population was divided using the auto‐selected best value of GAD1 mRNA expression as the cutoff.
To validate this result, we performed survival analysis by drawing Kaplan‐Meier survival curves using KM plotter (Figure 2B). A total of 9 studies from the GEO dataset were included (Table S5). In a total of 720 patients with LADC from 9 cohorts, high GAD1 mRNA expression also significantly correlated with worse OS. In subgroup analysis of OS using datasets of KM plotter, heterogeneous results were obtained among different cohorts. Larger cohorts such as GSE31210 and GSE50081 consistently showed that higher GAD1 mRNA expression was a poor prognostic factor, whereas cohorts with a smaller number of cases showed varying results (Figure S2). The results of univariate Cox regression analysis confirmed these results (Figure 2C).
3.4. Immunohistochemical staining pattern of GAD1 and its association with prognosis in LADC tumors
To further validate the prognostic significance of GAD1 expression status, we further examined the correlation between GAD1 protein expression and clinicopathological features including prognosis in patients with LADC. We performed immunohistochemical staining of GAD1 in tissue samples from our cohort of 162 patients with LADC (Table S3). Cytoplasmic GAD1 staining was observed in LADC tumor cells with higher mRNA expression, whereas nearly no staining was observed in normal lung epithelial cells and either tumorous or non‐tumorous epithelial cells in LADC with lower mRNA expression (Figure 3A). According to the staining score (Table S6B), 112 patients (69.1%) were classified into the group with tumors showing GAD1 protein overexpression (positive GAD1 immunoreactivity). Among the various clinicopathological factors, the pathological stage, pleural invasion, and lymph vessel invasion were identified as factors significantly and positively associated with positive GAD1 immunoreactivity (Table 2). Lymph node metastasis also tended to be more frequently observed in the positive GAD1 immunoreactivity group.
Figure 3.
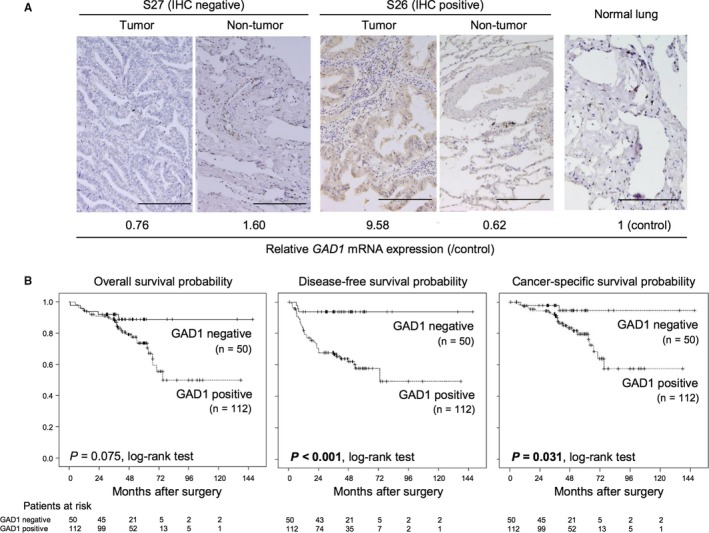
Association between GAD1 protein expression status and prognosis in patients with LADC. (A) Representative images of immunohistochemically detected GAD1 protein in tumors and non‐tumorous lesions of LADC samples and normal lung tissue. Scale bars, 200 μm. The relative GAD1 mRNA expression level of each sample as determined by rqRT‐PCR is also shown. (B) Kaplan‐Meier curves for overall survival, disease‐free survival, and cancer‐specific survival rates of 162 LADC patients according to the immunoreactivity of GAD1. P‐values were calculated using the log‐rank test. Statistically significant P‐values are in boldface type
Table 2.
Correlation between GAD1 immunoreactivity and clinicopathological factors in 162 patients with LADC
| Factor | GAD1 immunoreactivity (n = 162) | P‐valuea | |
|---|---|---|---|
| Negative (n = 50) | Positive (n = 112) | ||
| Male/Female | 26/24 | 55/57 | 0.865 |
| Ageb | 69.0 ± 9.6 | 67.4 ± 9.0 | 0.386 |
| Smoking historyc (+/−) | 22/27 | 55/56 | 0.603 |
| Brinkman indexb, c | 406.5 ± 536.4 | 485.0 ± 622.7 | 0.461 |
| Tumor sizeb, c | 23.5 ± 14.3 | 26.1 ± 13.4 | 0.226 |
| pStage (I/II + III) | 39/11 | 65/47 | 0.021 |
| Lymph node metastasis (±) | 8/42 | 36/76 | 0.054 |
| Pleural invasionc (±) | 5/40 | 35/72 | 0.005 |
| Vascular invasionc (±) | 5/40 | 22/79 | 0.284 |
| Lymph vessel invasionc(±) | 6/39 | 31/66 | 0.023 |
| EGFR mutationc(±) | 10/6 | 30/29 | 0.573 |
| Predominant histologic subtype (lepidic/papillary/acinar/ solid/ enteric) | 23/18/4/4/1 | 36/47/24/5/0 | 0.068 |
P‐values were calculated using Fischer's exact test for gender, smoking history, lymph node metastasis, pleural invasion, lymph vessel invasion, and vascular invasion, EGFR mutation, and using Student's t test for age, Brinkman index, and tumor size and using χ 2 test for trend for predominant histologic subtype. Statistically significant values (P < 0.05) are in boldface type.
Age, Brinkman index, and tumor size are expressed as the mean ± SD.
Data of these factors were not available for all patients.
According to the GAD1 protein expression status of LADC tumors, Kaplan‐Meier curves of estimated OS, disease‐free survival (DFS), and cancer‐specific survival (CSS) were generated. Patients with GAD1 protein‐overexpressing tumors showed significantly poorer DFS (P < 0.001, log‐rank test) and CSS (P = 0.031, log‐rank test) than those without GAD1 protein overexpressing tumors. Patients with GAD1 protein‐overexpressing tumors tended to show poorer OS, although the difference between groups was not significant (Figure 3B). Univariate Cox regression analysis confirmed that positive GAD1 immunoreactivity was significantly associated with a worse prognostic significance for DFS (Table 3). Multivariate Cox regression analysis in 162 patients revealed that GAD1 immunoreactivity was an independent prognostic factor for DFS (P = 0.011, hazard ratio = 6.424, Table 3), but not for OS and CSS (Tables S8 and S9).
Table 3.
Cox proportional hazard regression analysis for disease‐free survival in 162 patients with LADC
| Factor | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | P‐value | Hazard ratio | 95% confidence interval | P‐value | |
|
Sex Male (n = 81) vs. Female (n = 81) |
1.202 | 0.666‐2.170 | 0.541 | 2.459 | 0.520‐11.624 | 0.256 |
|
Age (years) >67 (n = 87) vs. ≤67 (n = 75) |
1.048 | 0.582‐1.887 | 0.875 | 0.995 | 0.515‐1.922 | 0.988 |
|
Smoking historya
Positive (n = 77) vs. Negative (n = 83) |
1.302 | 0.724‐2.344 | 0.378 | 0.324 | 0.068‐1.546 | 0.158 |
|
Pathological stage II, III (n = 58) vs. I (n = 104) |
7.466 | 3.769‐14.789 | <0.001 | — | — | — |
|
Tumor sizea
pT2‐4 (n = 39) vs. pT1 (n = 115) |
2.309 | 1.241‐4.296 | 0.008 | 2.033 | 0.961‐4.303 | 0.070 |
|
N stage (pN) pN1‐3 (n = 44) vs. pN0 (n = 118) |
7.100 | 3.837‐13.140 | <0.001 | 2.507 | 1.057‐5.949 | 0.037 |
|
Pleural invasiona
Positive (n = 40) vs. Negative (n = 112) |
4.926 | 2.637‐9.202 | <0.001 | 2.091 | 0.977‐4.478 | 0.058 |
|
Vascular invasiona
Positive (n = 27) vs. Negative (n = 119) |
4.706 | 2.529‐8.757 | <0.001 | 1.139 | 0.389‐3.341 | 0.812 |
|
Lymph vessel invasiona
Positive (n = 37) vs. Negative (n = 105) |
5.346 | 2.809‐10.175 | <0.001 | 1.355 | 0.478‐3.847 | 0.568 |
|
Adjuvant chemotherapya
With (n = 47) vs. Without (n = 106) |
2.972 | 1.614‐5.470 | <0.001 | — | — | — |
|
EGFR mutationa
Negative (n = 35) vs. Positive (n = 40) |
1.285 | 0.678‐2.433 | 0.442 | — | — | — |
|
Predominant subtype Non‐lepidic (n = 103) vs. Lepidic (n = 59) |
6.711 | 2.392‐ 18.868 | <0.001 | 2.725 | 0.861‐8.621 | 0.088 |
|
GAD1 immunoreactivity Positive (n = 112) vs. Negative (n = 50) |
9.341 | 2.248‐38.824 | 0.002 | 6.424 | 1.522‐27.108 | 0.011 |
Statistically significant values are in boldface type.
Data of these factors were not available for all patients.
4. DISCUSSION
In the present study, we focused on GAD1 as a hypermethylated gene at specific CpG sites in LADC tumors and demonstrated its overexpression in tumor‐specific and methylation level‐associated manners in LADC. We also demonstrated the prognostic significance of GAD1 mRNA and protein expression levels in resected LADC tumors using various independent publicly available datasets and our cohort, respectively. Our study suggested that GAD1 overexpression may be a useful biomarker for predicting the prognosis of patients with LADC.
GAD1 is known to catalyze the production of γ‐aminobutyric acid (GABA) from L‐glutamic acid, the principal inhibitory neurotransmitter in the brain.13, 14 GAD1 overexpression has been reported in various neoplastic tissues, but not in LADC. Moreover, the associations between clinicopathological characteristics and GAD1 expression have not been well‐established. The most striking finding in this study is the prognostic significance of GAD1 mRNA and protein expression in patients with LADC. Although a sufficient number of RNA samples suitable for expression analysis was not available in our cohort for survival analyses, we used various publicly available data and demonstrated that GAD1 mRNA overexpression in tumors was significantly associated with poor prognosis (OS) in independent TCGA and GEO datasets of LADC cases. In immunohistochemical analysis using our cohort, a positive cytoplasmic GAD1 staining pattern in tumor cells was significantly associated with poor prognosis, particularly DFS but not OS, in patients with LADC. Although the difference in the association between GAD1 expression and OS among datasets remains unclear, it may be explained by (a) variations in GAD1 mRNA and protein expression, (b) the smaller size of the cohort for immunohistochemical analysis compared to those of cohorts used for mRNA analysis used in our study, and (c) variations in GAD1 expression level and/or pattern among different ethnicities.
Our study also demonstrated that GAD1 protein expression in LADC was significantly associated with pleural invasion and lymph vessel invasion. These findings suggest that GAD1 overexpression might be closely associated with cellular invasion. This hypothesis is supported by previous reports of other cancers. Kimura et al6 demonstrated that GAD1 promotes the cancer cell invasion and metastasis of oral cancer by inducing the nuclear translocation of β‐catenin and secretion of MMP7,15, 16, 17, 18, 19, 20 although the regulatory mechanisms of GAD1 in β‐catenin translocation remain unclear. In a brain metastasis model, it was reported that the metastatic activity of tumor cells depends on the GAD1‐GABA synthesis pathway.21 Further studies are needed to clarify the tumor‐promoting activity of overexpressed GAD1.
Recently, Yan et al5 reported hypermethylation‐associated GAD1 overexpression in colorectal and liver cancers and found that this paradoxical effect was caused by the hypermethylation of the CTCF‐binding site within GAD1, which may prevent CTCF binding, inhibit CTCF‐mediated repressive Polycomb repressive complex 2 (PRC2) complex recruitment to the GAD1 promoter, inhibit PRC2‐induced trimethylation of histone H3 lysine 27 (H3K27m3), and eliminate the blocking activity H3K27m3 for GAD1 transcription.22, 23 These observations are contradictory to the well‐established paradigm that promoter DNA methylation represses transcription by inhibiting transcription factor binding and/or chromatin structure modification.24, 25, 26 In this study, we also detected hypermethylation at cg15126544 within the CTCF‐binding site in LADC tumors, and tumor‐specific GAD1 overexpression was positively associated with hypermethylation at cg15126544 in our cohort and the TCGA dataset. Therefore, methylation of CTCF‐binding sites may regulate GAD1 expression in LADC as well. However, it remains unknown whether the methylation of CGI or each CpG site around GAD1, particularly cg15126544, is the only mechanism underlying the regulation of its transcription. Interestingly, in brain metastatic tumor cells, it was reported that the downregulation of the DNA methyltransferase DNMT1 induced by the brain microenvironment‐derived clusterin resulted in decreased GAD1 promoter methylation and subsequent upregulation of GAD1 expression.21 Therefore, even the effect of methylation levels of CpG sites around GAD1 on its expression level may vary under different conditions or in different cell lineages. Indeed, MethSurv, a web tool for multivariable survival analysis using DNA methylation data obtained from TCGA datasets (https://biit.cs.ut.ee/methsurv/), failed to show the prognostic significance of CpG sites around GAD1, including cg15126544 for OS (data not shown). Therefore, the methylation status of some CpG sites around GAD1 may contribute to its gene expression at some stages of LADC development, but not to the progression of this tumor. The GAD1 mRNA expression level data in normal lung tissues available in public databases, such as the NIH Genotype‐Tissue Expression Project (https://www.gtexportal.org/), as well as our immunohistochemical staining results revealed no or low GAD1 expression in normal lung tissue, suggesting that GAD1 is specifically expressed in tumor cells and contributes to the progression of tumors in LADC. Because the gene expression status appears to more directly contribute to the establishment of clinicopathological phenotypes in tumor cells, it is necessary to investigate the detailed regulatory mechanisms of GAD1 expression in LADC cells at each developmental stage of the tumor.
There are some limitations to this study. First, we demonstrated the prognostic impact of GAD1 mRNA and protein statuses mainly in Caucasian and Japanese (Asian) populations, respectively, but no data are available to directly compare GAD1 mRNA and protein expression levels among different ethnicities. Because it has been reported that the frequency of acquired alterations, such as epidermal growth factor receptor mutation, in lung tumors can vary across different ethnicities,27, 28, 29 it is possible that the GAD1 expression pattern and/or levels differ between Caucasian and Asian populations. However, the prognostic significance of the GAD1 mRNA expression status in Japanese cases with LADC was demonstrated by GSE31210 in GEO datasets (Figure 2C and Figure S2). Meta‐analysis using 9 GEO datasets, including GSE31210 and 8 other studies from western countries (Table S5) also revealed the prognostic significance of the GAD1 mRNA expression status (Figure 2C), suggesting that GAD1 overexpression is a common prognostic factor in various populations. Second, our patient cohort was relatively small even for immunohistochemical analysis, and a sufficient number of samples were not available for mRNA expression analysis to perform survival analysis. Prospective multiinstitutional studies are needed to further validate the prognostic value of GAD1 overexpression in patients with LADC.
5. CONCLUSION
GAD1 overexpression appears to be a significant and independent prognostic indicator in patients with resected LADC at both the mRNA and protein levels. This information may be helpful for identifying patients at high risk of recurrence and overall survival after tumor resection of LADC.
CONFLICT OF INTEREST
All authors declare no conflicts of interest associated with this manuscript.
Supporting information
ACKNOWLEDGMENT
The authors would like to thank Dr. Yoshimi Bando, Division of Pathology, Tokushima University Hospital for her contributing to this study. This work was supported by JSPS KAKENHI (grant number JP18H02894). The funding source was not involved in the design of the study, in the collection, analysis, and interpretation of results, in the preparation of the manuscript, or in the decision to submit the article for publication.
Tsuboi M, Kondo K, Masuda K, et al. Prognostic significance of GAD1 overexpression in patients with resected lung adenocarcinoma. Cancer Med. 2019;8:4189–4199. 10.1002/cam4.2345
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson DH, Schiller JH, Bunn PA. Recent clinical advances in lung cancer management. J Clin Oncol. 2014;32(10):973‐982. [DOI] [PubMed] [Google Scholar]
- 4. Kajiura K, Masuda K, Naruto T, et al. Frequent silencing of the candidate tumor suppressor TRIM58 by promoter methylation in early‐stage lung adenocarcinoma. Oncotarget. 2017;10(2):2890‐2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan H, Tang G, Wang H, et al. DNA methylation reactivates GAD1 expression in cancer by preventing CTCF‐mediated polycomb repressive complex 2 recruitment. Oncogene. 2016;35(30):3995‐4008. [DOI] [PubMed] [Google Scholar]
- 6. Kimura R, Kasamatsu A, Koyama T, et al. Glutamate acid decarboxylase 1 promotes metastasis of human oral cancer by beta‐catenin translocation and MMP7 activation. BMC Cancer. 2013;13:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee Y‐Y, Chao T‐B, Sheu M‐J, et al. Glutamate decarboxylase 1 overexpression as a poor prognostic factor in patients with nasopharyngeal carcinoma. J Cancer. 2016;7(12):1716‐1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maemura K, Yamauchi H, Hayasaki H, et al. Gamma‐amino‐butyric acid immunoreactivity in intramucosal colonic tumors. J Gastroenterol Hepatol. 2003;18(9):1089‐1094. [DOI] [PubMed] [Google Scholar]
- 9. Matuszek M, Jesipowicz M, Kleinrok Z. GABA content and GAD activity in gastric cancer. Med Sci Monit. 2001;7(3):377‐381. [PubMed] [Google Scholar]
- 10. Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thoracic Oncol. 2007;2(8):706‐714. [DOI] [PubMed] [Google Scholar]
- 11. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart, 4th edn Lyon: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 12. Győrffy B, Surowiak P, Budczies J, Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non‐small‐cell lung cancer. PLoS ONE. 2013;18:e82241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16(3):215‐226. [DOI] [PubMed] [Google Scholar]
- 14. Young SZ, Bordey A. GABA's control of stem and cancer cell proliferation in adult neural and peripheral niches. Physiology (Bethesda). 2009;24:171‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kruck S, Eyrich C, Scharpf M, et al. Impact of an altered Wnt1/β‐catenin expression on clinicopathology and prognosis in clear cell renal cell carcinoma. Int J Mol Sci. 2013;14(6):10944‐10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sawada G, Ueo H, Matsumura T, et al. CHD8 is an independent prognostic indicator that regulates Wnt/β‐catenin signaling and the cell cycle in gastric cancer. Oncol Rep. 2013;30(3):1137‐1142. [DOI] [PubMed] [Google Scholar]
- 17. Abd El‐Rehim D, Ali MM. Aberrant expression of β‐catenin in invasive ductal breast carcinomas. J Egypt Natl Canc Inst. 2009;21(2):185‐195. [PubMed] [Google Scholar]
- 18. Ishikawa T, Kimura Y, Hirano H, Higashi S. Matrix metalloproteinase‐7 induces homotypic tumor cell aggregation via proteolytic cleavage of the membrane‐bound Kunitz‐type inhibitor HAI‐1. J Biol Chem. 2017;292(50):20769‐20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mylona E, Kapranou A, Mavrommatis J, Markaki S, Keramopoulos A, Nakopoulou L. The multifunctional role of the immunohistochemical expression of MMP‐7 in invasive breast cancer. APMIS. 2005;113(4):246‐255. [DOI] [PubMed] [Google Scholar]
- 20. Wang FQ, So J, Reierstad S, Fishman DA. Matrilysin (MMP‐7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer. 2005;114(1):19‐31. [DOI] [PubMed] [Google Scholar]
- 21. Schnepp PM, Lee DD, Guldner IH, et al. GAD1 upregulation programs aggressive features of cancer cell metabolism in the brain metastatic microenvironment. Cancer Res. 2017;77(11):2844‐2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128(4):735‐745. [DOI] [PubMed] [Google Scholar]
- 23. Li T, Hu J‐F, Qiu X, et al. CTCF regulates allelic expression of Igf2 by orchestrating a promoter‐polycomb repressive complex 2 intrachromosomal loop. Mol Cell Biol. 2008;28(20):6473‐6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Razin A, Riggs AD. DNA methylation and gene function. Science. 1980;210(4470):604‐610. [DOI] [PubMed] [Google Scholar]
- 25. Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187(4173):226‐232. [PubMed] [Google Scholar]
- 26. Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396‐398. [DOI] [PubMed] [Google Scholar]
- 27. Arrieta O, Cardona AF, Martín C, et al. Updated frequency of EGFR and KRAS mutations in non‐small‐cell lung cancer in Latin America: The Latin‐American consortium for the investigation of lung cancer (CLICaP). J Thorac Oncol. 2015;10(5):838‐843. [DOI] [PubMed] [Google Scholar]
- 28. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339‐346. [DOI] [PubMed] [Google Scholar]
- 29. Campbell JD, Lathan C, Sholl L, et al. Comparison of prevalence and types of mutations in lung cancers among black and white populations. JAMA Oncol. 2017;3(6):801‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


