Abstract
Object
To ascertain the treatment effect of concurrent chemotherapy (CCT) in stage II‐III nasopharyngeal carcinoma (NPC) patients with different Epstein‐Barr virus (EBV) DNA level in intensity‐modulated radiotherapy (IMRT) era.
Methods
A total of 2742 patients diagnosed with stage II‐III NPC were involved in this study. Patients received IMRT with/without CCT. Overall survival (OS) was the primary endpoint. Receiver operating characteristics curve was used to determine the cut‐off value of pre‐DNA based on OS. After propensity score matching, the role of CCT was explored in patients with different EBV DNA level.
Results
In our cohort, the cut‐off value of pre EBV DNA was 1460 copies/mL (area under curve [AUC], 0.695‐0.769; sensitivity, 0.766; specificity, 0.599). Patients with high EBV DNA level showed poor survival in OS, progression free survival (PFS), locoregional relapse‐free survival (LRFS) and distant metastasis‐free survival (DMFS). In patients with EBV DNA level >1460 copies/mL, the concurrent chemoradiotherapy (CCRT) group achieved higher 3‐year OS compared with IMRT groups. However, the CCRT and IMRT groups showed comparable OS in patients with EBV DNA ≤1460 copies/mL. In multivariate analyses, CCT was a protective factor for OS, PFS, and LRFS in high‐risk patients (EBV DNA level >1460 copies/mL), while not an independent prognostic factor among the low‐risk patients (EBV DNA level ≤1460 copies/mL).
Conclusion
Pre‐EBV DNA could be a useful tool to guide individualized treatment for stage II‐III NPC patients. Additional CCT to IMRT improved the survival for patients with high pre‐EBV DNA, while those with low pre‐EBV DNA could not.
Keywords: chemotherapy, Epstein‐Barr virus, nasopharyngeal carcinoma, overall survival
1. INTRODUCTION
Nasopharyngeal cancer (NPC) is an uncommon cancer, with an estimated 86,700 new cases in 2012, accounting for 0.6% of all cancers in China.1 However, it has a high incidence in the Guangdong Province, Fujian Province, and Hong Kong.2, 3 In endemic areas, the nonkeratinising undifferentiated NPC subtype comprises ~95% of cases and is inevitably correlated with Epstein‐Barr virus (EBV) infection.4
Radiotherapy (RT) is the only curative treatment for NPC because of its radiosensitivity.5 In two‐dimensional radiotherapy (2DRT) era, several studies demonstrated that concurrent chemoradiotherapy (CCRT) was recommended for locoregional advanced NPC.6, 7, 8 Recently, with the development of economics, mathematics, and computer science, intensity‐modulated radiotherapy (IMRT) replaced 2DRT in centers where this radiation technology was available. IMRT was superior to 2DRT in terms of locoregional control of cancer and it improved the patient's quality of life as a result of its spatial dose distribution in the target volume.9, 10, 11 Thus, the role of CCRT needed to be further verified in IMRT era. As the application of CCRT led to a higher incidence of late toxicities compared with RT alone,12 the individualized treatment was necessary according to different risk levels. Stratified by TNM staging system, previous study verified that the combination of concurrent chemotherapy (CCT) did not result in a survival benefit for stage II and low‐risk stage III NPC patients.13 Plasma EBV DNA levels was proven to be an important biomarker, which could monitor and predict the survival of NPC.14, 15 Our previous study established an effective prognostic nomogram with EBV DNA, which provided significantly better discrimination than TNM stage.16 Therefore, EBV DNA could be served as further supplement in risk stratification.
Based on these evidences, we carried out a retrospective analysis using a large cohort and long duration of follow‐up to identify the prognostic value of pre‐EBV DNA level for risk stratification among stage II‐III NPC patients and selected the candidates that might benefit from CCRT.
2. MATERIALS AND METHODS
2.1. Patients
Between 2008 and 2013, we assessed 2742 consecutive and unselected patients with stage II‐III NPC within the Cancer Center of Sun Yat‐sen University (Guangdong, China). All patients were restaged according to the seventh TNM staging manual from the American Joint Committee on Cancer.17 The inclusion criteria of this study were as follows: (a) newly diagnosed stage II‐III NPC; (b) age ≥18 years; (c) Karnofsky performance score ≥70; (d) no other malignancies; (e) receiving CCRT or IMRT alone; (f) CCT was single‐agent cisplatin. Flow chart of patient inclusion was shown in Figure 1. Before the diagnosis and treatment, a sequence of evaluations was conducted, including physical examination, magnetic resonance imaging (MRI) with contrast of the nasopharynx and neck, nasopharyngoscopy, radiography of the chest or contrast‐enhanced computed tomography (CT) of the chest, abdomen ultrasound or contrast‐enhanced CT of the abdomen, electrocardiography, and bone scintigraphy. PET/CT was applied optionally. The study protocol was approved by the Research Ethics Committee of the Cancer Center of Sun Yat‐sen University.
Figure 1.
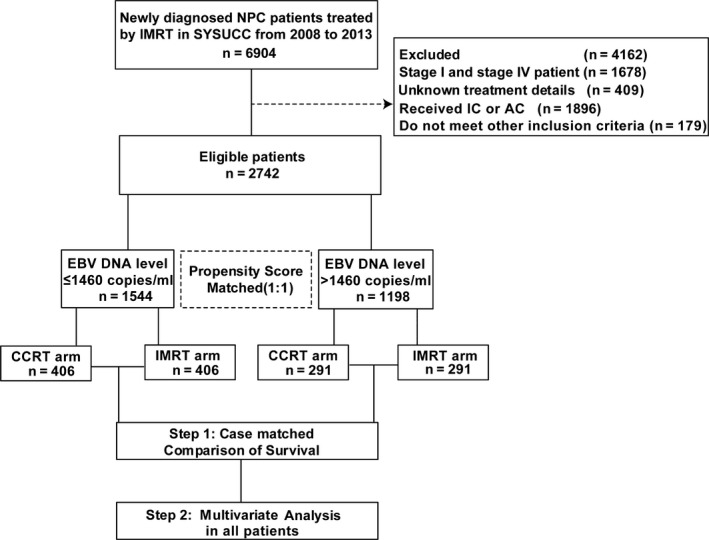
Flow chart used for patient inclusion
2.2. Quantification of plasma EBV DNA levels
Pretreatment EBV DNA levels was measured and subjected to real‐time quantitative polymerase chain reaction (qPCR) as mentioned in previous study.18 The BamHI‐W was the initially region of the qPCR system. The amplification primers involved W‐44F (5′‐AGTCTCTGCCTCCAGGCA‐3′), W‐119R (5′‐ACAGAGGGCCTGTCCACCG‐3′) and the dual‐labeled fluorescent probe W‐67T (5′‐[FAM]CACTGTCTGTAAAGTCCAG CCTCC[TAMRA]‐3′), which were consisted in this system. The β‐actin gene was served as a loading control, the primers 5′‐ACA GGCACCAGGGCGTGATGG‐3′ (forward), 5′‐CTCCATGTCGTCCCAGTTGGT‐3′ (reverse) and the dual labeled uorescent probe sequence 5′‐[FAM]CATCCTCACCCTGAAGTACCCCATC[TAMRA]‐3′ were applied. The cut‐off value of the EBV DNA level was defined by receiver operating characteristic (ROC) curve analysis for Overall survival (OS), which showed the best trade‐off between sensitivity and specificity.
2.3. Chemotherapy and RT
All patients received IMRT with or without CCT based on the treatment protocol for NPC patients at the Cancer Center of Sun Yat‐sen University. RT was performed 5 times a week at 1.8‐2.2 Gy per day by IMRT. The accumulated radiation dose to the planning target volume of a primary tumour was 66‐72 Gy. The design of the IMRT plan has been previously reported.19 CCT was administered using a cisplatin (80‐100 mg/m2, i.v.) regimen for 2‐3 cycles or dose of 30‐40 mg/m2 every week during RT.
2.4. Outcome and follow‐up
The primary endpoint of our study was OS. Progression‐free survival (PFS), locoregional relapse‐free survival (LRFS) and distant metastasis‐free survival (DMFS) served as secondary endpoints. OS was defined as the time from the date of diagnosis to the date of death from any cause. PFS was considered the time from the date of the diagnosis to the date of first failure or death from any cause. LRFS was determined as the time from the date of the diagnosis to the date of first local and/or regional failure. DMFS was the time from the date of the diagnosis to the date of distant metastasis. After treatment, patients were evaluated every 3 months in the first 3 years and every 6 months thereafter until death. Physical examination, nasopharyngoscopy, contrast‐enhanced MRI of the nasopharynx and neck, ultrasound of the abdomen, chest radiography, and measurement of plasma levels of EBV DNA were done routinely. PET/CT was considered if necessary.
2.5. Statistical analyses
Statistical analyses were conducted using SPSS v23 (IBM, Armonk, IL, USA). The propensity score for each patient was calculated to estimate their probability using multivariable logistic regression models given the following covariates: age, gender, smoking, family history of NPC, T stage, N stage, EBV‐DNA level, and overall stage. Matching was carried out by the nearest neighbor‐matching method with use of a 1:1 matching protocol with a calliper of 0.05. The Pearson χ2 test was used to assess the statistical relationship between the subgroups. Kaplan‐Meier curves were used to compare the survival in different treatment groups with log rank test. A Cox proportional hazards regression model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) in multivariate analysis. All statistical tests were 2‐tailed. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
The baseline characteristics of the original dataset were summarized in Table 1. The entire cohort carried a male‐to‐female ratio of 2.46, and the median age was 47 years old. The median follow‐up duration was 47.5 months (range, 1.3‐90.7 months). Overall, 923 (33.7%) patients received IMRT alone and 1819 (66.3%) received CCRT. The CCRT group had a higher percentage of T3, N2, and stage III disease (P < 0.001), more young patients and higher EBV DNA levels (Table S1).
Table 1.
Patient characteristics of the cohort
| Characteristic | No. of patients (%) |
|---|---|
| Age, y | |
| ≤47 | 1439 (52.5) |
| >47 | 1303 (47.5) |
| Gender | |
| Female | 792 (28.9) |
| Male | 1950 (71.1) |
| Smoking history | |
| No | 1731 (63.1) |
| Yes | 1011 (36.9) |
| NPC family history | |
| No | 2403 (87.6) |
| Yes | 340 (12.4) |
| T stageb | |
| T1 | 226 (8.2) |
| T2 | 866 (31.6) |
| T3 | 1650 (60.2) |
| N stageb | |
| N0 | 625 (8.2) |
| N1 | 1248 (31.6) |
| N2 | 869 (60.2) |
| Overall stage | |
| II | 779 (28.4) |
| III | 1963 (71.6) |
| EBV DNA levela | |
| ≤1460 copies/mL | 1554 (56.3) |
| >1460 copies/mL | 1198 (43.7) |
| Treatment method | |
| IMRT alone | 923 (33.7) |
| CCRT | 1819 (66.3) |
Pearson χ2 test was used to calculate the P‐value.
Abbreviations: CCRT, concurrent chemoradiotherapy; EBV, Epstein‐Barr virus; IMRT, intensity‐modulated radiotherapy; NPC, nasopharyngeal carcinoma.
The value of EBV‐DNA levels is based on receiver operating characteristic (ROC) curve analysis.
According to the seventh edition of UICC/AJCC staging system.
3.2. Cut‐off value of EBV DNA level
The median EBV DNA concentration for the 2742 patients was 556 copies/ml (range, 0‐44 500 000 copies/mL). Based on the ROC analysis, the cut‐off value of pre‐DNA was 1460 copies/mL for OS (sensitivity = 0.766, specificity = 0.599, 95% CI of area under curve [AUC] = 0.695‐0.769) (Figure 2). Thus, we used 1460 copies/mL as the threshold and divided patients into different risk subgroups according to EBV DNA level. We further comparatively evaluated the prognostic impact of EBV DNA. Undoubtedly, patients with EBV DNA ≤1460 copies/mL showed higher 3‐year OS and the same trend was found in PFS, LRFS, and DMFS (Figure S1). Therefore, this cut‐off value was valid, and patients with EBV DNA ≤1460 copies/mL were classified in the low‐risk group. Patients with EBV DNA >1460 copies/mL were included in the high‐risk group.
Figure 2.
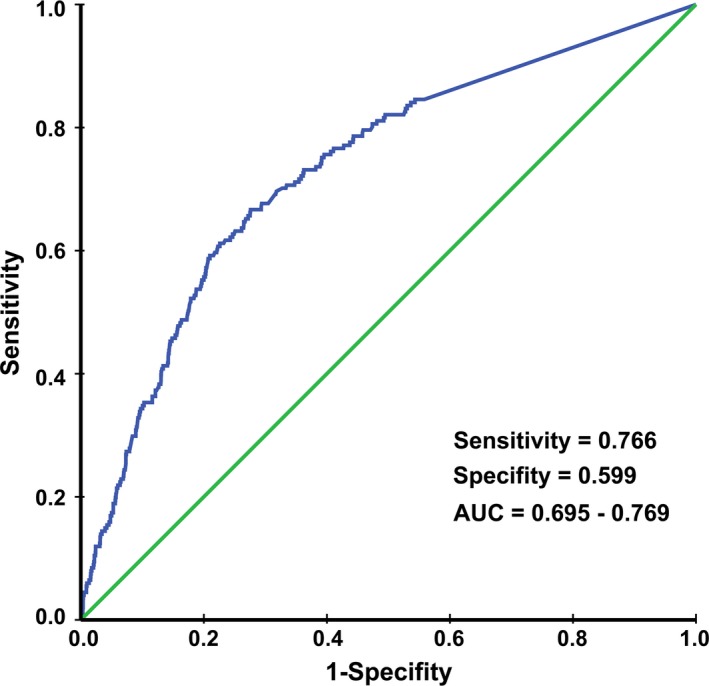
Receiver operating characteristic (ROC) curve analysis used to identify the cut‐off value of pretreatment Epstein‐Barr virus DNA
3.3. Multivariate analysis within the patient cohort
In the multivariate analysis of the patient cohort, the following variables were considered in the Cox proportional hazards model: age, gender, smoking, family history of NPC, T stage, N stage, level of EBV DNA, and treatment regimen. As shown in Table 2, application of CCRT was associated with a lower risk of death (HR, 0.582; 95% CI, 0.430‐0.788; P < 0.001), tumour progression (0.708; 0.562‐0.893; P = 0.004) and locoregional relapse (0.590; 0.404‐0.861; P = 0.006) than the application of IMRT alone. In addition, multivariate analysis revealed that the presence of high plasma EBV DNA remained an independent prognostic factor for poor OS (3.581; 2.503‐5.125; P < 0.001), PFS (3.353; 2.607‐4.311; P < 0.001), LRFS (3.049; 2.041‐4.555; P < 0.001) and DMFS (3.908; 2.794‐5.467; P < 0.001) in patients.
Table 2.
Multivariable analysis of prognostic factors for OS, PFS, LRFS, and DMFS
| Characteristic | HR (95% CI) | P‐value |
|---|---|---|
| Overall survival | ||
| Age | 1.592 (1.191‐2.130) | 0.002 |
| Gender | 2.419 (1.413‐3.268) | <0.001 |
| Smoking | 1.086 (0.925‐1.275) | 0.314 |
| Family history of NPC | 0.809 (0.519‐1.261) | 0.349 |
| T stage | ||
| T2 vs T1 | 1.439 (0.705‐2.938) | 0.317 |
| T3 vs T1 | 1.945 (0.980‐3.859) | 0.057 |
| N stage | ||
| N1 vs N0 | 1.033 (0.673‐1.585) | 0.883 |
| N2 vs N0 | 1.506 (0.967‐2.345) | 0.070 |
| EBV‐DNA level | 3.581 (2.503‐5.125) | <0.001 |
| Treatment method | 0.582 (0.430‐0.788) | <0.001 |
| Progression free survival | ||
| Age | 1.127 (0.911‐1.393) | 0.270 |
| Gender | 1.543 (1.167‐2.039) | 0.002 |
| Smoking | 1.025 (0.908‐1.159) | 0.687 |
| Family history of NPC | 0.881 (0.638‐1.217) | 0.442 |
| T stage | ||
| T2 vs T1 | 1.474 (0.889‐2.442) | 0.132 |
| T3 vs T1 | 1.675 (1.029‐2.727) | 0.038 |
| N stage | ||
| N1 vs N0 | 1.003 (0.737‐1.364) | 0.986 |
| N2 vs N0 | 1.191 (0.859‐1.653) | 0.295 |
| EBV‐DNA level | 3.353 (2.607‐4.311) | <0.001 |
| Treatment method | 0.708 (0.562‐0.893) | 0.004 |
| Loco‐regional relapse‐free survival | ||
| Age | 1.019 (0.718‐1.446) | 0.916 |
| Gender | 1.605 (1.018‐2.531) | 0.042 |
| Smoking | 0.991 (0.810‐1.213) | 0.931 |
| Family history of NPC | 1.256 (0.787‐2.005) | 0.340 |
| T stage | ||
| T2 vs T1 | 2.519 (0.997‐6.364) | 0.051 |
| T3 vs T1 | 2.028 (0.808‐5.088) | 0.132 |
| N stage | ||
| N1 vs N0 | 0.941 (0.589‐1.503) | 0.799 |
| N2 vs N0 | 0.924 (0.547‐1.562) | 0.768 |
| EBV‐DNA level | 3.049 (2.041‐4.555) | <0.001 |
| Treatment method | 0.590 (0.404‐0.861) | 0.006 |
| Distant metastasis‐free survival | ||
| Age | 1.061 (0.811‐1.387) | 0.667 |
| Gender | 1.479 (1.039‐2.103) | 0.030 |
| Smoking | 1.019 (0.872‐1.190) | 0.817 |
| Family history of NPC | 0.844 (0.556‐1.280) | 0.425 |
| T stage | ||
| T2 vs T1 | 1.064 (0.577‐1.963) | 0.842 |
| T3 vs T1 | 1.539 (0.866‐2.734) | 0.142 |
| N stage | ||
| N1 vs N0 | 1.246 (0.806‐1.927) | 0.323 |
| N2 vs N0 | 1.593 (1.015‐3.500) | 0.043 |
| EBV‐DNA level | 3.908 (2.794‐5.467) | <0.001 |
| Treatment method | 0.782 (0.580‐1.055) | 0.107 |
A Cox proportional hazard model was used to perform multivariate analyses. All variables were transformed into categorical variables. HRs were calculated for Age (years) (>47 vs ≤47); Gender (Male vs Female); Smoking (Yes vs No); Family history of NPC (Yes vs No); EBV DNA level (>1460 copies/mL vs ≤1460 copies/mL) and Treatment method (CCRT vs IMRT alone).
Abbreviations: CI, confidence interval; DMFS, distant metastasis‐free survival; EBV, Epstein–Barr virus; HR, hazard ratio; NPC, nasopharyngeal carcinoma; OS, Overall survival; LRFS, locoregional relapse‐free survival; PFS, progression free survival.
3.4. Survival outcomes within low EBV DNA group
We further evaluated the survival difference between the IMRT alone and CCRT groups among patients with low DNA. In total, 1544 patients demonstrated EBV DNA ≤1460 copies/mL. After matching, 406 pairs were selected and a well‐balanced cohort was created (Table 3). All reported parameters were balanced between the two groups, respectively, and no statistical differences were detected. The 3‐year OS, PFS, LRFS, and DMFS rates for IMRT alone vs CCRT were 97.7% vs 97.9% (P = 0.321), 93.3% vs 94.2% (P = 0.677), 95.6% vs 98.0% (P = 0.298) and 97.1% vs 96.9% (P = 0.992; Figure 3), respectively. Multivariate analysis found that there was no significant survival difference between IMRT alone and CCRT groups (P > 0.05 for all survival endpoints, Table 4). Therefore, IMRT alone and CCRT achieved similar outcomes in the low EBV DNA group.
Table 3.
Clinical characteristics of patients in different risk groups according to EBV‐DNA levels
| Characteristic | Low‐risk patientsa (n = 812) | High‐risk patientsa (n = 582) | ||||
|---|---|---|---|---|---|---|
| LRRT | CCRT | P‐value | LRRT | CCRT | P‐value | |
| Total | 406 | 406 | 291 | 291 | ||
| Age, y | ||||||
| ≤47 | 204 (50.2) | 227 (55.9) | 0.122 | 132 (45.4) | 156 (53.6) | 0.056 |
| >47 | 202 (49.8) | 179 (44.1) | 159 (54.6) | 135 (46.4) | ||
| Gender | ||||||
| Female | 112 (27.6) | 115 (28.3) | 0.876 | 83 (28.5) | 86 (29.6) | 0.855 |
| Male | 294 (72.4) | 291 (71.7) | 208 (71.5) | 205 (70.4) | ||
| Smoking history | ||||||
| No | 264 (65.0) | 285 (70.2) | 0.283 | 169 (58.1) | 163 (56.0) | 0.527 |
| Yes | 142 (35.0) | 121 (29.8) | 122 (41.9) | 128 (44.0) | ||
| NPC family history | ||||||
| No | 355 (87.4) | 357 (87.9) | 0.915 | 251 (86.3) | 250 (85.9) | 1.000 |
| Yes | 51 (12.6) | 49 (12.1) | 40 (13.7) | 41 (14.1) | ||
| T stageb | ||||||
| T1 | 45 (11.1) | 54 (13.3) | 0.606 | 26 (8.9) | 38 (13.1) | 0.288 |
| T2 | 167 (41.1) | 159 (39.2) | 94 (32.3) | 88 (30.2) | ||
| T3 | 194 (47.8) | 193 (47.5) | 171 (58.8) | 165 (56.7) | ||
| N stageb | ||||||
| N0 | 152 (37.4) | 158 (38.9) | 0.415 | 42 (14.4) | 36 (12.4) | 0.687 |
| N1 | 225 (55.4) | 228 (56.2) | 138 (47.4) | 147 (50.5) | ||
| N2 | 29 (7.1) | 20 (4.9) | 111 (38.1) | 108 (37.1) | ||
| Overall stage | ||||||
| II | 197 (48.5) | 199 (49.0) | 0.944 | 72 (24.7) | 32 (25.8) | 0.849 |
| III | 209 (51.5) | 207 (51.0) | 39 (75.3) | 29 (74.2) | ||
P‐value was calculated with the Pearson χ2 test.
Abbreviations: CCRT, concurrent chemoradiotherapy; LRRT, locoregional radiotherapy; NPC, nasopharyngeal carcinoma.
Low‐risk patients: EBV‐DNA levels ≤1460 copies/mL; High‐risk patients: EBV‐DNA levels >1460 copies/mL.
According to the seventh edition of UICC/AJCC staging system.
Figure 3.
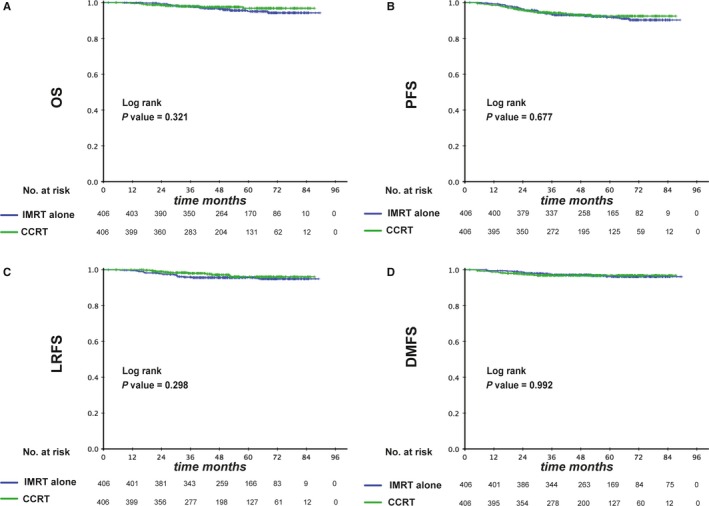
Kaplan‐Meier survival curves between the IMRT and CCRT groups in low‐risk patients (pre‐EBV DNA ≤1460 copies/mL). Shown are results for overall survival (A), progression free survival (B), locoregional relapse free survival (C), and distant metastasis free survival (D). P values were calculated using the unadjusted log‐rank test. EBV, Epstein–Barr virus; CCRT, concurrent chemoradiotherapy; IMRT, intensity‐modulated radiotherapy
Table 4.
Multivariate analysis of OS, PFS, LRFS, and DMFS in low‐ and high‐risk patients according to EBV DNA level
| Characteristic | Low‐risk patients | High‐risk patients | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Overall survival | ||||
| Age | 3.071 (1.561‐6.042) | 0.001 | 1.350 (0.975‐1.871) | 0.071 |
| Gender | 5.398 (1.590‐18.318) | 0.007 | 1.825 (1.160‐2.872) | 0.009 |
| Smoking | 1.270 (0.924‐1.745) | 0.140 | 1.036 (0.860‐1.248) | 0.709 |
| Family history of NPC | 0.341 (0.082‐1.409) | 0.137 | 0.955 (0.595‐1.532) | 0.848 |
| T stage | ||||
| T2 vs T1 | 0.911 (0.294‐2.823) | 0.872 | 1.871 (0.732‐4.782) | 0.191 |
| T3 vs T1 | 1.312 (0.441‐3.901) | 0.625 | 2.504 (1.014‐6.182) | 0.047 |
| N stage | ||||
| N1 vs N0 | 1.143 (0.582‐2.248) | 0.698 | 0.990 (0.566‐1.731) | 0.973 |
| N2 vs N0 | 2.258 (0.936‐5.446) | 0.070 | 1.400 (0.815‐2.406) | 0.223 |
| Treatment method | 0.603 (0.307‐1.181) | 0.140 | 0.575 (0.408‐0.811) | 0.002 |
| Progression free survival | ||||
| Age | 1.211 (0.806‐1.819) | 0.356 | 1.101 (0.858‐1.413) | 0.448 |
| Gender | 2.305 (1.304‐4.074) | 0.004 | 1.339 (0.970‐1.850) | 0.076 |
| Smoking | 1.021 (0.816‐1.276) | 0.858 | 1.039 (0.898‐1.202) | 0.609 |
| Family history of NPC | 0.701 (0.353‐1.391) | 0.310 | 0.935 (0.648‐1.350) | 0.720 |
| T stage | ||||
| T2 vs T1 | 1.214 (0.497‐2.969) | 0.670 | 1.623 (0.880‐2.995) | 0.121 |
| T3 vs T1 | 1.960 (0.825‐4.655) | 0.127 | 1.583 (0.877‐2.858) | 0.127 |
| N stage | ||||
| N1 vs N0 | 0.995 (0.633‐1.565) | 0.983 | 1.002 (0.654‐1.538) | 0.991 |
| N2 vs N0 | 1.293 (0.712‐2.348) | 0.399 | 1.163 (0.762‐1.774) | 0.484 |
| Treatment method | 0.763 (0.484‐1.201) | 0.242 | 0.672 (0.513‐0.880) | 0.004 |
| Loco‐regional relapse‐free survival | ||||
| Age | 0.866 (0.463‐1.618) | 0.651 | 1.080 (0.705‐1.652) | 0.724 |
| Gender | 1.523 (0.683‐3.396) | 0.304 | 1.655 (0.951‐2.880) | 0.074 |
| Smoking | 1.093 (0.770‐1.552) | 0.618 | 0.954 (0.746‐1.221) | 0.709 |
| Family history of NPC | 0.713 (0.254‐1.999) | 0.520 | 1.475 (0.866‐2.515) | 0.153 |
| T stage | ||||
| T2 vs T1 | 1.287 (0.363‐4.569) | 0.696 | 4.489 (1.075‐18.737) | 0.039 |
| T3 vs T1 | 1.781 (0.508‐6.236) | 0.367 | 2.838 (0.685‐11.763) | 0.150 |
| N stage | ||||
| N1 vs N0 | 1.158 (0.590‐2.272) | 0.669 | 0.740 (0.391‐1.401) | 0.355 |
| N2 vs N0 | 0.875 (0.301‐2.544) | 0.806 | 0.787 (0.416‐1.487) | 0.461 |
| Treatment method | 0.526 (0.263‐1.051) | 0.069 | 0.605 (0.384‐0.954) | 0.031 |
| Distant metastasis‐free survival | ||||
| Age | 1.252 (0.706‐2.218) | 0.442 | 1.019 (0.751‐1.382) | 0.904 |
| Gender | 2.647 (1.197‐5.856) | 0.016 | 1.243 (0.835‐1.851) | 0.284 |
| Smoking | 0.878 (0.637‐1.210) | 0.425 | 1.080 (0.903‐1.291) | 0.402 |
| Family history of NPC | 0.787 (0.312‐1.984) | 0.611 | 0.855 (0.536‐1.365) | 0.512 |
| T stage | ||||
| T2 vs T1 | 0.683 (0.212‐2.203) | 0.524 | 1.232 (0.598‐2.538) | 0.572 |
| T3 vs T1 | 1.596 (0.546‐4.661) | 0.393 | 1.507 (0.761‐2.981) | 0.239 |
| N stage | ||||
| N1 vs N0 | 1.028 (0.526‐2.006) | 0.937 | 1.397 (0.764‐2.552) | 0.277 |
| N2 vs N0 | 1.777 (0.808‐3.904) | 0.153 | 1.654 (0.912‐3.001) | 0.098 |
| Treatment method | 0.870 (0.455‐1.665) | 0.674 | 0.739 (0.528‐1.035) | 0.079 |
A Cox proportional hazard model was used to perform multivariate analyses. All variables were transformed into categorical variables. HRs were calculated for Age (years) (>47 vs ≤47); Gender (Male vs Female); Smoking (Yes vs No); Family history of NPC (Yes vs No); EBV DNA level (>1460 copies/mL vs ≤1460 copies/mL) and Treatment method (CCRT vs IMRT alone).
Abbreviations: CI, confidence interval; DMFS, distant metastasis‐free survival; EBV, Epstein–Barr virus; HR, hazard ratio; NPC, nasopharyngeal carcinoma; OS, Overall survival; LRFS, locoregional relapse‐free survival; PFS, progression free survival.
3.5. Survival outcomes within high EBV DNA group
Among the patients with EBV DNA >1460 copies/mL, 291 pairs were selected by PSM and baseline characteristics are presented in Table 3. The 3‐year OS, PFS, LRFS, and DMFS rates for IMRT alone vs CCRT were 88.5% vs 94.3% (P = 0.003), 78.3% vs 85.5% (P = 0.043), 91.1% vs 94.0% (P = 0.678) and 84.9% vs 88.8% (P = 0.126; Figure 4), respectively. When entered into multivariate analysis, treatment (IMRT alone vs CCRT) was identified as an independent prognostic factor for OS (HR, 0.575; 95% CI, 0.408‐0.811; P = 0.002), PFS (HR, 0.672; 95% CI, 0.513‐0.880; P = 0.004) and LRFS (HR, 0.605; 95% CI, 0.384‐0.954; P = 0.031; Table 3). Thus, CCRT was superior to IMRT alone among patients with high levels of EBV DNA.
Figure 4.
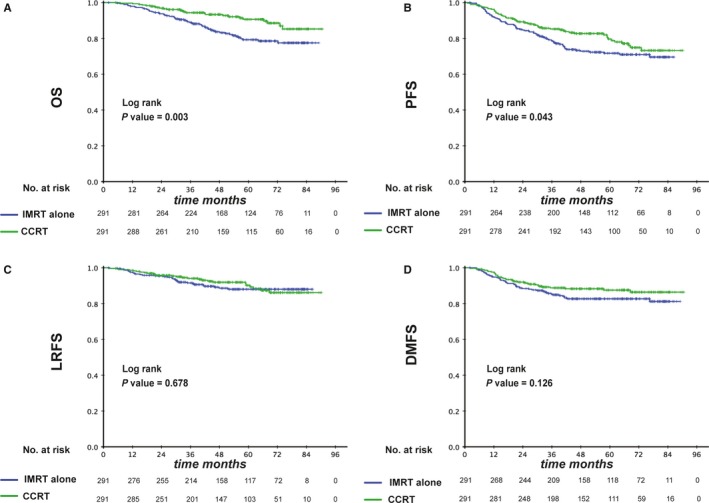
Kaplan‐Meier survival curves between IMRT and CCRT groups in high‐risk patients (pre‐EBV DNA >1460 copies/mL). Shown are the results for overall survival (A), progression free survival (B), locoregional relapse free survival (C), and distant metastasis free survival (D). P values were calculated using the unadjusted log‐rank test. EBV, Epstein–Barr virus; CCRT, concurrent chemoradiotherapy; IMRT, intensity‐modulated radiotherapy
4. DISCUSSION
To our knowledge, this is the first study to explore the role of CCT among stage II‐III NPC patients based on the pre‐EBV DNA level with a large cohort. A total of 2742 NPC patients were involved in this study. We eliminated the patients with stage I and stage IV for several reasons. Patients with stage I NPC showed low tumour burden and were treated with RT alone based on the principle of NCCN guideline.20 However, stage IV NPC was considered to be an advanced malignancy, which exhibited a higher tumour burden with a high‐risk of treatment failure. Thus, almost all patients received chemotherapy if the condition was tolerable. Besides, a considerable number of stage IV patients received induction chemotherapy to reduce the tumor size before RT, which was the optimum treatment model for these patients.21 We found that EBV DNA was an important biomarker to predict prognosis for the patients with stage II‐III, which could be used to make risk stratification. Furthermore, patients with low pre‐EBV DNA levels (≤1460 copies/ml) could not benefit from CCT, while the application of CCT could further improve the OS among high‐risk patients (pre‐EBV DNA level >1460 copies/mL).
CCRT was established as a standard treatment protocol in patients with locoregional advanced NPC because of the high‐risk of locoregional recurrence and distant metastasis.6, 7, 8 Among stage II NPC, we demonstrated in a phase‐III randomized study that CCT significantly improved the survival of patients with stage‐II NPC.22 Thus, addition of CCT during the RT period in patients with stage II‐III NPC seemed reasonable. However, all patients involved in the above studies received 2DRT. IMRT gradually replaced 2DRT. Compared with 2DRT, IMRT had the advantage of a higher dose delivery to the tumor area, decreasing the dose exposure of the normal organ.23, 24 As a result, a better locoregional control could be achieved.9, 10, 11
Several studies compared the survival of RT and RT plus CCT among NPC patients during the IMRT era.13, 25, 26 Tham et al evaluated 107 patients with stage‐IIb NPC and found no significant difference in survival between patients who underwent or did not undergo CCT.25 Zhang et al enrolled 661 low‐risk patients (T1N1M0, T2N0‐1M0 or T3N0M0) and similarly showed that patients receiving IMRT did not benefit from CCT.13 Conversely, Sun et al compared the efficacy of different treatment methods in advanced N‐stage (N2 and N3) and demonstrated that patients in the CCRT group achieved a higher 5‐OS rate compared with patients in the IMRT group.27 These studies proved that the application of CCT should be individualized according to the different tumor burdens.
Previous studies showed that patients with higher pre‐EBV DNA levels displayed poorer survival outcomes.14, 15 Chan et al demonstrated that with a cut‐off value of 4000 copies/mL, NPC patients could be divided into a poor‐risk group and a good‐risk group.15 Similarly, Lin et al found that patients with high levels of EBV DNA (more than 1500 copies/mL) suffered higher disease recurrence.14 Our results were consistent with these two studies, with the cutoff 1460 copies/mL, which was similar to Lin's report, supporting the prognostic value of EBV DNA in stage II‐III NPC.
In the entire cohort, the pre‐EBV DNA level showed great predictive value in all survival rates, indicating that it was credible to be used to stratify risk level. In stratified analysis according to pre‐EBV DNA level, a different scenario was observed. Among staged II‐III patients with low EBV DNA, CCRT and IMRT achieved similar survival outcomes; while for those with high EBV DNA, patients that received CCT achieved higher OS. Multivariate survival analysis showed that the application of chemotherapy was a protective factor for OS, PFS, and LRFS. Reasonably, patients with high EBV DNA suffered higher tumour burden; therefore, a more intensive treatment method, such as the application of induction chemotherapy was necessary for these patients to further eliminate tumors.
As the strong prognostic value of EBV DNA, our group launched two prospective clinical trials. In patients with stage III‐IV and EBV DNA >4000 copies/mL, we explored the effect of immunotherapy using tumour infiltrating lymphocytes after CCRT (NCT 02421640). Meanwhile, in low risk patients (stage III‐IV and EBV DNA <4000 copies/mL), we compared the survival rates between patients receiving 2 or 3 cycles cisplatin based CCT (100mg/m2) (NCT 02871518). This study showed the important basis for individualized treatment in stage II‐III NPC patients according to EBV DNA level. Moreover, it provided important theoretical support for another prospective clinical trial within low risk II‐III NPC patients, which investigate the role of CCT in these patients. The 1500 copies/mL was the proper cut‐off value to select the low risk patients based on this study and clinical application.
Several limitations existed in our study. First, this is a retrospective study and the selective bias is unavoidable. We used several methods to minimize the unbalance, such as PSM analysis and multivariate analysis. Second, all patients were from an NPC‐epidemic region in one treatment center. A multicentre prospective study is needed to validate our findings.
In conclusion, our study revealed that stage II‐III patients with high pre‐EBV DNA could benefit from additional CCT along with IMRT, whereas patients with low pre‐EBV DNA could not, indicating that pre‐EBV DNA could be a useful tool to help guide individualized treatment.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This retrospective study was approved by the Clinical Research Committee of Sun Yat‐sen University Cancer Center. Patients were required to provide written informed consent before enrolling in the study.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTERESTS
The authors declare no competing interests.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Supporting information
Sun X‐S, Chen W‐H, Liu S‐L, et al. Individualized concurrent chemotherapy by pretreatment plasma Epstein‐Barr viral DNA in II‐III stage nasopharyngeal carcinoma: A propensity score matching analysis using a large cohort. Cancer Med. 2019;8:4214–4225. 10.1002/cam4.2343
Funding information
This work was supported by grants from the National Key R&D Program of China (2017YFC0908500, 2017YFC1309003), the National Natural Science Foundation of China (No. 81425018, 81672868, 81602371), the Sun Yat Sen University Clinical Research 5010 Program (201707020039, 2014A020212103, 16zxyc02), the Sci‐Tech Project Foundation of Guangzhou City (201707020039), the National Key Basic Research Program of China (No. 2013CB910304), the Special Support Plan of Guangdong Province (No. 2014TX01R145), the Sci‐Tech Project Foundation of Guangdong Province (No. 2014A020212103), the Health & Medical Collaborative Innovation Project of Guangzhou City (No. 201400000001), the National Science & Technology Pillar Program during the Twelfth Five‐year Plan Period (No. 2014BAI09B10), the PhD Start‐up Fund of Natural Science Foundation of Guangdong Province, China (2016A030310221), the cultivation foundation for the junior teachers in Sun Yat Sen University (16ykpy28), the foundation for major project and new cross subject in Sun Yat Sen University (16ykjc38), and the Fundamental Research Funds for the Central Universities.
Data Availability Statement: The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Xue‐Song Sun, Wen‐Hui Chen, Sai‐Lan Liu and Yu‐Jing Liang contributed equally to this work
Contributor Information
Hai‐Qiang Mai, Email: maihq@sysucc.org.cn.
Lin‐Quan Tang, Email: tanglq@sysucc.org.cn.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30:114‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang E, Adami H. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765‐1777. [DOI] [PubMed] [Google Scholar]
- 4. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041‐2054. [DOI] [PubMed] [Google Scholar]
- 5. Choa G. Nasopharyngeal carcinoma. some observations on the clinical features and technique of examination. Pac Med Surg. 1967;75:172‐174. [PubMed] [Google Scholar]
- 6. Al‐Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310‐1317. [DOI] [PubMed] [Google Scholar]
- 7. Lin J‐C, Jan J‐S, Hsu C‐Y, Liang W‐M, Jiang R‐S, Wang W‐Y. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression‐free survival. J Clin Oncol. 2003;21:631‐637. [DOI] [PubMed] [Google Scholar]
- 8. Lee A, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally‐advanced nasopharyngeal carcinoma: NPC‐9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966‐6975. [DOI] [PubMed] [Google Scholar]
- 9. Zhang M, Li J, Shen G, et al. Intensity‐modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two‐dimensional radiotherapy: a 10‐year experience with a large cohort and long follow‐up. Eur J Cancer. 2015;51:2587‐2595. [DOI] [PubMed] [Google Scholar]
- 10. Peng G, Wang T, Yang K‐Y, et al. A prospective, randomized study comparing outcomes and toxicities of intensity‐modulated radiotherapy vs. conventional two‐dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286‐293. [DOI] [PubMed] [Google Scholar]
- 11. Lin S, Pan J, Han LU, et al. Update report of nasopharyngeal carcinoma treated with reduced‐volume intensity‐modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2014;110:385‐389. [DOI] [PubMed] [Google Scholar]
- 12. Du C‐R, Ying H‐M, Kong F‐F, Zhai R‐P, Hu C‐S. Concurrent chemoradiotherapy was associated with a higher severe late toxicity rate in nasopharyngeal carcinoma patients compared with radiotherapy alone: a meta‐analysis based on randomized controlled trials. Radiat Oncol. 2015;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang L‐N, Gao Y‐H, Lan X‐W, et al. Propensity score matching analysis of cisplatin‐based concurrent chemotherapy in low risk nasopharyngeal carcinoma in the intensity‐modulated radiotherapy era. Oncotarget. 2015;6:44019‐44029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin J‐C, Wang W‐Y, Chen KY, et al. Quantification of plasma Epstein‐Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461‐2470. [DOI] [PubMed] [Google Scholar]
- 15. Chan AT, Lo YM, Zee B, et al. Plasma Epstein‐Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94:1614‐1619. [DOI] [PubMed] [Google Scholar]
- 16. Tang L, Li C, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2016;108 10.1093/jnci/djv291 [DOI] [PubMed] [Google Scholar]
- 17. Edge S, Compton C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471‐1474. [DOI] [PubMed] [Google Scholar]
- 18. Shao J‐Y, Li Y‐H, Gao H‐Y, et al. Comparison of plasma Epstein‐Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100:1162‐1170. [DOI] [PubMed] [Google Scholar]
- 19. Zhao C, Han FLuL, et al. Intensity modulated radiotherapy for local‐regional advanced nasopharyngeal carcinoma. Ai Zheng. 2004;23:1532‐1537. [PubMed] [Google Scholar]
- 20. Pfister DG, Ang K‐K, Brizel DM, et al. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:917‐923. [DOI] [PubMed] [Google Scholar]
- 21. Sun Y, Li W‐F, Chen N‐Y, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509‐1520. [DOI] [PubMed] [Google Scholar]
- 22. Chen Q‐Y, Wen Y‐F, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. 2011;103:1761‐1770. [DOI] [PubMed] [Google Scholar]
- 23. Waldron J, Tin MM, Keller A, et al. Limitation of conventional two dimensional radiation therapy planning in nasopharyngeal carcinoma. Radiother Oncol. 2003;68:153‐161. [DOI] [PubMed] [Google Scholar]
- 24. Cheng JC, Chao KS, Low D. Comparison of intensity modulated radiation therapy (IMRT) treatment techniques for nasopharyngeal carcinoma. Int J Cancer. 2001;96:126‐131. [DOI] [PubMed] [Google Scholar]
- 25. Tham I, Lin S, Pan J, et al. Intensity‐modulated radiation therapy without concurrent chemotherapy for stage IIB nasopharyngeal cancer. Am J Clin Oncol. 2010;33:294‐299. [DOI] [PubMed] [Google Scholar]
- 26. Xu C, Sun R, Tang L‐L, et al. Role of sequential chemoradiotherapy in stage II and low‐risk stage III‐IV nasopharyngeal carcinoma in the era of intensity‐modulated radiotherapy: a propensity score‐matched analysis. Oral Oncol. 2018;78:37‐45. [DOI] [PubMed] [Google Scholar]
- 27. Sun X, Zeng L, Chen C, et al. Comparing treatment outcomes of different chemotherapy sequences during intensity modulated radiotherapy for advanced N‐stage nasopharyngeal carcinoma patients. Radiat Oncol. 2013;8:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.


