Abstract
BACKGROUND:
Urinary tract is subjected to a variety of disorders such as urethral stricture, which often develops as a result of scarring process. Urethral stricture can be treated by urethral dilation and urethrotomy; but in cases of long urethral strictures, substitution urethroplasty with genital skin and buccal mucosa grafts is the only option. However a number of complications such as infection as a result of hair growth in neo-urethra, and stone formation restrict the application of those grafts. Therefore, tissue engineering techniques recently emerged as an alternative approach, aiming to overcome those restrictions. The aim of this review is to provide a comprehensive coverage on the strategies employed and the translational status of urethral tissue engineering over the past years and to propose a combinatory strategy for the future of urethral tissue engineering.
METHODs:
Data collection was based on the key articles published in English language in years between 2006 and 2018 using the searching terms of urethral stricture and tissue engineering on PubMed database.
RESULTS:
Differentiation of mesenchymal stem cells into urothelial and smooth muscle cells to be used for urologic application does not offer any advantage over autologous urothelial and smooth muscle cells. Among studied scaffolds, synthetic scaffolds with proper porosity and mechanical strength is the best option to be used for urethral tissue engineering.
CONCLUSION:
Hypoxia-preconditioned mesenchymal stem cells in combination with autologous cells seeded on a pre-vascularized synthetic and biodegradable scaffold can be said to be the best combinatory strategy in engineering of human urethra.
Keywords: Scaffold, Tissue engineering, Urethral stricture, Urethral reconstruction
Introduction
Lower urinary tract, which has the responsibility of urine storage and evacuation, consists of urinary bladder, urethra and urinary sphincters. In addition, the male urethra, is also responsible for carrying sperm from the verumontanum to the external urethral orifice [1]. Both, the bladder and urethra are composed of two main functional tissues including urothelium layer and smooth muscle coat. Even though urine is highly concentrated with waste products, the specialized impermeable urothelium layer is able to inhibit reabsorption of toxic materials from urine [2].
There is a wide variety of urethral abnormalities, both congenital and acquired disorders such as hypospadias, epispadias and strictures which might necessitate extensive urethral reconstruction [1–3]. These abnormalities need to be corrected because they could cause voiding problems that lead to complications like pain, urinary tract infection and even renal damage.
Even though, currently there are more than 300 techniques available for repairing urethral stricture, complexity of the stricture condition, restricts the possible treatment options for each individual [4]. In the case of short and non-complex urethral strictures, the defect can be simply repaired by an end-to-end anastomosis. However the complex and long urethral defects can only be repaired by incorporating a graft or a flap in the site of injury [5].
The current available grafts using for urethral reconstruction are autologous graft or flap from genital skin and buccal mucosa. Even though skin grafts are readily used in genitourinary reconstructive surgery, but its application still can lead to number of complications such as development of infection due to hair growth and stone formation in neourethra. Moreover, there are limitations in utilizing non-hairy skin of patients suffering from lichen sclerosis [1, 6, 7] since it was proven that fibroblasts of skins affected by lichen sclerosis have an increased collagen secretion activity in compare to healthy skin, which per se, can intensify urethral narrowing condition [8].
Tissue engineering techniques recently emerged as an alternative approach in treating short and long urethral defects aiming to resolve complications associated with conventional treatment options in urethroplasty. Therefore wide variety of cells including progenitor cells and stem cells and biomaterials such as acellular tissue matrices, and synthetic polymers have been investigated for their applicability in urethral reconstructive surgery. However, lack of a desirable engineered-tissue construct to be used as an implant for treating long urethral stricture remains a troublesome challenge in urologic studies. The aim of this review is to provide a comprehensive coverage on the strategies employed so far, the efficacy and the translational status of urethral tissue engineering. Data collection and searching method were based on the key articles published in English language in years between 2006 and 2018 using the searching terms of urethral stricture AND tissue engineering on PubMed database. The list of articles resulted from the search and key papers related to urethral reconstruction are summarized in Table 1.
Table 1.
The basic characteristics of included studies
| Study model | Cell type | Scaffold | Surgical approach | Size of implanted construct | Follow-up/study period | Outcome measure | Limitations | Reference No. |
|---|---|---|---|---|---|---|---|---|
| Male New Zealand white rabbit | Rabbit foreskin epidermal cells | Rabbit BAM | Tubularised | 1.5 cm × 1 cm (length) × (width) | 6 months | Retrograde urethrogram/histology/immunocytochemistry | Adaptation of implanted epidermal cells into urethral environment is quite lengthy | [7] |
| Male white rabbit | Rabbit epidermal cells | Rabbit BSM | Tubularised | 1.5 cm (length) | 12 months | Histology/immunocytochemistry/urethrography | – | [75] |
| – | – | DF-assembled scaffold | Human urothelial cells | – | 28 days | Histology/cell viability/mechanical test/western blot | – | [61] |
| – | Rabbit urethral epithelial cells | Surface-modified non-knitted PLLA | – | – | 48 h | Electron microscope | – | [30] |
| – | Human keratinocytes and fibroblasts | PLGA | – | – | 3 months | Simple sterility test/scanning electron microscope/histology/biodynamic analysis/MTT assay | – | [33] |
| – | Human urothelial cells | Human amniotic membrane/PLCL | – | – | 14 days | Live/dead staining/water-soluble tetrazolium salts measurement/immunostaining | – | [51] |
| – | Human urothelial cells | Fibroblast- based self-assembled scaffold | Tubularised | – | 14 days | Histology/immunofluorescence, real-time PCR analysis, electron microscopy, permeation studies | – | [66] |
| Rabbit | Rabbit corporal smooth muscle cells and lingual keratinocytes | Porcine acellular corpus spongiosum matrices | – | – | 6 months | Retrograde urethrography/histochemical analysis | – | [37] |
| – | Human urothelial cells (HUCs) | Fibroblast-based self assembled scaffold | Tubularised | – | 14 days | Histology, immunofluorescence, real-time PCR analysis, electron microscopy, permeation studies | [66] | |
| Female Mongrel dog | Dog urethral epithelial cells and smooth muscle cells | Dog ABM | Tubularised | 3.0 cm (length) | 11 months | Retrograde urethrography/histopathological examination | Small number of dogs included/use of dog rather than rabbit as study model | [74] |
| Male New Zealand rabbi | Smooth muscle cells | BSM | Onlay |
0.5 cm × 3.5 cm patch |
3 months | Urethroscope/histological/immunochemical analysis | – | [45] |
| – | Rabbit oral keratinocytes and TGF-β1 siRNA transfected fibroblasts | BAMG | – | – | 7 days | Enzyme-linked immunosorbent assay/histology/scanning electron microscope assay | – | [72] |
| Male Beagle dog | Dog smooth muscle cells and urothelial cells | Porcine ABM | Tubularised | 6.0 cm (length) | 12 months | Retrograde and voiding computed urethrocystography/histology/immunohistochemical analysis | Small number of experimental animals evaluated each time point | [14] |
| Female beagle dog | Dog bladder urothelial cells | Silk fibroin | Onlay | 3 × 1 cm2 | 6 months | Scanning electron microscope/histology/immunohistochemical staining/retrograde urethrography | Sample size was not sufficiently large | [48] |
| Male New Zealand rabbit | Rabbit skeletal muscle and hUCMSC | Muscle patch | Onlay | 0.5 cm × 0.5 cm round patch | 3 months | Histopathology/urethroscope/ureterography analysis | Absence of the corpora spongiosum in the reconstructed urethra | [70] |
| Male New Zealand rabbit | Rabbit epithelial differentiated adipose derived stem cells | BAMG | Onlay | 2.0 cm × 0.8 cm (length × width) | 6 months | Retrograde urethrography/immunofluorescent/western blot/microscopic image analysis | In vitro epithelial induction need to be optimise to reach the mature epithelial differentiation of ASCs | [27] |
| Male New Zealand rabbit | – | Bi-layer silk fibroin | Onlay |
1.0 cm × 2.0 cm patch |
3 months | Retrograde urethrography/histology/immunohistochemistry/histomorphometric analysis | [3] | |
| Male New Zealand rabbit | Rabbit urethral epithelial cells | Denuded human amniotic scaffold | Onlay |
0.5 cm × 1.0 cm patch |
3 months | Histological/immunohistochemical analysis | Application of denuded human amniotic membrane seeded with rabbit urethral epithelium cells restricts initiation of human based investigations/longer follow-up period was required to reveal latent adverse effects and complications | [35] |
| – | – | Df and adipose derived stem cell base self assembled scaffold | – | – | 10 weeks | Histology/immunohistochemistry burst pressure, suture strength, failure load, elastic modulus, failure strain | – | [62] |
| Male chinchilla rabbit | Rabbit keratinocyte | Sponge + collagen gel containing human or rabbit fibroblast | Onlay | – | 3 months | Histological analysis | – | [7] |
| Male New Zealand rabbit | – | BSM + autologous urethral tissue | Onlay |
0.5 cm × 2.5 cm patch |
3 months | Retrograde urethrography/immunohisto-chemical/histological analysis | Small number of study rabbits/short follow-up period/lack of penile curvature evaluation to confirm sufficient elasticity to maintain erection/lack of uroflowmetry/lack of anatomic analysis with cystoscopy | [44] |
| – | Dog urothelial cells | Hybrid PCL/PLLA | – | – | 14 days | 3-(4,5-Dimethylthiazole-2,5-di-phenyltetrazolium bromide (MTT)/scanning electron microscope/histology/immunohisto-chemical analysis | – | [42] |
| Male New Zealand rabbit | Rabbit epithelial | ICG-001 delivering collagen-P(LL-CL) | Tubularised | 2.0 cm × 1.0 cm (length × width) | 3 months | Urethrography/histology/immunohisto-logical analysis | More appropriate animal model is needed to evaluate the treatment outcomes for the post-traumatic urethral stricture | [43] |
| – | Rabbit urothelial cells and smooth muscle cells | PCL/PLCL | Tubularised | 2.0 cm | 7 days | MTS assay/live, dead assay and immunohistochemistry | – | [59] |
| Nude athymic male rat | Bone marrow derived mesenchymal stem cells and CD34 + HSPC | Poly(1,8-octanediol-co-citric acid) | Onlay | 2 mm × 8 mm × 0.15 mm | 28 days | Immunohistochemistry, bright field and polarised microscope images, Picrosirius staining | limited information was collected only from specific aspects of the innate immune system | [23] |
| Male New Zealand rabbit | Undifferentiated human amniotic mesenchymal stem cells | Hybrid PLLA/PEG | Onlay |
1.5 cm × 2.0 cm patch |
3 months | Histology/immunohistochemistry/retrograde urethrography analysis | Unknown mechanism of hAMSCs-based urethral regeneration | [34] |
| Male New Zealand rabbit | – | Rat-tail collagen type I | Tubular | 2 cm | 9 months | Scanning electron microscopy/histology/immunohistochemistry | – | [38] |
| New Zealand white male rabbit | Autologous rabbit urine stem cells | Small intestinal submucosa | Onlay | 2 cm | 3 months | Retrograde urethrogram/histological analysis/immunohistochemistry | The bioactive factors secreted by urine stem cells that might play role in tissue regeneration were not analysed, urethral defects were created in healthy animals and the number of experimental animals was not sufficient. | [47] |
| Male New Zealand white rabbit | Rabbit mesothelial cells | Autogenous tissue granulated silastic tube | Tubularised | 2.0 cm | 6 months | Retrograde urethrography/histology/immunohisto-chemistry | Urethral defect was created in healthy urethra, small number of experimental animal, using of small animal which is not clinically informative and potential risk of pyogenic infection development | [12] |
| Male beagle dog | Dog’s adipose derived stem cells and oral mucosal epithelial cells | Fibroblast based self-assembled scaffold | Tubularised | 2.0 cm | 3 months | MRI/Retrograde urethrogram/histology/prussian blue staining/immunohisto-chemistry | Complicated and time consuming procedures, short follow up period, short-segment urethral reconstruction | [69] |
| Male New Zealand white rabbit | Bi-layer silk fibroin scaffold | Onlay | 5 mm × 10 mm (length × width) | 3 months | Urethroscopy, retrograde urethroplasty, histological and immunohisto-chemical analyses | Small number of rabbits, short-term implantation period and lack of functional assessment | [49] | |
| New Zealand white rabbit | Rabbit adipose derived stem cells | Silk fibroin | Onlay | 2.5 cm × 1 cm | 6 weeks | Urethrography/histology/ | – | [50] |
| – | Human urothelial cells | DF and adipose derived stem cell based self assembled scaffold | – | – | 21 days | Histology/immunohistochemistry/mechanical testing/scanning electron microscopy | – | [64] |
| – | Porcine urothelial cells | Df based self assembled scaffold | – | – | 7 days | Histology/immunohistochemistry/permeability test/mechanical test/ | – | [63] |
| Human (all suffering from lichen sclerosus) | Human buccal mucosa keratinocyte and fibroblast | Human de-epidermised dermis | Onlay | 9.0–11.0 cm (various length in different patients) | 32–37 months (mean: 33.6 months) | Cystoscopy | – | [13] |
| Human | Human muscle and epithelial cell | Polyglycolic acid:poly(lactide-co-glycolide acid) | Tubularised | 4.0–6.0 cm (length) | 72 months | Histology/immunohistochemistry/urine analysis/cystourethroscopy/cystourethrography/flow measurement | – | [5] |
| Male athymic mouse | Human umbilical vein endothelial cells | Fibroblast based self-assembled scaffold | Tubularised | 28 days | Histology, immunostaining | – | [68] |
ABM acellular bladder matrix, BAM bladder acellular matrix, BAMG bladder acellular matrix graft, BSM bladder submucosal matrix, DF dermal fibroblast, PEG poly ethylene glycol, PLC poly-l-lactide-co-ε-caprolactone, PLGA polylactic-co-glycolic acid, PLLA poly-l-lactic acid
Tissue engineering of urethra
Emergence of tissue engineering and regenerative medicine technologies offered a new and promising approach to compensate the shortage of autologous tissues and to eliminate complications of using common grafts [9]. Tissue engineering basically relies upon three pillars including (i) the cells to be seeded on the scaffold of choice, (ii) the biomaterial to act as a scaffold and (iii) the environmental parameters such as growth factors like vascular endothelial growth factor and cytokines [10]. There exist two main approaches in replacing urethra using tissue engineering techniques including the use of (i) acellular matrices and (ii) cell-seeded matrices.
Cell sources for engineering of urethral tissue
For engineering of urethral tissue, a wide variety of cell sources have been investigated and studied by researchers. Generally speaking two categories of cells have been used for engineering of urethral tissue namely (i) progenitor cells and (ii) stem cells.
Progenitor cells
Progenitor cells with restricted differentiation capacity such as (i) epithelial cells including autologous urothelial cells [5], autologous epidermal cells [11], autologous mesothelial cells [12], and autologous oral keratinocytes [13] and (ii) smooth muscle cells have been used as cell sources for engineering of bladder and urethra [1, 14].
The possibility of using keratinocytes and fibroblast-based living skin equivalent (LSE) for reconstructing urethral epithelium was investigated by Rogovaya et al. [7], by culturing rabbit keratinocytes on a LSE which was made by culturing collagen gel containing postnatal human or rabbit fibroblasts on a sponge surface. Results showed that as early as 4–7 days after transplanting keratinocyte seeded LSE on de-epithelialized urethra; all experimental rabbits were capable of unassisted urination with no evidence of developing urethral stricture or urethral narrowing. Moreover, 3 months after implantation, the urothelium in the injured site was fully restored functionally and structurally suggesting that LSE, besides of elimination complications associated with hair growth in neourethral lumen, can be used as a suitable graft in urethroplasty [7].
Epidermal cells isolated from rabbit foreskin were reported to be used successfully in reconstructing 1.5 × 1.0 cm of anterior urethra. The results of study showed that the tubularized acellular collagen matrix from rabbit’s bladder which was seeded by autologous 5-bromo-2′-deoxy-uridine labeled epidermal cells could restore urethral mucosal defect by formation of vascularized multi-layered epidermal cells within 6 months, resembling the original urethral tissue. The 5-bromo-2′-deoxy-uridine labeled foreskin epidermal cells further confirmed that the origin of restored cells is the implanted cells rather than migration of localized and native cells. However as opposed to animals treated with cell-seeded grafts, the control animals which were treated with non-seeded collagen matrix developed urethral stricture [11].
Recently application of mesothelial cells in regeneration of urethra was investigated. In this study a silastic tube was implanted in rabbit’s subcutaneous cavity and after 2 weeks, an autogenous granulated tubular graft which was encapsulated around the implanted silastic tube was harvested. It was found that the autogenous granulated tissue was made up of myofibroblasts and collagen bundles. The luminal surface of graft was seeded with mesothelial cells harvested from rabbit’s omentum biopsies. 1.5 cm of rabbit urethra was excised surgically and was replaced by 2.0 cm of seeded tubularized graft. Six months post-operation, the results revealed development of continuous epithelial layer and organized smooth muscle bundles with no evidence of urethral stricture in animals receiving seeded tubularized graft as opposed to control group, which was treated, by unseeded tubularized graft. Also severe fibrosis and progressive shrinkage were only observed in unseeded grafts [12].
Stem cells
Stem cells which are undifferentiated cells are another possible source of cells to be used for engineering of urethra and since they exhibit sufficient capability in differentiating into urothelial cells [15], they can be considered as an alternative option when progenitor cells are insufficient or they are from diseased or malignant sources that are not appropriate for tissue regeneration [16]. So far, application of embryonic stem cells and adult stem cells have been studied [1], however, application of embryonic stem cells is limited due to the restrictions associated with their biological recognition and rejection (non autologous embryonic stem cells), ethical considerations and their tendency to form teratomas when implanted in vivo [10]. Takahashi and Yamanaka [17] introduced induced pluripotent stem cells which were generated from adult cells by gene reprogramming, which can be an other potential candidate in urologic tissue engineering. It was shown that two-step in vitro differentiation of pluripotent stem cells into definitive endoderm and then into urothelial cells using chemically defined medium is possible. Using the two-step differentiation model, pluripotent stem cells derived from human foreskin and CD34+ hematopoietic stem cells, could generate urothelial cells with 64.9% and 58.7% expression of uroplakin-1a and uroplakin-1b, respectively [18].
Adult stem cells such as the stem cells derived from amniotic fluid [19], bone marrow [20], adipose tissue [21] and urine [22] are potential cell candidates to be used for urethral engineering. It was also shown that bone marrow-derived mesenchymal stem cells in combination with CD34+ hematopoietic stem/progenitor cells can offer inflammatory modulation, increase vascularization and improve wound healing by reduction of collagen III:I production [23]. However, adipose derived stem cells (ASCs), seem be superior over other cell sources [1]. Stem cells are about 500 times more abundant in adipose tissue in comparison to bone marrow [2], can be harvested by minimally invasive procedures and have multi-lineage differentiation capacity [1]. Moreover, ASCs have angiogenic [24] and anti-inflammatory effects and since they do not express HLA-DR (of the major histocompatibility complex class II, MHC II), ASCs are ideal source to be used as xenogeneic [25] and allogeneic transplants [26].
One successful study in using ASCs in urethra engineering was reported by Li [27], in which rabbit adipose-derived stem cells were successfully differentiated into epithelial cells in vitro using epithelial inductive medium containing all-trans retinoid acid under air–liquid interface culturing system. For producing urothelium substitute, the epithelial-differentiated rabbit ASCs were seeded on a bladder acellular matrix graft (BAMG) and was introduced in study group animals for treating 2.0 cm length and 0.8 cm width of ventral anterior urethral defect. Results revealed the formation of a normal urethral caliber with no recurrent stricture or other complications in study group for up to 6 months post-implantation as opposed to control groups receiving cell-free BAMG and undifferentiated rabbit ASCs/BAMG grafts [27].
To evaluate the possibility of differentiating human bone marrow mesenchymal stem cells into smooth muscle cells (SMCs) and urothelial cells (UCs) and by acknowledging the fact that the fate of cells can be modulated by the external signals and cytokines which are release by the neighboring tissues via paracrine signaling, Tian [20], and his colleagues tried to differentiate human bone marrow mesenchymal stem cells to SMCs and UCs, using both indirect co-culture system or induction by treating bone marrow mesenchymal stem cells with conditioned medium obtained from cultured human bladder SMCs and UCs respectively. The results showed that human bone marrow mesenchymal stem cells could successfully differentiate into SMCs (30–50%) and UCs (50–60%) using both of the approaches; however, using conditioned medium seemed to be more efficient [20].
Biomaterial scaffolds for engineering of urethral tissue
Scaffold facilitates the localization of cells and the regeneration of neo-tissue with a proper structure. An ideal scaffold must have porous structure in order to allow efficient diffusion of biomolecules [28]. It is also believed that ideal topographical features of a biomaterial, besides providing a large surface for cell attachment and growth, also play a role in facilitating proliferation, migration and organization of seeded cells, thereby granting a proper development of a potential tissue construct [29]. Scaffold surface roughness [30], pore size, shape and interconnection [31], and the mechanical properties of the biomaterial such as tensile strength and rigidity [32] are among the important features to be considered for having a desirable scaffold. The scaffold also should possess physicochemical features that allows them to be sterilized using conventional sterilization methods [33].
Generally speaking, three major types of biomaterials have been used so far for engineering genitourinary tissues including (i) decellularized tissue matrices such as bladder submucosal matrix (BSM) [27], small intestinal submucosa (SIS) [34], amniotic membrane (AM) [35], whole decellularized urethra [36], de-epidermised dermis (DED) [13], and acellular corpus spongiosum matrices (ACSM) [37], (ii) naturally derived scaffolds such as silk fibroin [3], collagen [38], and alginate [39, 40] and (iii) synthetic polymers such as polylactic acid (PLA) [41], poly-l-lactic acid (PLLA) [30], poly-ε-caprolactone (PCL) [42], poly(l-lactide-co-caprolactone) (P(LLA-CL)) [43] and poly(1,8-octanediol-co-cirtic acid) (POC) [23].
Decellularized tissue matrices
Porcine BSM, which is prepared by decellularization of submucosal layer of bladder, is a collagen-based non-immunogenic xenogeneic material with numerous favorable features such as being biocompatible and biodegradable. It was also suggested by Simões et al. [36] that decellularized porcine urethra can potentially be used in urethral regeneration purposes.
To further enhance the incorporation of implanted BSM graft into the damaged host tissue, Chun et al. [44], combined acellular BSM with a healthy autologous urethral muscle and endothelial tissues to treat urethral stricture in male rabbit models. Twelve weeks post-operation, well-organized incorporation of implanted graft and recipient tissue, and a normal urethral lumen regeneration with compact muscular layer, complete epithelialization and progressive infiltration of grafted tissue by blood vessels were observed which was indicative of a successful tissue engraftment, proving that combination of an autologous urethral tissue with BSM could enhance incorporation of implanted graft into the injured site [44].
Considering the fact that BSM is a widely used matrix in urethroplasty, the incorporation manner of seeded and non-seeded collagen matrices into urethral wall when they are implanted in an onlay fashion was further investigated by Sayeg et al. [45]. Rabbit autologous bladder smooth muscle cells were seeded onto a decellularized porcine BSM followed by implantation into ventral portion of the penile urethra. Results revealed detection of epithelial layer formation at day 7 post-implantation in both experimental and control group animals receiving cell-seeded and non-seeded BSM, respectively. It was also shown that the implanted grafts were completely detached from the urethral wall regardless of the matrix being seeded or not. As shown from the results, presence of collagen matrix at the beginning of scarring process and a healthy dorsal bed of original urethra were sufficient enough to trigger muscle and epithelial layer regeneration without needing collagen matrix to be incorporated into the urethral tissue. It was also shown that the presence of smooth muscle cells are not indispensable in reconstructing urethra and what really matters is the presence of the collagen matrix at the beginning of healing process [45].
In a comparative study which was done by Davis et al. [46], human urothelial cells (UCs) were isolated from human bladder biopsy and then were seeded on a decellularised porcine BSM and SIS, to evaluate and compare in vitro regenerative potential of BSM and SIS scaffolds with cultured human UCs. The results demonstrated that even though both of the scaffolds are widely used in urethroplasty, BSM grafts exhibited greater capability in supporting human UCs viability and proliferative activity compared to SIS grafts [46]. This suggests that the extracellular matrix components of BSM and SIS are different and these components affect cells differently.
However, most recently, successful application of SIS graft in combination with urine-derived stem cells was reported by Liu et al. [47]. In this study, after verifying the capability of stem cells isolated from rabbit’s urine to differentiated into UCs and SMCs in vitro, the prepared SIS graft was seeded with fluorescent labeled urine-derived stem cells. Using a rabbit model, 2.0 cm urethral replacement was performed using decellularised porcine SIS grafts seeded with autologous urine-derived stem cells, while control animals were treated with non-seeded SIS scaffold. Evaluation of treated animals after 3 months post-operation showed stricture development in only one of the animals receiving seeded SIS graft while stricture formation was apparent in all control animals treated with non-seeded SIS scaffold. Moreover, fluorescent analysis revealed that some of the seeded urine-derived stem cells were differentiated into SMCs and UCs in vivo. This study proved that stem cells isolated from urine, seeded on SIS graft have the potential to restore epidermal cellular layer without causing any immunoreaction in contrast to non-seeded SIS grafts, therefore it can be used as an alternative to urethroplasty [47].
Yuan [35] introduced a tissue-engineered urethtra using denuded human amniotic scaffold (dHAS). In this approach, the basement layer of AM was separated and became deprived of amniotic epithelial cells and then was seeded with autologous urethral epithelial cells harvested from the urethral mucous membrane of recipient rabbit. The results demonstrated that, 3 months after transplantation of cell-seeded dHAS in urethral damaged area in the rabbit model, the urethral injury was completely restored with apparent formation of smooth muscle layer and rich blood vessels and neither infection nor fistula was observed in rabbits receiving cell-seeded dHAS. The results suggested that well-incorporation of the graft into the injured site could be due to the reduced immunogenicity of dHAS as a result of removing human epithelial cells and seeding of the xenograft with autologous recipient epithelial cells. Accordingly, the same approach can be used for applying non-human AM in treating urethral injuries in human [35].
Naturally derived scaffolds
Due to the possibility of disease transmission and ethical issues associated with the use of animal-derived scaffolds [1], naturally derived scaffolds can be considered as a safe option for tissue engineering purposes. Silk fibroin which is a well-known biomaterial derived from Bombyx mori cocoon and contains up to 90% of the amino acids glycerine, alanine and serin, can be completely degraded by naturally occurring proteolytic enzymes and displays a great biocompatibility and low inflammatory properties. Silk fibroin supports the proliferation and stratification of the seeded epithelial cells, hence, in recent years the application of silk fibroin had been investigated in urologic filed. However, this biomaterial showed relatively poor mechanical properties when it is meant to be used as a electrospun matrices, therefore, Xie et al. [48] tried to enhance the mechanical strength of the material by stretching it in 90 vol% ethanol aqueous solution with 0.1 mm/s of stretch rate and the stretch ratio of 1.4 × followed by 30 min immersion in the same ethanol solution. Stretching the silk fibroin can also improve the alignments of molecules and helps the fibers to align parallel to the direction of the extension. The prepared fibers then were seeded with urothelial cells (UCs). In experimental female beagle dogs, a 3 × 1 cm2 section of urethral mucosa was excised surgically to create a urethral defect. The seeded construct was then anastomosed into defect area. The results of the study showed that the construct made by stretched silk fibroin was highly porous and interconnected with smooth and uniform fiber diameters. It was also showed that within 1 week of in vitro culture of UCs on the surface of silk fibroin, formation of multilayered urothelium with tight attachment of UCs in the surface of the scaffold was apparent. In vivo results showed no development of urethral narrowing, ulceration and fistula for up to 6 months post engraftment, with native-like epithelium development over 6 months of implantation. The control animals which did not receive any implant, in contrast developed inflammation, urethral stricture and fibrosis with urethral shrinkage [48]. In Chung et al. [3] study, an aqueous silk fibroin solution was prepared from Bombyx mori silkworm. The bi-layer fibroin silk scaffold consisting silk fibroin film and porous silk fibroin foam was fabricated using 8% wt/vol and 6% wt/vol of silk fibroin solution mixed with sieved granular NaCl, respectively. Sophisticated construction of bi-layered silk fibroin scaffold facilitates the ingrowth of surrounding host tissue in the porous foam while the silk fibroin film, provides a tight seal for retention of hollow organ contents (i.e. urine) during defect consolidation. The prepared graft was anastomosed to the site of urethral injury in a rabbit model. In a parallel experiment, the same quality of urethral injury was anastomosed with acellular SIS graft for comparison. Three months postoperation, minimal acute and chronic inflammatory reactions were detected in silk fibroin and SIS groups respectively and wide urethral caliber with no evidence of construct extravasation, stricture fistulas or stone formation were observed in both of the silk fibroin and SIS graft groups [3]. Hence, bi-layer silk fibroin scaffold can be used as an alternative to SIS as acellular grafts. In 2018, Algarrahi et al. using rabbit model of onlay urethroplasty reported use of the same scaffold, bi-layer silk fibroin scaffold. In that study, in contrast to Chung et al. [3], urethroplasty was performed on a 5 mm × 10 mm of urethra, which was intentionally injured by electrocoagulation. Electrocoagulation was used to encourage scar tissue formation and mimicking the disease pathology of targeted patients, which are suffering from urethral stricture. Findings of the research showed that as early as 1 month post-operation, regeneration of pseudostratified columnar epithelium in neourethra was apparent and was similar to control animal. However, up to 3 months of post-operation, only 50% of newly regenerated tissue exhibited smooth muscle formation with relatively lower densities of smooth muscle cells in comparison to the control animal. These finding suggest that even though bi-layer silk fibroin scaffold can be potentially used in treating urethral stricture and regeneration of epithelium layer, but presence of a prior damage in the area of treatment (which is an actual situation in clinic) can adversely affect the healing process of smooth muscle [49]. In such situation, seeding of biomaterials with smooth muscle cells or its progenitors would be desirable. It was also shown in another study that incorporation of mesenchymal stem cells with silk fibroin; compared to acellular silk fibroin, reduces the inflammatory reaction caused by the biomaterial and resulted in better epithelial and smooth muscle growth in the urethral defect [50]. As of the application of naturally derived scaffolds in urologic studies, Pinnagoda et al. [38] reported a research in 2016 in which a 2 cm urethral defect was treated with acellular tubular graft, which was made from collagen. In this study, a double-layered tube made from rat-tail type 1 collagen was used as a cell free construct to treat a previously introduced urethral defect in New Zealand white male rabbits. The histology analysis of the implanted grafts showed development of multi-layered urothelium as early as 1 months post operation. However, muscle bundle formation was detected only after 6 months post operation. It was stated by the authors that in the case of using acellular grafts, in spite of stenosis and fistula formation, yet a prolonged period of tissue regeneration is needed in order to achieve full recovery of urethral defect [38].
Synthetic scaffolds
Synthetic polymers are widely used in tissue engineering application since they are biocompatible and exhibit sufficient strength to support engineering of hollow constructs. An adequate porosity and ability of the scaffold to support cell adhesion, growth and proliferation have also a great impact in the success of constructing a desirable tissue [30].
The ability of acellular human amniotic membrane (hAM) and poly-l-lactic-co-ε-caprolactone (PLCL) in supporting viability, proliferation and phenotype maintenance of human urothelial cells UCs, were also compared by Sartoneva [51] The results revealed that 3 days after seeding scaffold with urothelial cells, majority of cells retained their viability on both of the PLCL and hAM scaffolds; but after 14 days, most of the seeded cells were found dead on hAM scaffold as opposed to PLCL scaffold. Moreover it was revealed that the number of cells kept increasing dramatically within 14 days of human UCs culture on PLCL, while the number of seeded cells on hAM did not show any growth from day 7 onward, confirming that hAM is less supportive than PLCL in regards to the cell proliferation. According to the level of cytokeratin (CK) 7/8, CK19 and uroplakin III (UPIII) expression, it was also shown that PLCL could maintain the phenotype of human UCs during 14 days of culture period as opposed to the hAM. Therefore, hAM can not be a suitable biomaterial for reconstructing human urothelial tissue while PLCL appeared to be a promising scaffold for urothelial tissue engineering [51].
An ideal synthetic polymer to be used in cell-based tissue engineering must be able to support cell attachment. However, providing an optimum condition for cells to adhere onto and then allowing them to proliferate can be complicated since the surface chemistry of synthetic polymers may not be suitable for the specific cells. Therefore, to enhance the properties of synthetic polymers, Fu et al. [30], introduced a novel surface-modification to the degradable non-knitted poly-l-lactic acid (PLLA) scaffold that enhances cell adhesion and proliferation of rabbit urethral epithelial cells. In this study, a hollow spiral cylinder construct was prepared by PLLA-chloroform solution and then was surrounded by external non-knitted PLLA filaments. To promote cell adhesion, the scaffold was treated with 2% chitosan and then with fibronectin. The results revealed that the engineered scaffold has more than 90% porosity, exhibited excellent biocompatibility and supported the adhesion, growth and proliferation of seeded urethral epithelial cells. The inner hollow part of the scaffold allows drainage of urethral discharge, hereby reduces possibility of infection while the outer non-knitted filaments provide enough friction to avoid construct from being displaced from the original site of implantation [30].
In another study reported by Naji et al. [42], urothelial cells were seeded on a hybrid scaffold fabricated from PLLA in combination with PCL. Prior to seeding with dog’s urothelial cells, the PCL/PLLA scaffold which had a porous structure, was treated with oxygen plasma in order to convert highly hydrophobic PCL/PLLA scaffold into extremely hydrophilic material with 100% wettability. According to the results, after 14 days of cell culture, an integrated and continuous layer of urothelium was formed, demonstrating that the PLLA/PCL scaffold could support urothelial cells viability and growth without altering their phenotype [42].
Lv et al. [19], fabricated PLLA/PEG hybrid scaffolds with various fractions of PEG (0%, 10%, 20%, 30%, 40%, and 50%) content in order to find the optimum PLLA/PEG composite to support urethral tissue regeneration. The results of this research revealed that the quantity of PEG content is in direct correlation with hydrophilicity of constructed hybrid scaffold while the mechanical properties (tensile strength and elongation) showed deterioration by increasing in the PEG content of the composite scaffold; therefore the PLLA/PEG with 30% PEG content was selected as the optimum composite. In the second phase of study, three groups of animals with urethral defect were treated with human amniotic mesenchymal stem cells-seeded PLLA/PEG, acellular PLLA/PEG construct and regular urethral reparation, respectively. Twelve weeks post-operation, the results showed formation of multilayered urothelium with similar characteristics to the native urethral tissue with no evidence of stricture and fistula formation in urethral defects treated with human amniotic mesenchymal stem cells—PLLA/PEG construct as opposed to animals treated with acellular construct and regular urethral reparation [19].
Three-dimensional (3D) printing or bioprinting is a newly introduced technique in the field of tissue engineering [52] which allows precise designing of complex and patient specific scaffolds [53]. 3D bioprinting technology makes it possible to fabricate a construct with specific placement of various cells and matrices, in order to mimic the complex architecture of native tissue, which otherwise is not achievable in vitro [54]. Bioprinting is based on using bio-inks such as collagen [55] or alginate [56] in fabrication of a scaffold. There is variety of techniques currently applied in 3D bioprinting including (i) multiphoton crosslinking which allows to control at micron-scale geometry of fabricated scaffold [57], (ii) inkjet technology which allows precise arrangement of multiple cell types and matrices in a pre-determined configuration and (iii) extrusion bioprinting which performs through simultaneous dispensing of cells and matrices, in order to fabricate a construct [58].
Combination of PCL/PLCL (50:50) was used by Zhang et al. [59] in the form of spiral scaffold for the 3D printing of urethral cells suspended in bioink. In this study, UCs and SMCs which were isolated from rabbit’s bladder biopsy were separately combined with fibrinogen, gelatin, hyaluronic acid and calcium-free cell growth medium in order to make an injectable hydrogel for 3D printing of a 2.0 cm length of urethra. Using the Integrated Organ Printing (IOP) System, outer and luminal surfaces of PCL/PLCL spiral scaffold was printed by cell-laden fibrin hydrogel (bioink) containing SMCs and UCs, respectively. The results of study showed that as early as 7 days after in vitro culturing of bioprinted 3D urethra, cells start to penetrate to the urethral scaffold pores and could retain their viability and proliferation ability. It was shown that infiltration of printed cells into the scaffold was essential for the survival of cells and for cell–cell communication [59].
The choice of technique used to sterilize a synthetic scaffold can alter the physical and mechanical properties of the synthetic scaffold material. Therefore, Selim et al. [33], besides introducing a potential alternative to human buccal mucosa to be used in urethral reconstructive surgery, compared three conventional sterilization methods namely peracetic acid (PAA), γ-irradiation and ethanol on a polylactide-co-glycolide (PLGA85:15) scaffold and assessed how these techniques might affect the physical and mechanical properties of PLGA scaffold. Upon sterilization, the PLGA scaffolds were seeded with keratinocytes and fibroblasts in either simultaneous or sequential culturing manner. The results showed that unsterilized and ethanol-sterilized scaffolds could maintain their aseptic condition only for 2–14 days while PAA and γ-irradiated scaffolds kept their sterility for 3 months. Moreover, it was shown that PAA and ethanol sterilization caused reduction in synthetic fibers diameter to about half of their original size while γ-irradiation did not affect fibers diameter. Also, the tensile strength of scaffolds was adversely affected by PAA and γ-irradiation methods. It was also shown that both of the simple co-culture and sequential culturing could successfully lead to the formation of epithelial tissue, however sequential culturing method seemed to accelerate epidermal layer formation, therefore according to the author the γ-irradiated and PAA sterilized scaffolds seeded on sequential manner seemed to be the most promising buccal mucosal alternative to be used in urethroplasty [33].
Self-assembled scaffolds, a new approach in engineering of urethra
Self-assembled scaffold model, was first introduced by Dr. Francois A. Auger and was meant to produce skin grafts for heavily burnt patients. Nevertheless this method proved to be also useful in reconstructing blood vessels [60]. Self-assembly method is based on the production of collagen sheets by the cells themselves, where a dense extracellular matrix is completely produced by fibroblasts or ASCs under the influence of ascorbic acid. The main advantage of this scaffold is the elimination of the biocompatibility concerns. Magnan et al. [61] investigated the possibility of using self-assembly technique in tissue engineering of urethra. In their study, dermal fibroblasts were used for production of self-assembled scaffold. The produced cell sheets then were tightly wrapped around a tubular support and were given 21 days for cell sheet maturation. Later, the tubular support was removed and luminal surface of produced tubular graft was seeded with urothelial cells. A group of tubular graft (with or without UCs seeding) were subjected to intraluminal flow of medium for 7 days. Later, the mechanical properties of experimental tubular grafts were examined. The results of the study showed that the produced tubular graft had uniform texture and could resist the pressure of internal flow of the medium in bioreactor. The formation of stratified urothelium was also apparent in luminal surface of tubular graft. However, it seemed that internal flow of medium enhances the growth and differentiation of seeded urothelial cells. It is also interesting that the produced tubular graft was strong enough to be sutured and also displayed mechanical strength better that porcine urethra [61]. In 2015, a research was conducted by Vallieres et al. [62], to investigate the feasibility of using ASCs to produce self-assembled scaffold for tissue engineering of vascular substitute. However the findings still can be applied in urologic studies. In this study, self-assembled scaffolds were made from either ASCs or dermal fibroblasts. The produced cell sheets were rolled around a mandrel for 7 and 14 revolutions to make 7 and 14-revolution thickness cells sheets respectively. To allow maturation, the rolled cell sheets produced from ASCs or dermal fibroblasts were maintained in culture medium for another 35 days. As it is expected the results showed that the thickness of 14-revolution cell sheets produced from both ASCs and dermal fibroblast were more that the 7-revolution counterparts, with no significant difference between the thicknesses of cell sheets produced from ASCs or dermal fibroblast with similar number of revolutions. However, Masson’s trichrome staining results showed that the cell sheet layers in 7-revolution self-assembled scaffolds seem more compact and uniform, and most probably due to more efficient diffusion of oxygen and nutrients into the inner layers, compared to 14-revolution self assembled scaffolds. The cell sheets produced from both ASCs and dermal fibroblasts showed similar mechanical properties including burst pressure, suture strength, failure load and elastic modulus. However, ASC based cell sheets proved better compliancy compared to dermal fibroblast-based cell sheets. Moreover in terms of the components presented in produced cell sheets, both ASC-based and dermal fibroblast-based cell sheets contained collagen type I, III, fibronectin and elastin, with higher expression of last two components in dermal fibroblast-based cell sheets [62]. It is noteworthy that Bouhout et al. [63] in 2011 proved the possibility of culturing urothelial cells on a dermal fibroblast-based self-assembled scaffold [63]. In an interesting research conducted by Rousseau et al. [64], the composition of extra cellular matrix produced by ASCs, dermal fibroblast and hybrid ASCs-dermal fibroblast-based self-assembled scaffolds were compared. Also their ability in supporting the growth and phenotype maintenance of seeded urothelial cells was compared. Their results showed almost identical expression of collagen type I, III, fibronectin and laminin in extra cellular matrix produced by all three groups. However, the expression of the component, laminin 5, was only detected in dermal fibroblast-based self-assembled scaffold and the hybrid one. The expression of keratin 8/18 was only detected in urothelial cells seeded on dermal fibroblast-based and hybrid self assembled scaffolds. Detection of urothelial cell differentiation and maturation markers, Uroplakin Ib, II, III and Zo-1 further proved the capability of dermal fibroblast-based self assembled scaffold and the hybrid one in supporting the growth and phenotype maintenance of seeded urothelial cells. Inability of ASC-based self assembled scaffold in supporting the phenotype maintenance of seeded urothelial cells might be associated with the absence of laminin 5 which is known as one the main components of basal membrane. Even though all three types of scaffolds studies in this study, showed sufficient mechanical properties to be used in clinic [64], but hybrid self-assembled scaffold seems to be the best among them due to its higher elasticity.
Engineered tissue pre-maturation via mechanical stimulation
Usage of mechanical stimulation in engineering of urethra to mimic the physiological condition of the original tissue is thought to be able to mature the engineered tissue further. Seivath et al. [65], developed a tubular ureteral construct from primary porcine smooth muscle cells embedded in a fibrin gel with a stabilising poly(vinylidene fluoride) mesh. A local and cyclical intraluminal pressure was applied to the tubular construct using a balloon kyphoplasty catheter. It was shown that mechanical stimulation of engineered ureter, leads to simultaneous axial and circumferential orientation of SMCs [65].
Cattan et al. [66] adopted a combination of self-assembled scaffold with in vitro mechanical stimulation of construct to develop tissue-engineered tubular genitourinary graft (TTGG). It was hypothesized by the author that mechanical stimulation leads to formation of a stratified urothelium which is critical for impermeability of urethra. Therefore, fibroblasts were isolated from human skin followed by 4 weeks of culturing under the influence of ascorbic acid in order to form a living tissue sheet, known as self-assembled scaffold. The prepared scaffold was then tubularized and seeded on its luminal side with human urothelial cells (UCs) isolated from renal pelvis biopsy. The seeded construct underwent a mechanical stimulation with internal pressure of 15 cm H2O and intraluminal flow of 15 ml/min of human UC medium as fluid. After 14 days of mechanical stimulation, development of a stratified and well-established urothelial layer, similar to the native tissue was accomplished in TTGG. In control construct, which was cultured under static condition, only a bilayer of HUCs was detected. Moreover, immunostaining analysis of TTGG construct under dynamic culture condition showed positive expression of CK 20 which is known to be the marker of terminally differentiated urothelial cells as opposed to TTGG constructs under static condition. While a weak expression of tight junction protein ZO-1 was observed in static culture condition, culturing under dynamic condition enhanced ZO-1 tight junction protein expression and consequently promoted barrier functionality of TTGG [66].
It was also shown by Vardar et al. [67], that mechanical stimulation can up-regulate expression of collagen type 1 and elastin as of major components of urinary tract extra cellular matrix [67].
Engineered tissue pre-vascularization strategies
Imbeault et al. [68] tried to incorporate human umbilical vein endothelial cells into self-assembled scaffold made from human dermal fibroblasts. To obtain endothelialized urethral model, endothelial cells were seeded on DFs sheet. The cell sheet was then tabularized and was left in culture medium for 21 days to allow maturation and fusion of cell sheets. After maturation, intraluminal cell seeding using UCs was performed. The prepared construct then were placed in bioreactor and subjected to intraluminal flow of fluid. To evaluate its vascularization potential, the prepared construct was implanted on a dorsal muscle of an athymic mouse. Histological analysis of endothelial cells-seeded construct before impanation showed presence of capillaries on the construct as opposed to the control construct (DF sheet), which was not seeded with endothelial cells. Even though growth of mouse capillaries in both ECs-seeded construct and the control construct were observed as early as 7 days post implantation, mouse erythrocytes were present in greater proportion in ECs-seeded construct. At day 7 post-implantation, mouse red blood cells were only detected on periphery of control construct while in endothelial cells -seeded construct; erythrocytes were found through entire thickness of the construct. Results of this study suggest that incorporation of endothelial cells into DFs sheet enhance rapid perfusion of oxygen and nutrients via earlier vascularization of construct, therefore the success rate of engraftment will be higher due to reduced possibility of ischemia, necrosis and fibrosis [68].
In a most recent study in 2017, a sheet composed of cells and extracellular matrix produced from dog’s autologous oral mucosa fibroblasts was used in formation of a three-layered urethra. In this study, three layers corresponding to native urethral tissue, including mucosa, submucosa and muscularis were made from dog’s oral mucosal epithelial cells, oral mucosal fibroblast cells, and myoblasts which were differentiated from dog’s autologous ASCs, respectively. The prepared layers were wrapped around a 4.0 mm silicon tube. To enhance the revascularization of the prepared construct, it was implanted in subcutaneous cavity for 3 weeks. The results, 3 months after performing reconstructive urethroplasty using a 2.0 cm length of prepared urethra in dogs showed no evidence of ulceration, stricture or fistula in treated area. There is no doubt that presence of extracellular matrix which is produced by fibroblasts plays an important role in success of urethral reconstruction since it is reported that extracellular matrix contains as much as 0.77 ng of vascular endothelial growth factor which enhances angiogenesis and has a great impact on increasing the possibility of tissue engraftment in the site of injury [69].
Human umbilical cord mesenchymal stem cells (MScs) which are believed to secrete cytokines such as vascular endothelial growth factor, have significant role in angiogenesis and their effect can sustain as long as the Human umbilical cord MSCs survive [70]. As it was proved, the hypoxia pretreatment of MSCs enhances autophagy which is known to be a kind of cell survival mechanism, by activation of adenosine monophosphate-activated protein kinase/mammalian target of rapamycin signaling pathway; therefore it can increase the survival rate of in vivo implanted MSCs. Subsequently, hypoxia activation of MSCs can help in sustaining the therapeutic effects (i.e. angiogenic effect) of MSCs after in vivo transplantation [71]. By knowing the fact that the minced muscle alone cannot survive in non-muscular environment, Sun et al. [70], proved that combination of hypoxia-activated Human umbilical cord MSCs with minced muscles which were then incubated in rabbit’s penile subcutaneous cavity for 3 weeks could result in formation of a pre-vascularized muscle flap. Implantation of constructed pre-vascularized muscle patch into urethral defect area could restore urothelium as early as 2 weeks. Moreover, in 12 weeks post-implantation wide urethral caliber with no evidence of stricture or fistulae were observed in experimental rabbits [70]. Recently, the same technique in preparing a pre-vascularized construct was practiced by Zhou et al. [69], in which a three-layered tissue engineered urethra was implanted subcutaneously for 3 weeks. It was believed by the author that insufficient formation of vascular network in vivo, often lead to epithelial cell necrosis and shedding due to insufficient supply of oxygen and nutrients, therefore, pre-vascularization of relatively thick tissues seems to be essential for the success of tissue engraftment. In this study, upon 3 weeks of subcutaneous implantation, formation of a dense blood vessel network was apparent in engineered construct [69].
Novel strategies for scar reduction
Developing urethral stricture is a frequently reported complication in reconstructive urethroplasty especially in the cases of using acellular tissue engineered constructs. Li et al. [72], who seeded oral mucosa keratinocytes and fibroblasts on acellular BSM to construct a tissue engineered urethra showed that even though, ideal results were obtained in a short-term follow up but development of extremely disordered collagen fibers in the engineered urethra persuaded researchers to investigate another alternative technique to inhibit or reduce the scar formation as a result of collagen fiber overproduction. Since it is believed that transforming growth factor β1 (TGF-β1) plays a critical role in over secretion of collagen type I by fibroblasts, Li et al. [72], examined the effect of TGF-β1 small interfering RNA-transfected fibroblasts in inhibition of expression of TGF-β1 and reduction in secretion of collagen type I. In their study, rabbit fibroblasts were transfected with TGF-β1 small interfering RNA and then were seeded onto BSM along with autologous oral keratinocytes. The results demonstrated that the scar formation and stricture recurrence rate in engineered tissue were reduced as a result of RNA interference which could effectively reduce the secretion of collagen type I by fibroblasts [72].
Zhang et al. [43] in 2015 introduced ICG-001 delivering collagen/poly(l-lactide-co-caprolactone) (P(LLA-CL)). TGF-β1 exerts its profibrotic effect through activation of Wnt signaling pathway; ICG-001, a small molecular weight modulator that blocks Wnt signaling pathway was used in their approach to prevent expression of TGF-β1. An electrospun nanofiber scaffold comprising of collagen/(P(LLA-CL)) was designed which was capable of delivering and releasing ICG-001 in a controlled manner. In vitro anti-fibrotic effect of ICG-001 delivering scaffold was assessed by culturing TGF-β1 treated and non-treated rabbit fibroblasts in conditioned medium (conditioned medium collected after 24 h of incubating ICG-001 delivering scaffold in complete culture medium). In vitro results showed decrement in expression level of collagen type I and III by both of the TGF-β1 treated and non-treated fibroblasts cultured in conditioned medium. For in vivo study, experimental rabbits received tubularized epithelial cell seeded—ICG-001 delivering scaffold for treating ventral urethral defect while control animals were treated with epithelial cell-seeded non-drug delivering scaffold. Three months post-operation, the results showed development of fistula and urethral narrowing along with discontinuous epithelial layer formation and large amount of collagen deposition in control rabbits while a successful reconstruction of urethral defect with apparent development of multi-layered epithelium with no evidence of restricted urethral lumen or fistula was reported in experimental rabbits, confirming that gradual release of ICG-001 can inhibit over secretion of collagen and reduce scar formation [43].
Grafting strategies using tissue engineered urethra
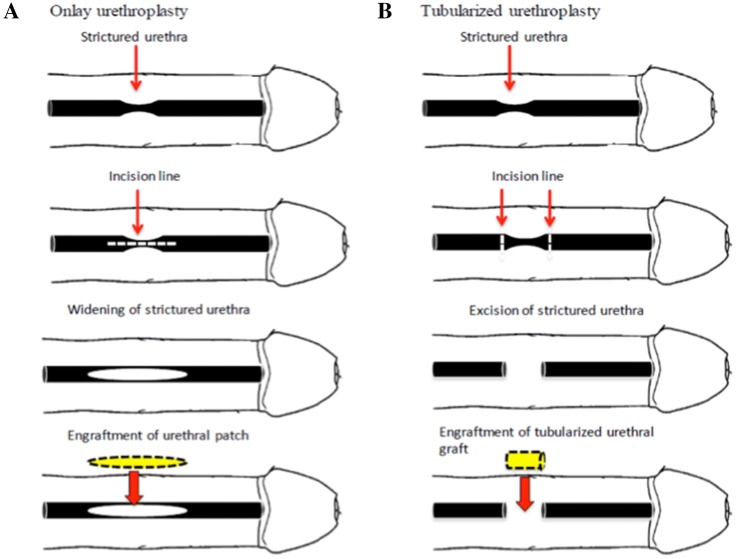
Even though many successful replacement of urethral defect using acellular matrices in an onlay fashion were reported but there exist some experimental data showing that the maximum distance of urethral tissue which can be regenerated by implanting acellular tubularized graft is limited and cannot exceed 0.5 cm [73], therefore cell seeding is necessary in the case of using tubularized graft for urethral replacement [74], moreover seeding scaffold with urothelial cells besides preventing urine leakage into surrounding tissues [65], can effectively inhibit scaring and formation of stone [30]. Similarly, it was shown by Feng et al., that seeding of porcine acellular corpus spongiosum matrices (ACSM) with both rabbit autologous corporal SMCs and lingual keratinocytes could successfully form stratified epithelial layer and organized muscle fiber bundles as opposed to ACSM alone which led to development of strictures with evidences of fibrosis and inflammation [37] (Fig. 1) shows simple onlay and tubularized model of substitution urethroplasty. It was shown that cell-seeded collagen-based tubularized grafts are able to repair small urethral defect in rabbit model. It was also shown that using a canine model, a 6.0 cm tubularized porcine ABM seeded with urothelial and smooth muscle cells isolated from dog’s bladder biopsy can successfully reconstruct urothelial coverage and muscle layer with no evidence of urethral narrowing for up to 12 months after urethroplasty [14].
Fig. 1.
A Onlay substitution urethroplasty, B tubularized substitution urethroplasty
However, in contrast to report of Orabi et al. [14], El-Tabey et al. [74], showed that cell-seeded tubularized tissue engineered construct failed to treat a 3.0-cm long urethral defect in female mongrel dog model with no obvious urothelial layer formation, extensive shrinkage (0.6–1.2 cm in length) of tubular graft and narrowing of the urethral lumen within 11 months post-implantation [74].
Among antithetical results reported by different researchers, the outcomes of Fu et al. [75], were in concordance with Orabi et al. [14] supporting the capability of treating short urethral defect with tubularised collagen scaffold seeded with epidermal cells in rabbit model which ended with formation of smooth muscle layer and no evidence of stricture in treated urethral defect area [75]. Moreover, it was shown that the detected transitional epithelial cells in the treated urethral defect areas were originated from implanted epidermal cells (keratinocytes) suggesting that the urethral environment may have directed transformation of implanted keratinocytes into transitional epithelial cells [75]. Recently a similar finding in supporting the regenerative capability of cell-seeded tubularised silastic graft in treating long urethral stricture was also reported by Jiang et al. [12] in 2017.
Clinical translation
From 2006 to July 2018, there are only two clinical studies reported in the literature. Bhargava et al. [13], in 2008, investigated the possibility of using tissue engineered buccal mucosa to treat urethral strictures of 9.0 to 11.0 cm in length in an onlay fashion in 5 patients which were all suffering from lichen sclerosis. Autologous keratinocytes and fibroblasts isolated from human buccal mucosa were seeded on human de-epidermised dermis. After 7 days of cell seeding, histological assessment confirmed that the tissue engineered buccal mucosa (TEBM) was similar morphologically to the native tissue. Within 32 to 37 months of follow up, results showed that 3 out of 5 patients could retain the grafted TEBM. The other 2 patients required entire or partial graft excision as a result of extensive fibrosis, which could be associated with the initial period of ischemia and lack of blood capillary network in TEBM. Moreover, in two of the patients which could retain TEBM, cystoscopy examination revealed development of stricture [13] at 6 and 9 months post-operation, respectively. However, in the long-term follow-up of patients published in 2014, satisfactory results were reported. Except for the two patients who required entire TEBM graft excision, the other four patients could retain the TEBM in situ for 9 years, and were able to void spontaneously [76].
In the second study, Raya-Rivara et al. [5], used a tubularized polyglycolic acid:poly (lactide-co-glycolide acid) scaffold seeded with autologous urothelial and smooth muscle cells on the luminal and outer surface of the scaffold, respectively. A total of 5 boys with urethral complications, received a tubular graft of 4.0 to 6.0 cm in length depending on their urethral defect measurement. According to the results, as soon as 3 months post-operation, normal urethra with apparent epithelial and smooth muscle layers was detected in all of the patients. And, within 72 months of follow-up, no evidence of stricture, diverticula, urinary tract infection and fistula were reported in any of the patients, proving that it is possible to reconstruct long segment of urethral defect in tubularised fashion with at least 6 years of post-operation patient satisfaction [5].
Discussion
This review paper aimed to summarize the accomplishment in the field of urethral tissue engineering in the last 10 years. Despite the range of approaches with regards to the scaffold materials, cells and tissue engineering techniques, there is still a lack of consensus in selecting the optimal parameters for engineering of urethra.
Although stem cells are readily available and have been demonstrated to differentiate into UCs and SMCs in vitro, few studies use stem cells as a potential cellular source and instead, there is a tendency to isolate the required cells from biopsies from patients. Based on this review, it was found that in both onlay and tubular urethroplasty, the preferred cellular sources for engineering of urethra are progenitor cells (i.e. UCs and SMCs). Regardless of the method used for urethroplasty, seeding of scaffold with at least one cell type is necessary because application of the unseeded scaffold often lead to post-operation stricture [11, 19, 27, 47]. In onlay grafting, angiogenesis, nutritional support and cell migration from the surrounding tissues seem to be easier, therefore using only one cell type for seeding the scaffold seems to suffice. Among the available cell sources, UCs are the best candidate to be seeded on the scaffold since mature urothelial cells are responsible for making a water-tight urethra [65, 66]. However, in tubularized urethroplasty, which is usually used for treating long segment of urethra, seeding of scaffold with both of the UCs along with SMCs is necessary, because SMCs provide additional support for the neourethra and prevent the engrafted scaffold from collapsing until complete recovery of injury. Moreover, since the initial ischemia upon grafting, is accused to be the reason for graft contraction and fibrosis, is the authors suggest that a combination of two cell types, incorporation of endothelial cells or hypoxia-activated MSCs that promote angiogenesis, and prevent inflammation may be required altogether. These cells should be derived from similar cell type autogenously or differentiated from autologous stem cells. Alternatively, allogenic MSCs, which were reported to be immunoprivileged (although still debatable), can be explored.
The added advantage of pre-maturation using mechanical stimulation leading to formation of water tight urethra with stratified urothelial layers and pre-vascularization of tissues for promotion of graft survival have been demonstrated [65, 69]. However, such strategies may be costly and laborious. It may be considered when critically long grafts are required. Novel approaches to inhibit the pathways leading to fibrosis and scarring have also been demonstrated to circumvent the frequently reported complication in urethral reconstruction surgeries, especially when unseeded scaffold is used. In the opinion of the authors, such problems may be circumvented when tissue regeneration can be accelerated, vascularization can quickly take place and inflammation can be suppressed with the seeding of appropriate cells.
Although there had been considerable successes reported in animal studies, very few made further progress into the clinical phase [14, 75]. In tissue engineering practices, mimicking the native environment of targeted tissue is a crucial fundamental principle, since, the proper cell-ECM interaction is the key element in urethral well-performance [36] and by knowing this fact that the exact components of the extracellular matrix of human urethra varies significantly between the distal, middle and proximal segments of the spongy urethra [77], lack of a detailed study in identification and detection of urethral endothelial matrix and the components which play role in directing natural urethral regeneration can be seen as a gap in this field which needs to be fulfilled.
Conclusion
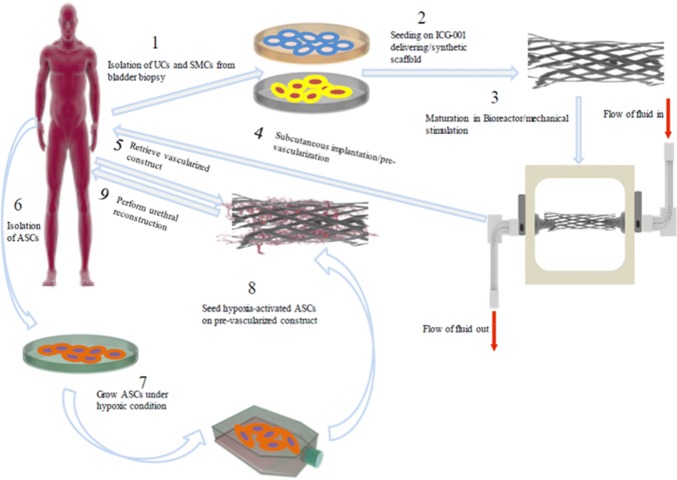
Even though, the possibility of differentiating MSCs into UCs or SMCs have been demonstration, in vitro differentiation of MSCs besides being complicated and tricky, does not offer any advantage over autologous UCs and SMCs. However, using hypoxia-preconditioned MSCs in their undifferentiated state in combination with autologous cells seeded on a synthetic and biodegradable scaffold with proper porosity and mechanical strength, and in vitro mimicking of the urethral environment by providing mechanical stimulation and pre-vascularization of prepared graft or incorporation of endothelial cells into the engineered construct, can be said to be the best combinatory strategy in engineering of human urethra. Figure 2 shows a schematic view of the proposed strategy for engineering urethral tissue in future.
Fig. 2.
A schematic view of proposed combinatory strategy for engineering human urethra in future
Acknowledgement
This review paper was supported by grants from Universiti Kebangsaan Malaysia (GUP-2017-092 and FF-2017-227).
Compliance with ethical standards
Conflict of interest
The authors declare that there are no financial conflicts of interest regarding the publication of this paper.
Ethical statement
There are no animal and human experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Orabi H, Bouhout S, Morissette A, Rousseau A, Chabaud S, Bolduc S. Tissue engineering of urinary bladder and urethra: advances from bench to patients. ScientificWorldJournal. 2013;2013:154564. doi: 10.1155/2013/154564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orabi H, Goulet CR, Fradette J, Bolduc S. Adipose-derived stem cells—are they the optimal cell source for urinary tract regeneration? In: Eberli D, editor. Cells and biomaterials in regenerative medicine. London: IntechOpen; 2014. [Google Scholar]
- 3.Chung YG, Tu D, Franck D, Gil ES, Algarrahi K, Adam RM, et al. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS One. 2014;9:e91592. doi: 10.1371/journal.pone.0091592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Kemp V, de Graaf P, Fledderus JO, Rudd Bosch JL, de Kort LMO. Tissue engineering for human urethral reconstruction: systematic review of recent literature. PLoS One. 2015;10:e0118653. doi: 10.1371/journal.pone.0118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–1182. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangera A, Chapple CR. Tissue engineering in urethral reconstruction—an update. Asian J Androl. 2013;15:89–92. doi: 10.1038/aja.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogovaya OS, Fayzulin AK, Vasiliev AV, Kononov AV, Terskikh VV. Reconstruction of rabbit urethral epithelium with skin keratinocytes. Acta Naturae. 2015;7:70–77. doi: 10.32607/20758251-2015-7-1-70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oikarinen A, Sandberg M, Hurskainen T, Kinnunen T, Kallioinen M. Collagen biosynthesis in lichen sclerosus et atrophicus studied by biochemical and in situ hybridization techniques. Acta Derm Venereol Suppl (Stockh) 1991;162:3–12. [PubMed] [Google Scholar]
- 9.Xue JD, Gao J, Fu Q, Feng C, Xie H. Seeding cell approach for tissue-engineered urethral reconstruction in animal study: a systematic review and meta-analysis. Exp Biol Med (Maywood) 2016;241:1416–1428. doi: 10.1177/1535370216640148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahfouz W, Elsalmy S, Corcos J, Fayed AS. Fundamentals of bladder tissue engineering. Afr J Urol. 2013;19:51–57. doi: 10.1016/j.afju.2013.01.006. [DOI] [Google Scholar]
- 11.Fu Q, Deng CL, Liu W, Cao YL. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007;99:1162–1165. doi: 10.1111/j.1464-410X.2006.06691.x. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S, Xu Z, Zhao Y, Yan L, Zhou Z, Gu G. Urethral reconstruction using mesothelial cell-seeded autogenous granulation tissue tube: an experimental study in male rabbits. Biomed Res Int. 2017;2017:1850256. doi: 10.1155/2017/1850256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol. 2008;53:1263–1271. doi: 10.1016/j.eururo.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 14.Orabi H, Aboushwareb T, Zhang Y, Yoo JJ, Atala A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: a preclinical study. Eur Urol. 2013;63:531–538. doi: 10.1016/j.eururo.2012.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mousa NA, Abou-Taleb HA, Orabi H. Stem cell applications for pathologies of the urinary bladder. World J Stem Cells. 2015;7:815–822. doi: 10.4252/wjsc.v7.i5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramaniam R, Hinley J, Stahlschmidt J, Southgate J. Tissue engineering potential of urothelial cells from diseased bladders. J Urol. 2011;186:2014–2020. doi: 10.1016/j.juro.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Osborn SL, Thangappan R, Luria A, Lee JH, Nolta J, Kurzrock EA. Induction of human embryonic and induced pluripotent stem cells into urothelium. Stem Cells Transl Med. 2014;3:610–619. doi: 10.5966/sctm.2013-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv X, Guo Q, Han F, Chen C, Ling C, Chen W, et al. Electrospun poly(l-lactide)/poly(ethylene glycol) scaffolds seeded with human amniotic mesenchymal stem cells for urethral epithelium repair. Int J Mol Sci. 2016;17:E1262. doi: 10.3390/ijms17081262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian H, Bharadwaj S, Liu Y, Ma PX, Atala A, Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. 2010;16:1769–1779. doi: 10.1089/ten.tea.2009.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Huang J, Lin T, Zhang C, Yin X. Cell-to-cell contact induces human adipose tissue-derived stromal cells to differentiate into urothelium-like cells in vitro. Biochem Biophys Res Commun. 2009;390:931–936. doi: 10.1016/j.bbrc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 22.Qin D, Long T, Deng J, Zhang Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res Ther. 2014;5:69. doi: 10.1186/scrt458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JS, Bury MI, Fuller NJ, Sturm RM, Ahmad N, Sharma AK. Bone marrow stem/progenitor cells attenuate the inflammatory milieu following substitution urethroplasty. Sci Rep. 2016;6:35638. doi: 10.1038/srep35638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blasi A, Martino C, Balducci L, Saldarelli M, Soleti A, Navone SE, et al. Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc Cell. 2011;3:5. doi: 10.1186/2045-824X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leto Barone AA, Khalifian S, Lee WP, Brandacher G. Immunomodulatory effects of adipose-derived stem cells: fact or fiction? Biomed Res Int. 2013;2013:383685. doi: 10.1155/2013/383685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Xu Y, Xie H, Li C, Song L, Feng C, et al. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: an animal model. Tissue Eng Part A. 2014;20:774–784. doi: 10.1089/ten.tea.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen F, Chang S, Toh YC, Teoh SH, Yu H. Development of poly (lactic-co-glycolic acid)-collagen scaffolds for tissue engineering. Mater Sci Eng C Mater Biol Appl. 2007;27:285–292. doi: 10.1016/j.msec.2006.05.007. [DOI] [Google Scholar]
- 29.Margolis G, Polyak B, Cohen S. Magnetic induction of multiscale anisotropy in macroporous alginate scaffolds. Nano Lett. 2018;18:7314–7322. doi: 10.1021/acs.nanolett.8b03514. [DOI] [PubMed] [Google Scholar]
- 30.Fu WJ, Wang ZX, Li G, Zhang BH, Zhang L, Hu K, et al. A surface-modified biodegradable urethral scaffold seeded with urethral epithelial cells. Chin Med J (Engl). 2011;124:3087–3092. [PubMed] [Google Scholar]
- 31.Stachewicz U, Szewczyk PK, Kruk A, Barber AH, Czyrska-Filemonowicz A. Pore shape dependence on cells growth into electrospun fiber scaffolds for tissue engineering: 2D and 3D analyses using SEM and FIB-SEM tomography. Mater Sci Eng C Mater Biol Appl. 2017;95:397–408. doi: 10.1016/j.msec.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 32.Figallo E, Flaibani M, Zavan B, Abatangelo G, Elvassore N. Micropatterned biopolymer 3D scaffold for static and dynamic culture of human fibroblasts. Biotechnol Prog. 2007;23:210–216. doi: 10.1021/bp0602092. [DOI] [PubMed] [Google Scholar]
- 33.Selim M, Bullock AJ, Blackwood KA, Chapple CR, MacNeil S. Developing biodegradable scaffolds for tissue engineering of the urethra. BJU Int. 2011;107:296–302. doi: 10.1111/j.1464-410X.2010.09310.x. [DOI] [PubMed] [Google Scholar]
- 34.Lv XG, Feng C, Fu Q, Xie H, Wang Y, Huang JW, et al. Comparative study of different seeding methods based on a multilayer SIS scaffold: which is the optimal procedure for urethral tissue engineering? J Biomed Mater Res Part B Appl Biomater. 2016;104:1098–1108. doi: 10.1002/jbm.b.33460. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Liu T, Yang L, Zhang G, Liu H, Yi X, et al. Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med Sci Monit. 2014;20:2430–2438. doi: 10.12659/MSM.891042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simões IN, Vale P, Soker S, Atala A, Keller D, Noiva R, et al. Acellular urethra bioscaffold: decellularization of whole urethras for tissue engineering applications. Sci Rep. 2017;7:41934. doi: 10.1038/srep41934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng C, Xu YM, Fu Q, Zhu WD, Cui L. Reconstruction of three dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng Part A. 2011;17:3011–3019. doi: 10.1089/ten.tea.2011.0061. [DOI] [PubMed] [Google Scholar]
- 38.Pinnagoda K, Larsson HM, Vythilingam G, Vardar E, Engelhardt EM, Thambidorai RC, et al. Acta Biomaterialia engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016;43:208–217. doi: 10.1016/j.actbio.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 39.Yan H, Zhong L, Jiang Y, Yang J, Deng J, Wei S, et al. Controlled release of insulin-like growth factor 1 enhances urethral sphincter function and histological structure in the treatment of female stress urinary incontinence in a rat model. BJU Int. 2018;121:301–312. doi: 10.1111/bju.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raddatz L, Lavrentieva A, Pepelanova I, Bahnemann J, Geier D, Becker T, et al. Development and application of an additively manufactured calcium chloride nebulizer for alginate 3D-bioprinting purposes. J Funct Biomater. 2018;9:E63. doi: 10.3390/jfb9040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang DJ, Li MY, Huang WT, Lu MH, Hu C, Li K, et al. Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect Tissue Res. 2015;56:434–439. doi: 10.3109/03008207.2015.1035376. [DOI] [PubMed] [Google Scholar]
- 42.Naji M, Rasouli J, Shakhssalim N, Dehghan MM, Soleimani M. Supportive features of a new hybrid scaffold for urothelium engineering. Arch Med Sci. 2015;11:438–445. doi: 10.5114/aoms.2015.50977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang K, Guo X, Zhao W, Niu G, Mo X, Fu Q. Application of Wnt pathway inhibitor delivering scaffold for inhibiting fibrosis in urethra strictures: in vitro and in vivo study. Int J Mol Sci. 2015;16:27659–27676. doi: 10.3390/ijms161126050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chun SY, Kim BS, Kwon SY, Park SI, Song PH, Yoo ES, et al. Urethroplasty using autologous urethral tissue-embedded acellular porcine bladder submucosa matrix grafts for the management of long-segment urethral stricture in a rabbit model. J Korean Med Sci. 2015;30:301–307. doi: 10.3346/jkms.2015.30.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayeg K, Freitas-Filho LG, Waitzberg ÂF, Arias VE, Laks M, Egydio FM, et al. Integration of collagen matrices into the urethra when implanted as onlay graft. Int Braz J Urol. 2013;39:414–423. doi: 10.1590/S1677-5538.IBJU.2013.03.16. [DOI] [PubMed] [Google Scholar]
- 46.Davis NF, Callanan A, McGuire BB, Flood HD, McGloughlin TM. Evaluation of viability and proliferative activity of human urothelial cells cultured onto xenogenic tissue-engineered extracellular matrices. Urology. 2011;77:1007.e1–7. [DOI] [PubMed]
- 47.Liu Y, Ma W, Liu B, Wang Y, Chu J, Xiong G, et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res Ther. 2017;8:63. doi: 10.1186/s13287-017-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie M, Song L, Wang J, Fan S, Zhang Y, Xu Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J Surg Res. 2013;184:774–781. doi: 10.1016/j.jss.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 49.Algarrahi K, Affas S, Sack BS, Yang X, Costa K, Seager C, et al. Repair of injured urethras with silk fibroin scaffolds in a rabbit model of onlay urethroplasty. J Surg Res. 2018;229:192–199. doi: 10.1016/j.jss.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian B, Song L, Liang T, Li Z, Ye X, Fu Q, et al. Repair of urethral defects by an adipose mesenchymal stem cell—porous silk fibroin material. Mol Med Rep. 2018;18:209–215. doi: 10.3892/mmr.2018.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sartoneva R, Haimi S, Miettinen S, Mannerström B, Haaparanta AM, Sándor GK, et al. Comparison of a poly-l-lactide-co-ε-caprolactone and human amniotic membrane for urothelium tissue engineering applications. J R Soc Interface. 2011;8:671–677. doi: 10.1098/rsif.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaye R, Goldstein T, Grande DA, Zeltsman D, Smith LP. A 3-dimensional bioprinted tracheal segment implant pilot study: rabbit tracheal restriction with graft implantation. Int J Pediatr Otorhinolaryngol. 2019;117:175–178. doi: 10.1016/j.ijporl.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: a technological marvel. J Clin Orthop Trauma. 2018;9:260–268. doi: 10.1016/j.jcot.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A. Biomaterials Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34:130–139. doi: 10.1016/j.biomaterials.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 55.Drzewiecki KE, Malavade JN, Ahmed I, Lowe CJ, Shreiber DI. A thermoreversible, photocrosslinkable collagen bio-ink for free-form fabrication of scaffolds for regenerative medicine. Technology (Singap World Sci) 2017;5:185–195. doi: 10.1142/S2339547817500091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Axpe E, Oyen ML. Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci. 2016;17:E1976. doi: 10.3390/ijms17121976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bell A, Kofron M, Nistor V. Multiphoton crosslinking for biocompatible 3D printing of type I collagen. Biofabrication. 2015;7:035007. doi: 10.1088/1758-5090/7/3/035007. [DOI] [PubMed] [Google Scholar]
- 58.Pati F, Jang J, Lee JW, Cho DW. Extrusion bioprinting. In: Atala A, Yoo J, editors. Essential of 3D biofabrication and translation. San Diego: Academic press Elisevier; 2015. p. 123–52.
- 59.Zhang K, Fu Q, Yoo J, Chen X, Chandra P, Mo X, et al. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017;50:154–164. doi: 10.1016/j.actbio.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Morissette A, Imbeault A, Cattan V, Bernard G, Taillon G, Chabaud S, et al. Strategies to reconstruct a functional urethral substitute by self-assembly method. Procedia Eng. 2013;59:193–200. doi: 10.1016/j.proeng.2013.05.110. [DOI] [Google Scholar]
- 61.Magnan M, Lévesque P, Gauvin R, Dubé J, Barrieras D, El-Hakim A, et al. Tissue engineering of a genitourinary tubular tissue graft resistant to suturing and high internal pressures. Tissue Eng Part A. 2009;15:197–202. doi: 10.1089/ten.tea.2007.0303. [DOI] [PubMed] [Google Scholar]
- 62.Vallières K, Laterreur V, Tondreau MY, Ruel J, Germain L, Fradette J, et al. Human adipose-derived stromal cells for the production of completely autologous self-assembled tissue-engineered vascular substitutes. Acta Biomater. 2015;24:209–219. doi: 10.1016/j.actbio.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Bouhout S, Gauvin R, Gibot L, Aubé D, Bolduc S. Bladder substitute reconstructed in a physiological pressure environment. J Pediatr Urol. 2011;7:276–282. doi: 10.1016/j.jpurol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Rousseau A, Fradette J, Bernard G, Gauvin R, Laterreur V, Bolduc S. Adipose-derived stromal cells for the reconstruction of a human vesical equivalent. J Tissue Eng Regen Med. 2013;9:E135–E143. doi: 10.1002/term.1717. [DOI] [PubMed] [Google Scholar]
- 65.Seifarth V, Grosse JO, Gossmann M, Janke HP, Arndt P, Koch S, et al. Mechanical induction of bi-directional orientation of primary porcine bladder smooth muscle cells in tubular fibrin-poly(vinylidene fluoride) scaffolds for ureteral and urethral repair using cyclic and focal balloon catheter stimulation. J Biomater Appl. 2017;32:321–330. doi: 10.1177/0885328217723178. [DOI] [PubMed] [Google Scholar]
- 66.Cattan V, Bernard G, Rousseau A, Bouhout S, Chabaud S, Auger FA, et al. Mechanical stimuli-induced urothelial differentiation in a human tissue-engineered tubular genitourinary graft. Eur Urol. 2011;60:1291–1298. doi: 10.1016/j.eururo.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 67.Vardar E, Engelhardt EM, Larsson HM, Mouloungui E, Pinnagoda K, Hubbell JA, et al. Tubular compressed collagen scaffolds for ureteral tissue engineering in a flow bioreactor system. Tissue Eng Part A. 2015;21:2334–2345. doi: 10.1089/ten.tea.2015.0048. [DOI] [PubMed] [Google Scholar]
- 68.Imbeault A, Bernard G, Rousseau A, Morissette A, Chabaud S, Bouhout S, et al. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can Urol Assoc J. 2013;7:E4–E9. doi: 10.5489/cuaj.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou S, Yang R, Zou Q, Zhang K, Yin T, Zhao W, et al. Fabrication of tissue-engineered bionic urethra using cell sheet technology and labeling by ultrasmall superparamagnetic iron oxide for full-thickness urethral reconstruction. Theranostics. 2017;7:2509–2523. doi: 10.7150/thno.18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun D, Yang Y, Wei Z, Xu Y, Zhang X, Hong B. Engineering of pre-vascularized urethral patch with muscle flaps and hypoxia-activated hUCMSCs improves its therapeutic outcome. J Cell Mol Med. 2014;18:434–443. doi: 10.1111/jcmm.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Hao H, Huang H, Tong C, Ti D, Dong L, et al. Hypoxia regulates the therapeutic potential of mesenchymal stem cells through enhanced autophagy. Int J Low Extrem Wounds. 2015;14:63–72. doi: 10.1177/1534734615573660. [DOI] [PubMed] [Google Scholar]
- 72.Li C, Xu YM, Liu ZS, Li HB. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology. 2013;81:1075–1080. doi: 10.1016/j.urology.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 73.Chen KL, Wu HC, Chang CH. Tissue-engineered constructs for urethral regeneration. Urol Sci. 2012;23:42–44. doi: 10.1016/j.urols.2012.04.003. [DOI] [Google Scholar]
- 74.El-Tabey N, Shokeir A, Barakat N, El-Refaie H, El-Hamid MA, Gabr M. Cell-seeded tubular acellular matrix for replacing a long circumferential urethral defect in a canine model: is it clinically applicable? Arab J Urol. 2012;10:192–198. doi: 10.1016/j.aju.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu Q, Deng CL, Song XF, Xu YM. Long-term study of male rabbit urethral mucosa reconstruction using epidermal cell. Asian J Androl. 2008;10:719–722. doi: 10.1111/j.1745-7262.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- 76.Osman NI, Patterson JM, MacNeil S, Chapple CR. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur Urol. 2014;66:790–791. doi: 10.1016/j.eururo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 77.da Silva EA, Sampaio FJ, Ortiz V, Cardoso LE. Regional differences in the extracellular matrix of the human spongy urethra as evidenced by the composition of glycosaminoglycans. J Urol. 2002;167:2183–2187. doi: 10.1016/S0022-5347(05)65125-7. [DOI] [PubMed] [Google Scholar]