Abstract
Many publications have investigated the ingestion and toxicity of metallic lead from hunting and the shooting sports. However, there is limited literature on toxicity associated with the ingestion of lead fishing weights, despite our knowledge of damage caused to many species from entanglement in lines, nets, and fish-hooks. This paper surveys current knowledge of species poisoned by ingestion of lead fishing gear and the types of gear that have been implicated. We review the impacts of lead fishing tackle on wildlife species and human health and describe the efficacy of efforts to reduce the use of lead tackle through voluntary, educational, and regulatory approaches to encourage adoption of non-toxic fishing gear. The authors emphasize the need for further research and policy initiatives to deal with this serious problem.
Electronic supplementary material
The online version of this article (10.1007/s13280-019-01179-w) contains supplementary material, which is available to authorized users.
Keywords: Jig, Loon, Lure, Sinker, Swan, Waterbird
Introduction
Lead has been used for fishing weights since ancient times (Galili et al. 2013; Tyrrell 2015). Such weights are used to sink nets and fishing lines below the water’s surface and also to add mass to lines and nets to facilitate casting. Nomenclature for the weights used in fishing tackle varies regionally but includes such objects as split shot, sinkers, jigs, lures, worm weights and trolling weights (Schroeder 2010).
Toxicity from ingested lead fishing tackle has been documented in many species including humans (Table 1; Blus 1994; Perry 1994; Scheuhammer and Norris 1995; Anderson et al. 2000; Scheuhammer et al. 2003; Franson et al. 2003). It is well documented as a leading cause of death for common loons (great northern divers, Gavia immer) (Pokras and Chafel 1992; Stone and Okoniewski 2001; Sidor et al. 2003; Strom et al. 2009; Grade et al. 2018) and swans (Cygnus spp) (Sears and Hunt 1991; Kirby et al. 1994; Newth et al. 2016).
Table 1.
Animals documented to ingest lead fishing gear (modified from Perry 1994)
| Avian species | |
|---|---|
| Trumpeter swan, Cygnus buccinator | |
| Mute swan, Cygnus olor | |
| Tundra (whistling) swan, Cygnus columbianus | |
| Whooper swan, Cygnus cygnus | |
| Canada goose, Branta canadensis | |
| Wood duck, Aix sponsa (Scheuhammer et al. 2003) | |
| Mallard, Anas platyrhynchos | |
| American black duck, Anas rubripes | |
| Redhead, Aythya americana | |
| Greater scaup, Aythya marila | |
| White-winged scoter, Melanitta deglandi | |
| Long-tailed duck, Clangula hyemalis (Schummer et al. 2011) | |
| Red-breasted merganser, Mergus serrator | |
| Common merganser, Mergus merganser | |
| Great blue heron, Ardea herodias | |
| Great egret, Ardea alba | |
| Snowy egret, Egretta thula | |
| Green heron, Butorides virescens (Scheuhammer et al. 2003) | |
| Black-crowned night-heron, Nycticorax nycticorax (Franson et al. 2003) | |
| White ibis, Eudocimus albus | |
| Double-crested cormorant, Phalacrocorax auritus | |
| Sandhill crane, Antigone canadensis (Windingstad et al. 1984) | |
| Brown pelican, Pelecanus occidentalis | |
| American white pelican, Pelecanus erythrorhynchos | |
| Northern gannet, Morus bassanus (Pokras, unpubl.) | |
| Laughing gull, Leucophaeus atricilla | |
| Herring gull, Larus argentatus | |
| Royal tern, Thalasseus maximus (Scheuhammer et al. 2003) | |
| Common loon, Gavia immer | |
| Red-throated loon, Gavia stellata (Twiss 1997) | |
| Little penguin, Eudyptula minor (Harrigan 2016) | |
| Bald eagle, Haliaeetus leucocephalus | |
| Great horned owl, Bubo virginianus (MI DNR, unpubl.) |
| Non-avian species | |
|---|---|
| Humans, Homo sapiens (Mowad et al. 1998, St. Clair and Benjamin 2008) | |
| Domestic dog, Canis lupus familiaris (Bengfort and Carithers 1976) | |
| Harbor seal, Phoca vitulina (Zabka et al. 2006) | |
| Snapping turtle, Chelydra serpentina (Borkowski 1997) | |
| Painted turtle, Chrysemys picta (Scheuhammer et al. 2003) |
The purpose of this paper is to review current knowledge of the impact of lead fishing tackle on wildlife species and human health and to investigate the efficacy of efforts to reduce the use of lead tackle through voluntary and regulatory approaches. After reviewing estimates of rates of loss of lead tackle into the environment, we examine the impact of lead tackle on wildlife, using swan species and common loons as case studies. We then review the sublethal impacts of lead tackle on wildlife and human health before examining voluntary and regulatory efforts to limit the use of lead tackle. We conclude by calling for increased and more coordinated documentation of wildlife ingestion of lead tackle and suggest approaches to reduce the input of lead tackle into the environment.
In conducting our review of current literature on lead poisoning from fishing gear, we searched initially for the following keywords in Web of Science, Google Scholar, PubMed, and SORA: ‘avian’ or ‘lead poisoning’, ‘lead toxicosis’ and ‘fishing’, ‘fishing gear’, ‘sinker’. This initial search allowed us to determine the most commonly reported endpoints. We then conducted subsequent searches with ‘avian’ or ‘bird’ and ‘sinker’ or ‘weight’ and each endpoint of interest. References from the publications collected from these searches were also collected until we were satisfied that all relevant references were included. We included both field and experimental studies that examined correlations between tissue or blood concentrations of lead and endpoints of interest. Although we did not set a limitation to how far back we went in time, we only collected articles that were available electronically via the portals listed above.
Loss of lead fishing tackle
By their very nature, lead fishing weights are designed to be used in aquatic environments where they can be irretrievably lost. This often occurs when the line to which they are attached becomes caught or entangled and then breaks or is cut. But it can also occur when larger fish break the line, or when smaller lead tackle (e.g., split shot) are inadvertently dropped and not retrieved.
Studies have documented that significant amounts of lead can be deposited into lakes and rivers by the loss of lead fishing tackle. Although not differentiating among the various types of lead fishing weights, Duerr (1999) found, “Along heavily fished shorelines, we found an average of 0.05 sinkers/sqm…. Anglers lost, on average, 0.2 sinkers… per hour spent fishing.” Twiss and Thomas (1998) stated, “An estimated average of 125 to 187 million lead sinkers are deposited in Canadian waters annually, with about half in Ontario.” A recent Canadian study (ECCC 2018) stated, “An average Canadian angler can lose 11 to 15 jigs and sinkers per year while fishing due to snags and other reasons. This adds up to about 460 metric tons of lead jigs and sinkers lost every year into Canada’s lakes and waterways. This represents the most significant source of lead releases into Canadian waters.” Radomski et al. (2006) estimated that during a single walleye (Sander vitreus) fishing season, one metric ton of lead fishing weights entered five Minnesota waterbodies. Scheuhammer et al. (2003) calculated that approximately 4384 tons of lead fishing tackle were lost each year in U.S. waterways, and Jacks et al. (2001) reported that, in Sweden, 100–200 metric tons of lead sinkers are estimated to be lost annually. Similarly, in Great Britain, Birkhead (1982) reported an estimated annual loss of 250 metric tons of fishing sinkers each year. At local sites in Great Britain, studies reported > 15 000 lead split shots lost per hectare annually, with anglers losing 2–7 split shot sinkers per visit (Bell et al. 1985; Forbes 1986; Cryer et al. 1987). Lloret et al. (2014) document that lead fishing weights accounted for 36% of lost fishing gear recovered from the seabed in a coastal Mediterranean area.
Lead fishing gear ingestion in animals
To date, more than 30 species of birds have been documented to have ingested lead fishing tackle, along with 3 mammal and 2 reptile species (Table 1). It is estimated that 75 North American bird species may be at risk of lead tackle ingestion due to their foraging behavior (US EPA 1994). However, documentation of the extent of lead tackle ingestion both across and within species has been generally poor, due to the difficulty of detecting lead-poisoned animals as well as limitations of funding and research priorities. Regarding the former, Pain (1991) referred to the “invisibility” of waterfowl that have died from lead poisoning, because ailing birds tend to hide in thick vegetation and carcasses are quickly scavenged. Large numbers of birds generally do not die in a single location from lead, also making carcasses less conspicuous (Franson and Cliplef 1992; Newth et al. 2013). As a result, lead poisoning is likely underrepresented as a cause of mortality among wildlife (Franson and Cliplef 1992; Franson et al. 2003; Strom et al. 2009; Newth et al. 2013; Grade et al. 2018). Due to the difficulties of detecting lead-poisoned wildlife, lead tackle ingestion has been best documented in high profile, charismatic, and intensively studied large species such as swans and common loons. Because of this, we use these species as case studies for the impact of lead tackle ingestion on wildlife.
Case studies: Lead tackle ingestion in swans and common loons
Acquisition of lead tackle in swans and common loons
Methods of ingestion of lead fishing tackle for loons and swans vary from acquiring tackle from current fishing activity to ingesting lost tackle as grit. For common loons in New Hampshire, Grade et al. (2018) found a peak of lead tackle mortalities in July and August, coinciding with a peak of fishing activity, and that the majority of loons that died from lead fishing tackle ingestion also had ingested non-lead associated tackle (i.e., hooks, fishing line, swivels, leaders). This evidence suggests that current fishing activity (e.g., eating a fish that has ingested a lead jig or sinker and broken the line, or striking at tackle or a fish being retrieved by an angler) is a primary mechanism by which loons ingest lead fishing tackle (Grade et al. 2018). This is in contrast to speculations in previous studies (Pokras and Chafel 1992; Scheuhammer et al. 2003; Pokras et al. 2009; Haig et al. 2014). These studies noted that lead fishing gear ingested by common loons is typically close in size to the pebbles which these birds ingest to help break down food, suggesting that loons ingest lost lead tackle from lake substrates (Franson et al. 2001).
Similar to mortality patterns in common loons, mute swan (Cygnus olor) mortality from ingested lead tackle and median blood lead levels in England peaked during fishing season, prior to legislation banning lead fishing weights (Birkhead 1982, 1983; Sears 1988). Sears (1988) suggested that, in addition to swans ingesting tackle as grit, some swans likely ingested tackle from fishing lines, possibly after becoming entangled in line caught in vegetation or from eating bait attached to tackle. Fourteen percent of dead swans in the Thames Valley had associated tackle in their gizzards (Sears 1988). Subsequent to the ban, the previously documented spike in lead exposure during fishing season became less evident, suggesting that swans may be ingesting lead weights as grit that were lost prior to the ban rather than those recently lost or in current use (Sears and Hunt 1991; Perrins et al. 2002; Kelly and Kelly 2004).
Lead tackle ingestion in swans
The problem of mortality in wildlife from lead fishing tackle ingestion was first extensively documented in mute swans in the United Kingdom (UK). Goode (1981) reported that lead fishing tackle accounted for 50% of documented swan mortalities throughout England in 1980–1981, and estimated that approximately 3000–3500 swans in the UK died annually as a result of lead poisoning. Researchers also documented declines in local populations amid high rates of mortality from lead tackle ingestion (Goode 1981; Kirby et al. 1994). The majority (> 70%) of documented lead poisoned swans had ingested split shots (Birkhead 1982; Sears 1988; Sears and Hunt 1991) and ~ 7% had ingested larger weights (Sears and Hunt 1991). Less than 2% of cases of lead poisoning among mute swans in the UK were attributable to ingested lead shot ammunition (Sears and Hunt 1991). Lead tackle ingestion impacted both adult swans and cygnets (Birkhead 1982; Sears 1988; Kirby et al. 1994; Wood et al. 2019).
After legislation took effect in 1987 in England and Wales to ban the sale and use of lead fishing weights, mute swan deaths from lead poisoning declined from 34% of documented mortalities between 1971 and 1986 to 6% between 1987 and 2014 (Wood et al. 2019). The mute swan population rebounded, more than doubling according to a population index in the years following the legislation, with a model including the legal status of lead explaining 82% of the variation in population size (Wood et al. 2019). Despite this, after an initial decline in median blood lead levels among non-breeding flocks (Sears and Hunt 1991), subsequent sampling of swans brought to rescue centers in England (1994–2002) showed > 60% of birds still had lead levels that exceeded levels considered elevated for lead (> 1.21 µmol/l; Perrins et al. 2002; Kelly and Kelly 2004). Researchers concluded that lead poisoning remains a threat to swans (Perrins et al. 2002; Newth et al. 2013; Wood et al. 2019), but the regulation of lead tackle has resulted in the recovery of the population (Wood et al. 2019).
Mortality from lead fishing tackle ingestion has been documented in other swans, including trumpeter swans (Cygnus buccinator; Blus et al. 1989; Blus 1994; Degernes et al. 2006), tundra swans (Cygnus columbianus; Owen and Cadbury 1975; Blus 1994), and whooper swans (Cygnus cygnus; Spray and Milne 1988; Perry 1994). However, the majority of documented lead poisoning in these species has resulted from ingested lead ammunition (Blus 1994; O’Connell et al. 2008). Mute swans may be more susceptible to poisoning from ingested lead fishing tackle and less from ingested lead ammunition owing to their preference for foraging in aquatic habitats over agricultural fields in comparison with other swan species (Ciaranca et al. 1997; Bowen and Petrie 2007). The susceptibility of mute swans to lead fishing tackle ingestion can also likely be attributed to their habitation in urban areas, where they are fed by humans and may be attracted to anglers’ baits and areas in which lost lead tackle may accumulate (Sears 1989).
Lead tackle ingestion in common loons
Lead poisoning from ingested fishing tackle in common loons was first documented in 1976 (Locke et al. 1982) and has subsequently been documented to be a leading cause of mortality for this species (Pokras and Chafel 1992; Stone and Okoniewski 2001; Sidor et al. 2003; Strom et al. 2009; Grade et al. 2018). Across the range of common loons in North America, lead poisoning accounts for 11–49% of documented mortality (Table 2). Differences among regions should be interpreted with caution and are likely a function of differing reporting methods, collection efforts, and sample sizes, as well as real differences in fishing pressures and loon populations. Mortality from ingested lead fishing tackle primarily occurs among adult loons on the summer breeding lakes (Ensor et al. 1992; Daoust et al. 1998; Sidor et al. 2003), although loon deaths from ingested lead tackle have been documented on wintering grounds (Sidor et al. 2003; Pokras, unpubl.; Loon Preservation Committee, unpubl.) and among migrating loons in Washington and on the Great Lakes (Cooley and Melotti, pers. com.; Poleschook, pers. com.). Datasets that combine wintering and migrating adults generally result in lower rates of lead tackle ingestion than datasets reporting mortality among the breeding population. Lead poisoning has not been documented in loon chicks (Sidor et al. 2003), although it does occur in generally low rates among juvenile and sub-adult loons. An exception to this is in Michigan, where 15 of 60 juvenile/sub-adult loons were recorded to have died from lead poisoning (numbers not included in totals reported for Michigan in Table 2, which includes only adult loons; Cooley and Melotti, unpubl.). In regions where mortality numbers reported in Table 2 include age classes other than adults, rates of lead tackle mortality are diluted by younger age groups.
Table 2.
Mortality from lead poisoning in common loons in North America. Unless specified otherwise, “Total Mortalities Collected” include all age classes
| State/country/region | % Lead mortalities | Total mortalities collected | Population size (in most recent year of study) | Years of study | Source |
|---|---|---|---|---|---|
| New Hampshirea | 48.6 | 253 (NH AD population only) | 638 | 1989–2012 | Grade et al. (2018) |
| Maine | 25.2 | 480 (AD only) | 4100 (in 2010) | 1990–2017 | B. MacDonald, pers. com.; Evers et al. (2010) |
| New Yorkb | 20 | 261 | 1900–2300 | 1972–2017 | Stone and Okoniewski (2001); J. Okoniewski, pers. com. |
| New England | 44 | 254 (Breeding AD only) | 1987–2000 | Sidor et al. (2003) | |
| Canada | 15.0 | 433 (AD only) | ~ 500,000 | 1992–2018 | E.J. Parmley, pers. com.; CLLS (2019) |
| Michiganc | 14.1 | 340 (AD only) | 700–800 breeding pairs | 1987–2017 | J. Melotti, pers. com. |
| Wisconsin | ~ 20 | ~ 100 | 4350 | 2006–2017 | Strom et al. (2009); S. Strom, pers. com. |
| Minnesota | 11.4 | 132 | 12,000 | 1976–1991, 2009–2015 | C. Henderson, pers. com. |
| Washington | 38% from 1996 to 2010; 0% post-2010 | 21 (AD only) 1996–2010; 5 AD post-2010 | 50 | 1996–2018 | D. Poleschook and V. Gumm, pers. com. |
AD adult
aCommon loon mortality from lead poisoning is underrepresented in New Hampshire because the Grade et al. (2018) study included only loons that were clearly from the New Hampshire loon population and for which multiple lines of evidence indicated that birds died from lead fishing tackle ingestion. Thus, cases of lead-poisoned loons were excluded from this study that would have been included in studies and reporting from other regions
bTwenty-one additional loons that died from ingested lead fishing tackle were collected during type E botulism outbreaks on the Great Lakes in New York. Because the total number of dead loons collected during these outbreaks in New York is unknown, these lead mortalities are not included in the numbers reported in this table
cNote that “% Lead mortalities” and “Total mortalities collected” in Michigan include loons collected from type E botulism events on the Great Lakes
Collection efforts for dead loons are known to vary widely among different areas in North America (Table 2). In New Hampshire, the Loon Preservation Committee (LPC) conducts intensive monitoring of the state’s loon population and has extensive public outreach to encourage reporting of moribund or dead loons. LPC estimated that its recovery of deceased loons on breeding grounds is ~ 60%, resulting in mortality rates that are representative of causes of death (Grade et al. 2018). Less intensive collection efforts in other regions depend on a variety of factors, such as available funding and differing agency priorities and levels of public outreach, resulting in smaller sample sizes and less representative samples of loon mortality. In some states and regions, mortality sampling may occur in association with outbreaks of type E botulism in migratory birds. In general, increased rates of lead fishing tackle mortality are associated with more intense collection efforts and sample sizes that are more representative of overall mortality rates (Stone and Okoniewski 2001; Strom et al. 2009).
Fishing pressure in a given region appears to play a significant role in the rate of lead fishing tackle mortality. Although collection effort can be a confounding factor, resulting in areas with high fishing pressure but low documented rates of lead tackle mortality, the role of angling pressure in rates of lead tackle mortality is evident. Portions of southern Ontario with high rates of fishing pressure had the most frequent incidence of lead fishing tackle mortality among common loons in Canada (Scheuhammer et al. 2003). Similarly, in New England, New Hampshire’s high rate of lead tackle mortality compared with the rate in Maine is likely accounted for by high fishing pressure in the state (Scheuhammer et al. 2003), although a more intense effort to collect loon cadavers in New Hampshire likely plays a role. The correlation between the annual peak in lead tackle mortality in New Hampshire with months of peak fishing activity (Grade et al. 2018) likewise suggests the role of fishing pressure on loon mortality from lead poisoning.
Differences in body size across the range of the common loon may contribute to regional differences in mortality rates from lead tackle ingestion as well. Larger loons inhabiting regions near the coasts (Gray et al. 2014) may be more likely to ingest larger fish, which, in turn, may be more likely to break fishing lines and ingest tackle. The role of body size in lead tackle ingestion may also be reflected in the skewed sex ratio towards males (Grade 2011), which average > 20% larger than females (Gray et al. 2014). For the datasets represented in Table 2 for which the sex ratio of lead tackle mortalities is known, an overall average of 66.1% of lead mortalities are males, 28.6% are females, and 5.3% are unknown or unrecorded sex (total n = 469; range: males = 56.9–77.5%, females = 12.5–36.6%, and unknown sex = 0.0–33.3%).
While many factors influence rates of lead tackle mortality, results reported in Table 2 need to be interpreted with caution due to varying collection efforts, differences in reporting methods, and different criteria for including loons in studies (e.g., breeding vs. migrating and/or wintering loons) and assigning cause of death as lead poisoning (e.g., required presence of lead object vs. other criteria). Given high collection rates and a focus on adults of the breeding population, the studies for New Hampshire (Grade et al. 2018) and New England (Sidor et al. 2003) likely provide the most accurate assessment of the impact of mortality from lead fishing tackle ingestion on breeding loons. In New Hampshire, Grade et al.’s (2018) data indicate that toxicosis from ingested lead fishing tackle has had a population-level effect on the state’s common loons, reducing the population by 43% during the years of the study (1989–2012), and has inhibited the recovery of loons in the state. For all regions, the numbers presented in Table 2 likely underestimate the impact of lead poisoning from fishing tackle ingestion on loon populations.
Tackle types and sizes reported in loons
Jigs and sinkers account for the majority of tackle objects ingested by loons (Table 3), although loons are also known to ingest swim baits, internal weights from lures, and other types of tackle (Grade et al. 2018). Typical sizes of eroded tackle documented in loons ranged from 0.3 to 30.4 g for sinkers and 0.3 to 20.9 g for eroded jigs (Stone and Okoniewski 2001; Pokras et al. 2009; Grade et al. 2018), although loons can ingest much larger tackle. An eroded sinker weighing 78.2 g was reported by Franson et al. (2003) and several previously unreported eroded, saltwater jigs each exceeding 100 g were removed from loons recovered on the coasts of Massachusetts and California (Fig. 1; Grade et al. 2018; Pokras unpubl.).
Table 3.
Types of fishing tackle removed from loons that died from lead poisoning. Data from the same mortality datasets in Table 2. Pokras et al. (2009) uses the mortality dataset from Sidor et al. (2003)
| State/country/region | % Jigs | % Sinkers | % Unknown/other lead object | Total n of lead tackle objects | Source |
|---|---|---|---|---|---|
| New Hampshire | 52.6 | 38.8 | 8.6 | 116 | Grade et al. (2018) |
| New York | 68.7 | 20.9 | 10.5 | 67 | Stone and Okoniewski (2001); J. Okoniewski, pers. com. |
| New England | 19.0 | 60.0 | 21.0 | 222 | Pokras et al. (2009) |
| Canada | 25.6 | 39.5 | 34.9 | 43 | E.J. Parmley, pers. com. |
| Michigan | 53.2 | 25.5 | 21.3 | 47 | J. Melotti, pers. com. |
Fig. 1.
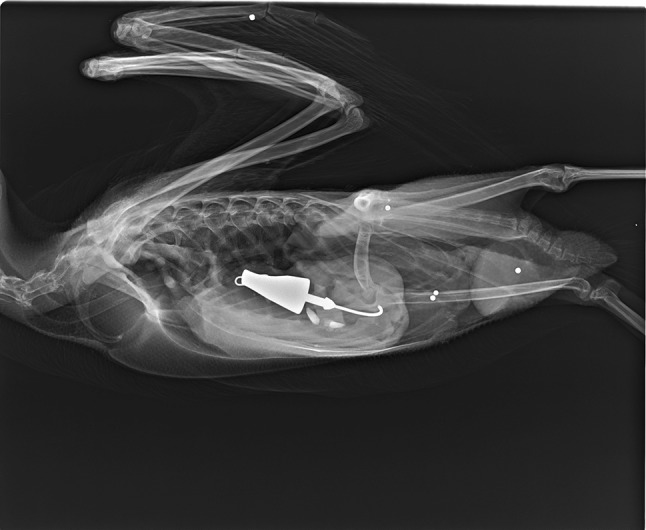
Lateral radiograph of large (116 g) lead jig ingested by common loon. (Steel shot identified on radiograph and recovered at necropsy had been present for an extended period and were not associated with significant pathology.)
The high proportion of ingested jigs recorded from across the range of the common loon highlights the importance of including lead-headed jigs in legislation restricting lead tackle to ensure effective protections for loons and other wildlife. Grade et al. (2018) recommend substituting non-lead alternatives for lead sinkers and jigs weighing ≤ 28.4 g to protect loons and other wildlife. While erosion rates of many metal objects in avian gizzards are unknown, studies of erosion of lead shot (Cook and Trainer 1966; Finley et al. 1976) and lead tackle suggest that erosion may take place fairly rapidly due to the grinding action with the pebbles that are virtually always present in adult loon gizzards (Franson et al. 2001; Pokras, pers. comm.).
Sublethal effects of lead in wildlife
While direct mortality from lead ingestion can be significant for wildlife, it is also important to consider the sublethal impacts to individuals and populations. The insidious effects of sub-acute lead exposure can add to the multiple stressors already affecting wildlife health, and even low levels of lead exposure may contribute to mortalities attributed to other causes (Newth et al. 2016; Ecke et al. 2017). Veterinarians and wildlife professionals are just beginning to investigate the potential effects of sublethal lead levels in animals, so some of our most detailed understanding of the sublethal effects from lead comes from the human medical literature where low level lead toxicosis is documented to impair a wide variety of metabolic processes (Wani et al. 2015).
In light of associations between low level lead exposure and impaired neuropsychological function in humans, similar cognitive effects of sublethal lead poisoning are beginning to be studied in wildlife. In herring gulls (Larus argentatus), effects on locomotion, food begging, feeding, treadmill learning, thermoregulation, and individual recognition were observed in chicks dosed with lead (Burger and Gochfeld 1994). The development of aggressive behaviors has been documented in great tits (Parus major) and northern mockingbirds (Mimus polyglottos) exposed to heavy metals (Janssens et al. 2003; McClelland et al. 2019). Just as lead exposure has been found to affect the human humoral immune response (Metryka et al. 2018), mallards (Anas platyrhynchos) experimentally exposed to lead shot were found to have impaired antibody production following antigen challenge compared to controls (Trust et al. 1990). Immunosuppression secondary to lead exposure may contribute to lowered disease resistance in wildlife.
Although subtle effects of sub-lethal lead exposure in wildlife species have been best documented in controlled laboratory settings, some studies are beginning to investigate how lead exposure may affect the complex behaviors of animals in their natural environment. For example, Ecke et al. (2017) identified lead-induced behavioral effects in a population of free-ranging golden eagles (Aquila chrysaetos). Sublethal lead concentrations were associated with impaired flight performance and increased mortality risk. A retrospective study of mute swans admitted to a wildlife care center for rehabilitation suggested that birds with elevated but moderate blood lead levels suffered an increased risk of collision with powerlines or other overhead cables. Those with intermediate to high levels had a reduced risk of collision, possibly because they were too weak to fly (Kelly and Kelly 2005). Karstad (1971), Hunter and Haigh (1978), and de Francisco et al. (2016) documented significant effects of lead on the cardiovascular and nervous systems of birds.
Pattee and Pain (2003) documented an increasing use of lead worldwide and state that ‘‘lead concentrations in many living organisms may be approaching thresholds of toxicity for the adverse effects of lead.’’ Environmental lead exposure, even at low levels, could very well contribute to wildlife mortality by impairing organ functions, increasing susceptibility to trauma and disease, and hindering the complex mental processes and social behaviors required for reproductive success and survival.
Lead in fishing gear and human health
In regard to consumer lead products and public health, we are at a critical moment where regulations urgently need to catch up with the science. In human health, current science asserts that no safe exposure level exists for lead, which contributes to 0.6% of the global burden of disease (WHO 2009). Given the growing body of evidence that even low doses of lead exposure over time can lead to multiple health and cognitive impairments, one should not underestimate the human health hazards associated with handling lead fishing gear.
Sahmel et al. (2015) found that simply handling fishing sinkers resulted in deposition of lead on the skin and that an average of 24% of this lead could be transferred from the hands to the mouth. Practices such as biting lead split-shot to secure onto the line and melting down scrap lead to produce home-made fishing weights are both examples of significant public health concerns directly related to lead fishing weights. Molds to cast homemade sinkers, jigs, bullets, lead soldiers, and other items are readily available for purchase, and there are numerous internet videos illustrating such techniques without providing any meaningful safety and health information. Indeed, many sources document significant lead exposure from the melting of lead at home to make fishing gear and other objects (Olivero-Verbel et al. 2007; Khan 2014). These cases expose people to lead via fumes and small particulates that can be inhaled or may contaminate food and water.
The ingestion hazard to humans posed by small fishing weights should not be overlooked. Poison control centers are commonly consulted on cases of ingestion of lead foreign bodies, and previous studies have noted that some of these are fishing weights (Cole et al. 2010). In 2016, 2412 of the poisoning cases reported to poison control centers in the US were due to single exposures to lead, typically due to the ingestion of small lead items (Gummin et al. 2017). In many cases the lead item ingested was not defined. However, in 38 cases reported to US poison control centers in 2016 the item ingested was specifically recorded as lead fishing tackle and most of these (28 cases) were due to ingestion by children under 6 years of age (Gummin et al. 2017). Note that not all ingestions of lead sinkers will result in reports to poison control centers and the toxic impacts of the exposure may not be immediately evident. It is likely that the poison control center numbers underestimate of the total number of children exposed to lead via this route. Significantly elevated blood lead levels have been documented in children exposed to lead for very short periods of time. For example, blood lead levels in a 4-year old child were found to exceed 65 µg/dl the day following ingestion of a single fishing sinker (Cole et al. 2010). Retention of lead fishing sinkers in the stomach and intestines of children following ingestion has been demonstrated and can result in long-term elevation of lead levels (Mowad et al. 1998).
Concerns regarding the public health impacts of lead exposure have resulted in regulations on other lead products including paint, toys, and gasoline additives (Stroud 2015). The human health perspective should also inform the risk management strategy for other lead products including lead fishing gear (Health Canada 2013).
Voluntary and legislative approaches for regulating lead fishing gear
Over more than three decades and in multiple jurisdictions, many approaches have been used to try to reduce the toxic impacts of lead fishing gear on wildlife. In our ESM (electronic supplementary materials), we summarize the effectiveness of key voluntary and legislative measures that have been used thus far. We assessed the effectiveness of each measure in terms of reduced uses of lead tackle and/or reduced mortality wherever data are available (Table S1). We then used this review to develop recommendations for the design of a risk management strategy to reduce the toxic impact of lead fishing gear on wildlife (Table 4).
Table 4.
Recommendations for risk management strategy development for regulating lead fishing tackle. Developed from the review of existing international voluntary and legislative approaches found in Table S1 for this publication
| Conclusions based on international efforts to date | Recommended instrument design features |
|---|---|
| Voluntary/education only approaches ineffective | Use a combination of legislation (regulatory restriction on lead sinker/jig sales and uses) with education to support regulation |
| Limited product restrictions based on size can be inadequate | If a size range is specified in the regulation, ensure that it covers ingestion hazard for all sizes that are typically ingested by receptors of concern, or restrict all sizes of lead terminal tackle (note that sizes specified in most existing legislation are based on heavily impacted species such as loons but other wildlife species and children can also ingest these lead products) |
| Risk management strategies that are very limited in geographical scope have little, if any, impact on the overall market for lead fishing gear. If the scope of the restriction excludes large numbers of the angling community, it will be ineffective in driving change | Restrictions should be applied at the state or national level wherever possible to ensure the fishing tackle market transitions from lead to lead-free non-toxic alternatives. Restrictions should apply equally to all anglers |
| Stockpiles of existing lead sinkers/jigs continue to be problematic years following introduction of restrictions | Restriction should be applied to both sale and use and be combined with effective enforcement. Enforcing a ban on uses also prevents the continued manufacture and use of home-made lead fishing weights and prevents purchasing from other jurisdictions that do not have restrictions on sales. Use education and enforcement, combined with buy-back programs, to ensure anglers cease use of lead in existing supplies. In some jurisdictions effective enforcement at the point of use may require cooperation between different levels of government |
| Lack of availability of non-lead alternatives for purchase by anglers and higher cost of alternatives can be a deterrent for switching to non-lead | Restrictions on sale ensure a guaranteed market for non-lead alternatives, hence manufacturers will produce them and retailers will stock them. Costs of non-lead alternatives expected to fall in any market with effective regulatory restrictions on lead due to increased economy of scale for non-lead options |
| Exclusions for coated lead products in some restrictions not supported by science, as coating is readily eroded after ingestion and is ineffective in limiting exposure or toxicity | Restrictions should not include exclusions for coated products |
| Wildlife are exposed to lead from multiple sources, toxicosis and mortalities occur from many | Coordinated action on a variety of lead products may be required for a comprehensive and effective risk management strategy |
In reviewing data on effectiveness of risk management measures, it is important to note that there can be high year to year variability in the number of mortalities recorded in any population, and many animals killed by lead tackle ingestion may not be recovered or subject to post mortem examination (Pain 1991). Long term monitoring programs and assessment of trends over many years are essential to determine the impact of risk management measures. Such data are not available for all jurisdictions. Several case studies are discussed in detail as these examples have the advantage of long-term monitoring data linked to voluntary and legislative approaches that evolved over time, allowing us to learn from their sustained efforts and experience. As Thomas and Guitart (2003) said, “…resolving lead exposure and toxicosis of wildlife is more about the development of appropriate social and governmental policy than the state of science.” This was reinforced by Arnemo et al. (2016) who stated, “Our understanding of the deleterious impacts of … lead exposure on wildlife and humans will change little with further scientific research, no more evidence is required….This is now a socio-political issue.”
Voluntary and education-only approaches to manage risks from lead fishing gear have proved ineffective, including efforts in the UK, Sweden, Denmark, and several US states (LPC 2012; Wood et al. 2019). As a result, legislative restrictions have been introduced in many jurisdictions (Table S1). In Washington State, it was noted that a sizable portion of anglers given on-site education about the toxicity of lead fishing tackle indicated that they would change in the future but only when an actual ban was in place (Poleschook and Gumm 2009). This attitude has also been noted in other jurisdictions. For example, following a 15-year outreach effort in Sweden to encourage the sale and use of non-toxic tackle, retailers stated that they did not intend to start selling alternatives until legislation banning lead tackle was introduced (KEMI 2007). In Minnesota, a well-funded 10-year outreach program was initiated to reduce mortalities from ingested of lead fishing tackle. This program was described as, “one of the most ambitious in the country” (LPC 2012). The campaign included over 200 tackle exchange programs which collected 8000 lbs. of lead, the distribution of 50 000 sample packages of lead-free tackle, displays at retail stores, and extensive media coverage. Despite such efforts, this program failed. At the end of the program, the supervisor of the Sustainable Development Unit of the Minnesota Pollution Control Agency concluded, “I believe no one knowledgeable about our concerted and sustained educational efforts in Minnesota would make the claim that education alone will sufficiently reduce or eliminate avoidable loon deaths as a result of lead ingestion” (LPC 2012).
This review of voluntary and legislative approaches concurs with the conclusion that, “a comprehensive solution involving legislation backed by intensive educational efforts will be required to address this issue” (LPC 2012). It is also clear that legislation developed must be appropriately designed to effectively address the issue (see Table 4). For example, legislation that fails to ban all sizes and types of lead tackle documented to be regularly ingested by wildlife will fail to adequately address the issue. Legislation that bans sale but not use will be much less effective since existing stocks of lead tackle will continue to be used, may continue to be purchased from jurisdictions outside the ban, and may continue to be cast at home (also resulting in public health concerns regarding lead exposure). Conversely legislation that bans use but not sale would leave lead tackle readily available, make enforcement difficult and discourage anglers from adopting nontoxic alternatives. Legislation that is limited in geographical scope and does not include both sale and use bans will have little, if any, impact on overall market demand for lead fishing gear. Enforcement throughout supply-chains is also critical. Even in Denmark, a jurisdiction with complete bans on importation and sale of all sizes of lead fishing tackle since 2002, lead fishing gear was found on sale in 2012 and 2013 (Danish EPA 2014). In this case, active enforcement and fines applied in recent years appear to be gradually reducing violations.
Ideally one might wish to advocate for elimination of all fishing tackle containing lead. But given historical opposition to attempts to limit lead sinkers and jigs by some fishing groups, tackle retailers, and tackle manufacturers, regulators have been reticent to extend bans beyond the sizes and types of ingested tackle documented to harm wildlife. Elected officials find themselves trying to reconcile the sometimes conflicting goals represented by the scientific data and jurisdictional economic priorities and political realities. Therefore, it is important to document sizes and types of ingested tackle and to press for science-based restrictions to protect wildlife.
The most effective risk management instrument is expected to be one that includes a prohibition on importation, manufacturing (including home casting), sale, and use of fishing tackle items made from lead. This needs to be combined with educational outreach to support the legislation and effective enforcement throughout the supply chain (at domestic manufacturing facilities, at importation, at points of sale, and at points of use).
Discussion: Seeking solutions through education and policy change
The role of human dimensions: Social science research and communication
Management decisions regarding lead fishing tackle have the potential to be very controversial, and legislation designed to protect wildlife is often met with resistance (Kneeland and Pokras 2008). Stakeholders are diverse, as this issue concerns government agencies, conservation non-profits, anglers, wildlife-viewers, fishing tackle retailers, manufacturers, and others. Stakeholder involvement in decision-making processes has increased the demand for human dimensions research in order to understand and predict stakeholder positions (Vaske and Manfredo 2012). Also, since human behavior is the root cause of lead in freshwater environments from fishing tackle, understanding angler behaviors is essential for accomplishing conservation goals (Ross-Winslow and Teel 2011), such as increased legislative awareness and elimination of lead tackle use.
Human dimensions research applies social psychology to understand stakeholder thoughts and actions towards wildlife (Vaske and Manfredo 2012). With this understanding, agencies can create more targeted outreach initiatives and increase message effectiveness, improve conservation strategies, as well as managing conflicts among stakeholders (Redpath et al. 2015). Leszek (2015) found that anglers not using lead-free fishing tackle believe it is too expensive and were unsure if it would perform as well as lead. In this case, it may be more effective to frame communication messages that minimize perceived barriers (Ross-Winslow and Teel 2011), rather than focus solely on traditional educational efforts involving lead toxicity or wildlife conservation.
Other approaches measure connections between attitudes and broad value scales, such as altruistic (i.e., caring about others), egoistic (maximizing individual outcomes), and biospheric (caring for non-human nature and the biosphere itself) (Stern et al. 1999; DeGroot and Steg 2007). Altruistic and biospheric values tend to be positively related to environmental policy acceptability, while egoistic values appear to be negatively related (Stern et al. 1999). Changes to values are unlikely to occur after education and informational campaigns because values are central to one’s identity and are relatively stable over the course of a lifetime (Fulton et al. 1996). Therefore, rather than attempting to change environmental values, another strategy is to promote messages that match those values. In the case of lead fishing tackle, it may be beneficial to focus on messages that appeal to egoistic values in addition to biospheric. Implementing message campaigns that focus on the human health hazards of lead, for example, might appeal to those expressing fewer concerns about wildlife health but are more concerned about their own personal well-being.
Many anglers may simply be unaware that lead fishing tackle causes ecological harm (Kneeland and Pokras 2008), but cognitive-based outreach approaches (i.e., presenting scientific information to the public) may not always be effective. Human behaviors are also influenced by beliefs, attitudes, value orientations, emotions, social norms, experience, and many other complex factors (Vaske and Manfredo 2012). An understanding of these factors is essential for designing effective communication messages. Since behaviors and attitudes of stakeholders regarding lead fishing tackle are still largely unknown (Thomas 1997; Ross-Winslow and Teel 2011), the authors recommend future studies to explore these relationships.
The taxonomy of fishing gear
In crafting educational and regulatory efforts on lead fishing gear, one of the problematic issues has been that manufacturers, marketers and anglers use a wide variety of terms for different fishing weights. Lead can be cast in many forms for fishing including items such as split shot, worm weights, trolling weights, jigs, ad infinitum. Initial attempts to regulate lead fishing gear focused on “sinkers,” but the term “sinker” only applies to certain types of fishing weights. In the U.S., many fishing groups were able to avoid proposed regulations by claiming that other types of fishing weights are not “sinkers.” The gear identified as “jigs” or “jigheads” in the U.S. is referred to as “lures” in the UK. Thus, in developing regulatory and educational materials, we should either make exhaustive lists of types of fishing gear that contain lead or consider a more inclusive terminology; perhaps something like, “any tackle, weights or lures containing lead used for fishing”.
The authors also note that there is potential for mis-classification of lead objects retrieved from avian GI tracts. Larger or more intact objects like bullets, jigs and sinkers are unlikely to be confused. But there is significant opportunity for inaccurate classification of smaller deformed or eroded objects, and it can sometimes be hard or impossible to tell if original objects were of fishing or shooting origin. Some of us have spent significant time using dissecting microscopes, magnets and other tools in attempts to differentiate eroded split shot or small jigs from gunshot, bullet fragments, or lead fragments from non-sporting origins. Over time we have improved our skills, but there are still items that end up being classified as “unknown Pb.” Because of the challenges associated with the identification of some deformed lead objects, we suspect that some things identified in other studies as being firearm projectiles may in fact have been fishing gear.
Coatings for lead fishing gear
Some groups have claimed that coating fishing gear with paint or other materials would prevent lead from being absorbed after ingestion. USFWS (1986) detailed extensive testing (some going back to the 1940s and 1950s) that had been done to see if lead shot could be coated to make them non-toxic when ingested by waterfowl. Those experiments concluded that for most practical purposes, coatings were uniformly unsuccessful and were quickly ground off in waterfowl gizzards. Thomas et al. (2015) reinforce the ineffectiveness of coatings for gunshot. To model what takes place with coated fishing gear ingested by common loons, Pokras (unpubl.) is currently testing commercially available painted or coated fishing weights in rock tumblers containing simulated gastric acid and the types of pebbles usually found in loon gizzards (Franson et al. 2001). Work to date has found that within 24 h, even heavily applied, multi-layer paint coatings are eroded enough to expose the metallic lead to gastric fluids. Thus, any legislation excluding coated or painted fishing tackle will be ineffective in preventing mortalities from lead poisoning.
Alternative materials
It may be that only metals have suitable characteristics for the temperatures and pressures encountered inside firearms. Thomas (2019) reviews the advantages and disadvantages of a variety of metals for ammunition and angling. For fishing, a variety of non-metallic materials can be suitable substitutes including natural rock and porcelain products.
An internet search for topics such as “rock fishing sinkers,” “biodegradable sinkers,” or “non-metal fishing weights” shows numerous alternative products. Most prices are similar to those of lead products. Sinkers and jigs made of non-toxic metals are also on the market, including tackle made of tungsten, tin, bismuth, and steel. Tungsten’s high density makes it a preferred material among professional anglers. However, fishing weights containing other toxic metals including zinc and cadmium have been found in U.S. stores, often bearing labels such as, “This Product Does Not Contain Lead.” Regulators and educators must be aware of this practice and take steps to avoid having these or other toxic substitutes enter the marketplace. Appendix S1 for this paper contains information on some sources for non-toxic fishing gear.
Other fishing-related issues
Clearly fishing activities deposit large amounts of metallic lead and other materials into a variety of aquatic environments (Bell et al. 1985; Forbes 1986). In addition to ingestion of such fishing gear by non-target species, under some conditions a great deal of lead can enter sediments and the water. Jacks et al. (2001) discuss the erosion of lead fishing gear in a Swedish river. Binkowski (2017) discusses environmental conditions, especially low pH, under which lead from spent gunshot or fishing gear may be transferred to sediments and the water column. The effects of this lead on aquatic organisms is deserving of further study but should be similar to lead deposited into aquatic systems from mining activities, shooting, industrial effluent, or other sources.
Effects of lead on individual animals
Traditionally wildlife managers have primarily been concerned about threats to animal health in two circumstances. First, if such threats are shown to have population-level effects on the species in question, and second, if these threats may serve a sentinel function to protect human health. There is no doubt that both of these are good reasons to replace lead in fishing gear with non-toxic alternatives.
But the authors would be remiss if we did not point out the significant benefits to individual animals of switching to non-toxic fishing gear. Hunters and anglers have long been some of our most ardent conservationists and traditionally abhor the unnecessary killing of non-target animals (Reiger 1975). Even if lead poisoning is not having a population-level effect on a particular species, it is killing large numbers of animals in a manner that is often prolonged, painful, and cruel. This flies in the face of two of the historic central tenets of sporting traditions: first, that we should avoid harm to non-target species, and second, that wild animals being taken for food or sport should, whenever possible, be afforded a quick death. Lead poisoning is inhumane and causes unnecessary stress, pain, and suffering in a wide variety of species including people, dogs, horses, ruminants, and birds. There is abundant literature over many years to demonstrate acute abdominal pain, peripheral muscle pain and weakness, incoordination, seizures, anemia and weakness, gout, and other clinical problems seen in many taxa (Oliver 1914; Walker 1981; Nriagu 1983; Needleman 2000; Blakley 2019). It is worth considerable money and effort to eliminate this poison from our outdoors activities.
Conclusions
There is a significant need to improve the development, marketing, adoption and regulatory approaches for non-toxic fishing gear. Those of us interested in reducing the use of lead need to develop strategies to increase the acceptance of non-toxic alternatives and educate anglers about:
The dangers of lead fishing gear to human and animal health
The availability and costs of non-toxic alternatives
The fact that non-lead fishing gear is suitable for their angling goals. This may include funds for demonstration activities such as lead fishing gear exchange programs, lead-free fishing derbies, and other programs
Dramatically improve the marketing of non-lead fishing gear.
Part of the solution may be developing novel business models. One suggestion, based on the regulatory desire to reduce the health threat from tobacco use, would be introduce a significant “sin tax” on the manufacture or sale of lead (or other toxic) fishing gear. Funds generated from such taxes could be dedicated to such things as research on non-toxic alternatives, public education, and other goals.
Compiling information for this paper has also made it clear that further efforts should be made to improve our knowledge and data collection about the ingestion of lead fishing gear. To paraphrase Sainsbury et al. (2001), with few exceptions, current programs to investigate morbidity and mortality of wildlife are fragmented and uncoordinated, often being limited to specific narrow taxonomic foci, large-scale outbreaks, or focal geographic areas. Due to limited time, personnel and funding, data collected in one jurisdiction are often not easily comparable with those collected elsewhere. Enhancements in citizen science efforts (including some STEAM education programs—Science, Technology, Engineering, the Arts, and Math) may provide opportunities to correct some of these deficiencies in the future.
We recommend that the following points can be important when developing scientific, educational and regulatory efforts for managing the risks associated with lead fishing gear:
It is important to specify what alternative materials are safe and non-toxic based on best available science and not simply to say “non-lead”
Scientists and agencies should work collaboratively with anglers’ groups, retailers, manufacturers and regulators to accelerate the development, marketing and acceptance of nontoxic fishing tackle
Anglers, manufacturers, sellers and regulators should be helped to understand that coatings will not render lead fishing gear safe and non-toxic
To enhance long-term data compatibility and sharing, researchers and agencies should consider more widely circulating their study plans, priorities and protocols. This will have the effect of more rapidly advancing science and accelerating the development of sound, science-based policies.
Voluntary and educational approaches alone are not effective for risk management and must be combined with legislative approaches which incorporate the features summarized in Table 4 of this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the following people for contributing their data and perspectives to the development of this paper: Eric Corneau, Environment and Climate Change Canada, Gatineau, Que., Canada. Susan Gallo, Maine Audubon Society, Falmouth, ME, USA. Meghan Hartwick, Univ. of New Hampshire, Durham, NH USA. Carrol Henderson, Minnesota Dept. of Natural Resources, St. Paul, MN, USA. Erica LeMoine, Sigurd Olson Inst., Northland College, Ashland, WI, USA. Daniel and Ginger Poleschook, loon biologists, Loon Lake, WA, USA. Sean Strom, Wisconsin Department of Natural Resources, Madison, WI, USA. Jillian Whitney, Massachusetts Department of Conservation and Recreation, West Boylston, MA, USA.
Biographies
Tiffany Grade
is a biologist at the Loon Preservation Committee (LPC). Her research interests include causes of mortality in loons, particularly issues related to lead fishing tackle mortality, and impacts of contaminants on loon survival and breeding success.
Pamela Campbell
is a Principal at ToxEcology – Environmental Consulting Ltd. Research interests include chemical risk assessment and risk management, chemical lifecycle and cost–benefit analyses, and science-based policy development.
Thomas Cooley
is a Wildlife Biologist/Pathologist at Michigan Department of Natural Resources. His research interests include wildlife disease/pathology.
Michelle Kneeland
is a veterinarian and Director of the Wildlife Health Program at Biodiversity Research Institute. Her research interests include implementing a One Health approach to wildlife health, and network building among professionals across the realms of human, environmental, and wildlife health.
Elaine Leslie
is Chief of Biological Resources for the US National Park Service, overseeing wildlife conservation and health, at-risk species, non-native species and their impacts, landscape-level restoration and issues affecting ecological integrity in the national park system. Elaine has long been involved in issues of lead toxicity, including work on California condors, bald and golden eagles.
Brooke MacDonald
is a master’s student in Ecology and Environmental Sciences at the University of Maine, Orono. Her research interests are in applied ecology and human dimensions of conservation biology and resource management.
Julie Melotti
is a Laboratory Technician with the Michigan Department of Natural Resources, Wildlife Disease Laboratory (WDL). Her duties include performing gross necropsies on birds and mammals for cause of death determinations, monitoring reports of sick and dead wildlife from the public, and conducting disease surveillance for bovine tuberculosis and chronic wasting disease.
Joseph Okoniewski
is a wildlife biologist recently retired after 37 years of necropsies and investigations. Research interests included the effects of environmental contaminants on wildlife.
Elizabeth Jane Parmley
is a veterinarian and epidemiologist. She works in surveillance programs, risk assessments, and wildlife conservation and management projects. Research interests include emerging threats and opportunities at the human–animal-environmental interface.
Cyndi Perry
is a biologist, retired from the US Fish & Wildlife Service. She serves on the Board of Directors of the Wildlife Center of Virginia. Her research interests include working in communities to understand the challenges they face while living in close connection with wildlife and how they can benefit.
Harry Vogel
is a Senior Biologist/Executive Director at the Loon Preservation Committee. His research interests include effects of anthropogenic stressors on loon survival and reproductive success, and efficacy of management and education to mitigate stressors and recover loon populations.
Mark Pokras
is an Associate Professor Emeritus at the Cummings School of Veterinary Medicine at Tufts University. His research interests include medicine and surgery of native wildlife, birds as indicators of environmental health, and conservation biology.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tiffany Grade, Phone: (603) 476-5666, Email: tgrade@loon.org.
Pamela Campbell, Email: campbellpm@toxecology.com.
Thomas Cooley, Email: cooleyt2@michigan.gov.
Michelle Kneeland, Email: mkneel02@gmail.com.
Elaine Leslie, Email: elaine_leslie@nps.gov.
Brooke MacDonald, Email: brooke.hafford@maine.edu.
Julie Melotti, Email: melottij@michigan.gov.
Joseph Okoniewski, Email: joseph.okoniewski@dec.ny.gov.
Elizabeth Jane Parmley, Email: jparmley@uoguelph.ca.
Cyndi Perry, Email: cmperry353@gmail.com.
Harry Vogel, Email: hvogel@loon.org.
Mark Pokras, Email: mark.pokras@tufts.edu.
References
- Anderson WL, Havera SP, Zercher BW. Ingestion of lead and nontoxic shotgun pellets by ducks in the Mississippi Flyway. Journal of Wildlife Management. 2000;64:848–857. doi: 10.2307/3802755. [DOI] [Google Scholar]
- Arnemo JM, Andersen O, Stokke S, Thomas VG, Krone O, Pain DJ, Mateo R. Health and environmental risks from lead-based ammunition: Science versus socio-politics. EcoHealth. 2016;13:618–622. doi: 10.1007/s10393-016-1177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DV, Odin N, Torres E. Accumulation of angling litter at game and coarse fisheries in South Wales, UK. Biological Conservation. 1985;34:369–379. doi: 10.1016/0006-3207(85)90041-2. [DOI] [Google Scholar]
- Bengfort, J., and R.W. Carithers. 1976. Lead poisoning in dogs. Iowa State University Veterinarian, 38. https://lib.dr.iastate.edu/iowastate_veterinarian/vol38/iss3/4.
- Binkowski LJ. The influence of environmental conditions on lead transfer from spent gunshot to sediments and water: Other routes for Pb poisoning. Chemosphere. 2017;187:330–337. doi: 10.1016/j.chemosphere.2017.08.103. [DOI] [PubMed] [Google Scholar]
- Birkhead M. Causes of mortality in the mute swan, Cygnus olor, on the River Thames. Journal of Zoology. 1982;198:15–25. doi: 10.1111/j.1469-7998.1982.tb02057.x. [DOI] [Google Scholar]
- Birkhead M. Lead levels in the blood of mute swans, Cygnus olor, on the River Thames. Journal of Zoology. 1983;199:59–73. doi: 10.1111/j.1469-7998.1983.tb06117.x. [DOI] [Google Scholar]
- Blakley, B.R. 2019. Overview of lead poisoning. Merck Veterinary Manual (online). https://www.merckvetmanual.com/toxicology/lead-poisoning/overview-of-lead-poisoning
- Blus LJ. A review of lead poisoning in swans. Comparative Biochemistry and Physiology Part C. 1994;108:259–267. [Google Scholar]
- Blus LJ, Stroud RK, Reiswig B, McEneaney T. Lead poisoning and other mortality factors in trumpeter swans. Environmental Toxicology and Chemistry. 1989;8:263–271. doi: 10.1002/etc.5620080308. [DOI] [Google Scholar]
- Borkowski R. Lead poisoning and intestinal perforations in a snapping turtle (Chelydra serpentina) due to fishing gear ingestion. Journal of Zoo and Wildlife Medicine. 1997;28:109–113. [PubMed] [Google Scholar]
- Bowen JE, Petrie SA. Incidence of artifact ingestion in mute swans and tundra swans on the Lower Great Lakes, Canada. Ardea. 2007;95:135–142. doi: 10.5253/078.095.0115. [DOI] [Google Scholar]
- Brown MJ, Linton E, Rees EC. Causes of mortality among wild swans in Britain. Wildfowl. 1992;43:70–79. [Google Scholar]
- Burger J, Gochfeld M. Behavioral impairments of lead-injected young herring gulls in nature. Fundamental and Applied Toxicology. 1994;23:553–561. doi: 10.1006/faat.1994.1140. [DOI] [PubMed] [Google Scholar]
- Ciaranca, M.A., C.C. Allin, and G.S. Jones. 1997. Mute swan (Cygnus olor), version 2.0. In The Birds of North America, eds. A.F. Poole and F.B. Gill. Ithaca, NY: Cornell Lab of Ornithology. 10.2173/bna.273.
- CLLS (Canadian Lakes Loon Survey). 2019. The Canadian lakes loon survey: Science for lake and loon conservation. https://www.birdscanada.org/volunteer/clls/resources/CLLSBrochure.pdf.
- Cole JB, Stellpflug SJ, Karpas A, Roberts DJ. Ingestion of one lead fishing sinker resulting in toxic lead levels within hours [abstract] Clinical Toxicology. 2010;48:622. [Google Scholar]
- Cook RS, Trainer DO. Experimental lead poisoning of Canada geese. Journal of Wildlife Management. 1966;30:1–8. doi: 10.2307/3797877. [DOI] [Google Scholar]
- Cryer M, Corbett JJ, Winterbotham MD. The deposition of hazardous litter by anglers at coastal and inland fisheries in South Wales. Journal of Environmental Management. 1987;25:125–135. [Google Scholar]
- Danish EPA (Environmental Protection Agency). 2014. Report on lead fishing gear sales in Denmark. Copenhagen, DK: Ministry of Environment and Food of Denmark. https://mst.dk/service/nyheder/nyhedsarkiv/2014/maj/miljoestyrelsen-paa-jagt-efter-bly-i-fiskegrej/
- Daoust P-Y, Conboy G, McBurney S, Burgess N. Interactive mortality factors in common loons from Maritime Canada. Journal of Wildlife Diseases. 1998;34:524–531. doi: 10.7589/0090-3558-34.3.524. [DOI] [PubMed] [Google Scholar]
- de Francisco ON, Feeney D, Armién AG, Wuenschmann A, Redig PT. Correlation of brain magnetic resonance imaging of spontaneously lead poisoned bald eagles (Haliaeetus leucocephalus) with histological lesions: A pilot study. Research in Veterinary Science. 2016;105:236–242. doi: 10.1016/j.rvsc.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Degernes LA, Heilman S, Trogdon M, Jordan M, Davison M, Kraege D, Correa M, Cowen P. Epidemiologic investigation of lead poisoning in trumpeter and tundra swans in Washington state, USA, 2000–2002. Journal of Wildlife Diseases. 2006;42:345–358. doi: 10.7589/0090-3558-42.2.345. [DOI] [PubMed] [Google Scholar]
- DeGroot J, Steg L. Value orientations and environmental beliefs in five countries: Validity of an instrument to measure egoistic, altruistic, and biospheric value orientations. Journal of Cross-Cultural Psychology. 2007;38:318–332. doi: 10.1177/0022022107300278. [DOI] [Google Scholar]
- Duerr, A.E. 1999. Abundance of lost and discarded fishing tackle and implications for waterbird populations in the United States. MS Thesis. Tucson, AZ: Univ. of Arizona. http://hdl.handle.net/10150/278698.
- Ensor KL, Helwig DD, Wemmer LC. Mercury and lead in Minnesota common loons (Gavia immer) St. Paul, MN: Minnesota Pollution Control Agency; 1992. [Google Scholar]
- ECCC. 2018. Study to gather use pattern information on lead sinkers and jigs and their non-lead alternatives in Canada. Gatineau, QC: Prepared by ToxEcology Environmental Consulting Ltd. for Environment and Climate Change Canada (ECCC). https://www.canada.ca/en/environment-climate-change/services/management-toxic-substances/list-canadian-environmental-protection-act/lead/using-more-lead-free-fishing-tackle.html.
- ECHA. 2018. A review of the available information on lead in shot used in terrestrial environments, in ammunition and in fishing tackle. Helsinki, Finland: European Chemicals Agency, Annex XV Investigation report. https://echa.europa.eu/documents/10162/13641/lead_ammunition_investigation_report_en.pdf/efdc0ae4-c7be-ee71-48a3-bb8abe20374a.
- Ecke F, Singh NJ, Arnemo JM, Bignert A, Helander B, Berglund AMM, Borg H, Bröjer C. Sublethal lead exposure alters movement behavior in free-ranging golden eagles. Environmental Science and Technology. 2017;51:5729–5736. doi: 10.1021/acs.est.6b06024. [DOI] [PubMed] [Google Scholar]
- European Commission. 2004. Advantages and drawbacks of restricting the marketing and use of lead in ammunition, fishing sinkers and candle wicks. European Commission Enterprise Directorate-General COWI CONTRACT NUMBER – ETD/FIF.20030756 Final Report.
- Evers, D.C., J.D. Paruk, J.W. McIntyre, and J.F. Barr. 2010. Common loon (Gavia immer). No. 313. The Birds of North America Online, ed. A Poole. Ithaca, NY: Cornell Laboratory of Ornithology. http://bna.birds.cornell.edu/bna/species/313.
- Finley MT, Dieter MP, Locke LN. Lead in tissues of mallard ducks dosed with two types of lead shot. Bulletin of Environmental Contamination and Toxicology. 1976;16:261–269. doi: 10.1007/BF01685887. [DOI] [PubMed] [Google Scholar]
- Forbes IJ. The quantity of lead shot, nylon fishing line and other litter discarded at a coarse fishing lake. Biological Conservation. 1986;38:21–34. doi: 10.1016/0006-3207(86)90017-0. [DOI] [Google Scholar]
- Franson JC, Cliplef DJ. Causes of mortality in common loons. In: Morse L, Stockwell S, Pokras M, editors. Proceedings from the 1992 conference on the loon and its ecosystem: Status, management, and environmental concerns. Concord, NH: U.S. Fish and Wildlife Service; 1992. pp. 2–12. [Google Scholar]
- Franson JC, Hansen SP, Pokras MA, Miconi R. Size characteristics of stones ingested by common loons. Condor. 2001;103:189–191. doi: 10.1650/0010-5422(2001)103[0189:SCOSIB]2.0.CO;2. [DOI] [Google Scholar]
- Franson JC, Hansen SP, Creekmore TE, Brand CJ, Evers DC, Duerr AE, DeStefano S. Lead fishing weights and other fishing tackle in selected waterbirds. Waterbirds. 2003;26:345–352. doi: 10.1675/1524-4695(2003)026[0345:LFWAOF]2.0.CO;2. [DOI] [Google Scholar]
- Fulton DC, Mangredo MJ, Lipscomb J. Wildlife value orientations: A conceptual and measurement approach. Human Dimensions of Wildlife. 1996;1:24–47. doi: 10.1080/10871209609359060. [DOI] [Google Scholar]
- Galili E, Zemer A, Rosen B. Ancient fishing gear and associated artifacts from underwater explorations in Israel—A comparative study. Archaeofauna. 2013;22:145–166. [Google Scholar]
- Goode, D.A. (ed). 1981. Lead poisoning in swans. Report of the Nature Conservancy Council’s Working Group.
- Grade, T.J. 2011. Effects of lead fishing tackle on common loons (Gavia immer) in New Hampshire, 1989–2010. MS Thesis. Madison, WI: Univ. of Wisconsin.
- Grade TJ, Pokras MA, LaFlamme EM, Vogel HS. Population-level effects of lead fishing tackle on common loons. Journal of Wildlife Management. 2018;82:155–164. doi: 10.1002/jwmg.21348. [DOI] [Google Scholar]
- Gray CE, Paruk JD, DeSorbo CR, Savoy LJ, Yates DE, Chickering MD, Gray RB, Taylor KM, et al. Body mass in common loons (Gavia immer) strongly associated with migration distance. Waterbirds. 2014;37(sp1):64–75. doi: 10.1675/063.037.sp109. [DOI] [Google Scholar]
- Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W. 2016 Annual report of the American Association of Poison Control Centers’ national poison data system (NPDS): 34th annual report. Clinical Toxicology. 2017;55:1072–1254. doi: 10.1080/15563650.2017.1388087. [DOI] [PubMed] [Google Scholar]
- Haig SM, D’Elia J, Eagles-Smith C, Fair JM, Gervais J, Herring G, Rivers JW, Schulz JH. The persistent problem of lead poisoning in birds from ammunition and fishing tackle. The Condor. 2014;116:408–428. doi: 10.1650/CONDOR-14-36.1. [DOI] [Google Scholar]
- Harrigan K. Causes of mortality of little penguins, Eudyptula minor, in Victoria. Emu-Austral Ornithology. 1991;91:273–277. doi: 10.1071/MU9910273. [DOI] [Google Scholar]
- Health Canada. 2013. Risk management strategy for lead. Ottawa, ON. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewhsemt/alt_formats/pdf/pubs/contaminants/prms_lead-psgr_plomb/prms_lead-psgr_plomb-eng.pdf.
- Hunter B, Haigh JC. Demyelinating peripheral neuropathy in a guinea hen associated with subacute lead intoxication. Avian Diseases. 1978;22:344–349. doi: 10.2307/1589548. [DOI] [PubMed] [Google Scholar]
- Jacks G, Bystron M, Johansson L. Lead emissions from lost fishing sinkers. Boreal Environment Research. 2001;6:231–236. [Google Scholar]
- Janssens E, Dauwe T, Van Duyse E, Beernaert J, Pinxten R, Eens M. Effects of heavy metal exposure on aggressive behavior in a small territorial songbird. Archives of Environmental Contamination and Toxicology. 2003;45:0121–0127. doi: 10.1007/s00244-002-0133-7. [DOI] [PubMed] [Google Scholar]
- Karstad L. Angiopathy and cardiopathy in wild waterfowl from ingestion of lead shot. Connecticut Medicine. 1971;35:355–360. [PubMed] [Google Scholar]
- Kelly A, Kelly S. Fishing tackle injury and blood lead levels in mute swans. Waterbirds. 2004;27:60–68. doi: 10.1675/1524-4695(2004)027[0060:FTIABL]2.0.CO;2. [DOI] [Google Scholar]
- Kelly A, Kelly S. Are mute swans with elevated blood lead levels more likely to collide with overhead power lines? Waterbirds. 2005;28:331–334. doi: 10.1675/1524-4695(2005)028[0331:AMSWEB]2.0.CO;2. [DOI] [Google Scholar]
- KEMI. 2007. Lead in articles. Stockholm, SE: Reported by the Swedish Chemicals Agency and the Swedish Environmental Protection Agency, Report 5/07. https://www.kemi.se/global/rapporter/2007/rapport-5-07-lead-in-articles.pdf.
- Khan, H. 2014. Exposure of lead amongst primary school children in fishing communities in South Africa. MS Thesis. Univ. of Witwatersrand, Johannesburg, South Africa. http://146.141.12.21/bitstream/handle/10539/15464/MMed587333FINAL.pdf?sequence=1&isAllowed=y.
- Kirby J, Delany S, Quinn J. Mute swans in Great Britain: A review, current status, and long-term trends. Hydrobiologia. 1994;279:467–482. doi: 10.1007/BF00027878. [DOI] [Google Scholar]
- Kneeland M, Pokras M. Lead poisoning: Using transdisciplinary approaches to solve an ancient problem. EcoHealth. 2008;5:379–385. doi: 10.1007/s10393-008-0177-x. [DOI] [PubMed] [Google Scholar]
- Leszek, M. 2015. Changing angler behavior to reduce the impacts of lead fishing tackle in New Hampshire: Applied social science using community based social marketing. MS Thesis. Plymouth State University, Plymouth, NH.
- Lloret J, Garrote A, Balasch N, Font T. Estimating recreational fishing tackle loss in Mediterranean coastal areas: Potential impacts on wildlife. Aquatic Ecosystem Health & Management. 2014;17:179–185. doi: 10.1080/14634988.2014.910070. [DOI] [Google Scholar]
- Locke LN, Kerr SM, Zoromski D. Lead poisoning in common loons (Gavia immer) Avian Diseases. 1982;26:392–396. doi: 10.2307/1590110. [DOI] [PubMed] [Google Scholar]
- LPC (Loon Preservation Committee). The efficacy of education to address loon mortality from ingested lead fishing tackle. Moultonborough, NH: Loon Preservation Committee; 2012. [Google Scholar]
- MacDonald JW, Goater R, Atkinson NK, Small J. Further causes of death in Scottish swans, Cygnus olor. State Veterinary Journal. 1990;44:81–93. [Google Scholar]
- McClelland SC, Ribeiro RD, Mielke HW, Finkelstein ME, Gonzales CR, Jones JA, Komdeur J, Derryberry E, et al. Sub-lethal exposure to lead is associated with heightened aggression in an urban songbird. Science of the Total Environment. 2019;654:593–603. doi: 10.1016/j.scitotenv.2018.11.145. [DOI] [PubMed] [Google Scholar]
- Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, Baranowska-Bosiacka I. Lead (Pb) exposure enhances expression of factors associated with inflammation. International Journal of Molecular Sciences. 2018;19:1813. doi: 10.3390/ijms19061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPA (Minnesota Pollution Control Agency). 2013. Toxics and pollution prevention evaluation report. St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/lrp-p2s-2sy13.pdf.
- Mowad E, Haddad I, Gemmel DJ. Management of lead poisoning from ingested fishing sinkers. Archives of Pediatric and Adolescent Medicine. 1998;152:485–488. doi: 10.1001/archpedi.152.5.485. [DOI] [PubMed] [Google Scholar]
- Needleman HL. Human lead exposure. Boca Raton, FL: CRC Press; 2000. [Google Scholar]
- Newth JL, Cromie RL, Brown MJ, Delahay RJ, Meharg AA, Deacon C, Norton GJ, O’Brien MF, et al. Poisoning from lead gunshot: Still a threat to wild waterbirds in Britain. European Journal of Wildlife Research. 2013;59:195–204. doi: 10.1007/s10344-012-0666-7. [DOI] [Google Scholar]
- Newth JL, Rees EC, Cromie RL, McDonald RA, Bearhop S, Pain DJ, Norton GJ, Deacon C, et al. Widespread exposure to lead affects the body condition of free-living whooper swans Cygnus cygnus wintering in Britain. Environmental Pollution. 2016;209:60–67. doi: 10.1016/j.envpol.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Nriagu JO. Lead & lead poisoning in antiquity. New York: Wiley; 1983. [Google Scholar]
- O’Connell MM, Rees EC, Einarsson Ó, Spray CJ, Thorstensen S, O’Halloran J. Blood lead levels in wintering and moulting Icelandic whooper swans over two decades. Journal of Zoology. 2008;276:21–27. doi: 10.1111/j.1469-7998.2008.00462.x. [DOI] [Google Scholar]
- Oliver T. Lead poisoning: From the industrial, medical, and social points of view. New York: Paul B. Hoeber; 1914. [Google Scholar]
- Olivero-Verbel J, Duarte D, Echenique M, Guette J, Johnson-Restrepo B, Parsons PJ. Blood lead levels in children aged 5–9 years living in Cartagena, Colombia. Science of the Total Environment. 2007;372:707–716. doi: 10.1016/j.scitotenv.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Owen M, Cadbury CJ. The ecology and mortality of swans at the Ouse Washes, England. Wildfowl. 1975;26:31–42. [Google Scholar]
- Pain DJ. Why are lead-poisoned waterfowl rarely seen?: The disappearance of waterfowl carcasses in the Camargue, France. Wildfowl. 1991;42:118–122. [Google Scholar]
- Pattee OH, Pain DJ. Lead in the environment. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr, editors. Handbook of ecotoxicology. 2. New York: Lewis Publishers; 2003. [Google Scholar]
- Pennycott TW. Causes of mortality in mute swans Cygnus olor in Scotland 1995–1996. Wildfowl. 1999;50:11–20. [Google Scholar]
- Perrins, C.M., P. Martin, and B. Broughton. 2002. The impact of lost and discarded fishing line and tackle on mute swans. Bristol, UK: Environment Agency R&D Technical Report W1-051/TR.
- Perry, C. 1994. Lead sinker ingestion in avian species. U.S. Fish and Wildlife Service, Division of Environmental Contamination Information Bulletin. 94-09-01, Arlington, VA.
- Pokras MA, Chafel R. Lead toxicosis from ingested fishing sinkers in adult common loons (Gavia immer) in New England. Journal of Zoo and Wildlife Medicine. 1992;23:92–97. [Google Scholar]
- Pokras MA, Kneeland M, Ludi A, Golden E, Major A, Miconi R, Poppenga RH. Lead objects ingested by common loons in New England. Northeastern Naturalist. 2009;16:177–182. doi: 10.1656/045.016.0202. [DOI] [Google Scholar]
- Poleschook D, Gumm G. Recommendation to ban the use of lead fishing tackle in Washington. Olympia, WA: Report to Washington Fish and Wildlife Commission; 2009. [Google Scholar]
- Radomski P, Heinrich T, Jones TS, Rivers P, Talmage P. Estimates of tackle loss for five Minnesota walleye fisheries. North American Journal of Fisheries Management. 2006;26:206–212. doi: 10.1577/M05-121.1. [DOI] [Google Scholar]
- Redpath AM, Gutierrez RJ, Wood KA, Young JC. Conflicts in conservation: Navigating towards solutions. Cambridge, UK: Cambridge University Press; 2015. [Google Scholar]
- Reiger JF. American sportsmen and the origins of conservation. New York: Winchester Press; 1975. [Google Scholar]
- Ross-Winslow DJ, Teel TL. The quest to eliminate lead from units of the National Park System: Understanding and reaching out to audiences. The George Wright Forum. 2011;28:34–77. [Google Scholar]
- Sainsbury AW, Kirkwood JL, Bennett PM, Cunningham AA. Status of wildlife health monitoring in the United Kingdom. Veterinary Record. 2001;148:558–563. doi: 10.1136/vr.148.18.558. [DOI] [PubMed] [Google Scholar]
- Sahmel J, Hsu EI, Avens HJ, Beckett E, Devlin KD. Estimation of hand-to-mouth transfer efficiency of lead. Annals of Work Exposures and Health. 2015;59:210–220. doi: 10.1093/annhyg/meu088. [DOI] [PubMed] [Google Scholar]
- St. Clair WS, Benjamin J. Lead intoxication from ingestion of fishing sinkers: A case study and review of the literature. Clinical Pediatrics. 2008;47:66–70. doi: 10.1177/0009922807304135. [DOI] [PubMed] [Google Scholar]
- Scheuhammer, A.M. 2009. Historical perspective on the hazards of environmental lead from ammunition and fishing weights in Canada. In Ingestion of lead from spent ammunition: Implications for wildlife and humans, eds. R.T. Watson, M. Fuller, M. Pokras, and W.G. Hunt. Boise, ID: The Peregrine Fund. 10.4080/ilsa.2009.0105.
- Scheuhammer, A.M., S.L. Money, D.A. Kirk, and G. Donaldson. 2003. Lead fishing sinkers and jigs in Canada: Review of their use patterns and toxic impacts on wildlife. Ottawa, ON: Canadian Wildlife Service. Occasional Paper #108.
- Scheuhammer, A.M., and S.L. Norris. 1995. A review of the environmental impacts of lead shotshell ammunition and lead fishing weights in Canada. Ottawa, ON: Canadian Wildlife Service Occ. Paper # 88.
- Schroeder, R.R. 2010. Lead fishing tackle: The case for regulation in Washington State. MS Thesis. Olympia, WA: Evergreen State College.
- Schummer ML, Fife I, Petrie SA, Badzinski SS. Artifact ingestion in sea ducks wintering at northeastern Lake Ontario. Waterbirds. 2011;34:51–58. doi: 10.1675/063.034.0106. [DOI] [Google Scholar]
- Sears J. Regional and seasonal variations in lead poisoning in the mute swan Cygnus olor in relation to the distribution of lead and lead weights, in the Thames area, England. Biological Conservation. 1988;46:115–134. doi: 10.1016/0006-3207(88)90095-X. [DOI] [Google Scholar]
- Sears J. Feeding activity and body condition of mute swans Cygnus olor in rural and urban areas of a lowland river system. Wildfowl. 1989;40:88–98. [Google Scholar]
- Sears, A., and J. Hunt. 1991. Lead poisoning in mute swans, Cygnus olor, in England. In Proceedings of the third IWRB international swan symposium, eds. J. Sears, and P. Bacon. Wildfowl Supplement 1: 383–388.
- Sidor IF, Pokras MA, Major AR, Poppenga R, Miconi RM. Mortality of the common loon in New England, 1988 to 2000. Journal of Wildlife Diseases. 2003;39:306–315. doi: 10.7589/0090-3558-39.2.306. [DOI] [PubMed] [Google Scholar]
- Spray C, Milne H. The incidence of lead poisoning among whooper and mute swans Cygnus cygnus and C. olor in Scotland. Biological Conservation. 1988;44:265–281. doi: 10.1016/0006-3207(88)90020-1. [DOI] [Google Scholar]
- Stern P, Dietz T, Kalof L, Guagano G. A value-belief-norm theory of support for social movements: The case of environmental concern. Human Ecology Review. 1999;6:81–97. [Google Scholar]
- Stone WB, Okoniewski JC. Necropsy findings and environmental contaminants in common loons from New York. Journal of Wildlife Diseases. 2001;37:178–184. doi: 10.7589/0090-3558-37.1.178. [DOI] [PubMed] [Google Scholar]
- Strom, S.M., J.A. Langenberg, N.K. Businga, and J.K. Batten. 2009. Lead exposure in Wisconsin birds. In Ingestion of lead from spent ammunition: Implications for wildlife and humans, eds. R.T. Watson, M. Fuller, M. Pokras, and W.G. Hunt. Boise, ID: The Peregrine Fund. 10.4080/ilsa.2009.0104.
- Stroud, D.A. 2015. Regulation of some sources of lead poisoning: A brief review. In Proceedings of the Oxford lead symposium, eds. R.J. Delahay and C.J. Spray, 8–26. Oxford: Edward Grey Institute.
- Thomas, V.G. 2019. Chemical compositional standards for non-lead hunting ammunition and fishing weights. In Lead in hunting ammunition: Persistent problems and solutions, eds. N. Kanstrup, V.G. Thomas, and A.D. Fox, Ambio vol. 48, Special Issue. 10.1007/s13280-018-1124-x. [DOI] [PMC free article] [PubMed]
- Thomas, V.G, N. Kanstrup, and C. Gremse. 2015. Key questions and responses regarding the transition to use of lead-free ammunition. In Proceedings of the Oxford lead symposium. Lead ammunition: Understanding and minimising the risks to human and environmental health. Edward Grey Institute, The University of Oxford. http://www.oxfordleadsymposium.info/wp-content/uploads/OLS_proceedings/papers/OLS_proceedings_thomas_kanstrup_gremse.pdf.
- Thomas VG, Guitart R. Lead pollution from shooting and angling, and a common regulative approach. Environmental Policy and Law. 2003;33:143–149. [Google Scholar]
- Thomas VG. Attitudes and issues preventing bans on toxic lead shot and sinkers in North America and Europe. Environmental Values. 1997;6:185–199. doi: 10.3197/096327197776679176. [DOI] [Google Scholar]
- Trust KA, Miller MW, Ringelman JK, Orme IM. Effects of ingested lead on antibody production in mallards (Anas platyrhynchos) Journal of Wildlife Diseases. 1990;26:316–322. doi: 10.7589/0090-3558-26.3.316. [DOI] [PubMed] [Google Scholar]
- Twiss, M.P. 1997. Preventing lead poisoning of Ontario’s piscivorous birds through policy and regulative reform. MS Thesis. Univ. of Guelph, Ontario, Canada.
- Twiss MP, Thomas VG. Preventing fishing-sinker-induced lead poisoning of common loons through Canadian policy and regulative reform. Journal of Environmental Management. 1998;53:49–59. doi: 10.1006/jema.1998.0190. [DOI] [Google Scholar]
- Tyrrell, R. 2015. Lead weights. In Heybridge: A late iron age and Roman settlement, excavations at Elms Farm 1993-5 eds. M. Atkinson and S.J. Preston. Internet Archaeology 40. 10.11141/ia.40.1.tyrrell8. [DOI]
- USEPA (Environmental Protection Agency) Lead fishing sinkers; response to citizens’ petition and proposed ban; proposed rule, 40 CFR part 745. Federal Register. 1994;59:11122–11143. [Google Scholar]
- USFWS (US Fish and Wildlife Service). 1986. Final supplemental environmental impact statement on the use of lead shot for hunting migratory birds. Office of Migratory Bird Management, FES 86-16.
- Vaske, J.J., and M. Manfredo. 2012. Social psychological considerations in wildlife management. In Human dimensions of wildlife management, 2nd ed., eds. D.J. Decker, S.J. Riley, and W.F. Siemer. Baltimore, MD: The Johns Hopkins University Press.
- Walker RE. Malleus and podagra: Lead poisoning in horse and man. Veterinary History. 1981;1:118–136. [PubMed] [Google Scholar]
- Wani AL, Ara A, Usmani JA. Lead toxicity: A review. Interdisciplinary Toxicology. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2009. Global health risks: Mortality and burden of disease attributable to selected major risks. Geneva, CH. https://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf.
- Windingstad RM, Kerr SM, Locke LN, Hurt JJ. Lead poisoning of sandhill cranes (Grus canadensis) Prairie Naturalist. 1984;16:21–24. [Google Scholar]
- Wood KA, Brown MJ, Cromie RL, Hilton GM, Mackenzie C, Newth JL, Pain DJ, Perrins CM, et al. Regulation of lead fishing weights results in mute swan population recovery. Biological Conservation. 2019;230:67–74. doi: 10.1016/j.biocon.2018.12.010. [DOI] [Google Scholar]
- Zabka TS, Haulena MM, Puschner B, Gulland FMD, Conrad PA, Lowenstine LJ. Acute lead toxicosis in a harbor seal (Phoca vitulina richardsi) consequent to ingestion of a lead fishing sinker. Journal of Wildlife Diseases. 2006;42:651–657. doi: 10.7589/0090-3558-42.3.651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


