Abstract
Background:
Human adipose tissue is routinely discarded as medical waste. However, this tissue may have valuable clinical applications since methods have been devised to effectively isolate adipose-derived extracellular matrix (ECM), growth factors (GFs), and stem cells. In this review, we analyze the literature that devised these methods and then suggest an optimal method based on their characterization results.
Methods:
Methods that we analyze in this article include: extraction of adipose tissue, decellularization, confirmation of decellularization, identification of residual active ingredients (ECM, GFs, and cells), removal of immunogens, and comparing structural/physiological/biochemical characteristics of active ingredients.
Results:
Human adipose ECMs are composed of collagen type I–VII, laminin, fibronectin, elastin, and glycosaminoglycan (GAG). GFs immobilized in GAG include basic fibroblast growth factor (bFGF), transforming growth factor beta 1(TGF-b1), insulin like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), BMP4 (bone morphogenetic protein 4), nerve growth factor (NGF), hepatocyte growth factor (HGF), and epithermal growth factor (EGF). Stem cells in the stromal-vascular fraction display mesenchymal markers, self-renewal gene expression, and multi-differentiation potential.
Conclusion:
Depending on the preparation method, the volume, biological activity, and physical properties of ECM, GFs, and adipose tissue-derived cells can vary. Thus, the optimal preparation method is dependent on the intended application of the adipose tissue-derived products.
Keywords: Human adipose tissue, Extracellular matrix, Growth factors, Adipose-derived stem cell, Optimum method
Introduction
Human adipose tissue is removed safely and easily with local anesthesia using simple techniques, and the amount of material removed is sufficient for many clinical applications without further manipulation [1, 2]. Once removed via clinical liposuction, body contouring, breast reduction, or abdominoplasty procedures, the tissue is typically discarded as medical waste [3]. However, human adipose tissue is an ideal biomaterial for implantation because it contains large amounts of bioactive constituents such as extracellular matrix (ECM) components, growth factors (GFs), and stem/progenitor cells [4, 5].
ECM components that can be found in human adipose tissue include collagen, fibronectin, elastin, laminin, and glycosaminoglycan (GAG). These components have minimal immunogenicity (i.e. no xenogeneic antigens, such as α-Gal epitopes), induce angio-/adipogenesis, and promote long-term retention of soft-tissue [6]. GFs that can be found in human adipose tissue include bFGF, TGF-b1, IGF-1, VEGF, PDGF, BMP4, NGF, HGF, and EGF. They are persistent even after the decellularization process and can promote cell differentiation into several target lineages [7]. The fractioned cell types include smooth muscle, endothelial, fibroblasts, lipogenic, hematopoietic, and stem cells. Adipose-derived stem cells display mesenchymal stem cell characteristics [8], and cell quality is not dependent on donor age [9].
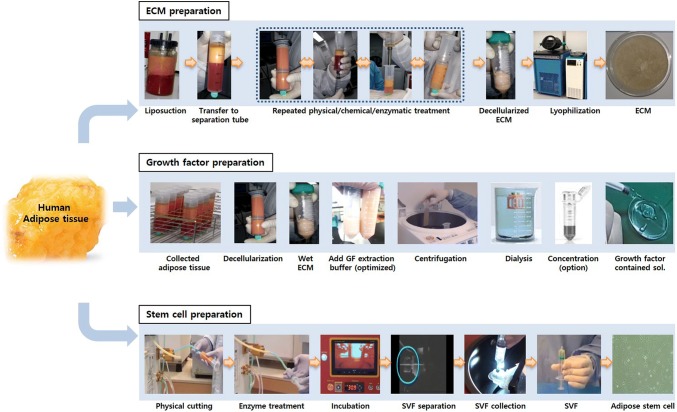
However, the type, components, volume, biological activity, and physical properties of adipose-derived ECM, GFs, and cells vary depending on how they are extracted. This can result in a variety of regeneration effects during in vivo application [10, 11]. Thus, this review surveys the characteristics that ECM, GFs, and cells can acquire based on the method used to extract the original adipose tissue (Fig. 1). Such information is critical to maximizing target tissue regeneration capabilities.
Fig. 1.
Scheme for preparation of human adipose tissue-derived extracellular matrix (ECM), growth factors (GFs), and stem cells. Human adipose-derived ECM is collected from liposuction, decellularized, and then manufactured to the required form. The GFs are prepared from decellularized ECM using optimized extraction buffer, then collected through dialysis and concentration. The stem cells are isolated from stromal-vascular fraction (SVF) through physical and enzymatic process
Human adipose-derived ECM
To collect ECM, human adipose tissue is obtained via liposuction using a commercially available device. The extracted tissue is washed with distilled water to remove blood, and then the tissue/water mixture is homogenized and centrifuged. The oil contained upper layer is discarded, and the remaining gel-like suspension is washed with distilled water and decellularized to obtain ECM [12]. During the decellularization step, the cellular and nuclear materials are removed because they can cause an in vivo inflammatory response and immune rejection [13].
Decellularization methods
There are three common decellularization methods: physical (e.g. freezing, pressure, agitation, sonication, and radiation) [14], chemical (e.g. detergent, acid, and alkaline), and biological treatment with enzymes [15]. To increase the efficiency of decellularization, these treatments are often combined. For example, freeze–thaw treatments loosen connective tissue, and chemical and enzymatic agents facilitate the removal of cellular components [15, 16].
When choosing a decellularization protocol, several factors are considered such as origin, thickness, density, cellularity, and lipid content [17]. This is also true for human adipose tissue, which is composed of loose connective tissue; therefore, a mild method is recommended over a harsher method. For example, extensive freezing (− 80 °C) and thawing (37 °C) to remove lipid tissue causes severe alterations in the matrix architecture. Therefore, this physical method has been replaced by chemical methods that use polar solvents such as acetone, ethanol, or isopropanol (with isopropanol being the most effective) [18, 19]. Some chemical detergents such as (sodium dodecyl sulfate, SDS) have a strong decellularization effect resulting in the disruption of the matrix architecture. Usually, SDS is applied to dense, compact organs such as the heart or porcine adipose tissue decellularization [13]. But human adipose tissue is a gel-like substance [19], and SDS treatment causes dramatic swelling of the ECM and irreversible macroscopic degradation of its structure [19]. Furthermore, SDS strongly interacts with ECM components and renders them difficult to remove at the end of processing. SDS is also cytotoxic when reseeding cells on a matrix [20]. For these reasons, SDS has been replaced with the milder detergent EDTA when decellularizing human adipose tissue [19]. The ECM extraction method was visualized on Fig. 1.
Adipose ECM composition
After the decellularization process, the volume of ECM extracted accounts for approximately 5% of the original adipose tissue [16]. The types of ECM in human adipose tissue include collagen type I–VII, laminin, fibronectin, elastin, and GAGs [21, 22]. Collagens are the most abundant proteins after decellularization [23], and there are four different types: the densely-packed type, the lumen type with vascular structure, the void space type with interwoven fiber, and the network type [19]. Type I collagen surrounds adipocytes with thin fibers and thick bundles composed of heterotrimers in a triple helix and provides the major ECM framework necessary to sustain the structure [24]. Collagen type III also surrounds adipocytes [10]. Collagen type IV serves as a basement membrane for the adipocyte area and is located under the vascular endothelial cell layer [10]. Fibrillar collagen type IV contains various binding sites for bioactive molecules that modulate cellular behavior; thus, it acts as a functional and structural protein [25]. Collagen type V and VI form micro-fibers composed of heterotrimers and are located between type I collagen fibers [26]. Collagen type VII is located within the matrix between adipocytes and near blood vessels in small amounts [10].
Non-collagen ECM proteins found in adipose tissue include laminin, fibronectin, elastin, and GAGs. Laminin composes trimeric α-, β-, and γ-chain structures [27]; promotes epithelial cell attachment to the basal lamina; and is involved in cell migration and growth [28]. Laminin also forms a network with type IV collagen α-chain [27]. Fibronectin dictates cytoskeletal and ECM organization, which controls cell shape and contractility [29]. Also, fibronectin maintains the fibrillary organization of collagen type I by cross-linking to an a1(I) chain [30]. Elastin is related to collagen type VI [31], and its network density increases with obesity [32]. In visceral fat, elastin fiber forms a mesh-like net, and in subcutaneous fat, it is linear [33] and involved in the elasticity of adipose tissue [34, 35]. Sulfated GAG sequesters growth factors and presents them to cells creating a path for bioactive molecule delivery [36, 37].
Extracted adipose ECMs are quantified with commercial kits. For collagen quantification, the Sircol Soluble Collagen Assay Kit is used [38]. Collagen types that are rich in adipose tissue are I, II, and IV [15]. Masson’s trichrome and Picrosirius (Sirius) red are used for collagen fiber staining. For laminin and fibronectin quantification and identification, commercial ELISA kits and immunohistochemical staining are available, respectively. For elastin quantification, the Fastin Elastin Assay Kit is used [13], and elastic fiber is stained with Verhoeff’s-Van Gieson elastic stain. For GAG quantification, the Blyscan Sulfated GAG Assay Kit is used [38]. Reported GAG content is approximately 40 μg per 1 g of adipose tissue [15]. Sulfated GAG is stained with Alcian blue.
Confirmation of decellularization
The inefficient removal of cellular contents such as cell membranes, nucleic acids, lipids, and cytosolic components can cause inflammatory responses and low regeneration of target tissue in vivo [39, 40]. DNase, RNase, and lipase are added during the decellularization process to remove these immunogens. Quantification of residual DNA is performed with a commercial kit, and DNA content is normalized to the initial wet weight of the sample. The elimination of DNA is also validated by polymerase chain reaction (PCR) and histological staining [16]. For PCR, primers designed to amplify the glyceraldehyde-3-phosphate dehydrogenase (GAPDH, NM002046) gene are used with total DNA serving as the template. For end-point PCR, the amplified product is analyzed with agarose gel electrophoresis. When real-time PCR is used, Ct values are observed. Standard criteria state that residual DNA fragments should be < 200 bp in length, and the total DNA content should be < 50 ng of double-stranded DNA/mg of ECM (dry weight) [10]. For histological staining, ECM is fixed in paraformaldehyde, embedded in paraffin or frozen, and stained with 4,6-diamidino-2-phenylindole (DAPI) or acridine orange (AO). Routine hematoxylin and eosin staining (H&E) can also show residual cytosolic and nuclear components [14]. The residual lipids within the ECM are observed with Oil red O staining; the red color indicates the presence of lipids [10].
Mechanical properties
Hydrated human adipose ECM exhibits a whitish gel-like appearance, and the final product volume is 30–40% of the original tissue mass [38]. Freeze-dried ECM resembles a porous sponge where the pore sizes can range between 20 and 200 μm, and the average porosity is approximately 90% [15]. The porosity can be controlled by temperature; pore size is relatively decreased as the temperature [22]. The collagen structure is interconnected with much finer, network-type collagen [19]. The extensive surface area and median pore size achieved by freeze-drying are favorable for cell adhesion, penetration, proliferation, oxygen/nutrient transport, and waste exchange [15]. With respect to mechanical properties, initial tensile strength is approximately 0.2 MPa, Young’s modulus is about 65 MPa, and the maximum elongation at the breakage point is approximately 65%. ECM swells in a time-dependent manner, and the absorption ratio is approximately six times [15]. Gel-type ECM stiffness is approximately 1.8 MPa, and Young’s modulus is between 1 and 2.5 kPa [41].
Clinical application
When compared to xenogeneic ECM-based scaffolds, human adipose-derived ECM is superior in terms of biocompatibility, biodegradability, and/or bioinductivity because it has lower immunogenicity and is less likely to transmit pathogens during clinical application [12, 42]. In a comparison of naïve autologous adipose tissue transplantation, an adipose ECM graft could maintain volume, structure, strength, and/or stiffness, which promoted mechanical support, cell shape/function stability, the transport of chemical signals, angiogenesis, and vascularization of the graft [12, 43, 44]. Adipose ECM can form 3D scaffolds with a variety of shapes such as sheet, microsphere, square, film, hollow tube, bead [16], or injectable gel type [12, 45]. The above contents were summarized in Table 1.
Table 1.
Human adipose-derived ECM
| Category | Contents | References | |
|---|---|---|---|
| Decellularization methods | Physical | Freezing, pressure, agitation, sonication and radiation | [13, 15–20] |
| Chemical | Detergent, acid, and alkaline | ||
| Biological | Enzymes | ||
| Adipose ECM composition | Collagens | Type I–VII | [10, 16, 21–26] |
| Non-collagen | Laminin, fibronectin, elastin and GAGs | [27–37] | |
| Confirmation of decellularization | DNA quantification with PCR, IHC |
< 50 ng ds DNA/mg < 200 bp in length |
[10, 16] |
| H&E stain | DAPI, acridine orange | [14] | |
| Mechanical property (lyophilized) | Pore size | 20 and 200 μm | [15] |
| Porosity | ≒ 90% | ||
| Tensile strength | ≒ 0.2 MPa | ||
| Young’s modulus | ≒ 65 MPa | ||
| Maximum elongation | ≒ 65% | ||
| Possible 3D scaffolds types | Sheet, microsphere, square, film, hollow tube, bead, injectable gel | [12, 16, 45] | |
Human adipose-derived growth factors
Adipose tissue is a major organ of the endocrine system, and adipocytes secrete various paracrine factors to induce cell adhesion, proliferation, migration, and differentiation [19, 46]. These bioactive molecules, including GFs, exist in ECM and retain their activity even after the decellularization process [7]. This phenomenon is associated with the binding with GAG. The heparan sulfate in GAG immobilizes GFs rendering them resistant to degradation by protein hydrolytic enzymes. Decorin and biglycan also help bind bioactive molecules to ECM [47].
Extraction steps
Extraction of GFs from decellularized adipose ECM consists of 6 steps: (1) place the ECM in an extraction buffer containing a protease inhibitor. Commonly used extraction buffers are acetic acid, urea, NaCl, or Triton X100. (2) Stir the solution for 1–3 days at 4 °C. (3) Centrifuge the solution at 3000–12,000 g for 30 min at 4 °C. (4) Dialyze the supernatant using an MWCO 3–15 k membrane and 80–100 volumes of ddH2O at 4 °C over 2 days. (5) Centrifuge the dialyzed product at 3000–12,000 g for 30 min at 4 °C. (6) Collect the supernatant and lyophilize [38]. The quantified GFs (per g of dry human adipose ECM) are transforming growth factor-beta1 (TGF-b1, approximately 8 ng), insulin growth factor-1 (IGF1, approximately 13 ng), basic fibroblast growth factor (bFGF, approximately 80 ng), and vascular endothelial growth factor (VEGF, approximately 25 ng) [38]. The GF extraction method was summarized on Fig. 1.
Importance of solvent selection
The type, concentration, and activity of extracted GF’s can vary depending on the solvent used in the extraction. TGF-b1, IGF-1, VEGF, PDGF, BMP4, HGF and EGF are extracted in large amounts with acetic acid [38]. For extraction of bFGF, NaCl is preferred. NGF is only extracted with urea, but BMP4 cannot be detected in urea (Table 2). The assumed reason for these differences are: (1) depending on the decellularization detergent used on ECM, the remaining GF’s type, concentration, and activity can vary [7]; and (2) the solubility and stabilization of each GF can vary depending on the solvent’s pH, composition, and excipients [48]. For example, each GF is stabilized at a different pH. bFGF is stabilized at pH 7 [48], EGF is stabilized at pH 5.3 [49], and VEGF is stabilized at pH 5.5-6 [50]. NaCl electrostatically bonds with proteins in aqueous solution, thus the binding force increases according to the degree of ionization of the GF [51]. Triton X100 readily reacts with hydrophobic proteins and NGF [51]. In addition, GF stability is affected by the ECM structure. Acetic acid damages collagen and weakens the extracellular matrix but does not affect GAG [52]. Urea damages collagen but does not affect elastin [53]. Triton X100 effectively conserves the structure and concentration of GAG [54]. Therefore, a GF extraction solution should be carefully selected with the end-purpose in mind because each target tissue requires different growth factors. For example, chondrogenesis requires TGF-b1, IGF-1, bFGF, and VEGF; adipogenesis requires insulin and insulin-like growth factor (IGF) [55]; myogenesis requires IGF [56]; osteogenesis and neurogenesis requires growth hormone (GH) and IGF-I [57, 58]; and endothelial differentiation requires VEGF and hepatocyte growth factor (HGF).
Table 2.
Human adipose-derived growth factors
Human adipose-derived stem/progenitor cells
To increase the yield of adipose-derived cells, collection of fat en bloc (mass) is more efficient than liposuction. Liposuction yields 1 L of adipose tissue per donor while collection en bloc yields 2 L of adipose tissue per donor. Thus, the en bloc adipose collection method effectively increases the yield by increasing the total volume of adipose tissue per donor [14].
Extraction method
To fractionate cells from adipose tissue, the tissue is washed with PBS (containing penicillin/streptomycin) to remove blood. It is then treated with collagenase to digest connective fibers and filtered through 100-μm filter to suspend into single cell. The collagenase activity is stopped with media containing 10% FBS. The mixture is then centrifuged at 200 g for 10 min to obtain the stromal-vascular fraction (SVF) [12]. The stem cells are contained in the SVF [8]. If a milder fractionation is necessary, mechanical force can be used instead of collagenase treatment. Fractionation with mechanical force reduces processing and possibly increases cell survival rate and stem cell yield [8, 44]. The SVF extraction method using automated machine was visualized on Fig. 1.
Characterization of fractionated cells
Morphology and growth features
Adhered cells display fibroblastic-like features when cultured (diameter up to 120 μm) [59], and hematopoietic and lipogenic cells are removed through washing and culture expansion. The late adherent cells (15 h-1 week) to plastic surface exhibits higher self-renewal, proliferation rate, differentiation potency, and cytokine secretion than the early adherent cells (before 3 h) [60]. The cells are used within passages 5–6 because of the accumulation of cytotoxic effects by the digestive enzymes used in passaging and cellular senescence induced by in vitro culture conditions.
Cell surface phenotype
Expression of classical mesenchymal markers (CD44, CD73, CD90, CD105, and CD166) is high while expression of endothelial (CD31, CD34, CD144, CD146) and hematopoietic (CD45, CD133) markers is low [61–63].
Self-renewal
OCT4, SOX2, and NANOG expression are identified [8].
Secreted cytokines
HGF, granulocyte and macrophage colony stimulating factors, IL-6, -7, -8 and -11, TNF-α, VEGF, BDNF, NGF, adipokines, MMP-1, CXCL8, Ang-1, Ang-2, FGF-1, TIMP-1, CCL2 [41, 64].
Multi-differentiation potential
Stem cells from adipose tissue can differentiate into adipocytes, fibroblasts, smooth muscle cells, endothelial cells, immune cells [65], pancreatic cells, hepatocytes, neurons, cardiomyocytes [66, 67], osteocyte [68], or chondrocyte [69].
The above contents were summarized in Table 3.
Table 3.
Human adipose-derived stem/progenitor cells
| Category | Contents | References | |
|---|---|---|---|
| Morphology | Fibroblastic-like | [59, 60] | |
| Diameter | Up to 120 μm | ||
| Adherent period | 15 h–1 week | ||
| Stem cell characters | Self-renewal | OCT4, SOX2, NANOG expression | [8] |
| High proliferation rate | [60–64] | ||
| Multi-differentiation potential | Adipocyte, fibroblasts, smooth muscle cells, endothelial cells, immune cells, pancreatic cells, hepatocytes, neurons, cardiomyocytes, osteocytes, chondrocyte | [63, 65–69] | |
| Secreted cytokines | HGF, granulocyte and macrophage colony stimulating factors, IL-6, -7, -8 and -11, TNF-α, VEGF, BDNF, NGF, adipokines, MMP-1, CXCL8, Ang-1, Ang-2, FGF-1, TIMP-1, CCL2, TGF, VCAM, EGF, FGF | [41, 64, 78] | |
| Recommended passage limitation | ≒ 5–6 | [60] | |
| Cell surface phenotype | Mesenchymal markers | CD44+, CD73+, CD90+, CD105+, CD166+ | [61, 62] |
| Endothelial markers | CD31−, CD34−, CD144−, CD146− | ||
| Hematopoietic markers | CD45−, CD133− | ||
Clinical application
For clinical application, cells are combined with SVF to mimic the cells’ native microenvironment [70]. This increases graft survival and vessel formation while decreasing inflammatory cell migration and fibrosis. The positive effects of SVF are demonstrated by histological changes in the introduced fat mass into the body. If an in vivo immune response is stimulated, inflammatory cells are penetrated and form cysts, fat cells’ size became larger than normal, and the transplanted fat volume decreases. As the inflammatory reaction progresses, granulomatous syncytial-like structures form and lobular panniculitis occurs. As necrosis progresses, connective bundles are formed, and the adipose tissue becomes an amorphous and disorganized mass. The occurrence of these histologic transformations is significantly reduced by SVF [8]. Thus, combining SVF with adipose tissue-derived cells is useful in cell therapy and fat enrichment [8].
Further considerations
Differences between subcutaneous and visceral adipose tissue
Depending on the deposition site, adipose tissue can be divided into two types: subcutaneous and visceral adipose tissue [71]. These adipose tissues display different physiological functions because they have different ECM compositions and secrete different bioactive molecules [72]. Visceral adipose tissue displays excess collagen deposition [71], which is indicative of severe inflammation and enhanced infiltration by neutrophils, lymphocytes, and macrophages when compared to subcutaneous adipose tissue [73]. Visceral adipose tissue displays a mesh-like, dense elastin fiber while subcutaneous adipose tissue displays a linear form of elastin, which is related to macrophage activity [33].
Loss of fat tissue and health
Excessive adipose tissue harvesting increases the risk of diabetes and cardiovascular disease because fat loss stimulates macrophage infiltration, inflammation, and fibrotic responses [74]. Body mass index (BMI) and the risk of these diseases have a U- or J-curve relationship [75, 76]. Thus, the importance of balanced adiposity needs to be considered for people with metabolic diseases.
Guidelines for ECM, GFs, and adipose tissue-generated cell as therapeutic agents
In order to use ECM, GFs, and adipose-generated cells in a clinical setting, it is necessary to follow the laws and regulations established by the appropriate regulatory body. For example, products must be manufactured according to mandated quality standards and be safe and effective when applied to a patient.
The required considerations are: (1) an analysis that explains the final form, function, and safety of the material being used; (2) a quality evaluation that examines the sampling method, the identification method, purity, potency, and stability of the material being used as well as cell viability within the scaffold; (3) a non-clinical evaluation that examines the morphological and functional characteristics of the material being used as well as their transplant duration, tumorigenicity, and safety; and (4) a clinical trial that includes a functional, structural, pharmacodynamic (functional), and pharmacokinetic (persistence) evaluation [77].
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Korean government (MSIT) (2014M3A9D3034164), (2016R1C1B1011180), (2018R1C1B5040264) (2019R1A2C1004046), and the Ministry of Trade, Industry and Energy (MOTIE), Korea, under the “Regional Industry Infrastructure and R&D Support Program” (R0005886) supervised by the Korea Institute for Advancement of Technology (KIAT), and 2017–2019 Medical Cluster R&D Support Project through Daegu Gyeongbuk Medical Innovation Foundation funded by the Ministry of Health & Welfare, the Republic of Korea (HI16C2505).
Compliance with ethical standards
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
So Young Chun and Jeong Ok Lim have contributed equally to this work.
Contributor Information
Bongsu Jung, Phone: 82-53-790-5690, Email: bsjung@dgmif.re.kr.
Tae Gyun Kwon, Phone: 82-53-200-3012, Email: tgkwon@knu.ac.kr.
References
- 1.Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27:6052–6063. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Patrick CW., Jr Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001;263:361–366. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 3.Banyard DA, Borad V, Amezcua E, Wirth GA, Evans GR, Widgerow AD. Preparation, characterization, and clinical implications of human decellularized adipose tissue extracellular matrix (hDAM): a comprehensive review. Aesthet Surg J. 2016;36:349–357. doi: 10.1093/asj/sjv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Q, Desta T, Fenton M, Graves DT, Amar S. Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect Immun. 2005;73:935–943. doi: 10.1128/IAI.73.2.935-943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 6.Young DA, Christman KL. Injectable biomaterials for adipose tissue engineering. Biomed Mater. 2012;7:024104. doi: 10.1088/1748-6041/7/2/024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun SY, Oh SH, Yoo JJ, Kwon TG. Fabrication and characterization techniques for decellularized organ scaffolds. Tissue Eng Regen Med. 2014;11:S1–S10. [Google Scholar]
- 8.Gentile P, Piccinno MS, Calabrese C. Characteristics and potentiality of human adipose-derived stem cells (hASCs) obtained from enzymatic digestion of fat graft. Cells. 2019;8:E282. doi: 10.3390/cells8030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufrane D. Impact of age on human adipose stem cells for bone tissue engineering. Cell Transplant. 2017;26:1496–1504. doi: 10.1177/0963689717721203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown BN, Freund JM, Han L, Rubin JP, Reing JE, Jeffries EM, et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods. 2011;17:411–421. doi: 10.1089/ten.tec.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH. An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg. 2012;129:1247–1257. doi: 10.1097/PRS.0b013e31824ec3dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JS, Yang HJ, Kim BS, Kim JD, Kim JY, Yoo B, et al. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J Control Release. 2009;139:2–7. doi: 10.1016/j.jconrel.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Choi YC, Choi JS, Kim BS, Kim JD, Yoon HI, Cho YW. Decellularized extracellular matrix derived from porcine adipose tissue as a xenogeneic biomaterial for tissue engineering. Tissue Eng Part C Methods. 2012;18:866–876. doi: 10.1089/ten.tec.2012.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Dittman B, Atkinson B, Stilwell RL. Cell repopulated collagen matrix for soft tissue repair and regeneration. US Patent 20140286911A1, 9 Sept 2014 (2014).
- 15.Song M, Liu Y, Hui L. Preparation and characterization of acellular adipose tissue matrix using a combination of physical and chemical treatments. Mol Med Rep. 2018;17:138–146. doi: 10.3892/mmr.2017.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi JS, Kim BS, Kim JY, Kim JD, Choi YC, Yang HJ, et al. Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering. J Biomed Mater Res A. 2011;97:292–299. doi: 10.1002/jbm.a.33056. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715–4724. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 20.Rieder E, Kasimir MT, Silberhumer G, Seebacher G, Wolner E, Simon P, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127:399–405. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Chung YC, Su YP, Chen CC, Jia G, Wang HL, Wu JC, et al. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sin. 2004;25:932–936. [PubMed] [Google Scholar]
- 22.Choi JS, Yang HJ, Kim BS, Kim JD, Lee SH, Lee EK, et al. Fabrication of porous extracellular matrix scaffolds from human adipose tissue. Tissue Eng Part C Methods. 2010;16:387–396. doi: 10.1089/ten.TEC.2009.0276. [DOI] [PubMed] [Google Scholar]
- 23.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun TH. Peri-adipocyte ECM remodeling in obesity and adipose tissue fibrosis. Adipocyte. 2012;1:89–95. doi: 10.4161/adip.19752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999;11:634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 26.Niyibizi C, Fietzek PP, van der Rest M. Human placenta type V collagens. Evidence for the existence of an alpha 1(V) alpha 2(V) alpha 3(V) collagen molecule. J Biol Chem. 1984;259:14170–14174. [PubMed] [Google Scholar]
- 27.Noro A, Sillat T, Virtanen I, Ingerpuu S, Bäck N, Konttinen YT, et al. Laminin production and basement membrane deposition by mesenchymal stem cells upon adipogenic differentiation. J Histochem Cytochem. 2013;61:719–730. doi: 10.1369/0022155413502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkinson JJ, Adair-Kirk TL, Kelley DG, Demello D, Senior RM. Clara cell adhesion and migration to extracellular matrix. Respir Res. 2008;9:1. doi: 10.1186/1465-9921-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 30.Mosher DF, Schad PE. Cross-linking of fibronectin to collagen by blood coagulation Factor XIIIa. J Clin Invest. 1979;64:781–787. doi: 10.1172/JCI109524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96(12):E1990–E1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Santibanez G, Singer K, Cho KW, DelProposto JL, Mergian T, Lumeng CN. Obesity-induced remodeling of the adipose tissue elastin network is independent of the metalloelastase MMP-12. Adipocyte. 2015;4:264–272. doi: 10.1080/21623945.2015.1027848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 35.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28:1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 36.Doran MR, Markway BD, Aird IA, Rowlands AS, George PA, Nielsen LK, et al. Surface-bound stem cell factor and the promotion of hematopoietic cell expansion. Biomaterials. 2009;30:4047–4052. doi: 10.1016/j.biomaterials.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 37.Mullen LM, Best SM, Brooks RA, Ghose S, Gwynne JH, Wardale J, et al. Binding and release characteristics of insulin-like growth factor-1 from a collagen-glycosaminoglycan scaffold. Tissue Eng Part C Methods. 2010;16(6):1439–1448. doi: 10.1089/ten.tec.2009.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi JS, Kim BS, Kim JD, Choi YC, Lee HY, Cho YW. In vitro cartilage tissue engineering using adipose-derived extracellular matrix scaffolds seeded with adipose-derived stem cells. Tissue Eng Part A. 2012;18:80–92. doi: 10.1089/ten.tea.2011.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raeder RH, Badylak SF, Sheehan C, Kallakury B, Metzger DW. Natural anti-galactose alpha1,3 galactose antibodies delay, but do not prevent the acceptance of extracellular matrix xenografts. Transpl Immunol. 2002;10:15–24. doi: 10.1016/s0966-3274(01)00044-2. [DOI] [PubMed] [Google Scholar]
- 40.Daly KA, Stewart-Akers AM, Hara H, Ezzelarab M, Long C, Cordero K, et al. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng Part A. 2009;15:3877–3888. doi: 10.1089/ten.TEA.2009.0089. [DOI] [PubMed] [Google Scholar]
- 41.van Dongen JA, Getova V, Brouwer LA, Liguori GR, Sharma PK, Stevens HP, et al. Adipose tissue-derived extracellular matrix hydrogels as a release platform for secreted paracrine factors. J Tissue Eng Regen Med. 2019;13:973–985. doi: 10.1002/term.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–1053. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 43.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Kokai LE, Schilling BK, Chnari E, Huang YC, Imming EA, Karunamurthy A, et al. Injectable allograft adipose matrix supports adipogenic tissue remodeling in the nude mouse and human. Plast Reconstr Surg. 2019;143:299e–309. doi: 10.1097/PRS.0000000000005269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011;7:1040–1049. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shan X, Choi JH, Kim KJ, Lee YJ, Ryu YH, Lee SJ, et al. Adipose stem cells with conditioned media for treatment of acne vulgaris scar. Tissue Eng Regen Med. 2018;15:49–61. doi: 10.1007/s13770-017-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988;107:743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santana H, González Y, Campana PT, Noda J, Amarantes O, Itri R, et al. Screening for stability and compatibility conditions of recombinant human epidermal growth factor for parenteral formulation: effect of pH, buffers, and excipients. Int J Pharm. 2013;452:52–62. doi: 10.1016/j.ijpharm.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 49.Senderoff RI, Wootton SC, Boctor AM, Chen TM, Giordani AB, Julian TN, et al. Aqueous stability of human epidermal growth factor 1-48. Pharm Res. 1994;11:1712–1720. doi: 10.1023/a:1018903014204. [DOI] [PubMed] [Google Scholar]
- 50.Elias AP, Dias S. Microenvironment changes (in pH) affect VEGF alternative splicing. Cancer Microenviron. 2008;1:131–139. doi: 10.1007/s12307-008-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoener MC, Varon S. Effects of sodium chloride, Triton X-100, and alkaline pH on the measurable contents and sedimentability of the nerve growth factor (NGF) antigen in adult rat hippocampal tissue extracts. J Neurosci Res. 1997;49:508–514. [PubMed] [Google Scholar]
- 52.Dong X, Wei X, Yi W, Gu C, Kang X, Liu Y, et al. RGD-modified acellular bovine pericardium as a bioprosthetic scaffold for tissue engineering. J Mater Sci Mater Med. 2009;20:2327–2336. doi: 10.1007/s10856-009-3791-4. [DOI] [PubMed] [Google Scholar]
- 53.Wong ML, Wong JL, Horn RM, Sannajust KC, Rice DA, Griffiths LG. Effect of urea and thiourea on generation of xenogeneic extracellular matrix scaffolds for tissue engineering. Tissue Eng part C Methods. 2016;22:700–707. doi: 10.1089/ten.tec.2015.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vavken P, Joshi S, Murray MM. TRITON-X is most effective among three decellularization agents for ACL tissue engineering. J Orthop Res. 2009;27:1612–1618. doi: 10.1002/jor.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niemelä S, Miettinen S, Sarkanen JR, Ashammakhi N. Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. In: Ashammakhi N, Reis R, Chiellini F, editors. Topics in tissue engineering. Vol. 4; 2008. Chap. 4. p. 1–26.
- 56.Florini JR, Magri KA. Effects of growth factors on myogenic differentiation. Am J Physiol. 1989;256:C701–C711. doi: 10.1152/ajpcell.1989.256.4.C701. [DOI] [PubMed] [Google Scholar]
- 57.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aberg D. Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocr Dev. 2010;17:63–76. doi: 10.1159/000262529. [DOI] [PubMed] [Google Scholar]
- 59.von Heimburg D, Serov G, Oepen T, Pallua N. Fat tissue engineering. In: Ashammakhi N, Ferretti P, editors. Topics in tissue engineering. 2003. Chap. 8. p. 1–16.
- 60.Park JH, Kim KJ, Rhie JW, Oh IH. Characterization of adipose tissue mesenchymal stromal cell subsets with distinct plastic adherence. Tissue Eng Regen Med. 2016;13:39–46. doi: 10.1007/s13770-015-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maurer MH. Proteomic definitions of mesenchymal stem cells. Stem Cells Int. 2011;2011:704256. doi: 10.4061/2011/704256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmelzer E, McKeel DT, Gerlach JC. Characterization of human mesenchymal stem cells from different tissues and their membrane encasement for prospective transplantation therapies. Biomed Res Int. 2019;2019:6376271. doi: 10.1155/2019/6376271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 65.Dmitrieva LA, Elizova LA. Structural changes in human dentin with the use of modern filling materials. Stomatologiia (Mosk). 1991;5:21–23. [PubMed] [Google Scholar]
- 66.Strem BM, Zhu M, Alfonso Z, Daniels EJ, Schreiber R, Beygui R, et al. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7:282–291. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 67.Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 68.Shenaq DS, Rastegar F, Petkovic D, Zhang BQ, He BC, Chen L, et al. Mesenchymal progenitor cells and their orthopedic applications: forging a path towards clinical trials. Stem Cells Int. 2010;2010:519028. doi: 10.4061/2010/519028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murata D, Akieda S, Misumi K, Nakayama K. Osteochondral regeneration with a scaffold-free three-dimensional construct of adipose tissue-derived mesenchymal stromal cells in pigs. Tissue Eng Regen Med. 2018;15(1):101–113. doi: 10.1007/s13770-017-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dicker A, Le Blanc K, Aström G, van Harmelen V, Götherström C, Blomqvist L, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283–290. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 71.Mori S, Kiuchi S, Ouchi A, Hase T, Murase T. Characteristic expression of extracellular matrix in subcutaneous adipose tissue development and adipogenesis; comparison with visceral adipose tissue. Int J Biol Sci. 2014;10:825–833. doi: 10.7150/ijbs.8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome: an allostatic perspective. Biochim Biophys Acta. 2010;1801(3):338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci U S A. 2010;107(1):240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–729. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ministry of Food and drug safety of KOREA. Guidelines for evaluating cell therapeutic agents including scaffolds. B1-2014-3-011. 2014.
- 78.Noverina R, Widowati W, Ayuningtyas W, Kurniawan D, Afifah E, Laksmitawati DR, et al. Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs) Clin Nutr Exp. 2019;24:34–44. [Google Scholar]