Abstract
The consumption of teas has been increasing with the dissemination of information regarding the health benefits of its constituents. Obtaining food products with healthier profiles is already a reality for industry with the increasing development of new functional ingredients, including the use of tea and its derivatives (extracts). This work aimed to evaluate the encapsulation of green tea extract powder in lipid microparticles (LMP) by the spray chilling method and in ionic gelation microparticles (IGMP) by the ionic gelation method to obtain polyphenol-rich water insoluble components. Microparticles were adequately obtained in both methods, with typical physical characteristics consistent with the results in literature results, 83.5 ± 2.8% encapsulation efficiency for LMP and 72.6 ± 0.4% for IGMP, and antioxidant activity (IC50 μg/mL) of 33,169.4 ± 123.8 (IGMP) and 2099.7 ± 35.3 (LMP). The microparticles samples were considered suitable as ingredients for add polyphenols in foods.
Keywords: Microencapsulation, Polyphenols, Antioxidant activity, Double emulsion, Camellia sinensis
Introduction
The search by consumers for healthy habits has exerted a great influence on the consumption of beverages due to increased awareness related to the health impacts of carbonated drinks. In this context, sales of regular and diet soda, as well as milk and fruit beverages have declined, and at the same time, there has been an increase in sales of teas (FAO 2018).
After water, teas are the most widely consumed beverages in the world. According to Food and Agriculture Organization (FAO) data, world tea production has grown by 4.4% per year in the last decade, reaching 5.73 million tons in 2016 (FAO 2018).
Researchers evaluated the perceptions of people regarding the habits of herb and tea use, and it was observed that this practice is usually adopted to achieve results such as those obtained through medication, as in a medical treatment (Faller and Marcon 2013).
Camellia sinensis is a plant native to Southeast Asia but is currently cultivated in more than 50 countries around the world, and three different types of tea can be obtained from this species, black, green and oolong, with the only difference among them being the way in which the leaves are processed (Pasrija et al. 2015; Meegahakumbura et al. 2018).
Among the three types of teas obtained from C. sinensis, green tea stands out due to greater disclosure of its functional properties and antioxidant activity (Cheng 2006), with a greater number of studies focused on the addition of these bioactive components to food. Evidence of the beneficial properties of this type of tea is associated with increased consumption.
Among all foods rich in polyphenols and bioactive components, green tea stands out due to its high content of different types of polyphenolic components, such as methylxanthines, flavanols, flavonoids, flavonoids, and phenolic acids, which correspond to 30% of the dry weight of leaves. Most polyphenols found in green tea are flavanols, and the main flavanols are epicatechin, epicatechin gallate, epigallocatechin and epigallocatechin gallate (Bora et al. 2018).
Polyphenols are one of the groups of biologically active components that have been widely applied in foods (Alvim et al. 2016; Šaponjac 2016b; Cutrim and Cortez 2018). Polyphenols have been highlighted for their antioxidant activities, in addition to other biological activities such as reducing the effect on cholesterol and glycemia and providing anticarcinogenic and cardioprotective effects, which have previously been demonstrated in in vitro and in vivo studies (Castro-Acosta et al. 2017; Liu et al. 2018; Vetrani et al. 2018).
According to estimates by Faller and Fialho (2009), Brazilians ingest approximately 48.3 mg of polyphenols per day as a result of low consumption of fruits and vegetables (average of 66.8 g of fruit per day), compared to the recommended amount by FAO (400 g of fruit per day) that would lead to the recommended intake of polyphenols at a value of 292 mg per day. Despite the previously confirmed beneficial effects of polyphenols, there is concern about their use in free form, as low bioavailability attributed to their low solubility and low intestinal permeability observed in the gastrointestinal tract (Liang et al. 2017; Cai et al. 2018). In addition, poor stability is associated with passage through the stages of the digestive process (salivary, gastric and upper small intestine phase) (Shim et al. 2012).
Furthermore, some modifications in polyphenols have also been reported, such as epimerization, polymerization and oxidation related to the pH, temperature and presence of oxygen during food processing and storage, with polyphenols more stable at a lower pH, at lower temperatures and with lower exposure to oxygen (Ananingsih et al. 2013).
In addition to the factors that cause changes in the physicochemical characteristics and the difficulty in absorption, many potential natural substances rich in polyphenols have a typical taste, with a predominance of bitterness and astringency, which may limit their incorporation into foods (Munin and Edwards-Lévy 2011). Thus, strategies to protect polyphenols, such as microencapsulation of these compounds, can help reduce the negative effects on their stability, improve the absorption process, and reduce the perception of unpleasant taste (Ananingsih et al. 2013).
Microencapsulation technology is a tool based on the formation of a protective structure (“wall material”) covering a substance of interest (“core material”) for its protection and controlled release (Alvim et al. 2016).
Several techniques for encapsulation of polyphenols have been reported in the literature (Munin and Edwards-Lévy 2011; Bora et al. 2018; Moura et al. 2018). Ionic gelation is a physical–chemical method for the encapsulation of substances and is considered mild for most core material, but it has a porous wall structure that can compromise the retention of some soluble compounds (Silvério et al. 2018). The spray chilling technique forms a lipid wall that acts as a good barrier to moisture (Fadini et al. 2018). Both structures formed are insoluble in aqueous systems.
The microencapsulation of polyphenols can help in the process of adding these components to processed foods, preserving their benefits and avoiding their undesirable characteristics, which would contribute to an increase in the daily intake of phenolic compounds by consumers (Pasrija et al. 2015; Šaponjac et al. 2016a).
In this context, the objective of this research was to evaluate microencapsulation of green tea extract rich in polyphenols to obtain promising substances for food application. Two encapsulation methods were used: spray chilling (lipid microparticles) and ionic gelation (polysaccharide microparticles). In this phase of the study, ways of obtaining microparticles and their characteristics regarding encapsulation efficiency, amount of total phenolic compounds and antioxidant activity were evaluated.
Materials and methods
Material
To meet the requirements of the research, which considers the use of plant extracts with a high content of polyphenols, the green tea extract (C. sinensis) was chosen to start the tests.
Core material The Heide Extratos Vegetais company, located in Pinhais—PR, donated samples of green tea liquid extract (GTLE, solids content of 9.2% and 2.9% of polyphenols, data provided by the company) and green tea extract powder (GTEP).
IG microparticle wall GENU amidated low methoxyl pectin (LM 102 AS-Z; CP Kelco, Limeira, Brazil); calcium chloride for analysis (CAL, Synth, São Paulo, Brazil); food grade citric acid (CA, MIX, Duas Rodas, São Bernardo do Campo, Brazil); commercial rapeseed oil (CO, Liza oil, Cargill Agrícola S.A., Mairinque, Brazil); and polyglycerol polyricinoleate (PGPR) analytical grade (Concepta, São Paulo, Brazil).
Lipid microparticle wall 100% hydrogenated palm oil (PO) (melting point 58.32 ± 0.16 °C; A. Azevedo Óleos Vegetais, São Paulo, Brazil) and fat obtained from fully hydrogenated and interesterified vegetable oils (VF) (melting point 35.83 ± 0.15 °C) (AL Lette K39LT™, Cargill Agrícola S.A., Mairinque, Brazil). All the reagents used were of analytical grade.
Microparticle production
Ionic gelation microparticles (IGMP)
Particles were prepared according to the method described by Moura et al. (2018), which consisted of the association of a double emulsion (Water/Oil/Water; W/O/W) with ionic gelation to optimize the retention of the water-soluble compounds of green tea.
Core preparation: The GTEP was reconstituted (GTEP-R) at a ratio of 1 g:1 g (d.b) in filtered water and homogenized on a magnetic stirrer (RTC Basic, IKA, Germany) for 10 min.
First emulsion (FEm, Water/Oil; W/O): A mixture with a ratio of 3 g:7 g (GTEP-R:canola oil, added with PGPR in 4 g/100 g emulsion) was homogenized using an Ultra-Turrax® (T18, IKA, Germany) at 15,000 rpm for 15 min.
Second emulsion (SEm, W/O/W): A mixture with a ratio of 2 g:8 g (Fem:pectin solution—3 g/100 g, d.b.) was homogenized using Ultra-Turrax® (T18, IKA, Germany) (15,000 rpm/5 min).
The final emulsion was sprayed through a double-fluid atomizer (Mini Spray Dryer B-290, Büchi, Flawil, Switzerland, nozzle 0.7 mm) on the CaCl2 crosslinking solution (3 g/100 g, d.b.) acidified with citric acid (pH 3). The atomizing air pressure was 0.15 bar, and the feeding rate was 1.3 ml/min. The distance between the atomizer and the surface of the CaCl2 solution was 18 cm. After atomization, the microparticles were stirred (250 rpm) for 15 min for hardening and then filtered on commercial filter paper (n.102, Melitta Brazil, São Paulo, Brazil).
Spray chilling: solid lipid microparticle production
The solid lipid microparticles (LMP) obtained by the spray chilling method were produced according to the equipment configuration presented in Alvim et al. (2016) and process conditions and formulations in Fadini et al. (2018) with modifications. The wall material was a mixture of hydrogenated palm oil (PO) and vegetable fat fully hydrogenated and interesterified (VF) at the ratio of 2 g:3 g. A dispersion of green tea powder extract and lipid mixture (3 g:7 g) heated at 80 ± 0.5 °C was sprayed in a refrigerated environment in the Mini Spray Dryer B-290 (Büchi, Flawil, Switzerland). The cold air used to solidify the lipid particles was produced by a B296 dehumidifier (Büchi, Flawil, Switzerland). The main production parameters of SLM were as follows: Inlet temperature: 6 ± 2 °C; Outlet temperature: 15 ± 2 °C; Nozzle diameter: 2.0 mm; Atomization pressure: 1.35 bar. After production, both samples of LMP and IGMP were stored in plastic containers under refrigeration for further characterization.
Characterization of microparticles
Determination of total polyphenol content (TPC)
The total polyphenol content of the extract and the particles was determined by the Folin–Ciocalteau method (Singleton and Rossi 1965). The absorbance reading (in triplicate) was performed in a UV/visible spectrophotometer (SP-220, Biospectro, São Paulo, Brazil). Microparticles without the bioactive compound were used as a control sample to determine possible interferences in the analysis.
Encapsulation efficiency (EE%)
The encapsulation efficiency of microparticles containing green tea extract was determined by the ratio (%) between the amount of core material (polyphenols in green tea extract) retained in the microparticles and the amount of this core originally used at the beginning of the process (Sartori et al. 2015; Alvim et al. 2016).
Particle size distribution (PSD)
The particles produced were analyzed by the laser scattering method in a laser particle size distribution analyzer (LA-950V2, Horiba, Tokyo, Japan). The samples were prepared in specific dispersants: the IGMP were analyzed in water and the LMP in a 0.5 g/100 g Tween 20 solution (polyoxyethylene 20 sorbitan monolaurate) (adapted from Moura et al. 2018; Alvim et al. 2016). The results obtained from equipment software were expressed in D10, D50 (median size) and D90, which are the diameters for the accumulated size distribution of 10, 50 and 90% of particles. The polydispersity index (span) was also determined by Eq. 1 (Fadini et al. 2018).
| 1 |
D10, D50 and D90 correspond to the diameters relative to 10, 50 and 90% of the accumulated size distribution.
Morphology
The appearance of GTEP, the single (W/O) and double (W/O/W) emulsions, and the LMP and IGMP particles were evaluated under an optical microscope (model BX41, Olympus, Tokyo, Japan) at 10 × and 100 × magnification, depending on the sample. The images were taken by a digital camera (QColor 3, Olympus, Tokyo, Japan). For the IGMP particles, the illumination of the samples was performed by incident light using an external source of optical fiber (Silvério et al. 2018). GTEP, emulsions and LMP particles were illuminated by transmitted light using the conventional microscope source (adapted from Alvim et al. 2013).
Colorimetry
Colorimetric analysis was performed using Chromameter CR-400 (Konica-Minolta Sensing Inc., Osaka, Japan) with the CieLab System. Decaplate readings of the color of the GTEP and of the particles were carried out. The equipment was calibrated with the white calibration plate before any readings. The parameters of the total color difference (ΔE) for the microparticles were calculated by Eq. 2.
| 2 |
ΔE represents color difference between microparticles and GTEP (reference)
Moisture and water activity (aw)
The moisture content of the green tea extract and microparticles was determined by oven drying at 105 °C for 20 h (adapted from AOAC 2006). The water activity of the powder extract and particles was determined in triplicate using a water activity meter (AquaLab 4TEV, Decagon Devices Inc., Pullman, USA) at 25.0 ± 0.5 °C.
Determination of antioxidant activity
The antioxidant activity of GTEP and microparticles was evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenger activity method (von Gadow et al. 1997). The absorbance reading at 517 nm (in triplicate) was performed in a UV/visible spectrophotometer (SP-220, Biospectro, São Paulo, Brazil). The result of antioxidant activity was expressed as IC50, which indicates the concentration of antioxidant required to quench 50% of the initial DPPH radicals under the given experimental conditions.
Statistical analysis
Analysis of variance (ANOVA) and Tukey’s test at the 5% level of significance were used to evaluate the data using XLSTAT 2014 software (Addinsoft, Paris, France)
Results and discussion
Microparticle production
Considering 9.2% of solids content and 2.9% of polyphenols in green liquid tea extract, it was decided to use GTEP (more concentrate in polyphenols) in an attempt to increase the core load in the microparticles of IG and LMP. The extract in the solid form (GTEP) was produced with the liquid extract and maltodextrin as a drying adjuvant agent using a spray drying process (information provided by the company). Both forms of the green tea tested (liquid concentrate extract and powder) were highly water soluble, which limited their application in water-rich food products, either by rapid solubilization (powdered material) or by exposure of the compounds of interest to the process conditions and the storage of this type of food matrix.
As a microencapsulation strategy, two methods were used to generate water insoluble particles: spray chilling (lipid matrix) and ionic gelation (insoluble gel formation) (Moura et al. 2018; Sartori et al. 2015).
To obtain IG particles containing polyphenols from green tea powder, the strategy used by Moura et al. (2018) was applied, which successfully encapsulated hibiscus extract (liquid, water-soluble) into IG particles using a double emulsion and pectin as a wall polymer. The method for the inclusion of core underwent preliminary testing. Initially, the powder extract added to the oil was used, but due to the rapid decantation, this strategy could not be applied. It was chosen to rehydrate the powdered extract in a concentration higher than that of the original liquid extract, increasing the polyphenol load to be incorporated into the microparticles (Table 1). In preliminary tests, it was decided to reconstitute the 50% powdered extract with an incorporation in the 1st emulsion of 30% of this rehydrated extract (GTEP-R), which added approximately 1.9 times more polyphenols to the particles than if the conventional liquid extract were used. After preliminary adjustments, the microparticles containing the GTEP-R were suitably produced by ionic gelation using calcium chloride as a crosslinking agent.
Table 1.
Physical–chemical results of microparticle characterization
| Analysis | GTEP1 | LMP | IGMP |
|---|---|---|---|
| Total polyphenols (mg/g) | 109.47 ± 1.97 a | 27.44 ± 0.02b | 2.38 ± 0.91c |
| Encapsulation Efficiency (%) | ND1 | 83.5 ± 2.8a | 72.6 ± 0.4b |
| Moisture (%) | 6.63 ± 0.06b | 1.63 ± 0.06c | 79.17 ± 0.06a |
| Aw | 0.5416 ± 0.0043b | 0.5498 ± 0.0005b | 0.9928 ± 0.0047a |
| Antioxidant activity | |||
| IC 50 (ug/mL) | 44.70 ± 0.50c | 33,169.4 ± 123.84a | 2099.69 ± 35.28b |
| Colorimetry | |||
| L* | 76.84 ± 0.01b | 75.14 ± 0.01c | 84.69 ± 0.01a |
| a* | 0.24 ± 0.00b | 0.16 ± 0.03c | 1.12 ± 0.02a |
| b* | 18.91 ± 0.00b | 20.17 ± 0.02a | 9.87 ± 0.02c |
| ∆E2 | – | 2.1 | 12.0 |
1Core material, 2GTEP as sample reference for ∆E calculation. Different letters in the same column indicate significant difference (p < 0.05)
The lipid microparticles containing the polyphenols from the green tea extract were produced following the procedures in Fadini et al. (2018), in which the spray drying dehydrated material (GTEP) was confined in a sphere constituted by a lipid matrix that was formed by spray chilling. The process proceeded uneventfully, and the particles were also produced properly.
Characterization of microparticles
Table 1 presents the results of part of the physical–chemical characterization of the microparticle samples obtained and the core material (GTEP).
Total polyphenol content (TPC) and encapsulation efficiency (EE%)
The TPC remaining in the microparticles with GTEP, as expected, was higher for LMP than IGMP. The ionic gelation process involved three dilutions of the powdered extract used: the rehydration of the powder (1 g of powdered extract: 1 g of water), the first emulsion (3 g of rehydrated extract: 7 g of oil, equivalent to 1.5 g of powdered extract: 8.5 g of other constituents) and finally the second emulsion (2 g of 1st emulsion: 8 g of pectin solution, equivalent to 0.353 g powdered extract: 9647 g other constituents). In addition, the IGMP had a high moisture content (Table 1), with a resulting low solids content in relation to LMP. For the lipid microparticles, the powder extract was added directly in the proportion of 3 g:7 g in relation to the lipid matrix, and thus, the GTEP content was higher for this sample.
Comparing both samples on a dry basis, LMP carried 2.78 g of polyphenols/100 g of microparticles, while IGMP carried 1.14 g of polyphenols/100 g of microparticles. These values should be taken into account when considering the use of these microparticles. Arriola et al. (2019) determined total polyphenol values, on a dry basis, in the range of 0.91 ± 0.01 to 1.82 ± 0.06 g/100 g of microparticles produced by ionic gelation of alginate containing an extract of stevia leaves.
Based on the content of polyphenols remaining in the microparticles relative to the initial content, the encapsulation efficiencies (Table 1) of the samples were calculated. LMP presented an EE of 83.5 ± 2.8%, a value considered satisfactory for this system when compared to those reported in the literature. Values of 37.5 ± 3.0 to 83.3 ± 1.9% for EE (volatile retention) were observed by Oriani et al. (2016) in the encapsulation of ginger resin oil by spray chilling. Consoli et al. (2016) also used the spray chilling method to encapsulate gallic acid in the form of a simple W/O emulsion (3:7 and 2:8 ratios of gallic acid solution:lipid matrix) and observed EEs ranging from 54.14 to 101.83%. Fadini et al. (2019), using the same encapsulation method used in this research (fish oil entrapped in spray drying microparticles, and this powder entrapped in a lipid matrix by spray chilling), observed losses of essential fatty acids (EPA + DHA) between 1.68 and 28.68%, equivalent to EEs between 98.3 and 71.32%.
For IG, the polyphenol encapsulation efficiency value was 72.6% (Table 1). This value was lower than that observed by Moura et al. (2018), who obtained results between 80 and 95.6% EE for microparticles obtained by ionic gelation by double emulsion spraying, the same procedure that was adopted here. Kim et al. (2016) reported results ranging from 29 to 41% efficiency for loaded catechin microparticles prepared by emulsion gelling using sunflower oil. Arriola et al. (2019) observed encapsulation efficiencies between 62.7 and 101.0% for encapsulation of stevia leaf extract by IG with alginate. Belščak-Cvitanović et al. (2016) evaluated the EE for ionic gelation particles with dandelion polyphenols and observed results ranging from 60.78 to 82.75% depending on the association with other proteins and the coverage with chitosan or pectin.
Wisuitiprot et al. (2011) observed that the percentage of retention of green tea catechins in chitosan microparticles prepared by the silicone water emulsion technique ranged from 3.5 to 39.25% for epigallocatechin, 13.06% and 24.34% for epicatechin, 15.26% and 59.94% for epigallocatechin-3-gallate and between 21.65 and 47.12% for epicatechin gallate, based on the use of different concentrations of the crosslinking agent and the pH of the assay.
Ionic gelation is a microencapsulation technique directly applied to hydrophobic and low-solubility substances (Arriola et al. 2019). For hydrophilic compounds, many methods that produce microparticles by ionic gelling employ some osmotic balance-based strategy, for example, for retention of the compounds within the polymeric structure (Belščak-Cvitanović et al. 2016; Arriola et al. 2019). This procedure is not always efficient for inclusion and retention of the substance in the microparticle and may result in wasting the compound of interest. The strategy employed in this work, by the application of a hydrophobic barrier between the core material and external environment, may be more advantageous in the encapsulation of hydrophilic compounds (Moura et al. 2018).
Moisture and water activity (aw)
The moisture content of the microparticles obtained by ionic gelling in this work was 79.37 ± 0.06% (Table 1). Moura et al. (2018) obtained similar results for particles obtained by ionic gelling through the atomization method with a value of 77.03 ± 0.01% humidity. Silvério et al. (2018) reported humidity values between 95 and 96% for IG particles containing paprika oleoresin. On the other hand, the particles obtained by spray chilling presented a moisture content of 1.73 ± 0.06% (Table 1), similar to that obtained by Fadini et al. (2018) in the spray chilling particles loaded with sacha inchi oil (1.71 ± 0.02 g/100 g). The extract used to obtain both particles presented 6.63 ± 0.06% humidity and probably contributed almost exclusively to the humidity of this sample since the wall of this system is made of lipid material. Fadini et al. (2019) observed the same behavior by attributing the moisture of the lipid microparticles to the spray drying material encapsulated by the lipid matrix.
The water activity is a relevant parameter for food components because it is associated with several degradation reactions and microbiological growth (Fadini et al. 2018). The microparticles of IG, due to the high content of water retained in the matrix, end up having high aw values, as was the case in this work (0.9928 ± 0.0047). For the lipid microparticle sample, the aw was 0.5498 ± 0.0005 (Table 1), which was higher than that observed by Fadini et al. (2018) but closer to that reported by Salvim et al. (2015) at 0.43 ± 0.03. de Matos-Jr et al. (2017), on the other hand, observed aw values higher than those of this work, varying between 0.631 ± 0.014 and 0.969 ± 0.005.
The methodology for obtaining microparticles by ionic gelation often involves the use of low solids polymer solutions (due to the high viscosity of the polymers in solution), which produces samples with high moisture content (Belščak-Cvitanović et al. 2016). The general composition of the formulation of oil and/or retained solids content in the polymer matrix also influences the total moisture content of the particles (Moura et al. 2018).
Particle size distribution
The size distribution of microparticles can determine their application in foods because very small particles can influence, for example, the viscosity and texture of products, while larger particles can be perceived sensorially (sandiness, lumps, etc.) and cause the rejection of the product (Fadini et al. 2018).
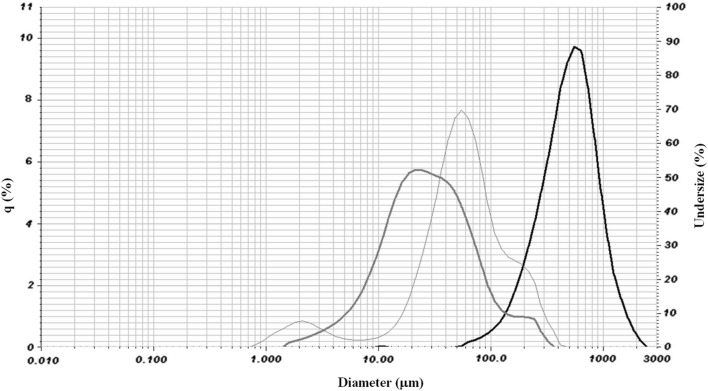
Table 2 presents the results of the size distribution of the particles generated in this work by the techniques of spray chilling and ionic gelation. Figure 1 also shows the granulometric distribution curves for the microparticles and the core material used.
Table 2.
Size distribution of green tea powder extract and the particles obtained
| Samples | D10 (μm) | D50 (μm) | D90 (μm) | Span |
|---|---|---|---|---|
| GTEP | 8.38 ± 0.09 | 26.96 ± 0.31 | 91.42 ± 6.68 | 3.08 ± 0.21 |
| LMP | 15.71 ± 0.72 | 55.19 ± 1.58 | 174.43 ± 10.64 | 2.87 ± 0.11 |
| IGMP | 219.62 ± 2.26 | 513.53 ± 11.37 | 1009.09 ± 38.53 | 1.54 ± 0.05 |
Fig. 1.
Size distribution of core material (GTEP—dark grey line), lipid microparticles (light grey line—LMP) and Ionic gelation microparticles (IGMP—black line)
Spray chilling particles, containing the powdered green tea extract, had an average diameter (D50) of 55.19 μm (Table 2). In the literature, several values were observed for this parameter. Procopio et al. (2018) obtained results between 8.42 ± 1.4 and 72.21 ± 11.27 μm for LMP containing cinnamon oleoresin. Oriani et al. (2016) reported D50 values of LMP containing ginger oleoresin from 31.0 ± 1.5 to 41.2 ± 3.0 μm. Sartori et al. (2015) produced LMP loaded with ascorbic acid with D50 values between 17 ± 1 and 35 ± 11 μm. Fadini et al. (2019), using the same double shell technique applied in this work, reported results with D50 values in the range of 14.67 ± 0.53 μm to 81.31 ± 1.15 μm.
In Fig. 1, it is possible to observe the size distribution curves for the powder extract (Fig. 1-dark grey line) obtained by spray drying and the LMP containing this extract (Fig. 1-light grey line). The distribution of LMP completely encompasses the distribution of the powdered extract, which may be evidence of the total encapsulation of the core material.
The IGMP had a mean particle diameter of 513.53 μm (Table 2). Moura et al. (2018), using the same production scheme, the same atomization pressure and similar formulation parameters, obtained a lower diameter of 165.03 μm, but the orifice of the atomizer nozzle used was 0.7 mm, while in our work, we used a 2.0 mm nozzle. Silvério et al. (2018) produced their IGMP by spraying with a 0.7 mm orifice atomizer and reported diameter values for their samples in the range of 154.7–205.1 μm. The size distribution of the IGMP was monomodal (Fig. 1-black line).
In relation to the polydispersity index (span), (Table 2) the LMP sample presented a higher value than the IGMP sample, indicating that the diameters of the lipid particles had a greater range of variation than the IGMP, in this case, less polydisperse. Span values in the range from 1.4 to 5.0 were observed by Fadini et al. (2019) for LMP containing spray dryer particles as the core material. Alvim et al. (2016) reported a span value of 1.45 ± 0.00 for LMP containing ascorbic acid in solid form, and Consoli et al. (2016) cited span values between 1.648 and 1.905 for their samples.
Morphology
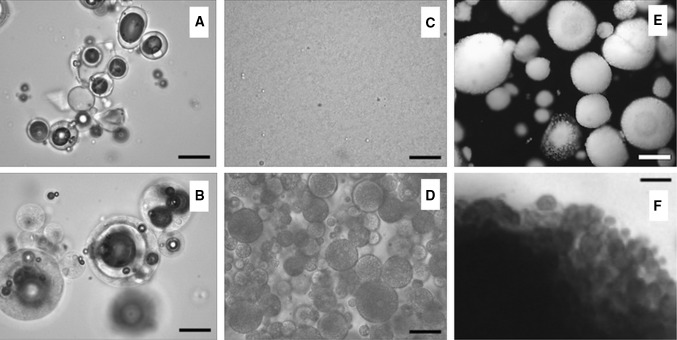
The appearance of the green tea powder particles (produced by spray drying), the LMP containing this powder, the emulsification stages and the microparticles generated in the ionic gelation process are presented in Fig. 2.
Fig. 2.
Morphology of samples. a Green tea extract powder (GTEP); b lipid microparticles containing green tea extract powder; c first single emulsion (W/O—GTEP Rehydrated + canola oil); d double emulsion (W/O/W—GTEP Rehydrated + canola oil + pectin solution); e IG microparticles (optical fiber light source); f Emulsion retention detail of IG microparticles (transmitted light source). Magnification: a–d × 1000X; e × 100; f image snip—original image captured in × 100. Bars: a–d 20 µm; e 200 µm; f 30 µm
The microparticles of green tea produced by spray drying (Fig. 2a) presented spherical shapes and polydispersities typical of materials obtained by this drying process. Alvim et al. (2016) and Fadini et al. (2018, 2019) obtained similar aspects of samples using the same equipment.
LMP also presented typical spherical shapes and polydispersities, with results similar to those of Consoli et al. (2016), Oriani et al. (2016), Alvim et al. (2016) and Fadini et al. (2019). The use of optical microscopy, with dispersion of the sample in mineral oil, allowed visualization of the spray dryer microparticles inside the lipid matrices. Images with similar information were reported by Fadini et al. (2019).
The production of the microparticles by ionic gelation was also monitored by optical microscopy in various stages (Fig. 2c–f), and it was possible to observe the formation of an emulsion with very small droplets in the first stage of inclusion of the tea extract powder reconstituted in canola oil (Fig. 2c). Subsequently, it was possible to observe the portions of this first W/O emulsion in the second W/O/W emulsion (Fig. 2d).
After crosslinking, the IGMP could be visualized and already contained the emulsion confined within (Fig. 2e, f). Moura et al. (2018) observed similar structures in IGMP obtained by the same procedure used in this work, and the micrographs of Silvério et al. (2018) using an optical fiber light source also allowed the observation of the surface and color details of the IGMP. The surface topography of IG particles with diameters between 500 and 1000 μm are often observed by scanning electron microscopy (SEM), requiring the dehydration of the samples, which may alter the observed information. The lightening feature of the particles above, as used in this work, can be very useful for observation of the morphology of particles in their natural, moist state, since it does not require dehydration and allows the visualization of the color and distribution of the core material within the structures.
Colorimetry
The color parameters (according to the CIELab system) obtained for the GTEP and for the microparticles produced by ionic gelling and spray chilling containing this material are described in Table 2.
GTEP was the sample with the lowest brightness value (L*), that is, it was the darkest, and statistically different (p < 0.05) from the microparticle samples. The LMP and IGMP samples were lighter than the core material sample, with little difference in the lipid microparticles (Table 2). The IGMP presented a larger L*, indicating a greater whiteness/luminosity of the sample. This may be due to the greater dilution of the core material in the formulation. For all samples, the color component a* was positive (red) with low values, while the value of b*, also positive (yellow), was more intense, indicating that the samples tended to have a light and slightly yellowish color. The parameters a* and b* were statistically different (p < 0.05) for all samples. Considering the color difference expressed by the ΔE value (Table 2), which in this case compared the color parameters of the microparticles to the GTEP, the LMP presented less color difference in relation to the core material after the encapsulation compared to the IGMP.
Moura et al. (2018) observed L* values between 49.73 and 70.40 for IGMP obtained by spraying. One suggestion to use for the microparticle samples generated in this work would be dairy products such as yogurt and ice creams, which, depending on the formulation, may have compatible microparticle coloration without major changes in appearance. Multicomponent products with cereal bars and fruit are also indicated for the application of the microparticles (considering the appearance/color) since these products already have intrinsic color heterogeneity.
Determination of antioxidant activity
In vitro analyses for the determination of antioxidant activity are necessary to verify the correlation between the total content of phenolic compounds and the antioxidant capacity of radical elimination of a sample (Huang et al. 2005).
DPPH (1,1-diphenyl-2-picrylhydrazyl) is a constant free radical that reacts with compounds capable of donating a hydrogen atom (Tengse et al. 2017). This method has been used by different researchers in the evaluation of microencapsulated green tea extracts (Noudoost et al. 2015; Tengse et al. 2017; Shetta et al. 2019) and was therefore chosen for comparison between the different methods of microencapsulation.
In this study, the results were expressed as the IC50, which is the necessary concentration of the antioxidant compound to reduce the DPPH radical by 50%, with lower IC50 values related to the higher antioxidant activity of the sample.
As expected, the green powder extract showed an IC50 value significantly (p < 0.05) lower than that of both microparticles produced (44.70 ± 0.50 μg/mL). The lipid particles obtained by spray chilling presented statistically lower IC50 values (p < 0.05) than those produced by ionic gelation at 2099.69 ± 35.28 and 33,169.40 ± 123.84 μg/mL, respectively.
Noudoost et al. (2015) observed lower values of IC50 for the free green tea extract (12.49 ± 0.6 μg/ml) and microencapsulated in nanoliposomes (1.78 ± 0.3 μg/ml). On the other hand, Pasrija et al. (2015) evaluated antioxidant activity in green tea extracts microencapsulated by freeze drying and spray drying and observed higher IC50 values (54,770 and 60,260 μg/ml, respectively) than those observed in this study.
Shetta et al. (2019) observed an increase in the antioxidant activity of essential oils of mint and green tea encapsulated in chitosan nanoparticles with IC50 values of 1610 and 340 μg/mL, respectively, while free oils presented IC50 values of 3250 and 810 μg/mL, respectively.
During the production of the microparticles, the pure extract is only a part of the formulation, in this way the amount of antioxidant material in the particles is smaller, thus providing, in the analysis, relative lower antioxidant activity compared with the GTEP. Another factor observed in the production of the microparticles is the loss of core material evidenced by the encapsulation efficiency, which certainly influences the final amount of extract available to act as an antioxidant. Losses during analysis in the extraction stage due to the presence of oxygen and exposure to light may also occur and influence the results (Lopes et al. 2007; Moura et al. 2018).
The useful properties of polyphenols microencapsulated are provide protection of the remaining compounds and masking of bitter/astringent flavors present in green tea and allow the controlled release of polyphenols for nutritional/biological proposes.
Conclusion
After some adjustments in the preliminary tests, the green tea extract powder was used in rehydrated form as the core material in encapsulation by the ionic gelation method. In the spray chilling procedure, it was possible to use the extract in the powder form directly as a core material. The particles were obtained successfully in both methods, with typical physical characteristics, such as morphology and size distribution, consistent with the results in literature. The encapsulation efficiencies were above 70% and considered good values. The double emulsion was a good strategy to retain the hydrophilic compounds of the extract in the rich water matrix of IGMP. Since it was observed a difference in antioxidant capacity of the free and microencapsulated extract its carrying in microparticles can give protection for the core, mask bitter/astringent flavors present in green tea and allow the controlled release of polyphenols for nutritional/biological proposes.
Acknowledgements
The authors are thankful for the technological support of the Instituto de Tecnologia de Alimentos (ITAL) for providing the microparticle laboratory. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alvim ID, Souza FDSD, Koury IP, Jurt T, Dantas FBH. Use of the spray chilling method to deliver hydrophobic components: physical characterization of microparticles. Food Sci Technol. 2013;33:34–39. doi: 10.1590/S0101-20612013000500006. [DOI] [Google Scholar]
- Alvim ID, Stein MA, Koury IP, Dantas FBH, Cruz CLDCV. Comparison between the spray drying and spray chilling microparticles contain ascorbic acid in a baked product application. LWT Food Sci Technol. 2016;65:689–694. doi: 10.1016/j.lwt.2015.08.049. [DOI] [Google Scholar]
- Ananingsih VK, Sharma A, Zhou W. Green tea catechins during food processing and storage: a review on stability and detection. Food Res Int. 2013;50:469–479. doi: 10.1016/j.foodres.2011.03.004. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of AOAC international. 18. Gaithersburg: Association of Official Analytical Chemists; 2006. [Google Scholar]
- Arriola NDA, Chater PI, Wilcox M, et al. Encapsulation of stevia rebaudiana Bertoni aqueous crude extracts by ionic gelation–Effects of alginate blends and gelling solutions on the polyphenolic profile. Food Chem. 2019;275:123–134. doi: 10.1016/j.foodchem.2018.09.086. [DOI] [PubMed] [Google Scholar]
- Belščak-Cvitanović A, Bušić A, Barišić L, et al. Emulsion templated microencapsulation of dandelion (Taraxacum officinale L.) polyphenols and β-carotene by ionotropic gelation of alginate and pectin. Food Hydrocoll. 2016;57:139–152. doi: 10.1016/j.foodhyd.2016.01.020. [DOI] [Google Scholar]
- Bora AFM, Ma S, Li X, Liu L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: review and recent advances. Food Res Int. 2018;105:241–249. doi: 10.1016/j.foodres.2017.11.047. [DOI] [PubMed] [Google Scholar]
- Cai ZY, Li XM, Liang JP, Xiang LP, et al. Bioavailability of tea catechins and its improvement. Molecules. 2018;23:2346. doi: 10.3390/molecules23092346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Acosta ML, Stone SG, Mok JE, et al. Apple and blackcurrant polyphenol-rich drinks decrease postprandial glucose, insulin and incretin response to a high-carbohydrate meal in healthy men and women. J Nutr Biochem. 2017;49:53–62. doi: 10.1016/j.jnutbio.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Cheng TO. All teas are not created equal: the Chinese green tea and cardiovascular health. Int J cardiol. 2006;108(3):301–308. doi: 10.1016/j.ijcard.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Consoli L, Grimaldi R, Sartori T, Menegalli FC, Hubinger MD. Gallic acid microparticles produced by spray chilling technique: production and characterization. LWT Food Sci Technol. 2016;65:79–87. doi: 10.1016/j.lwt.2015.07.052. [DOI] [Google Scholar]
- Cutrim CS, Cortez MAS. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int J Dairy Technol. 2018;71(3):564–578. doi: 10.1111/1471-0307.12515. [DOI] [Google Scholar]
- de Matos-Jr FE, Comunian TA, Thomazini M, Favaro-Trindade CS. Effect of feed preparation on the properties and stability of ascorbic acid microparticles produced by spray chilling. LWT Food Sci Technol. 2017;75:251–260. doi: 10.1016/j.lwt.2016.09.006. [DOI] [Google Scholar]
- Fadini AL, Alvim ID, Ribeiro IP, et al. Innovative strategy based on combined microencapsulation technologies for food application and the influence of wall material composition. LWT Food Sci Technol. 2018;91:345–352. doi: 10.1016/j.lwt.2018.01.071. [DOI] [Google Scholar]
- Fadini AL, Alvim ID, Paganotti KBDF, Bataglia da Silva L, Bonifácio Queiroz M, Miguel AMRDO, Rodrigues RAF. Optimization of the production of double-shell microparticles containing fish oil. Food Sci Technol Int. 2019 doi: 10.1177/1082013219825890. [DOI] [PubMed] [Google Scholar]
- Faller ALK, Fialho E. Disponibilidade de polifenóis em frutas e hortaliças consumidas no Brasil. Rev Saúde Públ. 2009;43:211–218. doi: 10.1590/S0034-89102009005000010. [DOI] [PubMed] [Google Scholar]
- Faller JW, Marcon SS. Práticas socioculturais e de cuidado a saúde de idosos em diferentes etnias. Esc Anna Nery Rev Enferm. 2013;17:512–519. doi: 10.1590/S1414-81452013000300015. [DOI] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) (2018) Current market situation and medium term outlook for tea to 2027. http://www.fao.org/3/BU642en/bu642en.pdf. Accessed 20 Dec 2018
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Kim ES, Lee JS, Lee HG. Calcium-alginate microparticles for sustained release of catechin prepared via an emulsion gelation technique. Food Sci Biotechnol. 2016;25:1337–1343. doi: 10.1007/s10068-016-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Yan H, Puligundla P, Gao X, Zhou Y, Wan X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: a review. Food Hydrocoll. 2017;69:286–292. doi: 10.1016/j.foodhyd.2017.01.041. [DOI] [Google Scholar]
- Liu S, You L, Zhao Y, Chang X. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res Int. 2018;107:73–83. doi: 10.1016/j.foodres.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Lopes T, Xavier M, Quadri MG, Quadri M. Antocianinas: uma breve revisão das características estruturais e da estabilidade. Curr Agric Sci Technol. 2007 [Google Scholar]
- Meegahakumbura MK, Wambulwa MC, Li MM, Thapa KK, Sun YS, Möller M, Xu JC, Yang JB, Liu J, Liu BY, Li DZ. Domestication origin and breeding history of the tea plant (Camellia sinensis) in China and India based on nuclear microsatellites and cpDNA sequence data. Front Plant Sci. 2018;8:2270. doi: 10.3389/fpls.2017.02270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura SC, Berling CL, Germer SP, Alvim ID, Hubinger MD. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: pigment stability during storage of microparticles. Food Chem. 2018;241:317–327. doi: 10.1016/j.foodchem.2017.08.095. [DOI] [PubMed] [Google Scholar]
- Munin A, Edwards-Lévy F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics. 2011;3:793–829. doi: 10.3390/pharmaceutics3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Noori N, Amo Abedini G, Gandomi H, Akhondzadeh Basti A, Jebeli Javan A, Ghadami F. Encapsulation of green tea extract in nanoliposomes and evaluation of its antibacterial, antioxidant and prebiotic properties. J Med Plant Res. 2015;3:66–78. [Google Scholar]
- Oriani VB, Alvim ID, Consoli L, Molina G, Pastore GM, Hubinger MD. Solid lipid microparticles produced by spray chilling technique to deliver ginger oleoresin: structure and compound retention. Food Res Int. 2016;80:41–49. doi: 10.1016/j.foodres.2015.12.015. [DOI] [Google Scholar]
- Pasrija D, Ezhilarasi PN, Indrani D, Anandharamakrishnan C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT Food Sci Technol. 2015;64:289–296. doi: 10.1016/j.lwt.2015.05.054. [DOI] [Google Scholar]
- Procopio FR, Oriani VB, Paulino BN, do Prado-Silva L, Pastore GM, Sant’Ana AS, Hubinger MD. Solid lipid microparticles loaded with cinnamon oleoresin: characterization, stability and antimicrobial activity. Food Res Int. 2018;113:351–361. doi: 10.1016/j.foodres.2018.07.026. [DOI] [PubMed] [Google Scholar]
- Salvim MO, Thomazini M, Pelaquim FP, Urbano A, Moraes ICF, Favaro-Trindade CS. Production and structural characterization of solid lipid microparticles loaded with soybean protein hydrolysate. Food Res Int. 2015;76:689–696. doi: 10.1016/j.foodres.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Šaponjac VT, Ćetković G, Čanadanović-Brunet J, et al. Sour cherry pomace extract encapsulated in whey and soy proteins: incorporation in cookies. Food Chem. 2016;207:27–33. doi: 10.1016/j.foodchem.2016.03.082. [DOI] [PubMed] [Google Scholar]
- Šaponjac VT, Čanadanović-Brunet J, Ćetković G, Jakišić M, Djilas S, Vulić J, Stajčić S. Encapsulation of beetroot pomace extract: RSM optimization, storage and gastrointestinal stability. Molecules. 2016;21:5–584. doi: 10.3390/molecules21050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori T, Consoli L, Hubinger MD, Menegalli FC. Ascorbic acid microencapsulation by spray chilling: production and characterization. LWT Food Sci Technol. 2015;63:353–360. doi: 10.1016/j.lwt.2015.03.112. [DOI] [Google Scholar]
- Shetta A, Kegere J, Mamdouh W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: encapsulation, thermal stability, in vitro release, antioxidant and antibacterial activities. Int J Biol Macromol. 2019;126:731–742. doi: 10.1016/j.ijbiomac.2018.12.161. [DOI] [PubMed] [Google Scholar]
- Shim SM, Yoo SH, Ra CS, Kim YK, Chung JO, Lee SJ. Digestive stability and absorption of green tea polyphenols: influence of acid and xylitol addition. Food Res Int. 2012;45:204–210. doi: 10.1016/j.foodres.2011.10.016. [DOI] [Google Scholar]
- Silvério GB, Sakanaka LS, Alvim ID, Shirai MA, Grosso CRF. Production and characterization of alginate microparticles obtained by ionic gelation and electrostatic adsorption of concentrated soy protein. Cienc Rural. 2018;48:12. doi: 10.1590/0103-8478cr20180637. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Tengse DD, Priya B, Kumar PAR. Optimization for encapsulation of green tea (Camellia sinensis L.) extract by spray drying technology. J Food Meas Charact. 2017;11:85–92. doi: 10.1007/s11694-016-9374-4. [DOI] [Google Scholar]
- Vetrani C, Vitale M, Bozzetto L, et al. Association between different dietary polyphenol subclasses and the improvement in cardiometabolic risk factors: evidence from a randomized controlled clinical trial. Acta Diabetol. 2018;55:149–153. doi: 10.1007/s00592-017-1075-x. [DOI] [PubMed] [Google Scholar]
- von Gadow A, Joubert E, Hansmann CF. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT, and BHA. J Agric Food Chem. 1997;45:632–638. doi: 10.1021/jf960281n. [DOI] [Google Scholar]
- Wisuitiprot W, Somsiri A, Ingkaninan K, Waranuch N. A novel technique for chitosan microparticle preparation using a water/silicone emulsion: green tea model. Int J Cosmet Sci. 2011;33:351–358. doi: 10.1111/j.1468-2494.2010.00635.x. [DOI] [PubMed] [Google Scholar]