Abstract
Biochar prepared from rice husk (rBC) was used as a nanosorbent for the sustained delivery of 2,4-dichlorophenoxyacetic acid (2,4-D) and for its potential use as an eco-friendly nanoherbicide formulation. For the biochar-based 2,4-D nanoformulation (DrBC), the loading efficiency of the herbicide onto the rBC nanocarrier was checked at various weight ratios, where a weight ratio of 1:0.25 (rBC:2,4-D) was found optimum. Further experiments were carried out to understand the 2,4-D sorption characteristics of rice husk biochar (rBC) and its sustained herbicidal release property in soil and water. When the adsorption mechanism was analyzed, the Freundlich isotherm and pseudo-second-order kinetic equation were found to be the best. The sustained release of 2,4-D from the DrBC nanoformulation was detected for about 26 days. The release profile of 2,4-D from DrBC was found to be a controlled diffusion mechanism based on Korsmeyer–Peppas model fit. The size of the DrBC nanoformulation was found to be 256.5 nm using dynamic light scattering (DLS) with a negative zeta potential of 28.8 mV. Fourier transform infrared spectroscopy (FT-IR) results indicated the involvement of carboxyl, aromatic carbon, and siloxane in 2,4-D adsorption onto rBC. The additional chlorine in the EDAX spectrum of scanning electron microscope (SEM) indicated the herbicide adsorption onto the rBC carrier. The DrBC nanoformulation exhibited enhanced herbicidal activity against the tested Brassica sp., but did not affect the growth of the non-target plant (Zea mays). The present study could be promising for achieving high herbicidal loading, sustained release, and reduced leaching of 2,4-D for its effectual usage in an eco-friendly fashion.
Keywords: 2,4-D; Biochar; Herbicide; Leaching; Nanoparticles; Release
Introduction
Restrained crop yield due to the competition for nutrients, light, and water, posed by the weeds is a universal problem faced by the farmers (Sun et al. 2017; Pereira et al. 2014). Herbicides come into play to aid in weed control as well for increasing the crop yield. 2,4-Dichlorophenoxyacetic acid (2,4-D) is a commonly used chlorinated aromatic herbicide due to its effectiveness against the broad-leaved weeds. Low cost availability and highly selective nature makes 2,4-D as the third most widely used herbicide in farms and gardens. Simultaneously paves way to its contamination of the ground and surface waters around the world (Tang et al. 2015). The herbicide falls under the toxicity class II referred as moderately toxic to humans and animals on continuous exposure and eventually has appeared as an emerging and potent carcinogen. Due to its global detection and lack of potent removal technology for removal from the contaminated water bodies, it calls for an urgent and immediate notice of the researchers to gaze upon a way to employ it effectively.
Nowadays, nanoformulation of pesticides has received enormous attention due to their bioefficacy and eco-friendly nature. Nanopesticide formulations using different biodegradable polymers seem to be dominating the field, due to their controlled release characteristics, reduced toxicity, and less leaching properties (Pereira et al. 2014). Unfortunately, the rate of biodegradability of these polymers is difficult to control due to acidic environment that would prevail in the polymer systems matrices upon pH decrease. This condition makes the polymer-based systems inappropriate for most herbicides (Qian et al. 2011). Therefore, the incorporation of herbicides into some good sorbents could be a practical way for achieving high bioefficacy as well to reduce its leaching to the surrounding waters. For instance, cyanobacteria blooms, an environmental waste exhibited high sorption of avermectin pesticide and was used as a carrier in the sustained release formulations of pesticide (Yan et al. 2013). So, the adsorption of highly water soluble herbicides like 2,4-D onto an appropriate carrier could enhance its bioefficacy in the fields. For reducing the 2,4-D ground water contamination, the carrier to be selected should improve its retention in soil for a longer period via sorption onto the soil particles.
The biochar technology which is gaining recent interest could be of great help in achieving the prime goal of effective sustained release of 2,4-D with high bioefficacy and less leaching (Xu et al. 2013). Biochar could be defined as non-liquefied, carbonized, porous biomass, produced by pyrolysis at limited oxygen environment. The introduction of biochar in soil aids in increasing the nutrient availability, water retention, crop yield, and microbial count. Upon amendment was reported to reduce soil erosion, greenhouse gasses emission, and the frequency of fertilizer application (Mohan et al. 2014). Although many kinds of biomass could be used for biochar formation via pyrolysis, biochar produced from rice husk is known for its high potassium, silicon, and nutrients concentration. So, due to its composition, the rice husk biochar (rBC) has great potential to act as a soil amendment (Lingamdinne et al. 2015). Biochar in its crushed form is known to increase the surface area which in turn increases its effectiveness (Vaccari et al. 2011). Additionally, the biochar prepared from rice husk is more resistant to degradation compared with the biochar prepared from other biomasses such as fruit wastes and wood (Abdulrazzaq et al. 2014).
In this study, the potential of rBC nanoparticles to act as a herbicide carrier as well its sustained release and leaching behavior was analyzed at the same time. For serving this purpose, biochar was prepared from rice husk by slow pyrolysis at 350 °C and was crushed to form rBC nanoparticles. The rBC nanoparticles were loaded with 2,4-D herbicide to yield DrBC nanoformulation, whose sustained release and leaching behavior were evaluated in water and soil. To the best of our knowledge, this is the first report on the use of nano-sized rice husk biochar as 2,4-D herbicide carrier for effectual delivery.
Materials and methods
Chemicals
Technical grade 2,4-D was purchased from Sigma Aldrich, Bangalore, India. All the other chemicals were procured from Himedia pvt. Ltd. India and were all of analytical grade. Rice husk was bought from a local mill, Vellore, Tamil Nadu, India and was sieved to get fine rice husk microparticles of size 150–175 µm and stored until further use.
Preparation of biochar from rice husk waste and characterization
For rice husk biochar (rBC) preparation, about 10 g of the collected rice husk microparticles were subjected to slow pyrolysis at 350 °C in a muffle furnace. To maintain a limited air supply, the rBC microparticles were placed in a ceramic pot and closed with a lid. After pyrolysis for 6 h, the obtained solid carbon residue was added into 100 ml of hydrochloric acid (1 mol/L) at room temperature and the dispersion was kept in contact for 4 h before washing with distilled water. The acid and water washings of the solid residue were repeated for four times, to enable the rBC to contain its stable minerals. After which, the solid char was dried at 70 °C in a hot air oven to achieve a constant weight. The resultant rBC was mechanically ground to yield rBC nanoparticles. The prepared rBC nanoparticles were utilized as adsorptive carriers for 2,4-D loading. Based on the initial dry weight of the rice husk microparticles, the percentage yield of rBC nanoparticles was determined.
Loading of 2,4-D onto the rice husk biochar
For the preparation of rBC nanoparticles-based 2,4-D nanoformulation, about 1 g of the biochar nanoparticles was added individually into 20 mL of methanol (pH 5.0) solution of various initial 2,4-D weight ratios (0.20, 0.25, and 0.30) at ambient temperature. At regular intervals, aliquots of sample were withdrawn to estimate the 2,4-D concentration in the solution with the help of high-pressure liquid chromatography (HPLC). After 24 h, the 2,4-D sorbed rBC was separated via centrifugation (600 rpm, 5 min) and was dried to be designated as DrBC nanoformulation.
Analytical determination of 2,4-D herbicide
For residual 2,4-D analysis, the supernatant collected at regular intervals was filtered with a 0.22 µm microporous membrane prior to analysis on reverse phase HPLC (C18 column, particle size of 5 µm) coupled with a UV detector at 230 nm, where methanol:water (70:30, pH 2.0) was used as mobile phase. The percentage of 2,4-D adsorbed onto the silica nanoparticle was calculated using standard formulae.
where C0 and Ct are the concentration (mg/L) of the solution at initial as well as time‘t’, respectively; Ad is the percentage of 2,4-D removal.
Characterization of biochar-based 2,4-dichlorophenoxyacetic acid nanoformulation
The DrBC nanoformulation was characterized for physiochemical stability with a dynamic light scattering (DLS) instrument (JUNO 10G-HO, nanoparticle analyser SZ-100) for size determination. The stability of the DrBC nanoformulation and rBC was analyzed using a zeta potential analyzer (nanoparticle analyser SZ-100). The interaction of the 2,4-D compound with the rBC nanoparticles was determined with the help of a Fourier transform infrared spectrophotometer (ALPHA-T, Bruker infrared) with a resolution of 4 cm−1 via the potassium bromide (KBr) method. The morphological analysis of the DrBC nanoformulation was visualized on a VEGA 3 TESCAN, scanning electron microscope (SEM) coupled with EDAX for elemental characterization.
Sustained release of DrBC nanoformulation in water
The sustained release of 2,4-D from the DrBC nanoformulation was calculated by evaluating the amount of 2,4-D released from 100 mg of DrBC nanoformulation in distilled water (10 ml), which was kept at constant stirring (100 rpm, pH 7.0). For estimating the released 2,4-D concentration, at designated time intervals, about 2 ml of the sample was withdrawn from the dispersion and as well replaced with equal volume of distilled water. The 2,4-D release profile was achieved by plotting the cumulative 2,4-D release (%) against time. The release data were fitted to a semi-empirical kinetic model, Korsmeyer–Peppas for understanding the release mechanism of 2,4-D from the rBC nanocarrier.
Sustained release of DrBC nanoformulation in soil
To study the sustained release behavior of DrBC in soil, about 100 g of red soil was placed onto a 0.5-mm-mesh-size nylon filter which was paved inside a Buchner funnel. The soil was amended with DrBC nanoformulation at different weight ratios viz. 0.5 and 1.0%, respectively. About 40 ml of water was sprayed onto the funnel for nine times at 1 h interval for estimating the sustained release of 2,4-D from the DrBC nanoformulation to the soil. The ratio of summation of the amount of herbicide released (Mi, mg) and the initial amount of herbicide (M0, mg) added into the soil was calculated for estimating the 2,4-D release (%) with respect to time. The experiments were performed in triplicates and the mean average data are presented.
Bioactivity of DrBC nanoformulation
For bioactivity determination, the DrBC nanoformulation was applied at the field application rate (2.5 kg/ha) to the target (Brassica sp.) and non-target plant (Zea mays) grown for about 4 days in soilrite mix plant substrate (600 g). Each pot contained about 15 seeds of Brassica sp. and 10 seeds of Zea mays. Control experiments were also setup, where the plants were tested for its growth in the presence of technical 2,4-D formulation and rBC nanoparticles, on the 4th day after sowing. The dry weight of all the experiments was measured after 14 days and the plants were photographed.
Leaching behavior of DrBC nanoformulation
To understand the leaching behavior of DrBC nanoformulation, red soil (sand 83%, slit 6%, clay 11%) with no history of pesticide application was collected and sieved to remove dirt and stones. The collected soil was filled in a column of 20 cm height constructed using PVC pipes of height and diameter of 4 cm, where each segment was separated with a filter paper in between. The top segment was amended with DrBC nanoformulation at field application rate of 2.5 kg/hectare. After amendment, the column was precipitated via stimulation by adding 25 ml of water for 24–48 h and the columns were dismantled for residual 2,4-D analysis at different depths.
For 2,4-D quantification, about 5 g of soil was added into equal volume of methanol and agitated for about 20 min at 30 °C for three times. The 2,4-D extracted into methanol was concentrated in a rotary evaporator, prior to its estimation, with the help of HPLC. All the experiments were conducted thrice and the mean data were presented for reliability.
Results and discussion
Loading of 2,4-D onto the rBC nanoparticles
The rBC nanoparticles prepared from pyrolysis at 350 °C was checked for its potential to act as 2,4-D adsorptive carrier at different rBC to 2,4-D weight ratios and the results are presented in Fig. 1. Higher adsorption (%) was recorded at the initial hours which reached equilibrium in 8 h. The quicker adsorption of 2,4-D molecules to the rBC nanoparticle surface could be due to the presence of more sorption sites and highlights the reason for choosing rBC as adsorptive 2,4-D nanocarrier. The 2,4-D loading capacity of rBC at the selected weight ratio was found to be 190.97, 238.09, and 279.91 mg/g, respectively. Based on the equilibrium data, a slight decrease in the 2,4-D adsorption (%) was noticed at a higher weight ratio of 1:0.30, which could be due to the presence of limited number of 2,4-D sorption sites on the rBC nanocarrier. So, the rBC nanoparticles loaded with 0.25 wt(%) ratio of 2,4-D was chosen and formulated to yield rBC-based 2,4-D nanoformulation designated as DrBC.
Fig. 1.
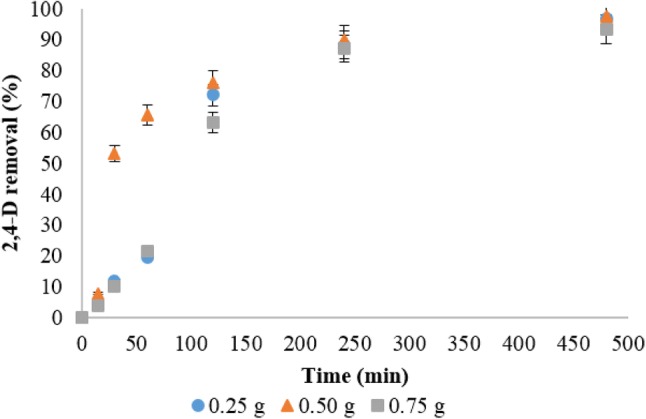
Loading pattern of 2,4-D onto rice husk biochar nanoparticles at various rBC:2,4-D ratios
The equilibrium data obtained during the adsorption of 2,4-D onto the rBC nanocarrier were fitted to the commonly employed adsorption isotherms and kinetic models (Table 1). The adsorption isotherm models can be used to understand the relationship between the concentrations of 2,4-D in liquid phase with that in the solid phase at equilibrium (Foo et al. 2012). The isotherm models such as Langmuir (Langmuir 1918), Freundlich (Freundlich 1906), Temkin (Temkin and Pyzhev 1940), and D–R (Dubinin 1960) isotherm were fitted with the obtained equilibrium data. Based on the highest regression coefficient, Freundlich isotherm was found to be the best-fitted model. The n value of Freundlich model which was greater than one indicated the adsorption process to be favorable (Table 2). So, it could be inferred that the 2,4-D molecules were attached onto the rBC nanoparticles via a heterogeneous reversible multilayer adsorption mode, which could have increased the 2,4-D sorption percentage.
Table 1.
Sorption and kinetics model equations used in the study
| Isotherm and kinetics | Linearized equation | Plot | Assumption upon best fit | References |
|---|---|---|---|---|
| Isotherm models | ||||
| Langmuir isotherm | 1/Ceq vs. 1/qe | Monolayer adsorption onto the adsorbent surface with no interaction between the adsorbed molecules | Mohan et al. (2014) | |
| Freundlich isotherm | log Ceq vs. log qeq | A heterogeneous surface and multilayer adsorption mode | Pereira et al. (2014) | |
| Temkin isotherm | ln Ceq vs. qe | Uniform distribution of binding energies | Qian et al. (2011) | |
| Dubinin–Radushkevich isotherm | ε2 vs. ln qe | Adsorption mechanism with a Gaussian energy distribution onto a heterogenous surface | Sivasamy et al. (2012) | |
| Kinetics models | ||||
| Pseudo-first-order kinetics | t vs. log (qe − qt) | A physisorption mode of mechanism | Sun et al. (2017) | |
| Pseudo-second-order kinetics | t vs. t/qt | A chemisorption mode of mechanism | Tang et al. (2015) | |
| Intraparticle diffusion kinetics | qt vs. log t | Intraparticle diffusion, the rate-limiting step or not | Temkin and Pyzhev (1940) | |
Table 2.
Sorption isotherm parameters of 2,4-D loading on biochar nanocarrier
| Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R 2 | Kf (L/g) | n | R 2 |
| 90.9 | 0.030 | 0.993 | 2.51 | 1.05 | 0.998 |
| Temkin isotherm | D–R isotherm | ||||
|---|---|---|---|---|---|
| BT | KL (L/mg) | R 2 | qm (mg/g) | β | R 2 |
| 0.0028 | 32.49 | 0.971 | 597.64 | 0.0038 | 0.991 |
The sorption kinetic modeling is mandatory for gaining insight into the mechanism of sorption and the rate controlling step (Vaccari et al. 2011), which was tested, in the present study with the pseudo-first-order kinetics, pseudo-second-order kinetics, and intraparticle diffusion kinetics. The obtained kinetic results (Table 3) revealed a better fit (R2 = 0.99) of the equilibrium data with pseudo-second-order model, depicting a chemisorption process. The equilibrium kinetics result was in support with the D–R isotherm result which predicted a chemisorption process with a mean energy value (E) of 9.71 kJ/mol.
Table 3.
Kinetic parameters of sorption for 2,4-D sorption onto rBC nanocarrier
| Initial 2,4-D concentration (mg/L) | Pseudo-first-order kinetics | Pseudo-second-order kinetics | Intraparticle diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | K1 (g/mg/min) | R 2 | Qe (mg/g) | K2 (g/mg/min) | R 2 | K i | C | R 2 | |
| 50 | 170.37 | 0.011 | 0.969 | 181.81 | 0.028 | 0.99 | 7.22 | 30.87 | 0.984 |
| 100 | 384.50 | 0.0050 | 0.966 | 434.78 | 0.018 | 0.998 | 11.23 | 99.16 | 0.990 |
| 150 | 492.03 | 0.0048 | 0.954 | 588.23 | 0.011 | 0.999 | 19.59 | 57.40 | 0.933 |
To analyze whether the intraparticle diffusion is a rate-limiting step or not, the equilibrium data were also fitted with the intraparticle diffusion kinetics. Based on the plot between qe and t0.5 (graph not shown), it was found to follow a rapid, medium, and slow phase of uptake which did not pass through the origin. So, the intraparticle diffusion was concluded to be not a rate-limiting step for 2,4-D sorption onto the rBC nanoparticles. So, based on the results, the adsorption of 2,4-D onto the rBC nanoparticles was found to be a multilayer mode via chemisorption mechanism which enabled the biochar to achieve high loading and strong reversible sorption.
Characterization of DrBC nanoformulation
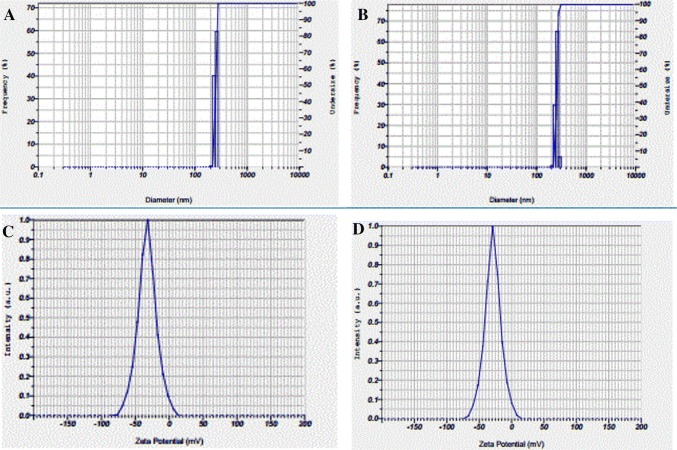
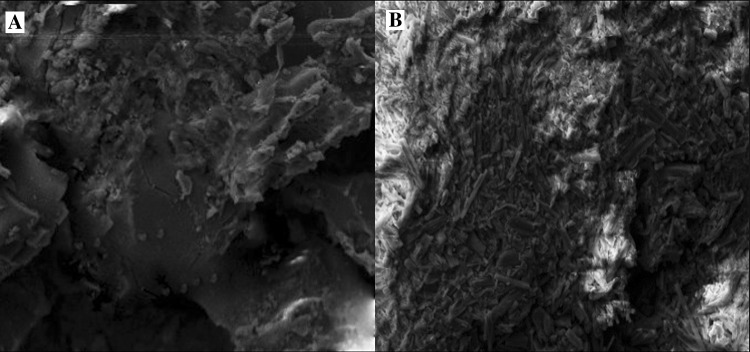
The size and zeta potential of the rBC before and after 2,4-D adsorption (DrBC) were checked and the results are presented in Fig. 2. The rBC nanoparticles formed by mechanical crushing was found to exhibit a hydrodynamic particle size of 251.9 nm (Fig. 2a) with a negative zeta potential of 32.5 mV (Fig. 2c). The obtained zeta potential reveals the stability of the rBC nanoparticles. On the other hand, the DrBC nanoparticles were found to show a hydrodynamic particle size of 256.5 nm (Fig. 2b) and a negative zeta potential of 28.8 mV (Fig. 2d). So, a stable DrBC nanoformulation was formulated in nanosize for enhanced herbicidal efficacy with high loading capacity. Similar reports where microbial nano-absorbents increased adsorption capacity were reported by other researchers (Ma et al. 2013; Dotto et al. 2012) as well. The involvement of functional groups that could have enabled the attachment of the herbicide to the surface of rBC was analyzed with the help of Fourier transform infrared analysis (FT-IR). The IR spectra of the rBC and DrBC are presented in Fig. 3a and b. No peaks corresponding to –OH stretch and aliphatic –CH stretch were detected in the range of 300–2500 cm−1 in case of rBC nanoparticle and DrBC nanoformulation. But the peak in the range of 1600–1800 cm−1 indicates that the aromatic carbon was high. This is an additional indication for the presence of aromatic C = C peaks of benzene-like rings. The presence of the above mentioned peak indicates the extra stability of the rBC in soil. The peaks at 1730.36 and 1738.50 cm−1 in rBC nanoparticle and DrBC formulation correspond to the presence of C = O stretch vibration of the ketones, aldehydes or carboxyl groups. The transmittance peak in the range of 1400–1500 cm−1 could be due to the bending vibrations of siloxane (Si–O–Si) bonds. The peaks at 682.99 and 673.91 cm−1 in rBC and DrBC spectra attribute to the presence of Si–O networks. The vibrational stretches in the region of 850–550 cm−1 contribute to the addition of C–Cl group of the herbicide to that of the silica networks present on the surface of rice husk biochar. So, the FT-IR result presents the involvement of the carboxyl, aromatic carbon, and siloxane in the adsorption of 2,4-D onto the rBC surface. The rBC was also checked for morphological changes before and after 2,4-D adsorption (Fig. 4a, b). The adsorption of 2,4-D on to the surface of the rBC nanoparticles could be visualized with a mild color change. And also, the rBC nanoparticles were found to be heterogeneous and porous in nature, which could have been responsible for enhancing the herbicide’s uptake. The EDX spectrum obtained before and after 2,4-D adsorption (figure not shown) exhibited the presence of the elements viz. carbon (C), oxygen (O), and silicon (Si), in case of DrBC formulation. Apart from the abovementioned elements, an additional peak indicated the presence of chlorine (Cl). The presence of ‘Cl’ peak in the spectra could be could due to the sorption of 2,4-D onto the rBC nanosorbent.
Fig. 2.
Size distribution and zeta potential of rBC nanoparticles before (a and c) and after (b and d) 2,4-D loading
Fig. 3.
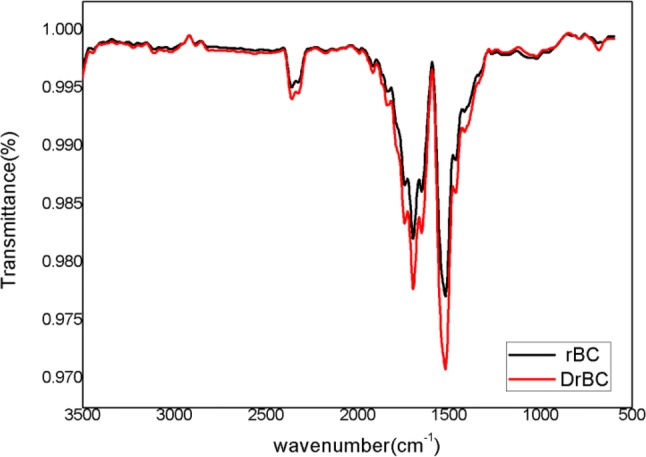
Fourier transform infrared (FT-IR) spectra of rBC nanoparticles DrBC nanoformulation
Fig. 4.
Scanning electron microscopic image of a rBC before and b after 2,4-D (DrBC) loading
Sustained release of DrBC nanoformulation in water and soil
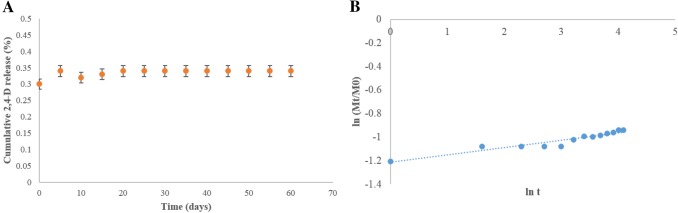
The release of 2,4-D from DrBC into water is shown in Fig. 5. The results demonstrated a sustained release of 2,4-D for about 55 days in water (pH 7.0) at room temperature. A burst release of 2,4-D which could have been loosely attached to the rBC nanocarrier was noticed in the initial hours after which the release rate was found to exhibit a steady state. On the other hand, the control experiment performed with free 2,4-D was found to get dispersed in the water immediate to its addition in water. The Korsmeyer–Peppas model was taken for analyzing the pattern of 2,4-D release from the rBC nanocarrier. The model exhibited a high regression coefficient (R2) of 0.99, where a low ‘n’ value of 0.06 revealed that the 2,4-D release from the carrier is via diffusion in spherical monolithic matrices. Additionally, a lower value of ‘k’ (0.16 day−1) indicated a slow release phenomenon of 2,4-D from the carrier. So, based on the Korsmeyer–Peppas kinetic model result, the release of 2,4-D from the DrBC formulation was understood to be a controlled diffusion process.
Fig. 5.
a Cumulative sustained release of 2,4-D from DrBC nanoformulation at optimum weight ratio of rBC:2,4-D in water; b kinetics of 2,4-D release via Korsmeyer–Peppas kinetic model
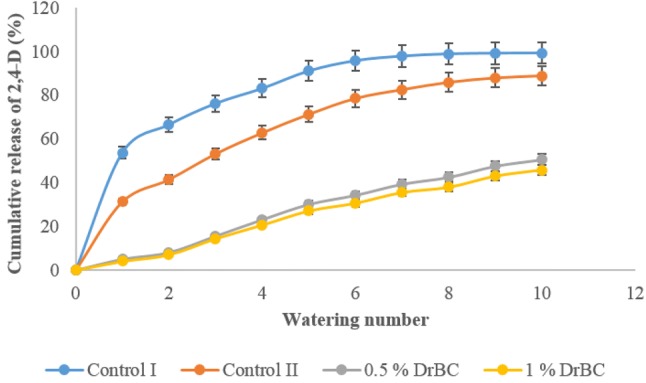
When checked for sustained release in soil, the DrBC nanoformulation exhibited a slow release pattern of 2,4-D than that of the free 2,4-D control experiment. The complete release of the added free 2,4-D was noticed by the end of five watering whereas even after nine waterings approximately only 50% of the adsorbed 2,4-D was found to be released from the DrBC nanoformulation. It was also noticed that the soil amended with 0.5 wt(%) DrBC released 2,4-D at a lesser rate than that of the soil amended with 1.0 wt(%) formulation. The release of 2.4-D from DrBC nanoformulation was slower in soil than in water, which could be due to the increased competition of the soil particles for 2,4-D sorption, enabling a lesser release at the end of nine waterings. These experiments could efficiently portray the role of rBC in aiding the sustained release of 2,4-D from DrBC nanoformulation in water and soil environments.
Bioactivity of DrBC nanoformulation
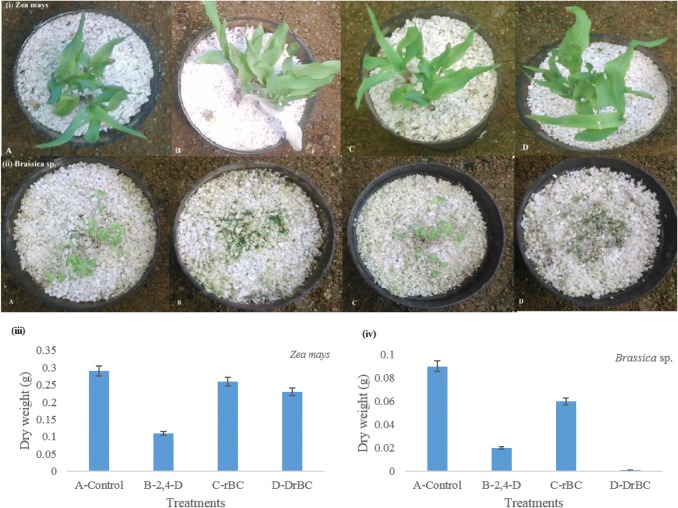
The herbicidal activity of the DrBC nanoformulation was investigated at the field application rate against the target plant (Brassica sp.) and non-target plant (Zea mays). The DrBC nanoformulation exhibited herbicidal activity similar to that of the free 2,4-D technical formulation (Fig. 6). The characteristic faster necrosis effect of 2,4-D herbicide was noticed in the Brassica sp. plants treated with DrBC nanoformulation. This serves as an evidence that the rBC nanosorbents used for the 2,4-D nanoformulation did not have any deteriorating effect on the herbicide’s efficacy, although it prolonged its presence in soil via sustained release.
Fig. 6.
Pre-emergent 2,4-D treatment of (i) non-target (Zea mays) and (ii) target plant (Brassica sp.); Dry mass of (iii) Zea mays and (iv) Brassica sp. Treatments as follows: a control, b free 2,4-D, c rBC nanoparticles, d DrBC nanoformulation
But in the case of Zea mays treated with DrBC nanoformulation, no notable effect was noticed in its growth or dry weight (Fig. 6). On the other hand, the plants exposed to unloaded rBC showed slightly higher dry weight than the plants grown without herbicide treatment. This exhibits the additional effect of using rBC nanoparticles as herbicide carrier. Apart from acting as an herbicide carrier, the rBC nanoparticles could also aid in stimulating the plant growth.
Leaching behavior of DrBC nanoformulation
The ability of DrBC nanoformulation to reduce the leaching of the herbicide was analyzed with a soil column experiment. The soil taken for the investigation was applied with 1.0 wt(%) of DrBC nanoformulation and watered. The residual 2,4-D concentration quantified at various depths of the column is presented in Fig. 7. Highest leaching was observed in case of the column applied with 2,4-D technical formulation followed by the soil-treated DrBC nanoformulation. The topmost soil column was detected with increased 2,4-D presence in all the treatments, whereas in case of the DrBC nanoformulation-treated column, 2,4-D concentration was very less in the lower segments of the column. This result exposes the increased retention of the herbicide in soil when applied in the form of DrBC nanoformulation. The soil column experiment demonstrates the ability of the DrBC nanoformulation to reduce the leaching potential of 2,4-D to the surface and ground water as well increase the herbicide efficacy against target weeds by making it available on the topmost layer of soil for a prolonged time in comparison with the free 2,4-D technical formulation.
Fig. 7.
Quantification of 2,4-D in the soil column amended with different 2,4-D formulations from the top to base: 0–4 cm, 4–8 cm, 8–12 cm, and 12–16 cm
Conclusions
In conclusion, the rice husk biochar was found to be good carrier for effectual and eco-friendly usage of 2,4-D. The formulated DrBC nanoformulation could both act as an herbicide carrier as well enable to reduce the leaching accompanied with sustained release properties. The nanoformulation enabled high loading of herbicide and increased its retention in soil and, thus, enabled the DrBC amended soil to prolong its activity for a longer duration. These results suggest that further investigations are noteworthy as the formulation could be the possibility for reducing the environmental pollution caused by the herbicides due to extensive application at a higher rate as well for extending their bioefficacy.
Acknowledgements
The authors would like to thank the Hindustan University management for the support and the VIT University, Vellore for helping in instrumental analysis.
Compliance with ethical standards
Conflict of interest
The author would like to declare that there exists no conflict of interest.
References
- Abdulrazzaq H, Jol H, Husni A, Abu-Bakr R. Characterization and stabilisation of biochars obtained from empty fruit bunch, wood and rice husk. Bioresources. 2014;9(2):2888–2898. doi: 10.15376/biores.9.2.2888-2898. [DOI] [Google Scholar]
- Dotto GL, Cadaval TRS, Pinto LAA. Use of spirulina platensis micro and nanoparticles for the removal synthetic dyes from aqueous solutions by biosorption. Process Biochem. 2012;47:1335–1343. doi: 10.1016/j.procbio.2012.04.029. [DOI] [Google Scholar]
- Dubinin MM. The potential theory of adsorption of gases and vapors for adsorbents with energetically non uniform surface. Chem Rev. 1960;60:235–266. doi: 10.1021/cr60204a006. [DOI] [Google Scholar]
- Foo LPY, Tee CZ, Raimy NR, Hassell DG, Lee LY. Potential Malaysia agricultural waste materials for the biosorption of cadmium(II) from aqueous solution. Clean Tech Environ Policy. 2012;14:273–280. doi: 10.1007/s10098-011-0398-5. [DOI] [Google Scholar]
- Freundlich HMF. Over the adsorption in solution. J Phys Chem. 1906;57:385–470. [Google Scholar]
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–1403. doi: 10.1021/ja02242a004. [DOI] [Google Scholar]
- Lingamdinne LP, Roh H, Choi YL, Koduru JR, Yang JK, Chang YY. Influencing factors on sorption of TNT and RDX using rice husk biochar. Ind: J Ind Eng Chem; 2015. [Google Scholar]
- Ma L, Peng Y, Wu B, Lei D, Xu H. Pleurotus ostreatus nanoparticles as a new nano-biosorbent for removal of Mn (II) from aqueous solution. Chem Eng J. 2013;225:59–67. doi: 10.1016/j.cej.2013.03.044. [DOI] [Google Scholar]
- Mohan D, Sarswat A, Ok YS, Pittman CU. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent: a critical review. Bioresour Technol. 2014;160:191–202. doi: 10.1016/j.biortech.2014.01.120. [DOI] [PubMed] [Google Scholar]
- Pereira AES, Grillo R, Mello NFS, Rosa AH, Fraceto LF. Application of poly(epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J Hazard Mater. 2014;268:207–215. doi: 10.1016/j.jhazmat.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Qian K, Shi T, Tang T, Zhang S, Liu X, Cao Y. Preparation and characterization of nano-sized calcium carbonate as controlled release pesticide carrier for validamycin against Rhizoctonia solani. Microchim Acta. 2011;173:51–57. doi: 10.1007/s00604-010-0523-x. [DOI] [Google Scholar]
- Sivasamy A, Nethaji S, Nisha LLJL. Equilibrium, kinetic and thermodynamic studies on the biosorption of reactive acid dye on Enteromorpha flexuosa and Gracilaria corticata. Environ Sci Pollut Res. 2012;19:1687–1695. doi: 10.1007/s11356-011-0666-2. [DOI] [PubMed] [Google Scholar]
- Sun X, Shan R, Li X, Pan J, Liu X, Deng R, Song J. Characterization of 60 types of Chinese biomass waste and resultant biochars in terms of their candidacy for soil application. GCB Bioenerg. 2017;9:1423–1435. doi: 10.1111/gcbb.12435. [DOI] [Google Scholar]
- Tang L, Zhang S, Zeng GM, Zhang Y, Yang GD, Chen J, Wang JJ, Zhou YY, Deng YC. Rapid adsorption of 2,4-dichlorophenoxyacetic acid by iron oxide nanoparticles-doped carboxylic ordered mesoporous carbon. J Colloid Interf Sci. 2015;445:1–8. doi: 10.1016/j.jcis.2014.12.074. [DOI] [PubMed] [Google Scholar]
- Temkin MJ, Pyzhev V. Recent modifications to Langmuir isotherms. Acta Phys Chim Sin. 1940;12:217. [Google Scholar]
- Vaccari FP, Baronti S, Lugato E, Genesio L, Castaldi S, Fornasier F, Miglietta F. Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur J Agron. 2011;34:231–238. doi: 10.1016/j.eja.2011.01.006. [DOI] [Google Scholar]
- Xu X, Cao X, Zhao L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere. 2013;92:955–961. doi: 10.1016/j.chemosphere.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Yan Y, Hou H, Ren T, Xu Y, Wang Q, Xu W. Utilization of environmental waste cyanobacteria as a pesticide carrier: studies on controlled release and photostability of avermectin. Colloid Surf B. 2013;102:341–347. doi: 10.1016/j.colsurfb.2012.07.043. [DOI] [PubMed] [Google Scholar]