Abstract
Cactus pear fruit consists of the peel, seeds and pulp. The peel is a major waste in cactus pear pulp based products accounting about 37.72% of the fruit weight. The aim of this study was to utilized and characterized the physicochemical and rheological properties of biscuits substituted with extracted cactus pear peel (CPP) and alcohol-insoluble solids (AIS) from cactus pear (Opuntia ficus-indica). To prepare AIS, peels were shredded and dropped in ethanol (70%) for 15 min. The mixture was boiled at 70 °C for 30 min, filtered and washed with ethanol 70% and the washing repeated until no sugars. The residue was washed and dried at room temperature. Changes in physiochemical and rheological properties of extracted CPP and AIS from cactus pear to qualify determine their use in the production of them to produce a fiber-rich food product. The water-holding capacity was 3.7 ml/g for the peel and 1.5 ml/g for the AIS, and the oil-holding capacity was approximately the same for both the CPP and AIS. The protein content was 3.5% for the CPP and 3.72% for the AIS. The CPP and AIS contained little fat (1.22% and 1.44%, respectively). Potassium and calcium in the AIS had the highest concentration, at 21.49 g/kg and 44.04 g/kg, respectively, and these minerals were found at 22.07 g/kg and 16.66 g/kg, respectively, in the CPP. The dominant phenolic compounds found in the CPP were pyrogallol, catechol, catechin, and alpha-coumaric acid. The results showed that the AIS contained pyrogallol (61.67 ppm), benzoic acid (10.68 ppm), vanillic acid (7.66 ppm), catechin (4.65 ppm) and salicylic acid (4.51 ppm). The CPP was rich in glucose (25.95%) and fructose (21.36%) compared to the AIS. The sensory evaluation indicated that 7.5% dried cactus pear peel or 7.5% AIS can be successfully used in substitution of wheat flour biscuits. It could be conducted cactus pear as major by-product can be important for the industrial utilization.
Keywords: Cactus pear, Peel, Alcohol-insoluble solids, Physicochemical, Biscuits
Introduction
Cactus pear fruit (Opuntia ficus-indica) is of great interest due to its nutritional and antioxidant properties, mainly due to the presence of vitamins, fibers, minerals and polyphenols (El-Said et al. 2010). The total world crop of cactus pear was estimated to be 200,000 tons of fruit (Lahsasni et al. 2004). The use of agro-industrial by-products with nutritional value to produce new food additives or supplements is growing. Peels from fruit account for 37.72% of fruit weight. Cactus pear peel is composed of 80.17% moisture, 1.6% ash, 0.9% protein, 9.6% total sugars, 2.85% sucrose, 8.5% reducing sugars, 14.6% alcohol-insoluble solids (AIS), 1.48% mannitol, 0.71% sorbitol, 0.05% arabinose and 2.23% galacturonic acid (El-Said et al. 2010).
Today’s consumers prefer healthy foods and a diet that is low in calories, cholesterol and fat but rich in fiber. Dietary fiber is resistant to digestive enzymes and includes cellulose, hemicellulose, pectin, lignin and gum. The benefits of consuming food rich in fiber content are well known, especially for preventing some illnesses (e.g., diabetes and gastrointestinal dis-orders). Many illnesses are associated with low dietary fiber intake, and fiber reduces glucose levels in the blood and has anti-hyperlipidemic and anti-hypercholesterolemia effects (Gebremariam et al. 2006). Fiber consumption is associated with reduced risk of coronary heart disease and certain types of cancer; the allowable amount of dietary fiber (DF) intake for adults should be 25–38 g. In addition, juices with fiber may enhance mouth feel and decrease blood sugar and plasma cholesterol levels (Thebaudin et al. 1997; Toma et al. 1979). Dietary fiber obtained from various sources has been used to replace wheat flour in the production of bakery products. Potato peel with high amounts of dietary fiber has been used in bread making (Toma et al. 1979). From a technological point of view the effect of apple pomace containing dietary fiber and polyphenols in cake making, dietary fiber may be used in the formulation of food products (e.g., bakery products, snacks, sauces, drinks, cereals, biscuits, dairy products, meat products), with benefits to texture modification and improvement of the stability of food during processing and storage (Thebaudin et al. 1997).
In the last five decades, there has been great interest in providing new sources of dietary fiber, but agronomic by-products are still undervalued. Dietary fibers from different sources have been used to replace wheat flour in the preparation of bakery products. (Pomeranz et al. 1977) used cellulose, wheat bran and oat bran in bread making. Potato peel, a by-product from potato industry, rich in dietary fiber, was used as a source of dietary fiber in bread making (Toma et al., 1979). Recently, Vergara-Valencia et al. (2007) tested fiber concentrate from mango fruit as a bakery product ingredient. Anil (2007), used hazelnut as a source of dietary fiber in bread making. Sudha et al. (2007) studied the influence of fiber from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Sudha et al. (2007) studied the effect of apple pomace by-product, as a source of dietary fiber and polyphenols, on cake making.
Therefore, the study extracted and evaluated the physiochemical properties of extracted peel and AIS (alcohol-insoluble solids) from cactus pear and determined their use in the production of biscuits.
Materials and methods
Materials
Raw material
Mature fresh cactus pear fruit (Opuntia ficus-indica) with yellow skin free from defects was harvested from a local farm located in Al-Behayrah Governorate, Egypt during the summer season (June 2015) and was transported to the laboratory.
Methods
Peel samples
Fruits were cleaned with a brush to remove glochids and then washed with water to remove any dirty particles. Cactus peels were separated by hand peeling using a sharp knife. Cactus pear peels were collected and divided into two portions; the first part was stored directly at − 18 °C until use. The second part was cut into slices, placed in a hot-air oven and dried at 45 °C until constant weight was achieved. The dried peel pieces were ground using a domestic coffee grinder and then sieved.
Separation of alcohol-insoluble solids (AIS)
According to the method of Thomas et al. (2000), AIS was prepared by homogenizing the peel sample in boiling alcohol (70%, v/w) using a Waring blender for 1 min at high speed. The peel to alcohol ratio was 1:30 (w/v). The suspension was boiled at 70 °C for 30 min and then filtered. The residue was washed with 95% ethanol, washed with acetone and dried at room temperature to remove acetone; samples were reweighed again to determine the percentage of AIS.
Processing of biscuits substituted with different level of cactus pear peel or cactus pear peel AIS powder
The straight dough method for biscuit production was carried out according to the method described by Nnam and Nwokocha (2003). Cactus pear peel and AIS powder at levels of 7.5% (on wheat flour 72% extraction) were used as substitutes. Biscuits were produced from each of the six ingredients: 300 g of composite flour, 100 g of margarine, 100 g of sugar, 35 ml of water, 6 g of double acting baking powder and 120 g of eggs. All the dry ingredients were blended together by stirring 12 strokes. Fat was rubbed into the flour mixture until the consistency of biscuits crumbs was achieved. The egg was whisked for 3 min and folded into the flour mixture. Water was added to the mixture and stir to get a homogenous dough. The biscuits were baked on an aluminum baking pan slightly greased with margarine in a gas oven at 200 °C for 15 min. The samples were removed from oven and cooled at room temperature on a rack before the sensory evaluation.
Proximate chemical analysis
Moisture was determined as the weight loss after air-oven drying at 105 °C to a constant weight A.O.A.C (2005). The pH was measured using a p107 Consort pH meter (Belgium) with temperature compensation at 20 °C according to Mertens (2005). The ash content was determined as the weight loss after overnight incineration at 600 °C (Mertens 2005). The crude protein was estimated by multiplying the total nitrogen value by a factor of 6.25 (Mertens 2005). The curd fat was determined using a Soxhlet apparatus with petroleum ether (40–60 °C) as a solvent for 16 h (Mertens 2005). Total carbohydrate content of fiber-rich fractions was estimated by the formula = 100 − (crude fat + crude protein + ash) (Mertens 2005). Mineral content was determined according to Mertens (2005) using a Perkin Elmer 2380 atomic absorption spectrometer (AAS) in the central laboratory of the Faculty of Agriculture, Zagazig. Phenolic compounds were determined by HPLC according to the method of Goupy et al. (1999) as follows: 5 g of sample was mixed with methanol and centrifuged at 10,000 rpm for 10 min, and the supernatant was filtered through a 0.2-µm Millipore membrane filter. Then, 1–3 ml was collected in a vial for injection into HPLC (Agilent series 1100) equipped with an auto-sampling injector, solvent degasser, and ultraviolet (UV) detector set at 280 nm and quaternary HP pump (series 1100). The column temperature was maintained at 35 °C. Gradient separation was performed with methanol and acetonitrile as a mobile phase at a flow rate of 1 ml/min. Phenolic acid standards from Sigma-Aldrich were dissolved in a mobile phase and injected into the HPLC. The retention time and peak area were used to calculate phenolic compound concentration using the data analysis software from Hewlett Packard.
The sugar composition of dried cactus pear peel or AIS powder was determined by HPLC according to, as follows: 1 g of sample was mixed with deionized water and centrifuged at 10,000 rpm for 10 min, and the supernatant was filtered through a 0.2-µm Millipore membrane filter. Then, 3 ml was collected in a vial for injection into HPLC (Agilent series 1100) equipped with an auto-sampling injector, solvent degasser and an IR-detector using an Aminex carbohydrate HPX-87 °C column (300 mm 7.8 mm). The column temperature was maintained at 85 °C. Gradient separation was performed with deionized water and an acetonitrile flow rate of 1 ml/min. Sugar standards from Sigma were dissolved in deionized water and injected into the HPLC. Retention time and peak area were used to calculate sugar compound concentration via data analysis in Hewlett Packard software. This assay was conducted at the Food Technology Research Institute of Cairo, Egypt.
Proximate physical analysis
Bulk density was determined according to the methods described in Parrott and Thrall (1978). Measurement utilized a calibrated graduated syringe (open end packed with cotton). The syringe was filled with 1 g of cactus pear peel and AIS powder. Pressure was applied manually until no further compression occurred. When the volume did not reduce further, the volume measurement was precisely recorded, and the result was expressed as grams per cubic centimeter.
The swelling property was measured using the bed volume technique (Kuniak and Marchessault 1972). Insoluble cactus pear peel and AIS powder (1 g) were hydrated in distilled water (10 ml) in a calibrated cylinder (1.5 cm diameter) at room temperature. After equilibration (24 h), the bed volume was recorded, and the swelling property was expressed as milliliters of swollen sample per gram of initial dry sample.
The water-holding capacity (WHC) of dried cactus pear peel and AIS powder was measured as described by (Borroto et al. 1995). One gram of dried cactus pear peel and AIS powder was soaked in 50 ml of distilled water for 1 h and centrifuged at 4500 rpm for 30 min. The supernatant was carefully decanted into a graduated cylinder, and the volume of excess water was recorded. WHC was expressed as milliliters of water per gram of sample.
Oil-holding capacity was measured according to Borroto et al. (1995). The method was essentially similar to the WHC method described previously, except a commercial corn oil was used (at a ratio of 50:1 v/m) instead of distilled water, and the difference in weight between the pellet with oil and sample was calculated and recorded as milliliters of oil per gram of sample.
The colour index values of cactus pear peel and AIS were measured using a Hunter Lab L optical sensor D25 (Reston, VA, USA) according to Askar and Treptow (2013). The colour parameter L* indicates the degree of lightness, a* indicates the degree of redness to greenness, and b* indicates the degree of yellowness to blueness (Hunter 1958). The chroma (C), hue angle (hab) and total colour difference (∆E) were calculated as C = (a*2 + b*2)0.5, hab= tan−1 (b*/a*) and ∆E = [(L L0)2 + (a a0)2 + (b b0)2]0.5, respectively, where L0, a0 and b0 were the L*, a* and b* values of the reference sample (Shih et al. 2003).
Mixolab test
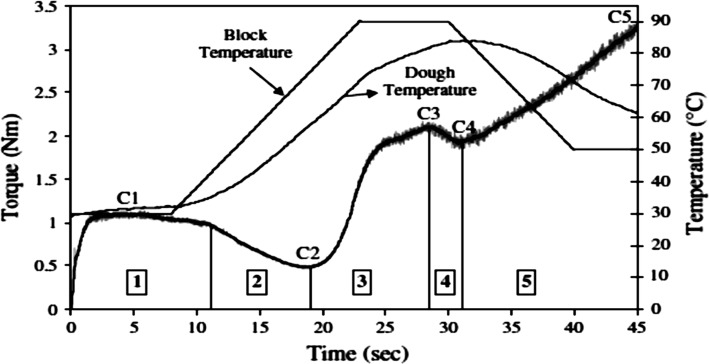
Dough rheological investigation were performed by mixolab (Chopin, Tripette et Renaud, Paris, France) according to methods of Hadnađev et al. (2011) and Shih et al. (2003) and Chopin + protocol with the slight modification in dough weight from 75 to 90 g. The running parameters of the Mixolab device during the tests are given in Table 1. Figure 1 shows the following parameters: water absorption (%) WA: the percentage of water required for the dough to produce a torque of 1.1 N m. Dough development time (min) DDT: the time to reach the maximum torque at 30 °C. Stability time until the loss of consistency is lower than 11% of the maximum consistency reacted during the mixing. Protein weakening: the torque difference between (Cl) and minimum consistency (C2) the minimum value of torque produced by dough passage while being subjected to mechanical and thermal constraints. Pasting ability (C3) the maximum torque produced during the heating stage. Minimum torque (C4) minimum torque reached during cooling to 50 °C. Breakdown torque calculated as the difference between (C3) and (C4). Final torque (C5) the torque after cooling at 50 °C. Setback torque: the difference between (C5) and (C4) torque. In addition, the angles between ascending and descending curves α, β and γ were calculated and defined as protein weakening rate, gelatinization rate and cooking stability rate, respectively.
Table 1.
Mixolab parameters used in Chopin + protocol (ICC 173, ICC Standards 2008)
| Settings | Values |
|---|---|
| Mixing speed | 80 rpm |
| Dough weight | 75 g |
| Tank temperature | 30 °C |
| Temperature 1st step | 30 °C |
| Duration 1st step | 8 min |
| 1st temperature gradient | 15 min—4 °C/min |
| Temperature 2nd step | 90 °C |
| Duration 2nd step | 7 min |
| 2nd temperature gradient | 10 min—4 °C/min |
| Temperature 3rd step | 50 °C |
| Duration 3rd step | 5 min |
| Total analysis time | 45 min |
Fig. 1.
Description of a typical curve obtained in the Mixolab
Sensory evaluation
Two samples of each treatment were served at random in porcelain dishes at room temperature to volunteer panels. A ten-member panel (five females and five males) from the professorial staff of the Food Science Department, Faculty of Agriculture, Zagazig University (Egypt) and ten-member panel (five females and five males) from the staff members of Hi-Food for Advanced Food Industries were performed the sensory evaluation. Panelists were asked to rate sensory attributes of the biscuits according to its appearance, color, taste, flavor and overall acceptability. The samples were evaluated using hedonic scale of 1–9 point, where (1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like nor dislike, 6 = like slightly, 7 = like moderately, 8, like very much, 9 = like extremely) as described by Hooda and Jood (2005).
Statistical analysis
Individual units were sampled in triplicate at 60 and 90 days of fermentation. All data were expressed as the mean SD. Statistical analyses were performed using one-way ANOVA followed by post hoc comparison of the mean (Duncan’s multiple range test) using SPSS 17. (p < 0.05) was considered statistically significant (Nie et al. 1970).
Result and discussion
Chemical composition of dried cactus pear peel and alcohol-insoluble solids (AIS)
The chemical composition of dried cactus pear peel and AIS is presented in Table 2. The moisture content of the dried peel and AIS was 11.2% and 10.03% respectively. The results were lower than those reported by El-Said et al. (2010) 18.5%.
Table 2.
Physicochemical characteristics of cactus pear peel and alcohol insoluble solids (AIS) prepared from cactus pear peel
| Physicochemical characteristics | Dried cactus pear peel (DCPP) | Cactus pear peel (AIS) |
|---|---|---|
| Moisture (%) | 11.2 ± 0.40 | 10.03 ± 0.14 |
| Ash (%) | 9.9 ± 0.30 | 9.05 ± 0.10 |
| Protein (%) | 3.5 ± 0.32 | 3.72 ± 0.20 |
| Fat (%) | 1.22 ± 0.18 | 1.44 ± 0.20 |
| pH | 5.7 ± 0.16 | 5.85 ± 0.10 |
| Total carbohydrate (%) | 85.38 ± 0.80 | 85.79 ± 0.50 |
| Minerals (g/kg) | ||
| Ca | 16.66 ± 0.12 | 44.04 ± 0.20 |
| K | 22.07 ± 0.22 | 21.49 ± 1.020 |
| Mg | 3.71 ± 0.18 | 5.69 ± 1.62 |
| Zn | 0.01 ± 0.02 | 0.02 ± 0.08 |
| Fe | 0.03 ± 0.01 | 0.06 ± 0.01 |
| Na | 0.94 ± 0.12 | 1.31 ± 0.18 |
| Se | 0.02 ± 0.08 | 0.02 ± 0.08 |
| L* | 60.02 ± 0.80 | 71.02 ± 1.02 |
| Colour value | ||
| a* | 3.75 ± 0.30 | 1.69 ± 0.71 |
| b* | 27.58 ± 0.27 | 20.22 ± 1.8 |
| c* | 27.90 ± 0.18 | 20.30 ± 1.4 |
| h* | 82.30 ± 1.2 | 85.20 ± 10 |
| ΔE | 83.70 ± 10 | 77.70 ± 0.80 |
| Bulk density (g/ml) | 1.14 ± 0.1 | 0.543 ± 0.11 |
| Swelling capacity (ml/g) | 3.50 ± 0.20 | 5.20 ± 0.18 |
| Water holding capacity (ml/g) | 3.70 ± 0.22 | 5.60 ± 0.24 |
| Oil holding capacity (ml/g) | 2.49 ± 0.14 | 2.95 ± 0.10 |
*The results are presented as the mean value ± SE. Values expressed with different treatments are significantly different at P < 0.05
Table 2 showed that the ash content of dried cactus pear peel and AIS was found to be 9.9% and 9.05%, respectively. Habibi et al. (2004) reported ash content in the cactus pear peel with a ranged from 11.5 to 12.1%. On the other hand, Namir et al. (2017) reported higher ash content (19.12%) in cactus pear peel snacks prepared by instant pressure drop texturing.
Table 2, the protein content of dried cactus pear peel was found to be (3.50%) and AIS (3.72%), this content was lower than that reported by El Kossori et al. (1998), who found that the protein content in cactus pear peel reached 8.3%. In addition, Namir et al. (2017) reported a amount of protein (5.71%) in cactus pear peel, while, Salim et al. (2009) presented lower protein content in cactus pear peel (1.45%).
The fat content of dried cactus pear peel was 1.22% and was slightly higher in AIS (1.44%). This may be due to the removal of the alcohol-insoluble component, which led to a concomitant increase in the chemical constituents of the AIS. The results from this study were lower compared to 3.33 gm/100 gm (peel) reported by Namir et al. (2017) and 3.43% in cactus pear peel (El Kossori et al. 1998).
A similar pH (5.4–5.8) was reported by Cerezal and Duarte (2005) for cactus pear peel powder.
The total carbohydrate content of cactus pear peel was 85.38% lower than that of AIS, at 85.79%. The major minerals were potassium (K), calcium (Ca) and magnesium (Mg) in peels and AIS. The quantities of potassium and calcium in AIS were the highest at 21.49 g/kg and 44.04 g/kg, respectively. These minerals were present at 22.07 g/kg and 16.66 g/kg, respectively, in the peel. Compared to all detected minerals, calcium (Ca) was highest in AIS, and its concentration was 44.04 g/kg. This high concentration of this mineral caciumin the peel could be because this mineral plays a role as a structural material that is normally associated with pectin substances that contribute to maintaining structure and shape (Sáenz and Berger 2006). The result agreed with the concentrations of calcium in cactus pear peel reported by El Kossori et al. (1998).
Bulk density is an important characteristic, as it reflects the behavior of a material in dry mixes and the volume occupied during packaging., dried cactus pear peel bulk density was approximately two-fold higher than that of AIS prepared from cactus pear peel, in agreement with Chau and Huang (2003), who reported that AIS prepared from dehydrated orange peel AIS was 0.48 g/ml.
The swelling capacities of highest value was for AIS (5.2 ml/g). The dried cactus pear peel had a lower value (3.5 ml/g). Namir et al. (2017) presented a lower value (2.89 ml/g) in cactus pear peel snacks. Result from Table 2, showed that AIS prepared from cactus pear peel had a lower value of swelling (5.20 ml/g) than that AIS (14.6 ml/g) from orange peel reported by Chau and Huang (2003). On the other hand, Namir et al. (2015) showed swelling capacity (4.2 ml/g) in AIS dehydrated from tomato waste.
Water-holding capacity, Oil-holding capacity and AIS of dried cactus pear peel were 3.7 ml/g and 5.6 ml/g and 2.49 ml/g and 2.95 ml/g, respectively. The dried cactus pear peel had a higher WHC value by twofold than that reported by Namir et al. (2017) for cactus pear peel snacks. Compared with the AIS from orange peel, the OHC of the obtained AIS was two times lower than that was presented by Chau and Huang (2003).
Colour analysis of food is an important field that is strongly related to marketability and consumer acceptability, as it controls the first impression of any food product. Colour changes caused by dietary fiber may limit the food application of certain products. The cactus pear peel showed lightness (60.02). The AIS prepared from cactus pear peel had higher lightness (71.02) but lower redness (1.69) and yellowness (20.22). Chroma followed the same trends in L* a* b* values. Chroma C* and ∆E were higher in cactus pear peel than AIS (27.9, 83.7, 20.3 and 77.7, respectively), but H* was lower in cactus pear peel than AIS (82.3 and 85.2, respectively).
Phenolic compounds
Twenty-four phenolic acids phenols are very important plant constituents due to their scavenging ability for free radicals via their hydroxyl groups. Twenty-four phenolic acids were identified according to their retention time compared to those of reference samples as given in Table 3. The total phenolic compounds of cactus pear peel and AIS were 2243.84 and 107.02 ppm, respectively. Table 3 showed the major components of cactus pear peel and AIS, were pyrogallol, catechol and catechin in the peel (48.52%, 9% and 6.68%, respectively) and pyrogallol, benzoic acid and E-vanillic acid in the AIS (57.58%, 9.97% and 7.15%, respectively). The subdominant phenolic compounds of dried cactus pear peel and AIS were alpha-coumaric acid, p-OH-benzoic acid, o-vanillic acid, ferulic acid, chlorogenic acid, epicatechin and gallic acid in the peel (4.02%, 3.93%, 3.85%, 3.8%, 3.07%, 2.49% and 2.13%, respectively) and catechin, salicylic acid, chlorogenic acid, epicatechin, p-OH-benzoic acid, caffeine and vanillic acid in the AIS (4.34%, 4.21%, 2.84%, 2.14%, 2.02%, 1.33% and 1.07%), respectively. The lowest total phenolic compounds in cactus pear peel and AIS were 4.13% and 3.32%, respectively. Anwar and Sallam (2016) found higher concentrations of components in dried cactus pear peel, with pyrogallol, benzoic acid, ellagic acid, chlorogenic acid and protocatechuic acid present at 1088.952 ppm, 982.37 ppm, 413.26 ppm, 271.1 ppm and 176.02 ppm, respectively. The components present at lower concentration in dried cactus pear peel were catechol (78.67 ppm), ferulic acid (39.27 ppm), Catechin (38.27 ppm), vanillic acid (11.14 ppm) and 4-Aminobenzoic (8.20 ppm). Anwar and Sallam (2016) agreed for the concentration of caffeic acid (13.10 ppm) and cinnamic acid (3.73 ppm).
Table 3.
Phenolic compounds of DCPP and cactus pear peel AIS
| Phenolic compounds (ppm) | Dried cactus pear peel (DCPP) | Cactus pear peel (AIS) |
|---|---|---|
| Pyrogallol | 1088.95 ± 3.7 | 61.67 ± 2.26 |
| Catechol | 201.96 ± 2.17 | 1.1 ± 0.20 |
| Catechein | 149.99 ± 20 | 4.65 ± 1.5 |
| Alpha-coumaric | 90.4 ± 0.20 | 0.1 ± 0.12 |
| P-OH-benzoic | 88.32 ± 1.96 | 2.16 ± 1.88 |
| E-vanillic | 86.6 ± 1.85 | 7.66 ± 0.20 |
| Ferulic | 85.41 ± 1.96 | 0.09 ± 0.08 |
| Chlorogenic | 68.9 ± 1.8 | 3.04 ± 0.20 |
| Epicatechein | 56.06 ± 20 | 2.3 ± 0.80 |
| Gallic | 48 ± 0.20 | 1.13 ± 0.22 |
| Benzoic | 44.1 ± 0.20 | 10.68 ± 1.74 |
| Iso-ferulic | 40.35 ± 1.97 | 0.36 ± 0.20 |
| Ellagic | 38.73 ± 1.8 | 0.68 ± 0.20 |
| P-coumaric | 32.03 ± 1.94 | 0.94 ± 0.80 |
| Caffeine | 31.32 ± 0.20 | 1.43 ± 0.90 |
| Vanillic | 19.88 ± 1.92 | 1.14 ± 0.24 |
| Salycilic | 18.15 ± 1.20 | 4.51 ± 1.0 |
| 4-Amino-benzoic | 14.91 ± 1.55 | 0.52 ± 0.20 |
| Caffeic | 14.32 ± 0.20 | 0.34 ± 0.30 |
| 3,4,5-methoxy-cinnamic | 9.13 ± 1.10 | 1.01 ± 0.20 |
| Protocatchuic | 7.77 ± 0.46 | 1.01 ± 0.20 |
| Cinnamic | 3.14 ± 0.20 | 0.07 ± 0.04 |
| Reversetrol | 3.13 ± 0.40 | 0.15 ± 0.10 |
| Coumarin | 2.25 ± 0.18 | 0.28 ± 0.08 |
| Total | 2243.84 | 107.02 |
*The results are presented as the mean value ± SE. Values expressed with different treatments are significantly different at P < 0.05
Osorio-Esquivel et al. (2011) reported that the reported high phenolic compounds and total flavonoid content in Opuntia joconostle fruits presented in the pericarp which accounted 2.07 mg Gallic acid equivalent (GAE)/g fresh weight (FW) and 0.46 mg (+) catechin equivalent (CE)/g FW. Seven phenolics were identified in sour prickly pears and included protocatechuic acid, 4-hydroxybenzoic acid, caffeic acid, vanillic and syringic acids, rutin, and quercetin. The consumption of cactus and their fruits is very common in the Mexican population; thus, it is important to investigate their composition. The benefits related to xoconostle pericarp intake include seric glucose control in individuals with type 2 diabetes mellitus and the prevention of hyperglycaemia, high cholesterol and high triglyceride levels in healthy people.
Table 4 show the separation of a large number of compounds, among which fifteen sugar compounds were identified. The sugar compounds were identified according to their retention time with comparison to reference samples. Table 4 showed that the major sugar components were glucose (25.95%) and (4.466%) for cactus pear peel, and fructose (21.36%) and (5.119%) for AIS. In concordance with data obtained by others (Habibi et al. 2002; Sáenz et al. 1998), Egyptian cactus pear peel was characterized by a slightly higher glucose content (25.95%) followed by fructose content (21.36%). The dried cactus pear peel had a lower sugar compound content than cactus pear peel snacks (Namir et al. 2017) containing galactose (7.17 mg/g), mannose (1.44 mg/g) and xylose (3.05 mg/g).
Table 4.
Sugar compounds of DCPP and cactus pear peel AIS
| Sugar compounds (%) | Dried cactus pear peel (DCPP) | Cactus pear peel (AIS) |
|---|---|---|
| Glucose | 25.95 ± 2.90 | 4.466 ± 0.20 |
| Fructose | 21.36 ± 2.10 | 5.119 ± 1.20 |
| Xylose | 0.829 ± 0.20 | 0.552 ± 0.16 |
| Sucrose | 0.632 ± 0.13 | 0.127 ± 0.15 |
| Manitol | 0.54 ± 0.20 | 0.066 ± 0.08 |
| Lactose | 0.505 ± 0.19 | 0.305 ± 0.19 |
| Stachyose | 0.459 ± 0.82 | 0.299 ± 0.62 |
| Glucuronic | 0.39 ± 0.08 | 0.233 ± 0.014 |
| Maltose | 0.35 ± 0.10 | 0.26 ± 0.08 |
| Galacturonic | 0.238 ± 0.12 | 0.182 ± 0.09 |
| Rhaminose | 0.232 ± 0.13 | 0.069 ± 0.14 |
| Galactose | 0.176 ± 0. 18 | 0.073 ± 0.02 |
| Mannose | 0.175 ± 0.02 | 0.07 ± 0.02 |
| Sorbitol | 0.064 ± 0.12 | 0.013 ± 0.02 |
| Ribose | 0.055 ± 0.02 | 0.032 ± 0.16 |
| Total | 51.955 | 11.866 |
*The results are presented as the mean value ± SE. Values expressed with different treatments are significantly different at P < 0.05
Complex polysaccharides can influence the pleasant flavour and serve as thickening agents to form viscous colloids. The sub-dominant sugar compounds of dried cactus pear peel and AIS are shown in Table 4. In the peel, xylose, sucrose, mannitol, lactose, stachyose and glucuronic acid were found (0.829, 0.632, 0.54, 0.505, 0.459 and 0.39%, respectively), and in the AIS, xylose, lactose, stachyose, maltose, glucuronic acid and galacturonic acid were detected (0.552, 0.305, 0.299, 0.26, 0.233 and 0.182%, respectively).
These unique characteristics make cactus pear peel sweeter and very suitable as a natural food or natural food additive for many categories of foodstuffs. However, further studies are required to completely characterize the hydrocolloid fraction of cactus pear.
Mixolab measurements
Protein characteristics of formulated dough
During mixing hydration of the compounds and the stretching and alignment of the proteins occurs, which lead to the formation of a three-dimensional viscoelastic structure. Data shown in Table 5, revealed that wheat flour substituted with 7.5% dried cactus pear peel was characterized with low water absorption (WA) by 58%. Which represents the percentage of water required for the dough to produce a torque of 1.1 N m. Wheat flour dough (72%), that served as a control sample and wheat flour substituted with 7.5% cactus pear peel AIS were characterized with higher (WA) 59.6% and 63.9%, respectively. On the other hand, WA (64%) was the highest in the dough substituted with 7.5% AIS. Gluten, dietary fiber and protein play a very important rolls water absorption capacity, through water hydration capacity and effect on dough gluten network (Rosell et al. 2010). Wheat flour substituted with 7.5% dried cactus pear peel or 7.5% AIS were characterized with higher DDT (4.77 and 6.27 min, respectively) compared to wheat flour 72% (control); 1.22 min but (DDT) was the highest in case of substitution of 7.5% AIS as shown in Table 5.
Table 5.
Protein and starch properties of biscuit substituted with 7.5% dried cactus pear peel and cactus pear peel (AIS)
| Characteristics | Control | Dried cactus pear peel (DCPP) | Cactus pear peel (AIS) |
|---|---|---|---|
| Protein | |||
| WA (%) | 59.6 ± 2.20 | 58 ± 0.20 | 63.9 ± 2.4 |
| DDT (min) | 1.22 ± 0.20 | 4.77 ± 1.26 | 6.27 ± 1.86 |
| Stability (min) | 8.62 ± 1.16 | 8.53 ± 1.74 | 9.30 ± 0.20 |
| C2 (N m) | 0.503 ± 0.54 | 0.477 ± 0.20 | 0.581 ± 0.20 |
| Protein weakening (Nm) | − 0.631 ± 0.20 | − 0.648 ± 0.20 | − 0.529 ± 0.02 |
| α (N m/min) | − 0.096 ± 0.08 | − 0.096 ± 0.08 | − 0.090 ± 0.14 |
| Starch | |||
| Pasting ability (C3) (N m) | 1.722 ± 0.20 | 1.665 ± 0.07 | 1.718 ± 0.20 |
| Gelatinization rate (β) N m/min | 0.368 ± 0.02 | 0.344 ± 0.04 | 0.364 ± 0.02 |
| Minimum torque (C4) (N m) | 1.405 ± 0.20 | 1.452 ± 0.96 | 1.361 ± 0.78 |
| Breakdown torque (N m) | 0.317 ± 0.04 | 0.213 ± 0.17 | 0.357 ± 0.02 |
| Cooking stability (γ) N m/min | − 0.054 ± 0.02 | − 0.026 ± 0.02 | − 0.002 ± 0.02 |
| Final torque (C5) (N m) | 2.425 ± 0.15 | 2.081 ± 0.2 | 1.920 ± 0.16 |
| Setback (N m) | 1.020 ± 0.20 | 0.629 ± 0.04 | 0.559 ± 0.04 |
WA water absorption, DDT dough development time, C2 minimum consistency, α protein breakdown
*The results are presented as the mean value ± SE. Values expressed with different treatments are significantly different at P < 0.05
It could be observed that the mixing time to form developed dough depends on the structure of fiber and the phenolic compounds that can form complexes with proteins and/or polysaccharides. Such a complexation can occur reversibly via hydrogen bonding between hydroxyl groups of phenols and the carbonyl group of peptide residue of proteins. The resultant complexes can be further stabilized via other types of bonds such as the covalent bonds and ionic bonds between the phenolate anion and cationic site of protein molecules and/or covalent links formed as a result of reaction between the oxidized phenolic groups and –SH, –OH, and –NH2 group of protein (Loomis 1974). However, the bending mechanism leading to the formation of phenolic-protein complex is still not well known.
These properties of wheat flour are related to its unique protein composition and quality. Regarding dough stability (ST), Table 5 shows that (ST) value of wheat flour 72% (control) was 8.62 min, data shows that substitution of 7.5% AIS into white wheat flour increased this parameter to 9.30 min. The increase in dough stability could be due to the increased interactions through hydrogen bounding involving the hydroxyl groups present in fibers molecules. On contrast, substitution of 7.5% dried cactus pear peel into white wheat flour decreased the (ST) value to 8.53 min, as consequence of gluten networks disruption.
During over-mixing stage, the dual mechanical shear and temperature constraint cause protein weakening and torque decrease to the minimum value C2 (Rosell et al. 2010). The control wheat flour 72% showed C2 value of 0.503 N m. The substitution of 7.5% AIS into white wheat flour led to increase this parameter (C2) to 0.581 N m, while, substitution with 7.5% dried cactus pear peel decreased the (C2) to 0.477 N m. The higher values of (C2) indicate that these doughs were more tolerant to mixing as compared to the other lowest samples as reported by Dhaka et al. (2012).
Protein weakening range (C2-C1) value of wheat flour 72% (control) was − 0.631 N m. Substitution of 7.5% AIS into white wheat flour led to increase (C2-C1) value to − 0.529 N m, while, substitution of 7.5% dried cactus pear peel into white wheat flour decreased the protein weakening to − 0.648 N m. As illustrated heating of white flour, aggregation and denaturation of the proteins occurs (Rosell and Foegeding 2007), which result in a decrease in dough consistency (C2 value). The increase in Table 5 of protein weakening range (C2–C1) and a faster protein breakdown rate (α) as in dried cactus pear peel. Nevertheless, the fibrous materials in AIS showed different behavior, this could be due to the fibers itself or these fibers may protect the gluten network.
Starch characteristics of formulated dough
As heating proceeded, protein changes have minor influence and the starch granules have predominant role in torque increase (Rosell and Foegeding 2007). The increase in viscosity and thus in the torque is the result of the starch granules swelling due to the water uptake and amylose chains leaching into the aqueous inter-granular phase (Rosell et al. 2010). Wheat flour (72%) was recorded the highest C3 value was 1.722 N m. Substitution of 7.5% AIS slightly decreased the C3 value to 1.718 N m but substitution of 7.5% dried cactus pear peel decreased the C3 value to 1.665 N m.
Concerning the stability of the hot gel (C4 values, C3–C4 values and γ values), value stability of wheat flour 72% (control) was 1.405 N m as see in Table 5. Substitution of 7.5% dried cactus pear peel increased the stability value to 1.452 N m but substitution of 7.5% AIS decreased the stability value to 1.361 N m as given Table 5. The further reduction in viscosity (C4 value) is the result of the physical breakdown of the granules due to the mechanical shear stress and the temperature constraint (Rosell and Foegeding 2007). Breakdown torque (C3–C4) is also a measure of amylase activity. Namely, the greater the difference between C3 and C4 is, the greater the amylase activity is subsequently, on cooling, starch retrogrades and the consistency increases (C5 value) (Collar et al. 2007). These results could be due to the higher content of α-amylases in the AIS causing the decrease of the C4 and the increase of the γ values.
On cooling, starch retrogrades and the consistency increases (C5 value) and the cooling setback (C5–C4) indicates the retrogradation ability of the starch (Collar et al., 2007). According to the results summarized in Table 5, C5 value of wheat flour 72% (control) was 2.425 N m. Substitution of 7.5% dried cactus pear peel or 7.5% AIS decreased C5 value to 2.081 and 1.920 N m, respectively. This behavior could be explained by the higher lipid contents of tested materials compared to white flour and lipid-amylase complex forming ability. The high lipid content causes changes in the re-association and recrystallization of the amylose molecules. Likewise, the mixolab characteristic of C5–C4, represent the shelf life of the end product as reported by Capouchová et al. (2012).
However, lower value of mixolab characteristic (C5) which represented the rate of retrogradation, confirm the worst quality of the starch part in the wheat grain (Collar et al. 2007).
Sensory evaluation
The effect of dried cactus pear peel or cactus pear peel AIS substitution on sensory characteristics (appearance, color, taste, flavor and overall acceptability) of wheat flour biscuits are shown in Table 6. Biscuits substituted with 7.5% dried cactus pear peel or 7.5% AIS had the highest scores of appearance, color, taste, flavor and overall acceptability being 8.1, 8.02, 8.15, 8.17, and 8.1 and 8.1, 8.15, 8.1, 8.05 and 8.15, respectively. The effect of cactus pear peel or AIS fortification on sensory characteristics (appearance, colour, taste, flavor and overall acceptability) of wheat flour biscuits are shown in Table 6. The appearance, colour, taste, flavor and Overall acceptability of control sample and biscuits with 7.5% cactus pear peel or 7.5% AIS were probability level (P > 0.05) superior to cactus pear peel or AIS biscuits. Biscuits substituted with 7.5% AIS had the highest scores of appearance (8.1), colour (8.15), taste (8.15), flavor (8.1) and overall acceptability (8.1). Biscuits substituted with 10% cactus pear peel or 10% AIS were not acceptable. The results of sensory evaluation indicated that 7.5% cactus pear peel or 7.5% AIS can be successfully used in fortification of wheat flour biscuits. Decreasing of protein weakening index improving the protein network formation and stability (Rosell and Foegeding 2007). while, increasing of the dough consistency during baking influencing the texture of the end product (Collar et al. 2007). Biscuits substituted with 10% dried cactus pear peel or 10% AIS were not acceptable because of the high fiber content of 10% influencing the biscuit’s appearance, texture and taste compared to control and the other substitution. The results of sensory evaluation indicated that 7.5% dried cactus pear peel or 7.5% AIS can be successfully used in substitution of wheat flour biscuits.
Table 6.
Sensory evaluation of biscuit substitute with different level of DCPP or cactus pear peel AIS
| Samples | Sensory evaluation | ||||
|---|---|---|---|---|---|
| Appearance (9) | Color (9) | Taste (9) | Flavor (9) | Overall acceptability (9) | |
| Control | 8.15a | 8.1a | 8.12a | 8.1a | 8.15a |
| Dried cactus pear Peel | |||||
| 2.5% | 7.31b | 7.25b | 7.22b | 7.25b | 7.19bc |
| 5% | 7.5ab | 7.45ab | 7.5ab | 7.4ab | 7.18bc |
| 7.5% | 8.1a | 8.02a | 8.15a | 8.17a | 8.1a |
| 10% | 6.45cd | 6.5cd | 6.35d | 6.5cd | 6.15d |
| Cactus pear peel AIS | |||||
| 2.5% | 7.34b | 7.34b | 7.3b | 7.25b | 7.1bc |
| 5% | 7.55ab | 7.6ab | 7.4ab | 7.4ab | 7.24b |
| 7.5% | 8.1a | 8.15a | 8.1a | 8.05a | 8.15a |
| 10% | 6.45cd | 6.6cd | 6.4cd | 6.4cd | 6.35d |
*LSD least significant difference (probability level 0.05)
Conclusion
This investigation shows the potential value of cactus pear peel as a good natural source of energy, nutritive components, sugar compounds and antioxidants, such as phenolic compounds. Based on its increased sweetness and attractive stable colors, cactus pear peel could be very suitable as a natural additive or substituted material in the production of many foodstuffs. The information obtained in the present investigation is useful for characterizing cactus pear peel and for the industrial utilization of the major byproduct of the fruit.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anil M. Using of hazelnut testa as a source of dietary fiber in breadmaking. J Food Eng. 2007;80:61–67. doi: 10.1016/j.jfoodeng.2006.05.003. [DOI] [Google Scholar]
- Anwar M, Sallam E. Utilization of prickly pear peels to improve quality of pan bread. Arab J Nucl Sci Appl. 2016;49:151–163. [Google Scholar]
- Askar A, Treptow H. Quality assurance in tropical fruit processing. Berlin: Springer Science & Business Media; 2013. [Google Scholar]
- Borroto B, Larrauri J, Cribeiro A (1995) Particle size influence on water holding capacity of citrus and pineapple fiber. Alimentaria (Spanish)
- Capouchová I, et al. Effect of different intensities of Fusarium infestation on grain yield, deoxynivalenol content and baking quality of winter wheat. Rom Agric Res. 2012;29:297–306. [Google Scholar]
- Cerezal P, Duarte G. Use of skin in the elaboration of concentrated products of cactus pear (Opuntia ficus-indica (L.) Miller) J Prof Assoc Cactus Dev. 2005;7:61–83. [Google Scholar]
- Chau C-F, Huang Y-L. Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L. Cv. Liucheng J Agric Food Chem. 2003;51:2615–2618. doi: 10.1021/jf025919b. [DOI] [PubMed] [Google Scholar]
- Collar C, Bollain C, Rosell CM. Rheological behaviour of formulated bread doughs during mixing and heating. Food Sci Technol Int. 2007;13:99–107. doi: 10.1177/1082013207078341. [DOI] [Google Scholar]
- Dhaka V, Gulia N, Khatkar B. Application of mixolab to assess the bread making quality of wheat varieties. Sci Rep. 2012;1:183. [Google Scholar]
- El Kossori RL, Villaume C, El Boustani E, Sauvaire Y, Méjean L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.) Plant Foods Hum Nutr. 1998;52:263–270. doi: 10.1023/A:1008000232406. [DOI] [PubMed] [Google Scholar]
- El-Said NM, Nagib AI, Rahman ZA, Deraz SF. Prickly pear [Opuntia ficus-indica (L.) Mill] peels: chemical composition, nutritional value, and protective effects on liver and kidney functions and cholesterol in rats. Funct Plant Sci Biotechnol. 2010;5:30–35. [Google Scholar]
- Gebremariam T, Melaku S, Yami A. Effect of different levels of cactus (Opuntia ficus-indica) inclusion on feed intake, digestibility and body weight gain in tef (Eragrostis tef) straw-based feeding of sheep. Anim Feed Sci Technol. 2006;131:43–52. doi: 10.1016/j.anifeedsci.2006.02.003. [DOI] [Google Scholar]
- Goupy P, Hugues M, Boivin P, Amiot MJ. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agric. 1999;79:1625–1634. doi: 10.1002/(SICI)1097-0010(199909)79:12<1625::AID-JSFA411>3.0.CO;2-8. [DOI] [Google Scholar]
- Habibi Y, Mahrouz M, Vignon MR. Isolation and structure of D-xylans from pericarp seeds of Opuntia ficus-indica prickly pear fruits. Carbohydr Res. 2002;337:1593–1598. doi: 10.1016/S0008-6215(02)00186-6. [DOI] [PubMed] [Google Scholar]
- Habibi Y, Heyraud A, Mahrouz M, Vignon M. Structural features of pectic polysaccharides from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr Res. 2004;339:1119–1127. doi: 10.1016/j.carres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Hadnađev TD, Torbica A, Hadnađev M. Rheological properties of wheat flour substitutes/alternative crops assessed by Mixolab. Procedia Food Sci. 2011;1:328–334. doi: 10.1016/j.profoo.2011.09.051. [DOI] [Google Scholar]
- Hooda S, Jood S. Organoleptic and nutritional evaluation of wheat biscuits supplemented with untreated and treated fenugreek flour. Food Chem. 2005;90:427–435. doi: 10.1016/j.foodchem.2004.05.006. [DOI] [Google Scholar]
- Hunter RS. Description and measurement of white surfaces. JOSA. 1958;48:597–605. doi: 10.1364/JOSA.48.000597. [DOI] [Google Scholar]
- Kuniak L, Marchessault R. Study of the crosslinking reaction between epichlorohydrin and starch. Starch-Stärke. 1972;24:110–116. doi: 10.1002/star.19720240404. [DOI] [Google Scholar]
- Lahsasni S, Kouhila M, Vighon M, Mahrouz M. Impact of convective solar drying on the color and the composition out of free sugars of the cladode, the peel and the fruit of a cactus inerme of Opuntia ficus indica. Phys Chem News. 2004;18:89. [Google Scholar]
- Loomis WD. Methods in enzymology. New York: Academic Press; 1974. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles; pp. 528–544. [DOI] [PubMed] [Google Scholar]
- Mertens D (2005) AOAC official method 975.03. In: Horwitz W, Latimer GW (eds) Metal in plants and pet foods. Official Methods of Analysis, 18th ed, pp 3–4
- Namir M, Siliha H, Ramadan MF. Fiber pectin from tomato pomace: characteristics, functional properties and application in low-fat beef burger. J Food Meas Charact. 2015;9:305–312. doi: 10.1007/s11694-015-9236-5. [DOI] [Google Scholar]
- Namir M, Elzahar K, Ramadan MF, Allaf K. Cactus pear peel snacks prepared by instant pressure drop texturing: effect of process variables on bioactive compounds and functional properties. J Food Meas Charact. 2017;11:388–400. doi: 10.1007/s11694-016-9407-z. [DOI] [Google Scholar]
- Nie NH, Bent DH, Hull CH. SPSS: statistical package for the social sciences. New York: McGraw-Hill; 1970. [Google Scholar]
- Nnam NM, Nwokocha MO. Chemical and organoleptic evaluation of biscuits madefrom mixtures of hungry rice, acha (Digitaria exilis), sesame (Sesamum indicum) and breadfruit (Artocarpus atilis) flours. Plant Foods Hum Nutr. 2003;58:1–11. [Google Scholar]
- Osorio-Esquivel O, Álvarez VB, Dorantes-Álvarez L, Giusti MM. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res Int. 2011;44:2160–2168. doi: 10.1016/j.foodres.2011.02.011. [DOI] [Google Scholar]
- Parrott ME, Thrall BE. Functional properties of various fibers: physical properties. J Food Sci. 1978;43:759–763. doi: 10.1111/j.1365-2621.1978.tb02412.x. [DOI] [Google Scholar]
- Pomeranz Y, Shogren M, Finney K, Bechtel D (1977) Fiber in breadmaking—effects on functional properties. Cereal Chem
- Rosell CM, Foegeding A. Interaction of hydroxypropylmethylcellulose with gluten proteins: small deformation properties during thermal treatment. Food Hydrocoll. 2007;21:1092–1100. doi: 10.1016/j.foodhyd.2006.08.003. [DOI] [Google Scholar]
- Rosell CM, Santos E, Collar C. Physical characterization of fiber-enriched bread doughs by dual mixing and temperature constraint using the Mixolab®. Eur Food Res Technol. 2010;231:535–544. doi: 10.1007/s00217-010-1310-y. [DOI] [Google Scholar]
- Sáenz C, Berger H (2006) Utilización agroindustrial del nopal, vol 162. Food and Agriculture Organisation
- Sáenz C, Estévez A, Sepúlveda E, Mecklenburg P. Cactus pear fruit: a new source for a natural sweetener. Plant Foods Hum Nutr. 1998;52:141–149. doi: 10.1023/A:1008033704523. [DOI] [PubMed] [Google Scholar]
- Salim N, Abdelwaheb C, Rabah C, Ahcene B. Chemical composition of Opuntia ficus-indica (L.) fruit. Afr J Biotechnol. 2009;8(8):1623–1624. [Google Scholar]
- Shih L, Chen L-G, Yu T-S, Chang W-T, Wang S-L. Microbial reclamation of fish processing wastes for the production of fish sauce. Enzyme Microb Technol. 2003;33:154–162. doi: 10.1016/S0141-0229(03)00083-8. [DOI] [Google Scholar]
- Sudha M, Baskaran V, Leelavathi K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007;104:686–692. doi: 10.1016/j.foodchem.2006.12.016. [DOI] [Google Scholar]
- Thebaudin J, Lefebvre A, Harrington M, Bourgeois C. Dietary fibres: nutritional and technological interest. Trends Food Sci Technol. 1997;8:41–48. doi: 10.1016/S0924-2244(97)01007-8. [DOI] [Google Scholar]
- Thomas M, Crépeau M, Rumpunen K, Thibault J-F. Dietary fibre and cell-wall polysaccharides in the fruits of Japanese quince (Chaenomeles japonica) LWT-Food Sci Technol. 2000;33:124–131. doi: 10.1006/fstl.1999.0628. [DOI] [Google Scholar]
- Toma R, Orr P, D’appolonia B, Dlntzis F, Tabekhia M. Physical and chemical properties of potato peel as a source of dietary fiber in bread. J Food Sci. 1979;44:1403–1407. doi: 10.1111/j.1365-2621.1979.tb06448.x. [DOI] [Google Scholar]
- Vergara-Valencia N, Granados-Pérez E, Agama-Acevedo E, Tovar J, Ruales J, Bello-Pérez LA. Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT - Food Sci Technol. 2007;40:722–729. doi: 10.1016/j.lwt.2006.02.028. [DOI] [Google Scholar]