Abstract
The present study investigates the effect of Acacia seed water extract (ASWE) at four levels (0, 50, 100, 150 mg/100 mL) in triplicate batch on the shelf-life and quality of chicken patties. Flavones, mainly (+)-catechin, were the predominant phenolic compounds in ASWE with high antioxidant activity. ASWE showed greater inhibition effects against gram-positive bacteria than gram-negative bacteria. ASWE incorporation had no significant effects on the chemical composition of chicken patties. The microbial load, and thiobarbituric acid reactive substances of chicken patties significantly decreased (P ≤ 0.05) and reached minimum values at 150 mg/100 mL but the pH decreased slightly. The cooking properties were significantly improved (P ≤ 0.05) at 150 mg/100 mL. Moreover, ASWE at high level (150 mg/100 mL) significantly (P ≤ 0.05) enhanced total phenolic content and free radical scavenging activity of chicken patties. The results showed that chicken patties with ASWE had better quality attributes compared to the unformulated. Shelf-life of chicken patties can therefore be prolonged for 15 days in refrigerated storage using ASWE especially at high concentration (150 mg/100 mL).
Keywords: Acacia seed water extract, Quality attributes, Antioxidant, Antimicrobial, Chicken patties
Introduction
Chicken meat products contain lower lipid amounts and higher contents of polyunsaturated fatty acids (PUFA) and thus have greater desirable nutritional characteristics than red meat (Petracci et al. 2014). However, some important factors such as lipid oxidation, spoilage by microorganisms and enzymatic changes can affect the storage quality of chicken products. According to Tang et al. (2001), high PUFA content in chicken products make them more susceptible to pro-oxidants compared to other meat products. Furthermore, processing methods such as mincing and cooking, affect the integrity of muscle tissues, causing exposure of lipid layers to metallic ions, thereby increasing the rate of interaction of the pro-oxidant with unsaturated fatty acids, causing the formation of free radicals and initiation of oxidation reaction (Asghar et al. 1988).
Oxidation of meat or its products during storage may reduce its shelf life and economic value as it modifies fat and muscle proteins and causes adverse effects on the quality and sensory properties of such products. Artificial antioxidants (butylated hydroxyl anisole and butylated hydroxyl toluene) have been applied against oxidation in food products. Consumers and health workers have raised concerns about the use of artificial antioxidants that are considered unsafe for consumption (Tang et al. 2001). This has prompted a search for alternative natural antioxidants that can be used to preserve food products, instead of unsafe synthetic antioxidants.
Various plants, such as spices and herbs, possess antimicrobial and antioxidant properties, as they are rich in different kinds of phytochemicals like phenolic acids, flavonoids, tannins, and polyphenols (Devatkal et al. 2010). Several studies have reported the effects of plant antioxidant extracts on the quality of food products; such as tea catechins in patties prepared from chicken meat (Mitsumoto et al. 2005), Argel leaf extract in chicken meatballs (Al-Juhaimi et al. 2018b), and kinnow peel extracts in chicken products (Devatkal et al. 2011). Moreover, the search for novel antioxidants from natural sources, particularly from underutilized plants such as Acacia, is on the rise (Ali et al. 2012).
Acacia is a genus in the Leguminosae family, including around 1350 species (Maslin et al. 2003). It is mainly found in the warm and arid regions of the world. Acacia species contain various kinds of secondary metabolites such as cyanogenic glycosides, condensed tannins, terpenes, cyclitols, alkaloids, and gums (Maslin et al. 2003). El Abbouyi et al. (2004) have reported the antioxidant, anti-inflammatory, antibacterial, antispasmodic, and astringent properties of Acacia. Sadiq et al. (2015) reported that leaves, bark, and pods of Acacia nilotica contain galloylated catechins and gallocatechin derivatives and possess antimicrobial and antioxidant activities.
Despite the antioxidant and antimicrobial potential of Acacia seeds, limited information on their application in the preservation of food products in the industry but used traditionally to preserve some foods like fish and yogurt. Therefore, the current study was conducted to determine the phenolic compounds, TPC and antioxidant activities of Acacia seed water extract (ASWE) and examine the effect of ASWE on the physicochemical, microbiological and oxidative stability of chicken patties stored at refrigeration temperature (4 ± 1 °C).
Materials and methods
Materials
Acacia seeds were purchased from a farm located in Sudan. Frozen minced chicken meat prepared under aseptic conditions was used in the same day after thawing to ensure low temperature during processing, and was purchased from a local market in Riyadh city. Other ingredients including vinegar, black pepper, table salt, chickpea, garlic, onion, and white pepper powders were also purchased from a local market in Riyadh, Saudi Arabia. For analytical purposes, chemicals of standard grade were used.
Preparation of Acacia seed water extracts
The preparation of Acacia seed water extracts was done as described by Al-Juhaimi et al. (2018b). ASWE was prepared by mixing 1.5 kg of seed powder in approximately 400 mL distilled water, followed by stirring using a magnetic stirrer (Fisher, 14-511-1A, USA) for 3 h, autoclaved for 21 min at 121 °C, and then allowed to cool. The slurry was thereafter filtered using filter paper (Whatman No. 1). The filtrate was then freeze-dried and kept at − 20 °C for further analysis. The extract was added to the chicken patties at concentrations of 50, 100, and 150 mg/100 mL according to the levels of bioactive compounds and antioxidant activity of ASWE.
Determination of phenolic compounds in ASWE
The quantification of phenolic compounds in ASWE was carried out using a high performance liquid chromatography (HPLC) system (Shimadzu Corporation, Kyoto, Japan) equipped with a Photometric Diode Array (PDA) detector and an Inertsil ODS-3 column (5 mm × 4.6 mm × 3250 mm). The mobile phase consisted of 0.05% acetic acid in water (A) and acetonitrile (B) and the flow rate was set at 1 mL/min. The gradient profile was 0–0.10 min 8% B; 0.10–2 min 10% B; 2–27 min 30% B; 27–37 min 56% B; 37–37.10 min 8% B; 37.10–45 min 8% B and 20 mL acetic acid and the temperature was set at 30 °C. The wavelengths of the PDA detector were set at 280 and 330 nm which were used for peak detection and measurement, after a 1-h sample run.
Determination of flavonoid content of ASWE
A colorimetric measurement method described by Kim et al. (2003) was used to analyze TFC of ASWE, using catechin as a standard. Briefly, ASWE (1 mL) was added to 4 mL distilled water and then sodium nitrite (5% solution, 0.3 mL) and aluminum chloride (10% solution, 0.3 mL) were added and kept at room temperature for 5 min. Thereafter, the pink mixture was vortexed and the absorbance was determined at 510 nm following the addition of 1 M NaOH (2 mL) and distilled water (10 mL). A standard curve was prepared using catechin, and the results were determined as mg catechin equivalents per gram sample (mg CE/g).
Antimicrobial activity of ASWE
The disc diffusion method of Boyanova et al. (2005) was used to assess the antimicrobial activity of ASWE. Briefly, pure cultures of indicator microorganisms (Escherichia coli ATCC 10536, Salmonella typhimurium ATCC 14028, Yersinia enterocolitica ATCC 27729, Klebsiella pneumonia ATCC 10031, Bacillus cereus ATCC 14579 and Staphylococcus aureus ATCC 29737) were cultivated on nutrient agar plates. Then, ASWE saturated filter paper discs (10 μg/mL) were suitability positioned on the surface of the cultures and incubated at 37 °C for 24 h. After that, the inhibition zones around the discs were measured. Penicillin (10 μg/discs) was used as control standard antibiotic.
Preparation of raw and cooked chicken patties using ASWE
Formulations of chicken patties were carried out in triplicate (three batches) by mixing minced chicken (72%) with various ASWE concentrations (0, 50, 100, and 150 mg/100 mL) and other ingredients (vinegar 0.4%, chickpea powder 2%, salt 2%, white pepper 0.4%, black pepper 0.2%, garlic powder 1%, onion powder 2%, ddH2O 20, 15, 10 and 0% for unformulated and ASWE incorporated patties, respectively) as reported previously (Al-Juhaimi et al. 2018a) with slight modifications. Stephan mixer (Stephan U. Sohner UM 12 GmbH and Co., Germany) was used to homogeneously mix the ingredients and a patty was formed from approximately 100 g of each of the blended formulations using a patty making machine (Expro. Co., Shanghai, China). The chicken patties were stored in sealed perforated polyethylene (PE) bags of low density at 4 °C for 15 days. Stored chicken patties were analyzed every 5 days. To study cooking properties, chicken patties were cooked using conventional oven (Hobart Corp., Troy, Ohio, USA) at 180 °C until the temperature at the center of the patties reached 80 °C. The temperature at the center was monitored using a digital probe thermometer (Oakton, Eutech Instruments, China). The patties were turned every 10-min during cooking to ensure uniform cooking. Various quality attributes of the raw and cooked ASWE-formulated and unformulated patties were determined during storage (15 days). To determine TPC, TFC, and free radical scavenging activity, raw patties at different storage period were freeze-dried (12525, Virtis Company, Gardner, New York).
Proximate composition of chicken patties
The analysis for the chemical composition of raw and cooked patties was carried out according to the AOAC (2003) methods.
Microbiological load and pH of chicken patties
The method of Harrigan and McCance (1976) was applied to determine microbial characteristics (plate count) of raw patties at different periods of storage. The pH of the samples was determined with a pH meter probe (Corning Scientific Products, New York, USA).
Evaluation of cooking properties chicken patties
Cooking yield (CY) represent the percentage of the weight of cooked patty to the weight of raw (uncooked) patty. Fat retention (FR) and moisture retention (MR) represent, respectively, the amounts of fat and moisture retained in the cooked patties and expressed as percent. Dimensional shrinkage (DS) specifies the differences in diameters and thicknesses between cooked and raw patties and expressed as percent. The evaluation of cooking properties (CY, FR, MR, and DS) of the cooked patties was done by the method described by Murphy et al. (1975).
Chicken patties extract preparation
A sample of 2.5 g of freeze-dried patties was thoroughly mixed with 20 mL distilled water and stirred overnight at 4 °C using a magnetic stirrer (Fisher, 14-511-1A, USA). The slurry was centrifuged at 4500×g for 30 min (Hermle 66110068, Germany) and the resultant supernatant was used to determine TPC, TFC, and free radical scavenging activity.
Total phenolic content determination of ASWE and patties
Analysis of TPC of the ASWE and patties extracts was performed according to the method described by Singleton and Rossi (1965) using Folin Ciocalteu (FC) solution.
Free radical scavenging activity (FRSA) determination
FRSA evaluation was done by the method of Lee et al. (1998). A diluted solution (1 mL) of the extract in methanol was mixed with DPPH solution (2 mL). As a control an equal volume of methanol and DPPH was used. The wavelength of the spectrophotometer was 518. Percentage inhibition was calculated as follows:
where A0 and A1 are the absorbance of the control and sample extract, respectively.
Thiobarbituric acid reactive substances (TBARS) determination
The value of TBARS of the stored raw patties was evaluated by the method of Rosmini et al. (1996). A standard curve was prepared using a standard solution of 1,1,3,3-tetraethoxypropane. The TBARS values were expressed as mg malonaldehyde/kg sample.
Color indices measurement
The color parameters (L*, a* and b*) of raw patties stored at different intervals were measured using Hunter Lab colorimeter (Miniscan® XE plus 4500L; Hunter Associates Laboratory, Inc., Reston, VA) according to Al-Juhaimi et al. (2016). Chroma was calculated as follows:
Statistical analysis
All measurements were carried out in triplicate and three batches of patties were produced on three different days. The effect of ASWE on parameters was analyzed statistically using SAS software (v 8.1, SAS Institute Inc., Cary, NC). Data of the parameters measured from various treatments, storage periods, and their interaction were analyzed using general linear model (Two-way ANOVA) and statistical differences were estimated using Duncan’s multiple range tests. Mean separation was done using least significant difference and data are reported as the means (n = 3) ± standard deviation (SD). The significance level was accepted at P ≤ 0.05.
Results and discussion
Phenolic compounds, total phenolic and flavonoid contents, and antioxidant activity of ASWE
The phenolic compounds, TPC, TFC, and antioxidant activity of ASWE are analyzed (data not shown). The findings of the present study revealed that ASWE contained sixteen different phenolic compounds categorized into four groups; flavones (quercetin, (+)-catechin, naringenin, kaempferol and isorhamnetin), phenolic acids (gallic acid, 3,4-dihydroxybenzoic acid, syringic acid, caffeic acid, p-coumaric acid, trans-ferulic acid and trans-cinnamic acid), polyphenols (1,2-dihydroxybenzene and resveratrol), and glycosylated flavonoids (rutin trihydrate and apigenin-7-glucoside). Among these, flavones and phenolic acids were present in highest levels with total of approximately 57.53 and 30.02 mg/100 g, respectively. They were followed by glycosylated flavonoids (20.09 mg/100 g) and polyphenols (15.29 mg/100 g). Out of the four groups, phenolic compounds with highest values were (+)-catechin (32.65 mg/100 g) and gallic acid (20.51 mg/100 g), followed by quercetin (14.13 mg/100 g) and apigenin-7-glucoside (13.50 mg/100 g), whereas 3, 4-dihydroxybenzoic acid had the lowest the value (0.14 mg/100 g). The ASWE assessment revealed high TPC (268.75 mg GAE/100 g) and antioxidant activity (92.63%) confirming that ASWE is rich in phenolic constituents. These results were within the broad range of TPC in several medicinal plants (19.00–10,133.00 mg GAE/100 g) (Li et al. 2013) and a previous study (Hannachi et al. 2011) reported a relatively high TPC in methanolic extracts of Acacia seeds (154.47–632.40 mg GAE/100 g). On the other hand, ASWE presented low amount of TFC (2.78 mg/100 g). The variance in the findings between these studies could be due to differences in some factors such as growing location, harvesting time, growth stage, genetic makeup, handling and storage methods, extraction methods and solvents used. Overall, the present findings showed high levels of phenolic compounds in Acacia seeds and high antioxidant activity. These results suggest that Acacia seeds may be suitable for various food-related applications such as extending the stability of foods like meat and meat products. Previous studies on the phytochemical composition of the bark of A. nilotica and A. leucophloea extracts revealed the presence of protocatechuic-acid-4-glucoside, quercetin 3-rhamnoside, quercetin 3-glucuronide, and p-coumaric acid (Sulaiman et al. 2014). Besides the phytochemicals previously discovered in Acacia, the majority of the phenolic compounds identified in this study are well known to possess strong antioxidant and antimicrobial activities. Previous reports have shown that (+)-catechin, quercetin, gallic acid, and apigenin-7-glucoside, which are the most abundant phytochemical (phenolic compounds) in ASWE possess antioxidant and antimicrobial activities similar to the synthetic antimicrobial and antioxidant compounds generally used to preserve meat and meat products (Roidoung et al. 2016).
ASWE antimicrobial activity
Since the antimicrobial effect of phenolic agents varies depending on the microorganism targeted, this study assessed the effect of ASWE on both gram-negative bacteria (E. coli, S. typhimurium, Y. enterocolitica, and K. pneumonia) and gram-positive bacteria (B. cereus and S. aureus) and compared with that of penicillin. The results in Table 1 show that the gram-negative bacteria had the lowest inhibition zone values compared to gram-positive bacteria, with the exception of E. coli ATCC 10536 that had highest inhibition zone value (20.0 mm). However, ASWE had lower inhibition zone compared to penicillin on both gram-positive and gram-negative bacteria. Previous study (Shan et al. 2007) has reported that the susceptibility of gram-positive bacteria to antibacterial compounds is greater than that of gram-negative bacteria. This was attributed to the resistance of the outer membrane of gram-negative bacteria that has high amount of lipopolysaccharide molecules and thus produces a buffer against the entry of different antibiotic molecules. In addition, the outer membrane of gram-negative bacteria contains perivascular enzymes that can breakdown the molecules crossing into the cell. However, such an external membrane is not present in the cell wall structure of gram-positive bacteria. The cell walls and the cytoplasmic membrane of some bacteria can still be destroyed by some antibiotics and this causes the cytoplasm to be released (Shan et al. 2007). Previous studies have shown high antibacterial and antifungal activities of Acacia leaf and bark extracts, respectively (Mahesh and Satish 2008). Therefore, the high antibacterial activity of ASWE found in the present study indicates that it can be a useful ingredient to prolong the shelf-life of patties.
Table 1.
Antimicrobial activity of ASWE compared to penicillin
| Bacterial species | Strains | Inhibition zone (mm) | |
|---|---|---|---|
| ASWE (10 μg/uL) | Penicillin (10 μg) | ||
| Escherichia coli ATCC 10536 | Gram −ve | 20.0 ± 0.15b | 32.0 ± 0.09a |
| Salmonella typhimurium ATCC 14028 | Gram −ve | 10.0 ± 0.05b | 19.0 ± 0.13a |
| Yersinia enterocolitica ATCC 27729 | Gram −ve | 6.0 ± 0.02b | 12.0 ± 0.04a |
| Klebsiella pneumoniae ATCC 10031 | Gram −ve | 6.0 ± 0.05b | 16.0 ± 0.08a |
| Bacillus cereus ATCC 14579 | Gram +ve | 12.0 ± 0.07b | 18.0 ± 0.18a |
| Staphylococcus aureus ATCC 29737 | Gram +ve | 18.0 ± 0.10b | 24.0 ± 0.12a |
Values presented as mean ± SD of triplicate samples
Means not sharing a common superscript(s) a and b in a row are significantly different at P ≤ 0.05 as assessed by Duncan’s multiple range test
Chemical composition of chicken patties
The chemical composition of raw and cooked patties prepared using varying concentrations of ASWE is presented in Table 2. The tests revealed that moisture of raw patties decreased when ASWE concentration increased. The reduction in moisture content could be due to the increase in total solids contents following the addition of ASWE as reported by Hawashin et al. (2016) for beef patties incorporated with destoned olive cake powder. The decrease, however, was not significant. Similarly, reduction in moisture of beef patties prepared with oat flour as a result of rise in solid contents have been reported (Serdaroglu 2006). In addition, moisture of beef patties prepared with Bambara groundnut flour has been reported to decrease with the increase in flour quantity (Alakali et al. 2010). No significant differences were observed in protein, ash contents of unformulated raw patties compared to ASWE-formulated, except fat content was observed to be significantly higher in unformulated sample than the formulated ones. The results of this study were similar to those of Baldin et al. (2016), who reported no significant difference in chemical composition of chicken sausage formulated with Myrciaria cauliflora extract. In addition, Soltanizadeh and Ghiasi-Esfahani (2015) found a similar result in the chemical composition of beef patties formulated with aloe-vera. Cooking of both formulated and unformulated chicken patties significantly (P ≤ 0.05) decreased the moisture content, whereas the protein content significantly (P ≤ 0.05) increased compared to uncooked patties. This could be caused by the increase in dry matter content, which may be due to leaching of water soluble components of the patties during cooking. Similar findings were observed by Al-Juhaimi et al. (2017) for patties preserved using pistachio hull water extract. Also, the protein content of meatballs of chicken prepared with Argel leaf extract increased significantly (P ≤ 0.05) after cooking (Al-Juhaimi et al. 2018b). In addition, Al-Juhaimi et al. (2018b) reported an increase in protein content of chicken meatballs formulated with Argel leaf water extract.
Table 2.
Chemical compositions (%) of freeze-dried chicken meatballs formulated with different concentrations of ASWE
| ASWE (mg/100 mL) | Chemical composition | |||
|---|---|---|---|---|
| Moisture | Ash | Protein | Fat | |
| Raw | ||||
| 0 | 8.03 ± 0.27a | 7.26 ± 0.15a | 57.99 ± 0.86b | 24.77 ± 0.46a |
| 50 | 7.27 ± 0.02a | 7.36 ± 0.05a | 56.81 ± 0.37b | 21.14 ± 0.32b |
| 100 | 7.71 ± 0.01a | 7.4 ± 1.09a | 56.57 ± 0.71b | 22.08 ± 0.41b |
| 150 | 7.97 ± 0.01a | 7.4 ± 0.11a | 56.48 ± 0.44b | 21.94 ± 0.71b |
| Cooked | ||||
| 0 | 4.12 ± 0.16b | 7.26 ± 0.15a | 65.57 ± 0.66a | 21.78 ± 0.75b |
| 50 | 3.44 ± 0.06b | 7.36 ± 0.05a | 65.09 ± 0.43a | 21.37 ± 0.38b |
| 100 | 3.79 ± 0.01b | 7.40 ± 1.09a | 64.81 ± 0.83a | 22.08 ± 0.81b |
| 150 | 3.80 ± 0.06b | 7.40 ± 0.10a | 64.70 ± 0.52a | 22.78 ± 0.53b |
Values presented as mean ± SE of triplicate samples
Means not sharing a common superscript(s) a, b, c, or d in a column are significantly different at P ≤ 0.05 as assessed by Duncan’s multiple range test
Cooking properties of chicken patties formulated using ASWE
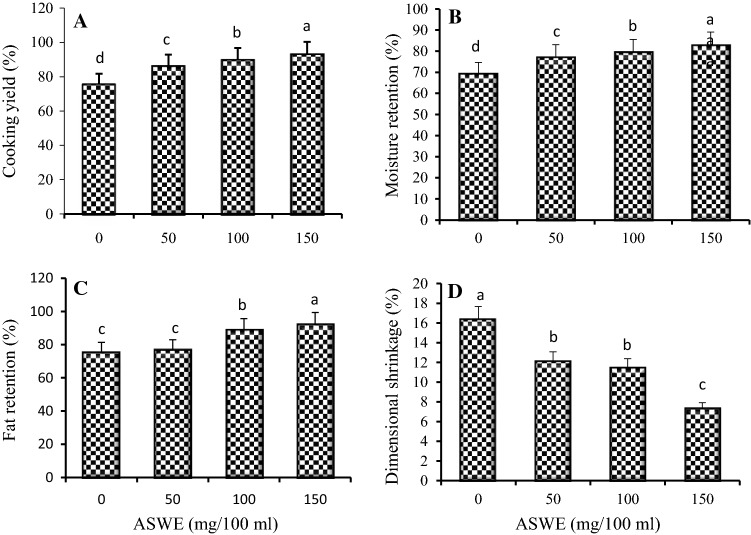
Figure 1A–D shows the cooking properties of fresh formulated and unformulated chicken patties. The cooking properties of the patties were significantly (P ≤ 0.05) influenced by the addition of ASWE. An increase in ASWE concentration increased CY, FR, and MR with maximum values obtained with a 150 mg/100 mL ASWE incorporation (Fig. 1A–C). The results obtained are similar to those observed in camel patties formulated with ginger extract and papain (Abdel-Naeem and Mohamed 2016) and chicken burger incorporated with pistachio seed hull extract (Al-Juhaimi et al. 2017). Better properties observed after cooking may be attributed to the ability of ASWE to retain moisture and fat. Quality attributes such as CY and structural binding of meat products are associated with water retention and fat binding in the protein matrix. Thus, the potential of ASWE to retain moisture in the patty matrix raised the CY of the patties. Higher MR and FR found in ASWE-formulated chicken patties could be attributed to moisture and fat absorption, as well as the swelling of starch and fiber, which results in their interaction with the protein matrix of minced meat. Consequently, it promotes fat and moisture retention. Serdaroglu (2006) highlighted that FR in the protein matrix ensures higher quality and sensory attributes of meat products resulting in increased consumer acceptability. An increase in ASWE concentration increased the capacity of the extracts to retain moisture; hence, the cooking properties were enhanced at 150 mg/100 mL ASWE concentration. However, significantly higher (P ≤ 0.05) DS value was observed in the unformulated patties than in those formulated with ASWE (Fig. 1D). It was also observed that the increase in the concentrations of ASWE enhanced the shape and size retention of chicken patties during cooking. Factors such as muscle protein denaturation, water evaporation, and loss of melted fat and juices can influence shrinkage of patties during the cooking process and thus affect textural quality of cooked patties (Alakali et al. 2010). The reduced shrinkage found in the patties prepared with ASWE could be related to the ASWE binding property. The binding ability of the extract helped the meat matrix to bind together, whereas the extract’s stabilizing property helped in retarding changes in moisture and juiciness. These two effects helped the patties to retain the shape (Muthukumar et al. 2014). This finding agrees with Hawashin et al. (2016) who observed an increase in DS as the concentration of destoned olive cake increase in formulated beef patties.
Fig. 1.
Cooking properties (A cooking yield; B moisture retention; C fat retention and D dimensional shrinkage) of chicken patties formulated with ASWE at different concentrations (0, 50, 100, and 150 mg/100 mL). Error bars indicate the standard deviation of three replicates. Means not sharing a common letter(s) a, b, c, or d are significantly different at P ≤ 0.05 as assessed by Duncan’s multiple range test
pH and microbial load of raw patties formulated with ASWE
The change in pH and microbiological load of raw patties stored for different periods of time are shown in Fig. 2. The values of pH for the unformulated and ASWE-formulated patties were not differed significantly at day 0 and 5 (Fig. 2A). Nonetheless, at day 10 and 15 of storage, the pH values in the unformulated sample decreased significantly (P ≤ 0.05) compared to the ASWE-prepared patties. This decrease in pH of the patties during storage could be due to the presence of acid producing bacteria, which may produce acidic substances responsible for lowering the pH (Wang et al. 2013). This is consistent with the observation that the total bacterial count of unformulated patties was significantly higher than the ASWE-formulated chicken patties (Fig. 2B). Similarly, Hawashin et al. (2016) reported lower pH in beef patties prepared using destoned olive cake compared to unformulated patties during cold storage. In addition, Al-Juhaimi et al. (2017) reported a reduction in pH values of chicken patties prepared using pistachio hull extract during cold storage. Interestingly, the incorporation of ASWE resulted in a significant (P ≤ 0.05) improvement of pH stability in the formulated patties which may be an indication that ASWE may play a protective role against spoilage causing microbes.
Fig. 2.
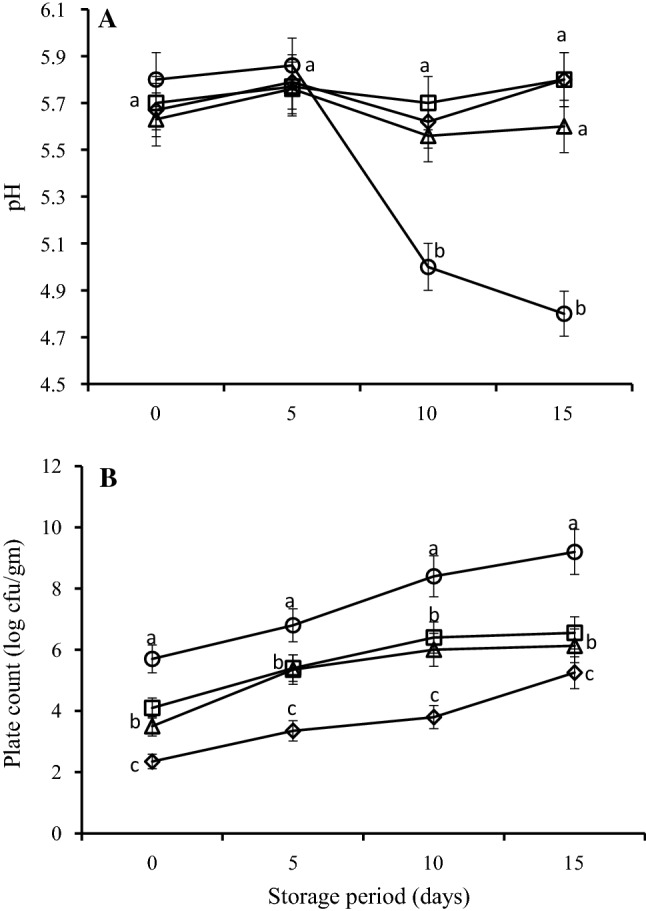
pH (A) and plate count (B) of raw chicken patties formulated with different concentrations (0, open circle; 50, open square; 100, open triangle and 150 mg/100 mL, open diamond) of Acacia seed water extracts during storage. Error bars indicate the standard deviation of three replicates. Means not sharing a common letter(s) a, b, c, or d are significantly different at P ≤ 0.05 as assessed by Duncan’s multiple range test
Figure 2B shows that at day 0, the unformulated patties had significantly (P ≤ 0.05) higher plate count than ASWE-formulated chicken patties. However, the plate count in both formulated and unformulated patties increased as storage period progressed. At the end of the 15-day, it was observed that the patties containing 150 mg/100 mL ASWE had significantly lower (P ≤ 0.05) plate counts compared to unformulated patties and better than those containing 50 and 100 mg/100 mL ASWE during day 5 and 10. This lower microbial count in chicken patties containing ASWE can be attributed to the antimicrobial properties of ASWE. The lower bacterial count together with pH stability in ASWE-formulated patties could decrease the rate of spoilage due to the protective role of ASWE against microbes. Furthermore, the lowest microbial load found in patties formulated with 150 mg/100 mL of ASWE can also be due to abundance of phenolic compounds in Acacia seed extract. Studies have reported that utilization of plant extract in the preparation of meat products results in prolonged stability and this has been attributed to the availability of natural antioxidants and phenolic compounds (with antimicrobial potential) in such extracts (Al-Juhaimi et al. 2017, 2018b; Hawashin et al. 2016).
TPC, FRSA, and TBARS of chicken patties prepared with ASWE
The TPC, FRSA, and TBARS results of formulated and unformulated chicken patties, as affected by cold storage, are shown in Table 3. An increase in ASWE concentration in chicken patties resulted in progressive increase (P ≤ 0.05) in the TPC and FSRA of the patties, with highest values (39.32 mg GAE/100 g sample and 62.82% inhibition, respectively) reported for chicken patties prepared with 150 mg/100 mL ASWE. An increase in TPC and FSRA after the addition of ASWE may be attributed to the significantly higher TPC (268.75 mg GAE/g sample) and FRSA (92.63% inhibition) of ASWE. Similarly, it has been reported that the addition of high concentration of plant extracts to meat products could substantially increase the TPC and FRSA of the products (Al-Juhaimi et al. 2017).
Table 3.
Oxidative characteristics of raw chicken patties formulated with different concentrations of ASWE during storage at 4 °C (± 1)
| Storage period (days) | ASWE (mg/100 mL) | |||
|---|---|---|---|---|
| 0 | 50 | 100 | 150 | |
| Total phenolic content (TPC) (mg GAE/100 g sample) | ||||
| 0 | 9.70 ± 0.61ap | 15.31 ± 1.83aq | 26.13 ± 0.26ar | 39.32 ± 2.45as |
| 5 | 7.55 ± 1.48bp | 13.95 ± 3.76bq | 24.75 ± 4.02br | 36.46 ± 2.62bs |
| 10 | ND | 11.79 ± 3.49cp | 22.17 ± 0.26cq | 35.12 ± 4.02cr |
| 15 | ND | 9.82 ± 0.44dp | 16.73 ± 0.45dq | 30.26 ± 0.88dr |
| Free radical scavenging activities (FRAS) (% inhibition) | ||||
| 0 | 36.17 ± 4.56ap | 57.71 ± 0.43aq | 58.32 ± 0.14ar | 62.82 ± 0.68as |
| 5 | 34.48 ± 4.13bp | 53.83 ± 0.87bq | 53.21 ± 0.29bq | 59.49 ± 2.14br |
| 10 | ND | 49.89 ± 0.74cp | 51.41 ± 0.27cq | 55.51 ± 0.14cr |
| 15 | ND | 48.76 ± 0.43dp | 50.56 ± 0.83cq | 53.37 ± 0.11dr |
| Thiobarbituric acid reactive substances (TBARS) in mg malonaldehyde/kg sample | ||||
| 0 | 3.02 ± 0.12ap | 2.86 ± 0.13bp | 2. 54 ± 0.23ap | 2.12 ± 0.41ap |
| 5 | 4.56 ± 0.34bp | 3.64 ± 0.22ap | 2.71 ± 0.52aq | 2.62 ± 0.45aq |
| 10 | ND | 3.71 ± 0.25ap | 2.78 ± 0.18aq | 2.72 ± 0.46aq |
| 15 | ND | 3.85 ± 0.005ap | 2.93 ± 0.01aq | 2.87 ± 0.35aq |
Values presented as a mean of triplicate samples (± SD)
ND not determined (spoil)
Means not sharing a common superscript(s) a, b, c, or d in a column or p, q, r, or s in a row are significantly different at P ≤ 0.05 as assessed by Duncan’s multiple range test
The TPC and FRSA of the formulated and unformulated chicken patties progressively reduced (P ≤ 0.05) with the storage time, and the minimum recorded value for the formulated patties was at day 15. However, further TPC and FRSA analysis was not conducted on the unformulated patties after day 5 of storage due to the high microbial load (> 1 × 107) observed in the patties and thus being unfit for human consumption. The decrease in TPC and FRSA during storage may be due to the hydrolysis and utilization of antioxidant compounds to prevent the product from undergoing oxidation. Similarly, previous studies have found reduction in the TPC and FRSA in pork (Muthukumar et al. 2014) and chicken (Al-Juhaimi et al. 2018a) during prolonged storage. High TPC and FRSA of ASWE-formulated patties for the entire duration of storage showed that addition of the extract prevented formation of free radicals, slowing down any undesirable reaction that may have negative impacts on the product during storage, thereby extending the stability of the patties.
Chicken patties formulated with ASWE had significantly (P ≤ 0.05) lower lipid peroxidation values (TBARS) than that of the unformulated patties. An increase in concentration of ASWE to 100 mg/100 mL in chicken patties progressively reduced the TBARS; the patties formulated with 150 mg ASWE exhibited the lowest TBARS value (2.12 mg malonaldehyde/kg sample). These findings indicated that ASWE confers lipid oxidation stability to formulated patties compared to unformulated ones. TBARS of the patties (0 and 50 mg ASWE/100 mL) increased with an increase in the storage period, indicating continuous formation of aldehydes in the products. Nonetheless, the lowest TBARS value (2.87 mg malonaldehyde/kg sample) was found in patties formulated with 150 mg/100 mL ASWE at the end of day 15. Incorporation of ASWE to patties lowered the rate of oxidation of lipids and could therefore prolong the stability of the product during refrigerated storage up to 15 days. Similarly, a decrease in the rate of oxidation of lipids after the addition of Argel leaf extract in chicken (Al-Juhaimi et al. 2018b) and destoned olive cake powder in beef patties (Hawashin et al. 2016) have been observed. Moreover, Al-Juhaimi et al. (2016) found that lipid oxidation in beef patties could be effectively controlled during cold storage by incorporating Moringa seed powder.
Color properties of raw chicken patties prepared with ASWE during storage
The color properties of formulated and unformulated chicken patties during storage are presented in Table 4. Lightness (L*), redness (a*), and yellowness (b*) values of formulated and unformulated patties varied depending on ASWE concentration and the storage period. As storage period progressed, a significant (P ≤ 0.05) reduction in the values of L* at the end of storage period but ASWE concentration had no effect. Previous studies of patties formulated with destoned olive cake powder (Hawashin et al. 2016), and Moringa seed flour (Al-Juhaimi et al. 2016) reported significant decrease in L*, and a* values and increase in b* value, which contradicts the present findings. This difference with the previous study could be attributed to direct incorporation of plant flours, as opposed to the aqueous extract used in this study, which may have a significant impact on the color of the patties. Moreover, the storage period had no effect on a* and b* values of formulated and unformulated patties but at higher concentration of ASWE (150 mg/100 mL), b* values were significantly (P ≤ 0.05) decreased compared to other levels of ASWE. Similarly, reduction in values of L* in meat patties formulated with different concentration of Moringa seeds has been previously reported (Al-Juhaimi et al. 2016). As storage time advanced, the b* values of the formulated and unformulated patties decreased but there was no significant difference, compared to day 0. However, previous studies report a decrease in b* values of beef patties formulated with different concentrations of defatted olive cake powder during cold storage, with unformulated patties showing the smallest values (Hawashin et al. 2016).
Table 4.
Color characteristics of raw chicken patties formulated with different concentrations of ASWE during storage at 4 °C (± 1)
| Storage period (days) | ASWE (mg/100 mL) | |||
|---|---|---|---|---|
| 0 | 50 | 100 | 150 | |
| Lightness (L*) | ||||
| 0 | 30.34 ± 0.54ap | 30.76 ± 0.35ap | 30.26 ± 0.26ap | 30.20 ± 0.31ap |
| 5 | 30.04 ± 0.35ap | 29.76 ± 0.20ap | 29.11 ± 0.005ap | 29.11 ± 0.05ap |
| 10 | ND | 29.83 ± 0.19ap | 28.61 ± 0.017bp | 28.25 ± 0.01bp |
| 15 | ND | 27.24 ± 0.05bp | 27.76 ± 0.16bp | 27.18 ± 0.06cp |
| Redness (a*) | ||||
| 0 | 0.07 ± 0.02ap | 0.07 ± 0.01ap | 0.13 ± 0.01ap | 0.35 ± 0.06aq |
| 5 | 0.06 ± 0.01ap | 0.06 ± 0.01ap | 0.13 ± 0.01ap | 0.32 ± 0.01aq |
| 10 | ND | 0.05 ± 0.01ap | 0.12 ± 0.01ap | 0.34 ± 0.01aq |
| 15 | ND | 0.05 ± 0.01ap | 0.13 ± 0.06ap | 0.30 ± 0.001aq |
| Yellowness (b*) | ||||
| 0 | 1.46 ± 1.61aq | 2.94 ± 0.43ap | 2.68 ± 0.21ap | 1.84 ± 0.10aq |
| 5 | 1.13 ± 0.01aq | 2.52 ± 0.01ap | 2.11 ± 0.01apq | 1.69 ± 0.06aq |
| 10 | ND | 2.68 ± 0.17ap | 2.24 ± 0.01ap | 1.71 ± 0.02aq |
| 15 | ND | 2.70 ± 0.005ap | 2.31 ± 0.01ap | 1.82 ± 0.01aq |
| Chroma (c*) | ||||
| 0 | 1.46 ± 0.11qa | 2.94 ± 0.43pa | 2.68 ± 0.35pa | 1.87 ± 0.32qa |
| 5 | 1.13 ± 0.02qa | 2.52 ± 0.23pa | 2.11 ± 0.67pa | 1.72 ± 0.45qa |
| 10 | ND | 2.68 ± 0.29pa | 2.24 ± 0.64pa | 1.74 ± 0.51qa |
| 15 | ND | 2.70 ± 0.18pa | 2.31 ± 0.78pa | 1.84 ± 0.28qa |
Values presented as a mean of triplicate samples (± SD)
ND not determined (spoil)
Means not sharing a common superscript(s) a, b, c, or d in a column or p, q, r, or s in a row are significantly different at P ≤ 0.05 as assessed by Duncan’s multiple range test
Chroma determines the quality of a color’s purity, intensity or saturation. Moreover, chroma is derived from a and b values correspond to the basic pigment of a color (yellow, red) and vividness of the color. The chroma (C*) value of the unformulated patties and those incorporated with ASWE was decreased slightly during the first 5 days and thereafter start to increase. Moreover, the C* value of the formulated patties reduced with increase in ASWE with minimum values obtained at a concentration level of 150 mg/100 mL throughout the storage periods. The reduction in C* could be due to incorporation of ASWE which had a gray color. The reduction in C* values indicated that the color’s purity, intensity or saturation start to decrease as reported by Pateiro et al. (2014) for pig liver pate formulated using chestnut, green tea and grape extracts during storage at 4 °C.
Conclusion
This study demonstrated that ASWE contains various amounts of phenolic compounds of which the majority were flavones, in addition to high TPC and FRAS. Incorporation of ASWE to chicken patties at higher concentrations (100 and 150 mg/100 mL) enhanced antioxidant activity, microbial stability, and prevented lipid oxidation without any adverse effect on the quality characteristics of the chicken patties. Hence, ASWE (≥ 100 mg/mL) could have a potential application as a functional ingredient as well as prolonging shelf-life of the patties.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research through the Research Group (RG-1435-049). “The authors also thank the Deanship of Scientific Research and the RSSU at King Saud University for their technical support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Naeem HHS, Mohamed HMH. Improving the physico-chemical and sensory characteristics of camel meat burger patties using ginger extract and papain. Meat Sci. 2016;118:52–60. doi: 10.1016/j.meatsci.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Alakali JS, Irtwange SV, Mzer MT. Quality evaluation of beef patties formulated with bambara groundnut (Vigna subterranean L.) seed flour. Meat Sci. 2010;85(2):215–223. doi: 10.1016/j.meatsci.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Ali A, Akhtar N, Khan BA, Khan MS, Rasul A, Khalid N, Waseem K, Mahmood T, Ali L. Acacia nilotica: a plant of multipurpose medicinal uses. J Med Plants Res. 2012;6(9):1492–1496. [Google Scholar]
- Al-Juhaimi F, Ghafoor K, Hawashin MD, Alsawmahi ON, Babiker EE. Effects of different levels of Moringa (Moringa oleifera) seed flour on quality attributes of beef pattiess. CyTA J Food. 2016;14(1):1–9. doi: 10.1080/19476337.2015.1034784. [DOI] [Google Scholar]
- Al-Juhaimi F, Adiamo OQ, Alsawmahi ON, Ghafoor K, Islam Sarker MZ, Mohamed Ahmed IA, Babiker EE. Effect of pistachio seed hull extracts on quality attributes of chicken patties. CyTA J Food. 2017;15(1):9–14. doi: 10.1080/19476337.2016.1193057. [DOI] [Google Scholar]
- Al-Juhaimi FY, Mohamed Ahmed IA, Adiamo OQ, Adisa AR, Ghafoor K, Özcan MM, Babiker EE. Effect of Argel (Solenostemma argel) leaf powder on the quality attributes of camel patties during cold storage. J Food Process Preserv. 2018 doi: 10.1007/s13197-018-3094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Juhaimi FY, Shahzad SA, Ahmed AS, Adiamo OQ, Mohamed Ahmed IA, Alsawmahi ON, Ghafoor K, Babiker EE. Effect of Argel (Solenostemma argel) leaf extract on quality attributes of chicken meatballs during cold storage. J Food Sci Technol. 2018;55:1797–1805. doi: 10.1007/s13197-018-3094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 14. Washington, DC: Association of Analytical Chemists; 2003. [Google Scholar]
- Asghar A, Gray JI, Buckley DJ, Pearson AM, Booren AM. Perspectives on warmed-over flavor. Food Technol. 1988;42:102–108. [Google Scholar]
- Baldin JC, Michelin EC, Polizer YJ, Rodrigues I, de Godoy SH, Fregonesi RP, Pires MA, Carvalho LT, Fávaro-Trindade CS, de Lima CG, Fernandes AM, Trindade MA. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016;118:15–21. doi: 10.1016/j.meatsci.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Boyanova L, Gergova G, Nikolov R, Derejian S, Lazarova E, Katsarov N, Mitov I, Krastev Z. Activity of Bulgarian propolis against 94 Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J Med Microbol. 2005;54:481–483. doi: 10.1099/jmm.0.45880-0. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010;85(1):155–159. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. The effect of salt, extract of kinnow and pomegranate fruit by-products on color and oxidative stability of raw chicken patties during refrigerated storage. J Food Sci Technol. 2011;48(4):472–477. doi: 10.1007/s13197-011-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Abbouyi A, Toumi M, El Hachimi Y, Jossang A. In vitro effects of aqueous seeds extract of Acacia cyanophylla on the opsonized zymosan-induced superoxide anions production by rat polymorphonuclear leukocytes. J Ethnopharmacol. 2004;91(1):159–165. doi: 10.1016/j.jep.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Hannachi H, Elfalleh W, Ennajeh I, Laajel M, Khouja ML, Ferchichi A, Nasri N. Chemicals profiling and antioxidants activities of Acacia seeds. J Med Plants Res. 2011;5(31):6869–6875. [Google Scholar]
- Harrigan WF, McCance ME. Laboratory methods in food dairy microbiology. London: Academic Press; 1976. pp. 753–850. [Google Scholar]
- Hawashin MD, Al-Juhaimi F, Ahmed IAM, Ghafoor K, Babiker EE. Physicochemical, microbiological and sensory evaluation of beef patties incorporated with destoned olive cake powder. Meat Sci. 2016;122:32–39. doi: 10.1016/j.meatsci.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. doi: 10.1016/S0308-8146(02)00423-5. [DOI] [Google Scholar]
- Lee SK, Mbwambo ZH, Chung H, Luyengi L, Gamez EJ, Mehta RG, Kinghorn AD, Pezzuto JM. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1998;1(1):35–46. [PubMed] [Google Scholar]
- Li S, Li SK, Gan RY, Song FL, Kuang L, Li HB. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind Crops Prod. 2013;51:289–298. doi: 10.1016/j.indcrop.2013.09.017. [DOI] [Google Scholar]
- Mahesh B, Satish S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World J Agric Sci. 2008;4(5):839–843. [Google Scholar]
- Maslin BR, Miller JT, Seigler DS. Overview of the generic status of Acacia (Leguminosae: Mimosoideae) Aust Syst Bot. 2003;16(1):1–18. doi: 10.1071/SB02008. [DOI] [Google Scholar]
- Mitsumoto M, O’Grady MN, Kerry JP, Buckley DJ. Addition of tea catechins and vitamin C on sensory evaluation, color and lipid stability during chilled storage in cooked or raw beef and chicken patties. Meat Sci. 2005;69(4):773–779. doi: 10.1016/j.meatsci.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Murphy EW, Criner PE, Gray BC. Comparisons of methods for calculating retentions of nutrients in cooked foods. J Agric Food Chem. 1975;23(6):1153–1157. doi: 10.1021/jf60202a021. [DOI] [PubMed] [Google Scholar]
- Muthukumar M, Naveena BM, Vaithiyanathan S, Sen AR, Sureshkumar K. Effect of incorporation of Moringa oleifera leaves extract on quality of ground pork patties. J Food Sci Technol. 2014;51(11):3172–3180. doi: 10.1007/s13197-012-0831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateiro M, Lorenzo JM, Amado IR, Franco D. Effect of addition of green tea, chestnut and grape extract on the shelf-life of pig liver pâté. Food Chem. 2014;147:386–394. doi: 10.1016/j.foodchem.2013.09.153. [DOI] [PubMed] [Google Scholar]
- Petracci M, Mudalal S, Babini E, Cavani C. Effect of white striping on chemical composition and nutritional value of chicken breast meat. Ital J Anim Sci. 2014;13(1):31–38. doi: 10.4081/ijas.2014.3138. [DOI] [Google Scholar]
- Roidoung S, Dolan KD, Siddiq M. Gallic acid as a protective antioxidant against anthocyanin degradation and color loss in Vitamin-C fortified cranberry juice. Food Chem. 2016;210:422–427. doi: 10.1016/j.foodchem.2016.04.133. [DOI] [PubMed] [Google Scholar]
- Rosmini MR, Perlo F, Pérez-Alvarez JA, Pagán-Moreno MJ, Gago-Gago A, López-Santoveña F, Aranda-Catalá V. TBA test by an extractive method applied to ‘paté’. Meat Sci. 1996;42(1):103–110. doi: 10.1016/0309-1740(95)00010-0. [DOI] [PubMed] [Google Scholar]
- Sadiq MB, Hanpithakpong W, Tarning J, Anal AK. Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind Crops Prod. 2015;77:873–882. doi: 10.1016/j.indcrop.2015.09.067. [DOI] [Google Scholar]
- Serdaroglu M. The characteristics of beef patties containing different levels of fat and oat flour. Int J Food Sci Technol. 2006;41(2):147–153. doi: 10.1111/j.1365-2621.2005.01041.x. [DOI] [Google Scholar]
- Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol. 2007;117(1):112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–158. [Google Scholar]
- Soltanizadeh N, Ghiasi-Esfahani H. Qualitative improvement of low meat beef patties using Aloe-vera. Meat Sci. 2015;99:75–80. doi: 10.1016/j.meatsci.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Sulaiman CT, Gopalakrishnan VK, Balachandran I. Phenolic compounds and antioxidant properties of selected Acacia species. J Biol Act Prod Nat. 2014;4(4):316–324. [Google Scholar]
- Tang S, Kerry JP, Sheehan D, Buckley JD. A comparative study of tea catechins and α-tocopherol as antioxidants in cooked beef and chicken meat. Eur Food Res Technol. 2001;213:286–289. doi: 10.1007/s002170100311. [DOI] [Google Scholar]
- Wang XH, Ren HY, Liu DY, Zhu WY, Wang W. Effects of inoculating Lactobacillus sakei starter cultures on the microbiological quality and nitrite depletion of Chinese fermented sausages. Food Control. 2013;32(2):591–596. doi: 10.1016/j.foodcont.2013.01.050. [DOI] [Google Scholar]