Abstract
In this study, the extraction yield, the mathematical modeling of pressurized liquid extraction (PLE) kinetics with sub- and supercritical carbon dioxide (SC-CO2) of olive leaves (Olea europaea) and the biological activity of the extracts were evaluated. The extraction with PLE was conducted isobarically (10.3 MPa), varying the temperature (20, 40 and 60 °C) and the solvent (ethyl acetate, acetone, ethanol, ethanol:water—80:20, v:v), solvent flow (2 mL min−1) and time (110 min) and the extractions with SC-CO2, varying the temperature between 20 and 60 °C and the pressure between 8 and 25 MPa, keeping the time constant (210 min) and the CO2 flow of 2 mL min−1. In the extracts, antioxidant activity, total phenolic and flavonoid contents and oleuropein were evaluated. The highest total extract yield in the PLE was 30.91% at 60 °C, 10.3 MPa using ethanol:water (80:20, v:v). The yield obtained using the supercritical fluid was 0.68% at 60 °C and 25 MPa. The PLE extract obtained with ethanol at 60 °C presented the highest concentration of total phenolic content (386.42 mg GAE g−1 extract), total flavonoids content (33.43 mg CAT g−1 extract), oleuropein (73.65 mg g−1 extract) and antioxidant activity (82.87%). The overall extraction curves were modeled using the well-established Sovová model and kinetic extraction model based on the Brunauer–Emmett–Teller theory of adsorption. Both kinetic models used were able to correlate well with the experimental data with slightly better results obtained by the former. The alternative PLE extraction technique investigated in this work was found to be suitable for the extraction of olive leaves after short times of extraction obtaining an extract with high biological activities.
Keywords: Pressurized liquid extraction, Sub- and supercritical CO2, Olive leaves, Antioxidant activity, Flavonoid, Sovová model, Pardo-Castaño model
Introduction
In the olive tree cultivation, the harvesting and pruning stages generate a considerable volume of biomass in the form of leaves and branches, considered as by-products or agro-industrial waste. In Spain, for example, about 6 million tons of biomass are generated per year. Most of these are destroyed immediately to avoid pests in the olive groves and eliminate additional difficulties in agricultural management (Guinda et al. 2015).
In addition to being a biomass-abundant plant, the olive leaf contains several phenolic compounds, secoiridoids and flavonoids, which have strong antioxidant activity, among other benefits reported in the literature (Rahmanian et al. 2015), such as antimicrobial, antiviral, anti-inflammatory and anti-carcinogenic activity, among others (Guinda et al. 2015).
The application of extraction techniques of bioactive compounds allows the addition of value to the raw material, reutilization of by-products, and generation of jobs throughout its productive cycle, making it a profitable and promising alternative to the academy, industry and farmers. In addition, increasing consumer concern on the use of synthetic substances in the industry has attracted a research interest in the field of biomaterial processing and pollution control for the development of clean technologies.
An integrated method of extraction of green olive leaves for concentrating oleuropein was developed using the combination of sub- and supercritical fluids and a pressurized liquid (Xynos et al. 2012). Obtaining bioactive compounds with pressurized liquid is an interesting alternative, as it allows extraction with higher temperatures and pressures, keeping the solvent always below its critical point and in the liquid state, reducing the viscosity and surface tension of the solvent and extraction time (Osorio-Tobón et al. 2014).
In this sense the extraction of olive leaves compounds has received special attention from researchers for the extraction of polar phenolic compounds using polar solvents such as ethanol, water and hydro-ethanolic mixtures, also being generally recognized as safe—GRAS. Besides these solvents, the use of acetone and ethyl acetate has also been reported, but with lesser interest compared to apolar compounds (Gil-Chávez et al. 2013).
All these propositions associated with the choice of an appropriate mathematical model capable of predicting the kinetic curves of extraction and the knowledge of the initial distribution of the solute inside the solid matrix is of fundamental importance in the optimization and the design of extractors in industrial scale, since they make it possible to adjust and simulate extraction processes in other scales and operating conditions (Abrahamsson et al. 2016).
In this context, the objective of this work was to conduct extraction experiments under different temperature and pressure conditions using pressurized liquid solvents and sub- and supercritical carbon dioxide of olive leaves (Olea europaea), evaluating the bioactive potential of the extracted/recovered compounds in addition to performing the mathematical modeling of the kinetic curves of the extractions, foreseeing the future use of an extraction processes in sequential and continuous mode with supercritical carbon dioxide (SC-CO2) and pressurized liquid extraction (PLE).
Materials and methods
Olive leaf samples
Olea europaea leaves (Negrinha de Freixo variety) were collected in March 2015 in the morning, in the Epagri experimental unit in Chapecó (SC Brazil, 27°05′4″S52°37′06″W). Adult leaves were collected from twelve individual, 8 to 12 year old trees. The collection took place randomly in various parts of plants, collecting small branches in the crown, lateral and inferior parts and were dried in an oven at 40 °C with forced air circulation (Solab model 102/1000) for 48 h, until constant weight. Subsequently, the leaves were grounded and sieved to obtain particles ranging from 1 to 3 mm and stored in vacuum sealed polyethylene bags at − 5 °C.
Experimental equipment and procedure
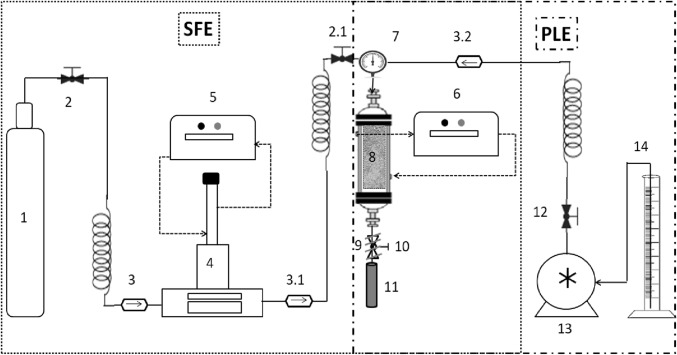
The experiments of high pressure extraction were conducted in the laboratory scale unit presented in Fig. 1 and reported in previous works (Dariva et al. 2003; Rodrigues et al. 2004; Freitas et al. 2008; Mazutti et al. 2008; Piva et al. 2018). This unit can be used to perform both SFE (with or without a cosolvent) and PLE. Both pressurized liquid extraction (PLE) and supercritical fluid extraction (SFE), consisted of the packaging of approximately 0.021 kg of olive leaves in the extraction vessel with static pressurization for 15 min with pressurized liquid solvent or CO2, and then the extraction was conducted in dynamic mode with a flow rate of 2 mL min−1 for 110 and 210 min, respectively for PLE and SFE.
Fig. 1.
Scheme of the SFE and PLE system. CO2 cylinder [1]; Needle type Valve [2, 2.1 and 12]; Anti reflux valve [3, 3.1 and 3.2]; High pressure syringe pump [4]; Ultra thermostatic recirculation bath [5 and 6]; Pressure gauge [7]; Extraction column [8]; Micrometric valve [9]; Heating tape [10]; Glass bottles collector [11]; HPLC pump [13]; Solvent cylinder [14]
Supercritical fluid Extraction (SFE)
The extraction experiments using SFE were carried out using the extraction unit shown in Fig. 1. The unit consists of a solvent reservoir (CO2, White Martins S.A. with purity of 99.9%) [1]. A needle valve (Hip 15-11AF1) [2] allowed CO2 to be fed to the syringe pump (ISCO 500D) [4]. Two anti-reflux valves (Hip 316SS HT) [3 and 3.1] prevent the reflux of CO2 and the needle valve (Hip 15-11AF1) [2.1] precludes carbon dioxide refluxing the extracting unit to the high pump pressure and isolate the extractor system in case of any leakage during extraction. Two thermostatic baths (Nova Ética 521/3D) were used to control the extraction temperature [6] and to control the pressurizing pump temperature [5]. All connections within the system are made of stainless steel tubing (Swagelok 1/16″ and 1/8″). A stainless steel extractor [8] with 20 cm length and 2 cm internal diameter was also used. Its ends are threaded, allowing the internal adaptation of two stainless steel screens (200 mesh) that prevent the access of solids, which is reinforced with the cotton layer coupling (approximately 1.5 cm thick) at the ends to prevent particles from obstructing the extraction system. To control the pressure in the extractor, a manometer is coupled (Warnig 1136120) [7]. The CO2 flow rate in the system was manually controlled by a micrometric valve (Swagelok SS-31RF2) [10]. Due to the CO2 expansion, a heating tape (Novus 300w) [9] was coupled, with temperature control being carried out through a potentiometer (Coel HW1440) [10]. The evaluated extraction temperatures were 20, 40, and 60 °C. The solvent was pumped into the extraction chamber at a constant flow rate of 2 mL min−1 and kept in contact with the samples bed until reaching the studied extraction pressure (8, 16.5 and 25 MPa). The extracted fractions were collected [11] up to 210 min, at time intervals of 10 min, and the amounts for each individual fraction were gravimetrically determined per yield. Extract yield (% wt) was calculated (Eq. 1) by the ratio between the extract amount and the amount of olive leaves placed in the extractor.
Pressurized liquid extraction (PLE)
The same experimental SFE system setup was used for PLE experiments (Fig. 1). In this method, the HPLC pump (SSI Series III) [13] was used to move the solvent stored in the container [14] to the extractor [8] at room temperature. Two valves were placed between the HPLC pump and the extractor, an anti-reflux valve (Hip 316SS HT) [3.2] which prevents solvent reflux and the needle valve [(Hip 15-11AF1) [12] system in the event of a leak in the extractor. In the PLE, the extractions were performed in isobaric conditions (10.3 MPa), varying the temperature (20, 40 and 60 °C) and the solvent (ethyl acetate, acetone, absolute ethanol, ethanol:water–Et:w (80:20, v:v). The collection of extracts were performed at 1, 2, 3, 5, 7, 10, 15, 20, 30, 40, 50, 65, 80, 85 and 110 min, and dried in oven at 40 °C and vacuum of 0.05 MPa to build the global yield curves. The yield curves for both extraction (SFE and PLE) procedures were calculated (Eq. 1) as the ratio between the mass of total extract (crude extract) and the mass of the raw material loaded in the extraction vessel (Sodeifian et al. 2016).
| 1 |
Bioactive contents and bioactivity analysis
To perform the analysis of antioxidant activity, total phenolic content and total flavonoids content of extracts obtained with carbon dioxide as solvent were directly diluted in DMSO 5 mg mL−1 (m/v). The extracts obtained by PLE were concentrated in rotaevaporator (Biotech®) at 40 °C. Subsequently, the extracts obtained by SFE and PLE were resuspended in 1 mL of absolute ethanol and diluted in distilled water at a concentration of 1 mg mL−1 (w/v).
Antioxidant activity (AA)
The antioxidant activity (AA) of all the extracts obtained were determined by the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) assay according the methods described by Brand-Williams (Brand-Williams et al. 1995). Each of the samples (0.1 mL) were added to 3.9 mL of a 6 × 10−5 mol L−1 DPPH solution in methanol. The absorbance of DPPH was measured spectrophotometrically (Nova®) at 515 nm.
Total phenolic content (TPC)
The total phenolic content was determined as described by Singleton et al. (1998) with slight modifications proposed by Blainski et al. (2013). Briefly, a 0.5 mL of extract properly diluted in 2.5 mL Folin–Ciocalteu reagent was left to rest for 5 min. An amount of 2 mL of Na2CO3 (5%, w/v) and 50 μL of lithium chloride (10%, w/v) was then added. The mixture was agitated and allowed to rest again for 2 h in incubation in a water bath at 25°C. The absorbance was measured using a spectrophotometer (Bioespectro®) at 760 nm. TPC was expressed as mg of gallic acid equivalents (mg GAE g−1 of olive leaf extract), according to a calibration curve (50–200 mg L−1; R2 = 0.983).
Total flavonoid content (TFC)
Total flavonoid contents (TFC) of the leave extracts were determined according to the colorimetric assay developed by Zhishen et al. (1999). The TPC results were expressed on a dry weight basis as mg CAT per g of extract, calculated after the construction of the calibration curve (100–600 mg L−1; R2 = 0.999).
Oleuropein quantification
The chromatographic analysis of oleuropein was performed following the methodology described by Al-Rimawi (2014), by high performance liquid chromatography (HPLC) (Agilent, series 1100) with a mobile phase composed of acetonitrile, distilled water and acetic acid in a volumetric ratio of 200:800:1 (v:v:v), C18 column: 150 × 4.60 mm and 5 micron (Phenomenex®). For detection, DAD (UV–VIS) detector was used at 280 nm (UV) with isocratic elution, a flow rate of 1.0 mL min−1 and the injection volume was adjusted to 20 μL. Standard oleuropein (Sigma-Aldrich) was used for the calibration curve (10 to 600 mg L−1, R2 = 0.99). The results were expressed as mg of oleuropein per g of extract.
Mathematical modeling
In this work, the model proposed by Sovová (1994) with fluidity of the solvent in the axial direction was applied in a cylindrical fixed bed extractor considered homogeneous in relation to the size of the particles (1–3 mm) and the initial solute distribution. The main parameters weighted for the model are: the initial concentration of the extract in the solid, the properties of the system (pressure, temperature and solvent flow), bed porosity, external and internal mass transfer coefficient, density of the fluid phase and solid phase.
Based on these weights it is possible to admit the following Eq. 2 for the Sovová model.
| 2 |
where the mass of the solute initially contained in the solid phase (O) is the sum of the mass of easily accessible solute (P) with the mass of the unreachable solute inside the particles of the solid phase (K).
The material balance for the process is represented by Eqs. 3 and 4, associated to the solid and liquid phases, respectively.
| 3 |
| 4 |
where h is the axial distance or bed length, ε is the porosity of the bed, ρ is the density of the solid phase, x is the mass fraction of the extract in the solid phase, ρε is the density of the fluid, y is the mass fraction of extract in the fluid and J(x,y) is the transfer rate of interfacial mass, while t is the time.
The accumulation term associated with the balance in the fluid phase of Eq. 5 () is neglected to facilitate the resolution of the systems of equations, resulting in:
| 5 |
For the calculation of the mass transfer rate, it is considered that the easily accessible solute is extracted first. When the concentration in the solid phase decreases to xk, the mass transfer is controlled by diffusion in the solid phase. The mass transfer rate is represented by Eqs. 6 and 7, for the fluid and solid phases, respectively:
| 6 |
| 7 |
The analytical solution of the differential equations was performed according to Sovová (1994) and presented for three periods of extraction, in terms of the mass of the extract relative to the mass of solid without counting the mass of solute (N). The author considers three distinct periods during the extraction: the first is related to the initial and linear part of the curve in which the extract is easily accessible, from the cells opened by the milling (q < qm). The second period is related to the transition band, where the extract can be extracted from both open and closed cells (qm ≤ q < qn) depending on the region of the bed in question. In the last period, only the hard-to-reach extract from the closed cells is being extracted (q ≥ qn).
| 8 |
where q is the specific amount of solvent spent up to a given extraction time, relative to N, and xo is the initial concentration of solute in the solid phase.
| 9 |
| 10 |
| 11 |
The parameters Z and W are directly proportional to the mass transfer coefficients of the fluid and solid phases (kfe ks), respectively, and inversely proportional to the mass flow of solvent () relative to N. Z is the parameter related to the fast period and W is related to the slow period.
| 12 |
| 13 |
where yr is the solubility and a0 is the specific area.
The mathematical modeling of the kinetic curves of extraction using the proposed model and presented by Pardo-Castaño et al. (2015) was based on the Brunauer–Emmett–Teller (BET) theory of absorption with some differences and considerations detailed by Correa et al. (2016). This model is based on the extraction of a solid substrate with a supercritical fluid in a compacted extractive bed, around a differential element along the axial direction of the extractor (Pardo-Castaño et al. 2015), described according to Eq. 14.
| 14 |
where Cf is the solute concentration in the supercritical fluid, u is the interstitial solvent velocity, ε is the empty bed fraction, D is the axial dispersion coefficient of the solute in the fluid phase, sf is the effective solid–fluid contact area for mass transfer, Ksf is the mass transfer coefficient for solute transport through the external fluid film surrounding the solid particles, C* is the solute concentration in the fluid phase film in equilibrium with the surface.
Pardo-Castaño et al. (2015), assumes that the term representing the axial dispersion, i.e. the first term [Dsf (∂2Cf/∂z2)] on the right side of Eq. 14 is negligible and this approximation is valid when the length of the extractor is 50 times less than the mean particle diameter and the Reynolds number is greater than 10. We also consider that the term representing the solute accumulation in the supercritical fluid, the first term on the left side [(∂C/∂z)], is negligible in comparison with the amount of solute in the solid material. With these assumptions, we obtain Eq. 15.
| 15 |
When K = [(1 − ε)/ε]asfksf1, y is the mass fraction of the solute in the supercritical fluid and y*f is the saturation condition related to the stagnant film at the solid–fluid interface.
It possible to obtain Eq. 16 from Eq. 15, when integrated in the conditions within the usual limits for an extractor packed in z = 0, yf = 0 and z = L, yf = yfL.
| 16 |
where yfL is the mass fraction of solute in the fluid phase up to the extractor outlet, L is the length of the extractor.
According to Correa et al. (2016), Eq. 16 can be written as a function of three dimensionless numbers: taking into account that there are changes in the flow conditions (Reynolds number—Re) and considering the mass transfer characteristics related to the properties of the solid (Biot number—Bi) or fluid properties (Schimidt number—Sc), then the exponential argument can be adjusted using an experimental condition or in this case, the diffusion limitations are insignificant (L → ∞ or k → ∞), then yL = ye. In addition to Eq. 16, the material balance of the solute in the solid matrix is necessary, given by Eq. 17.
| 17 |
where ms is the mass of the solute in the solid phase (matrix), is the mass flow rate and ml and msolv are the mass of solute and solvent until the extractor outlet (z = L).
Equation 18 can be written in terms of mass fraction.
| 18 |
where yfL is obtained using Eq. 16.
According to Correa et al. (2016) adapting the proposal of Pardo-Castaño et al. (2015) and Goto et al. (1998) proposing a BET equilibrium relation in which the solute interacts with the solid matrix, results in Eq. 19 (Brunauer et al. 1938).
| 19 |
where X is the ratio of the mass fraction of solute at equilibrium (ye) to the mass fraction of the solute in a saturated fluid phase (ySat), xm is the mass fraction in the first monolayer (mm/m0), where m0 is a mass of the extract in the solid matrix and K is the equilibrium coefficient of sorption. This equilibrium relation relates the concentration of the solute in a fluid film located at an infinitesimal distance from the surface of the solid substrate (ye) with the concentration of the solute in the solid matrix (xs). This relationship depends on the relative attraction forces exerted by both the solid and the solvent in the solute (Pardo-Castaño et al. 2015). After some algebraic manipulations, Eq. 19 can be written as:
| 20 |
where β = K(1 − xm/x) − 2 and γ = 1 − K.
Finally, Eq. 20 is applied in Eq. 16 with ye = xySat. Thus, Eq. 18 is numerically resolved and the parameters are related to solute solubility in the solvent phase, corrected with diffusion limitations, K is the ratio of the adsorption equilibrium constant in the first monolayer to the subsequent layers (if the interactions K → 0), and xm is the ratio between the mass of the solute present in the first monolayer and the initial mass of solute that can be extracted (Correa et al. 2016; Pardo-Castaño et al. 2015).
Both models were compared using the statistical parameters of root mean square deviation Eq. (21) and the determination coefficient (R2).
| 21 |
where NOBS represent the number of experimental data available, yexpi and ycalci are the experimental and predicted by the model extraction yield respectively, expressed in extracted mass (g) per leaf mass (g).
The two models were fitted using the least square objective function between the experimental and calculated values of the extraction yield, where the minimization was performed using the fminsearch sub-routine using the software MatLab® for Windows, version R2015a (Math works Inc., Natick, MA, USA).
Statistical analysis
The results were statistically treated by analysis of variance (ANOVA), followed by the average differences comparison by the Tukey’s test, with a 95% confidence level, using the software Statistica® version 7.0 (Stat soft Inc., USA). All experiments and analyses were performed in triplicate.
Results and discussion
Table 1 presents the results of total yield, antioxidant activity, flavonoids, total phenolics and oleuropein of extractions with sub- and supercritical CO2 at different pressures (8, 16.5 and 25 MPa) and pressurized liquid at a pressure of 10.3 MPa, using different solvents (ethyl acetate, acetone, absolute ethanol, ethanol:water 80:20 v:v). All extractions were conducted at temperatures of 20, 40 and 60 °C.
Table 1.
Extraction conditions (Pressure, T °C, solvent, density and solubility), yield, antioxidant activity (AA), total flavonoid content (TFC), total phenolic content (TPC) and oleuropein (OLE) by HPLC of olive leaf extracts obtained by SFE and PLE
| Treatment | ρsolvent (g cm−3)A | S (solubility) (g g−1 solvent)B | Yield (%)C | AA* (%) | TFC (mg CAT g−1 extract) | TPC (mg GAE g−1 extract) | OLE (mg g−1 extract) |
|---|---|---|---|---|---|---|---|
| SFE | |||||||
| 8.0 MPa/20 °C | 0.828 | 0.23E−3 | 0.24c ± 0.013 | 25.78b,c ± 2.59 | 7.92c,d ± 1.02 | 2.69d ± 0.17 | NI |
| 16.5 MPa/20 °C | 0.915 | 0.43E−3 | 0.33b,c ± 0.015 | 25.88b,c ± 2.38 | 20.48a ± 1.42 | 11.49a ± 0.25 | NI |
| 16.5 MPa/40 °C | 0.802 | 0.43E−3 | 0.31c ± 0.073 | 18.31d;e ± 1.90 | 7.97c,d ± 0.59 | 2.63d ± 0.23 | NI |
| 16.5 MPa/60 °C | 0.652 | 0.31E−3 | 0.30c ± 0.041 | 13.07e ± 2.55 | 12.97b ± 2.62 | 3.73c ± 0.25 | NI |
| 25.0 MPa/20 °C | 0.963 | 0.33E−4 | 0.34b,c ± 0.063 | 22.19c,d ± 0.67 | 11.10b,c ± 0.20 | 4.98b ± 0.15 | NI |
| 25.0 MPa/40 °C | 0.879 | 0.65E−3 | 0.50a,b ± 0.031 | 34.96a ± 0.59 | 7.91c,d ± 0.38 | 2.63d ± 0.23 | NI |
| 25.0 MPa/60 °C | 0.787 | 0.66E−3 | 0.68a ± 0.029 | 31.78a,b ± 1.35 | 4.66d ± 0.60 | 2.21d ± 0.16 | NI |
| PLE | |||||||
| EthylAc. 20 °C | 0.897 | 0.0241 | 5.60d ± 0.07 | 20.86h ± 0.85 | 28.28b ± 0.71 | 27.76g,h ± 1.43 | 23.24 |
| EthylAc. 40 °C | 0.872 | 0.0389 | 6.60e ± 1.98 | 22.74h ± 0.90 | 17.44f ± 0.11 | 17.12h ± 1.79 | 19.88 |
| EthylAc. 60 °C | 0.851 | 0.0373 | 7.17e ± 1.51 | 23.71h ± 1.36 | 21.47d ± 0.50 | 49.69g ± 3.60 | 29.05 |
| Acetone 20 °C | 0.791 | 0.0304 | 6.37e ± 0.68 | 28.57g ± 1.42 | 8.86i ± 0.34 | 79.74f ± 4.37 | 34.35 |
| Acetone 40 °C | 0.730 | 0.0447 | 7.2e ± 0.29 | 31.42g ± 1.11 | 10.71h ± 0.61 | 91.67f ± 2.98 | 33.86 |
| Acetone 60 °C | 0.713 | 0.0619 | 9.16d,e ± 0.15 | 39.72f ± 1.20 | 15.99f,g ± 0.40 | 140.79e ± 1.43 | 38.26 |
| PLE | |||||||
| Ethanol 20 °C | 0.790 | 0.0344 | 6.48e ± 0.39 | 47.26e ± 2.16 | 23.98c ± 0.30 | 259.55c ± 6.24 | 50.88 |
| Ethanol 40 °C | 0.775 | 0.0481 | 12.62c,d ± 0.46 | 56.97c;d ± 3.14 | 24.12c ± 1.05 | 251.44c ± 8.26 | 55.08 |
| Ethanol 60 °C | 0.759 | 0.0601 | 16.94b,c ± 2.44 | 82.87a ± 1.30 | 33.43a ± 0.20 | 386.42a ± 0.83 | 73.65 |
| Et:W 20 °C | 0.871 | 0.0609 | 21.16b ± 2.15 | 56.48d ± 1.60 | 14.80g ± 0.86 | 195.15d ± 8.71 | 34.61 |
| Et:W 40 °C | 0.853 | 0.0739 | 26.56a ± 2.78 | 61.16c ± 0.87 | 19.36e ± 0.40 | 256.69c ± 7.97 | 37.17 |
| Et:W 60 °C | 0.841 | 0.0787 | 30.91a ± 1.97 | 70.71b ± 1.02 | 22.07d ± 0.70 | 300.57b ± 6.07 | 43.92 |
Means (± standard deviations) followed by different letters on columns represent significant difference at 5% level (Tukey’s test)
NI not identified in HPLC analysis
ADensity estimated under conditions within the extractor (extraction conditions) which were calculated from Linstrom and Mallard (2000). 8.0 MPa/40 °C = 0.278 g/cm3; 8.0 MPa/60 °C = 0.192 g cm−3; *AA (%)—SFE: extract concentration (5 mg mL−1); PLE: extract concentration (1 mg mL−1)
BValues estimated by linear adjustment in the initial extraction period (R2 > 0.98)
CValues obtained at the 210 min of extraction in the SFE and 110 mm in the PLE (10.3 MPa)
Extraction yield
In the SFE, the highest yield (p < 0.05) was 0.68% in the extractions conducted at 25 MPa and 60 °C, followed by extraction at 25 MPa and 40 °C with 0.50%, showing a lower yield when compared to extractions with PLE (21.16 to 30.91% were obtained with solvent Et:W, 80:20 v:v) as seen in Table 1. For SFE in isobaric conditions of 8 MPa, it was only possible to carry out extraction with quantification of the yield at 20 °C. At 40 and 60 °C, the presence of the extracts was negligible (not possible to quantify). At the pressure of 16.5 MPa, there was a decrease in the yield as the extraction temperature was increased. At 8 MPa, and temperatures of 40 and 60 °C, the CO2 density is below the critical density of 0.467 g cm−3 (Jokić et al. 2015) and, 16.5 MPa and under the same temperature conditions, a decrease in density is also observed, explaining the absence or decrease in the overall yield in these treatments (Table 1) generated by reduction of solubility due to the decrease in density of the fluid caused by temperature increase (Brunner 2015).
Though not in the supercritical state (20 °C), substances in state conditions near or around this “critical area” may have similar properties as a supercritical fluid (carbon dioxide, 30.97 °C and 7.37 MPa—National Institute of Standards and Technology—NIST). In this case, the fluid is called subcritical (Brunner 1994) and allows extraction even at 20 °C. At 25 MPa and with increasing temperatures, a decrease in density is evident but the extraction yield increases. Therefore, the phenomenon known as “crossover” is observed, and occurs when the temperature rises and the density of CO2 in the supercritical state decreases, but the solubility of the solute rises as a result of the increased vapor pressure of the solute (Duba and Fiori 2015).
This phenomenon can also be explained by a reduction in viscosity and increase of the kinetic energy of the molecules, thus increasing the diffusivity of the solvent in the solid matrix. These changes confer better characteristics to the solvent, facilitating the mass transfer inside the plant matrix (Brunner 2015).
Thus, the increase or decrease in solubility of the solute in the supercritical fluid will depend on the operating pressure and temperature of the system. Near critical pressure (8 MPa), the effect of fluid density is predominant, thus a moderate increase in temperature leads to a large decrease in density of the fluid and hence a decrease in the solubility of the solute. However, between 16.5 and 25 MPa, the solubility increases with increasing temperature.
The use of CO2 in the extraction shows a reduction in the extraction yield, in the contents of TFC and TPC (Table 1). However, the extraction of waxy, non-polar, lipophilic compounds such as fatty acids, triglycerides, hydrocarbons and alcohols (Braga et al. 2003) may have adversely affected antioxidant activity (Table 1).
In the extractions with pressurized liquid solvent—PLE (Table 1), the yield was influenced by the solubility of the compounds present in the solid matrix in relation to the solvent used, that is, more polar solvents presented higher yields in the extract (Et:W > ethanol > acetone > ethyl acetate). This is associated with increased solvent diffusivity in the solid matrix, increasing the extraction rate and the final yield, which, even when allied to the PLE, sometimes afforded higher yields and/or extraction of compounds of interest. This is also reported in the literature, where the yields of plant extracts are dependent on the nature and polarity of the solvent, which should be similar to that of the analyte (Setyaningsih et al. 2015).
In the PLE extraction of olive leaves using hydro-alcohol solvent (20:80, v:v), yields between 21.16 and 30.91% were obtained (Table 1). Xynos et al. (2012) used an integrated system of extraction in olive leaves where the leaf matrix was initially degreased with SC-CO2 added with a co-solvent and later submitted to extraction with PLE. In the PLE, the authors obtained yields ranging from 15.5 to 41.5% under different temperature conditions and alcoholic solvent concentrations, hydro-alcoholic and sub-critical water, achieving a yield of 26.7% specifically for the H2O:EtOH mixture (40:60, v:v).
The presence of water in the solvent mixture can swell the matrix of the plant, which may justify the increase in the extractions by allowing the solvent to penetrate more easily into the solid matrix in addition to the extraction of sugars, proteins and organic acids that increase the yield (Xie et al. 2015).
Using only ethanol as solvent (Table 1), the extract yield (p < 0.05) in the present study ranged from 6.48 to 16.94%, being lower than the yields reported in literature, which ranged from 14.8 to 22.4% using PLE under static conditions of 150 °C for 20 min at 100 bar and ethanol as solvent in different cultivars of O. europaea (Taamalli et al. 2012). The highest yields obtained by the authors may have been influenced by the extraction conditions, the type of cultivar under study, geographic location, the time, the year of harvesting the olive leaves and the local climatic conditions (Bilgin and Sahin 2013).
In all PLE experiments, regardless of the solvent in use, the higher yields are associated with the higher temperatures (Table 1). This is related to a decrease in the viscosity and surface tension of the solvent. Increasing the diffusion coefficient and mass transfers rates, which improves the solvent diffusion in the matrix and facilitates the contact with the compounds. This behavior was also observed in other works that emphasize that the thermal energy involved can interrupt the strong interactions between the solute molecules and the active sites of the matrix, aiding in the release of target molecules and increasing extraction rates (Osorio-Tobón et al. 2014; Gil-Chávez et al. 2013; Setyaningsih et al. 2015).
In summary, increasing the temperature in the PLE increased (p < 0.05) the extraction yield and the concentration of the extracted bioactive compounds and consequently of the AA (Table 1), independent of the solvent used.
Bioactivity analysis
In the extractions with carbon dioxide, the highest levels (p < 0.05) were observed in the conditions of 20 °C and 16.5 MPa for the total phenolic content—TPC (11.49 mg GAE g−1 of extract) and total flavonoids content—TFC (20.48 mg CAT g−1 of extract) with a significant difference (p < 0.05) compared to other extraction conditions applied to SFE.
The antioxidant activity (AA) of the extracts obtained with sub- and supercritical carbon dioxide differed significantly (p < 0.05) from the other samples, indicating the highest AA (34.96%) in the condition of 25 MPa and 40 °C. The pressure exerted a positive significant effect (p < 0.05) within the study group for AA, TPC and TFC (data not shown). One of the most important compounds with antioxidant activity present in olive leaves are tocopherols, such as vitamin E, which are easily extracted by supercritical carbon dioxide at low temperatures (De Melo et al. 2014).
The higher levels for total phenolic content and total flavonoids content (Table 1) in the extracts were obtained at the lowest temperature (20 °C). On the other hand, when using CO2 at temperatures of 40 to 60 °C, a reduction in the TFC and TPC contents was observed. At high temperatures, there is the possibility of extraction of waxy compounds, non-polar, lipophilic such as fatty acids, triglycerides, hydrocarbons and alcohols that do not contribute much to the antioxidant activity (Braga et al. 2003). In addition, heat can affect stability of polyphenols due to chemical and enzymatic degradation causing the decrease in phenolic content and the loss of activity of some heat-sensitive antioxidants of low molecular weight. This is in agreement with Krishnaiah et al. (2012), in which a loss of 65% radical scavenging activity was found due to heating.
As observed in Table 1 for extractions with PLE, the best results were obtained using ethanol at 60 °C in relation to the TPC, oleuropein and AA, 386.42 mg GAE g−1 of extract, 73.65 mg OLE g−1 of extract and 82.87% AA, respectively, differing statistically (p < 0.05) when compared to the extracts obtained using the solvents Et:W, acetone and ethyl acetate. These results are in agreement with the studies carried out using the hexane, chloroform, ethyl acetate and methanol as solvents (Brahmi et al. 2012), and hexane, chloroform, ethyl acetate, water, 80% ethanol and butanol (Lee et al. 2009) with a decrease in total phenolic and total flavonoid content when more non-polar solvents are used in the extraction or fractionation of these compounds in olive leaves.
According to literature (Brahmi et al. 2012; Osorio-Tobón et al. 2014) the polarity of the solvent should be similar to or close to that of the target compound and the differences in the polarity of the extraction solvents may result in a wide variation in the polyphenolic content of the extract. This indicates that most of the phenolics of the present study are composed of polar phenolics due to the higher TPC content obtained in the polar solvent ethanol, followed by the hydro-alcohol, acetone and ethyl acetate solvents.
As mentioned earlier, these bioactivity results obtained using PLE with ethanol at 60 °C relative to the content of TPC (386.42 mg GAE g−1 extract), oleuropein (73.65 mg OLE g−1 extract), TFC (33.43 mg GAT g−1 extract) and AA (82.87%) are considered relevant when compared to other studies employing different conditions and/or extraction methods (Xie et al. 2015, Difonzo et al. 2017).
Similar to the total phenolics and total flavonoid content, the AA of the extracts obtained with ethanol and 60 °C in the PLE differed significantly (p < 0.05) from the other extracts, indicating the highest AA of 82.87% followed by the extract from Et:W at 60 °C with 70.71% AA. The lowest activities in the present study were observed in the extracts obtained with ethyl acetate (20.86 to 23.71%) and acetone (28.57 to 39.72%).
One of the advantages of extraction with PLE is the lower solvent consumption and higher extraction capacity because the oleuropein contents were considered high according to Table 1 (19.88 to 73.65 mg OLE g−1 of extract) when compared to the extractions with SFE and carbon dioxide in the present work, where the presence of oleuropein in the extract was not identified through HPLC analyses.
The bioactivity presented by the alcoholic and hydro-alcoholic extracts in the present study is associated to the compounds present in the olive leaf such as phenols, benzoic and cinnamic acids, secoiridoids, flavonoids and in a specifically greater proportion of oleuropein and its derivatives (Khemakhem et al. 2017), with redox properties of their hydroxyl-phenolic groups and the structure relation between the different parts of the chemical components (Ferreira et al. 2007).
Literature reports that in general, the higher the temperature and the more polar the solvent, the lower the selectivity of the extraction as a function of the variety of extracted compounds, besides mentioning the low solubility of the polyphenols in water, justifying the higher bioactivity of the alcoholic extract in relation to the hydro-alcoholic one (Rahmanian et al. 2015).
According to Basegmez et al. (2017) supercritical carbon dioxide extraction is capable of solubilizing only the lipophilic constituents and, therefore, other methods are required. It is not suitable for phenolic extractions or polar compounds, regardless of temperature and pressure. These data corroborate with those of the present study, showing lower yield, antioxidant activity, total flavonoid and phenolic contents in the extracts obtained with apolar solvents (ethyl acetate and acetone) and with sub- and supercritical fluid. However, supercritical CO2 can be useful in integrated extraction systems (SC-CO2 and PLE) removing apolar or lipophilic compounds, facilitating the isolation and concentration of major polar compounds of interest using PLE (Xie et al. 2015; Xynos et al. 2012).
Comparing the two methods of extraction, SFE and PLE, the extracts obtained by PLE, mainly with hydro-alcohol and ethanol solvents, showed better bioactivity, possibly facilitating the extraction of phenolic compounds, flavonoids and other categories of compounds with antioxidant properties.
Mathematical modeling
The extraction kinetics of the olive leaf (O. europaea) with sub- and supercritical carbon dioxide and pressurized liquid solvent was investigated by modeling the extraction curves using two models, Sovová (1994) and Pardo-Castaño et al. (2015). The experimental and calculated values of the adjusted kinetic models are presented in Tables 2 and 3 and grouped by different temperatures and solvent type. Besides the fitted parameter of each model, the maximum amount of available extract (m0: g extract g−1 of raw material) was also needed to be set, considering different extraction conditions, to assure a better fitting.
Table 2.
Adjusted parameters for the Sovová model for SFE and PLE extraction
| P (MPa) | T (°C) | Z | W | r | Rmsd (100) | R2 | tCER (min) | tFER (min) | kfa (min−1)(102) | ksa (min−1)(102) |
|---|---|---|---|---|---|---|---|---|---|---|
| CO 2 | ||||||||||
| 8.0 | 20 | 5.16 ± 4.19 | 0.027 ± 0.011 | 0.760 ± 0.070 | 0.007 | 0.992 | 16.60 | 127.98 | 9.74 | 5.80 |
| 16.5 | 20 | 4.55 ± 3.02 | 0.034 ± 0.006 | 0.670 ± 0.040 | 0.007 | 0.994 | 12.57 | 79.35 | 7.90 | 7.84 |
| 16.5 | 40 | 1.76 ± 0.45 | 0.021 ± 0.013 | 0.590 ± 0.060 | 0.013 | 0.984 | 39.17 | 113.85 | 3.56 | 4.93 |
| 16.5 | 60 | 5.15 ± 3.40 | 0.028 ± 0.006 | 0.680 ± 0.040 | 0.007 | 0.996 | 14.31 | 102.91 | 12.93 | 6.77 |
| 25.0 | 20 | 7.18 ± 6.96 | 0.009 ± 0.013 | 0.630 ± 0.070 | 0.011 | 0.993 | 16.70 | 146.77 | 11.59 | 2.29 |
| 25.0 | 40 | 5.94 ± 4.60 | 0.032 ± 0.007 | 0.550 ± 0.040 | 0.010 | 0.996 | 11.24 | 85.53 | 10.54 | 7.59 |
| 25.0 | 60 | 9.29 ± 12.56 | 0.099 ± 0.011 | 0.580 ± 0.080 | 0.009 | 0.998 | 6.02 | 81.48 | 20.56 | 26.00 |
| Ethyl acetate | ||||||||||
| 10.3 | 20 | 2.89 ± 0.92 | 0.100 ± 0.013 | 0.600 ± 0.035 | 0.080 | 0.998 | 5.98 | 25.11 | 5.73 | 24.00 |
| 10.3 | 40 | 2.22 ± 0.63 | 0.072 ± 0.048 | 0.273 ± 0.056 | 0.180 | 0.996 | 8.38 | 27.33 | 4.71 | 18.00 |
| 10.3 | 60 | 3.66 ± 1.04 | 0.092 ± 0.028 | 0.268 ± 0.032 | 0.090 | 0.999 | 5.29 | 25.22 | 8.02 | 23.10 |
| Acetone | ||||||||||
| 10.3 | 20 | 2.21 ± 0.4639 | 0.052 ± 0.016 | 0.504 ± 0.032 | 0.110 | 0.998 | 10.69 | 35.53 | 5.45 | 11.00 |
| 10.3 | 40 | 2.94 ± 0.7926 | 0.050 ± 0.020 | 0.364 ± 0.029 | 0.130 | 0.998 | 7.05 | 28.25 | 7.39 | 11.00 |
| 10.3 | 60 | 2.97 ± 0.9117 | 0.198 ± 0.057 | 0.293 ± 0.055 | 0.160 | 0.998 | 5.38 | 22.16 | 7.94 | 44.00 |
| Ethanol | ||||||||||
| 10.3 | 20 | 3.32 ± 1.83 | 0.332 ± 0.049 | 0.550 ± 0.077 | 0.110 | 0.998 | 3.84 | 19.13 | 7.82 | 71.90 |
| 10.3 | 40 | 9.99 ± 4.52 | 0.087 ± 0.029 | 0.547 ± 0.119 | 0.390 | 0.995 | 2.46 | 29.84 | 26.02 | 21.00 |
| 10.3 | 60 | 10.01 ± 4.23 | 0.212 ± 0.022 | 0.534 ± 0.055 | 0.160 | 0.999 | 1.99 | 26.03 | 27.08 | 51.00 |
| Et:W | ||||||||||
| 10.3 | 20 | 9.98 ± 3.61 | 0.068 ± 0.014 | 0.667 ± 0.065 | 0.420 | 0.998 | 2.88 | 37.73 | 30.88 | 19.10 |
| 10.3 | 40 | 9.48 ± 5.71 | 0.13 4 ± 0.016 | 0.658 ± 0.070 | 0.330 | 0.999 | 2.60 | 35.74 | 29.52 | 38.00 |
| 10.3 | 60 | 10.01 ± 6.95 | 0.225 ± 0.065 | 0.614 ± 0.102 | 0.690 | 0.997 | 2.59 | 41.01 | 31.90 | 63.90 |
Real density of the vegetable matrix: 1.3746 ± 0.0004 g cm−3 measured in helium gas pycnometer, model AccuPyc II 1340, (Micromeritics). Fitted parameters: Z, W and r
Table 3.
Adjusted parameters of the kinetic Pardo-Castaño model for extractions with SFE and PLE
| P (MPa) | T (°C) | y Sat | x m | K | Rmsd (100) | R 2 |
|---|---|---|---|---|---|---|
| Carbon dioxide | ||||||
| 8.0 | 20 | (0.221 ± 0.019)E−2 | 0.8 | 56.67 ± 23.57 | 0.67E − 2 | 0.992 |
| 16.5 | 20 | (0.289 ± 0.032) E−2 | 0.8 | 90.82 ± 19.30 | 0.68E − 2 | 0.995 |
| 16.5 | 40 | (0.259 ± 0.049) E−2 | 0.8 | 89.74 ± 34.65 | 1.18E − 2 | 0.983 |
| 16.5 | 60 | (0.188 ± 0.024) E−2 | 0.8 | 66.86 ± 19.61 | 0.77E − 2 | 0.993 |
| 25.0 | 20 | (0.156 ± 0.038) E−2 | 0.8 | 24.09 ± 13.24 | 1.13E−2 | 0.983 |
| 25.0 | 40 | (0.271 ± 0.087) E−2 | 0.8 | 14.01 ± 4.19 | 2.07E−2 | 0.977 |
| 25.0 | 60 | (0.210 ± 0.023) E−2 | 0.8 | 4.21 ± 1.08 | 1.25E−2 | 0.996 |
| Ethyl acetate | ||||||
| 10.3 | 20 | 0.175 ± 0.009 | 0.85 | 5272 ± 2198 | 0.07 | 0.992 |
| 10.3 | 40 | 0.252 ± 0.016 | 0.81 | 18,233 ± 8992 | 0.12 | 0.983 |
| 10.3 | 60 | 0.264 ± 0.029 | 0.81 | 8587 ± 3951 | 0.25 | 0.993 |
| Acetone | ||||||
| 10.3 | 20 | 0.199 ± 0.009 | 0.82 | 9085 ± 4548 | 0.09 | 0.999 |
| 10.3 | 40 | 0.342 ± 0.053 | 0.82 | 2192 ± 601 | 0.32 | 0.988 |
| 10.3 | 60 | 0.302 ± 0.017 | 0.75 | 5033 ± 1470 | 0.18 | 0.998 |
| Ethanol | ||||||
| 10.3 | 20 | 0.270 ± 0.013 | 0.85 | 1814 ± 318 | 0.08 | 0.999 |
| 10.3 | 40 | 0.159 ± 0.022 | 0.65 | 834 ± 455 | 0.65 | 0.988 |
| 10.3 | 60 | 0.199 ± 0.018 | 0.65 | 101 ± 37 | 0.35 | 0.997 |
| Et:W | ||||||
| 10.3 | 20 | 0.173 ± 0.015 | 0.60 | 22 ± 6 | 0.39 | 0.997 |
| 10.3 | 40 | 0.173 ± 0.007 | 0.60 | 6.5 ± 0.7 | 0.17 | 0.999 |
| 10.3 | 60 | 0.161 ± 0.019 | 0.60 | 2.3 ± 0.8 | 0.56 | 0.981 |
Considering the Sovová model for SFE, the m0 values were 0.008 and 0.006 at 25 and 16.5 MPa respectively and for the Pardo-Castaño model the value was fixed at 0.008 for all conditions. The adjustment of the Sovová model to PLE was only possible by varying the mass of the extract for each solvent (m0, expressed in % by mass of matrix). It considered the following values that allowed the best fit for each solvent. Ethyl Ac. m0 = 0.08 (mass of extract in solid matrix), acetone m0 = 0.10, ethanol m0 = 0.07 (T = 20 °C), ethanol m0 = 0.18 (T = 40 °C and 60 °C) and Et:W m0 = 0.33. For the Pardo-Castaño model and in the PLE, the extract mass available and extractable for each solvent was set at m0 = 0.33 and for SFE this was m0 = 0.008 for all treatments.
It can be seen that both kinetic models considered for the extraction of olive leaves with sub-supercritical CO2 and PLE were capable of being well-correlated with the extraction process at all considered conditions. Experimental and calculated values of the adjusted kinetic model for extractions with CO2 are shown in Fig. 2 and for extraction with PLE are shown in Fig. 3. The kinetic models adequately described the experimental results from the extraction process of olive leaf, in a fixed bed extractor using SC-CO2 and pressurized liquid under different conditions.
Fig. 2.
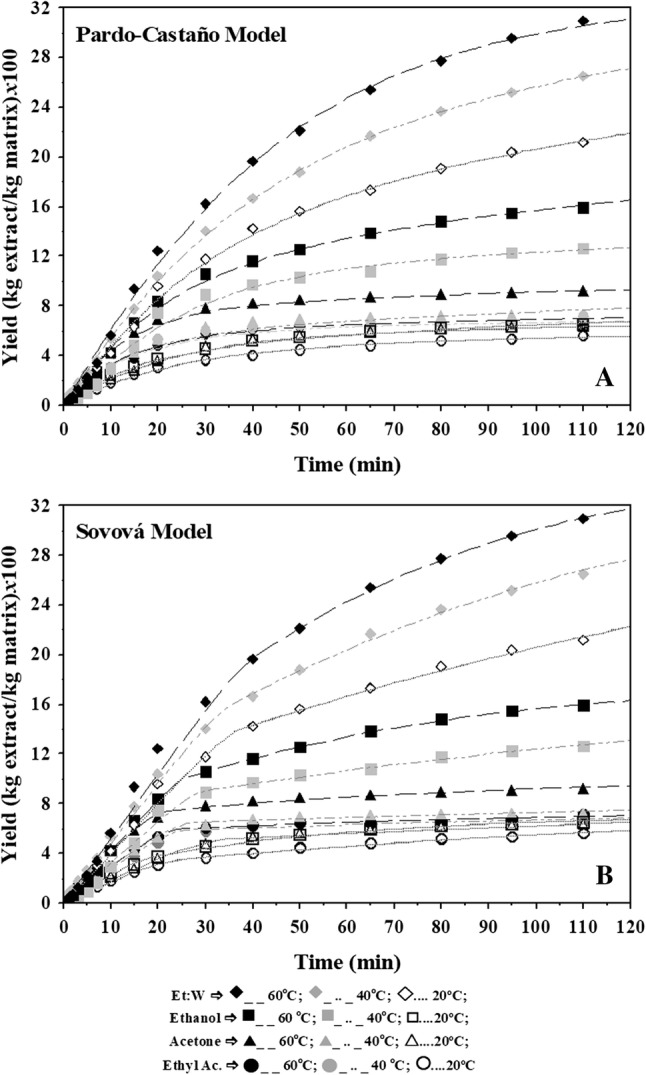
Modeling of the extraction curves with sub- and supercritical fluid using the Pardo-Castaño and Sovová models pooled pressure and at different temperatures
Fig. 3.
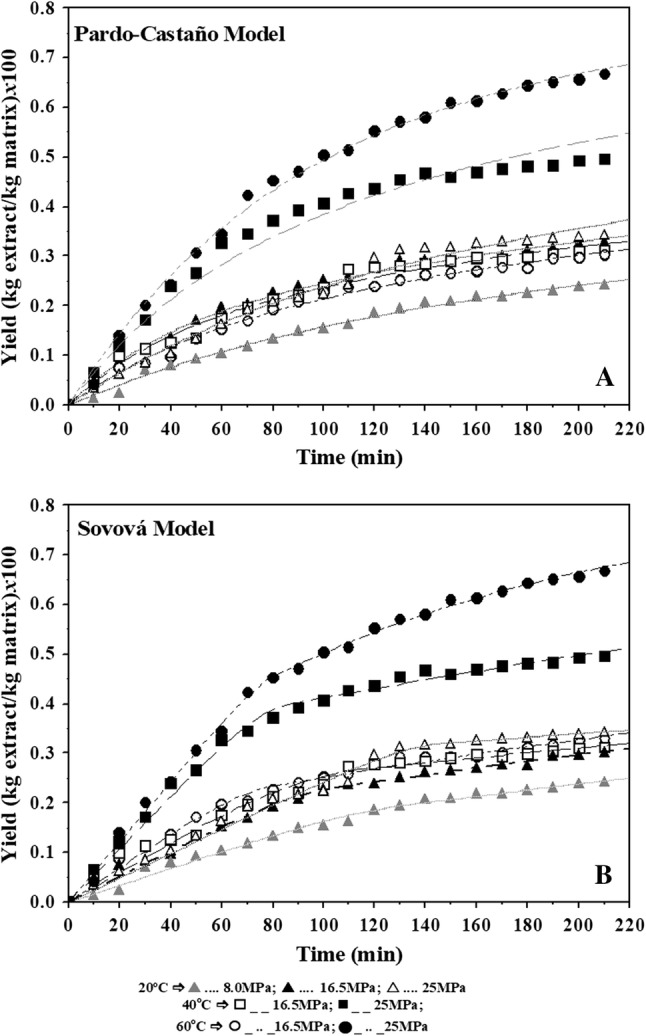
Modeling of the extraction curves with PLE using the Pardo-Castaño and Sovová models, with different solvents and temperatures
The Pardo-Castaño and Sovová kinetic models adequately described the experimental results of extraction under different conditions with R2 values ranging from 0.98 to 0.99 (Tables 2, 3, Figs. 2, 3). It was observed that all tested conditions had higher initial rates of extraction and the maximum extraction yield was achieved in approximately 120 min for extraction with SFE and 40 min for PLE. The influence of temperature on the kinetics of extraction can be seen in Fig. 2, with pressure for both models.
For the Sovová model in the SFE, the external mass transfer coefficient, kFa, is associated with the extractable fraction (r) in the first extraction period (tCER), which was faster with 6.02 min and kFa 20.56 min−1 in the extraction at 25 MPa and 60 °C. However the lower kFa values are associated to the conditions of 16.5 MPa and 40 °C with kFa 3.56 min−1. The internal mass transfer coefficient (kSa) represents the period where the extract is less accessible and refers to the last extraction period. In this period, the values of kSa ranged from 0.2289 to 2.6 × 103 min−1, with the highest values occurring under conditions of higher extraction, pressure and temperature (Table 2). Under these conditions, contact with the hard-to-reach extract are made possible by the association of these parameters to the vapor pressure of the solute and to the increase of the solvation power of the solvent. Similar results are reported in the literature by Sodeifian et al. (2016) and Ferreira et al. (2007).
In the PLE and using the Sovová model, the values of the mass transfers coefficient in the solid phase (kSa) were lower than the mass transfer coefficients in the fluid phase (kFa) for all curves evaluated (Table 3), these values indicate that the diffusion mechanism is slower than the convection mechanism in PLE extractions of olive leaves because the solute located internally in the particles is more difficult to remove compared to the solute located on the surface of the particles.
In Table 3, it is observed that the parameter K decreases and the parameter ySat increased as the extraction temperature increased when the ethanol and Et:W solvents were used. This is because K is directly related to the adsorption equilibrium constant of the solute in the first and subsequent layers and ySat is related to solubility of the solute and its solute-solid ratio (Pardo-Castaño et al. 2015). Under this situation, the use of higher temperatures in the extraction process causes an increase in molecular movement, aiding in perturbations of matrix-analyte interactions caused by Van de Waals bonds, hydrogen bonds and dipole attractions, besides reducing surface tension and viscosity of the solvent, facilitating the permeability in the matrix wall, making the increase of the diffusivity possible and consequently the increase of the extraction (Mustafa and Turner 2011).
The slope of the lines in Figs. 2 and 3 represent the mass transfer rate in a period of constant extraction rates (tCER) and can be considered the minimum time for the extraction process. In general, the values of the constant extraction rates (tCER) of Table 2 decreased with increasing temperature, which indicates that the time of the tCER is lower and the yield increases with increasing temperature. On the other hand, when the extraction rate fell (tFER) (Table 2), the extraction periods were longer and did not present a behavior trend with the temperature and solvent variation.
Conclusion
Due to growing interest in extracting bioactive compounds from plants and the parallel concern of using “greener” technologies, PLE is becoming a promising extraction technology to meet these demands. The PLE is an alternative extraction technique to SFE, investigated in this work and proved adequate for the extraction of bioactive compounds from the olive leaf.
The highest total yield using PLE, with ethanol:water, 10.3 MPa, 60 °C and 110 min was of 30.91%. The PLE extract obtained with ethanol at 60 °C presented the highest concentration of total phenolic content (386.42 mg GAE g−1 extract), total flavonoids content (33.43 mg CAT g−1 extract), oleuropein (73.65 mg g−1 extract) and antioxidant activity (82.87%).
Both Sovová and Pardo-Castaño kinetic models studied, for the extraction of olive leaves with supercritical CO2 and PLE were capable of correlating well with the extraction process at all conditions considered.
Acknowledgements
This study was financed in part by the National Council for Scientific and Technological Development—Brazil (CNPq), Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Finance Code 001 and Research Support Foundation of the State of Rio Grande of Sul—Brazil (FAPERGS).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abrahamsson V, Andersson N, Nilsson B, Turner C. Method development in inverse modeling applied to supercritical fluid extraction of lipids. J Supercrit Fluids. 2016;111:14–27. doi: 10.1016/j.supflu.2016.01.006. [DOI] [Google Scholar]
- Al-Rimawi F. Development and validation of a simple reversed-phase HPLC-UV method for determination of oleuropein in olive leaves. J Food Drug Anal. 2014;22:285–289. doi: 10.1016/j.jfda.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basegmez HIO, Povilaitis D, Kitrytė V, Kraujalien V, Šulniūtė V, Alasalvar C, Venskutonis PR. Biorefining of blackcurrant pomace into high value functional ingredients using supercritical CO2, pressurized liquid and enzyme assisted extractions. J Supercrit Fluids. 2017;124:10–19. doi: 10.1016/j.supflu.2017.01.003. [DOI] [Google Scholar]
- Bilgin M, Şahin S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J Taiwan Inst Chem Eng. 2013;44:8–12. doi: 10.1016/j.jtice.2012.08.008. [DOI] [Google Scholar]
- Blainski A, Lopes GC, De Mello JCP. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18:6852–6865. doi: 10.3390/molecules18066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MEM, Leal PF, Carvalho JE, Meireles MAA. Comparison of yield, composition, and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J Agric Food Chem. 2003;51:6604–6611. doi: 10.1021/jf0345550. [DOI] [PubMed] [Google Scholar]
- Brahmi F, Mechri B, Dabbou S, Dhibi M, Hammami M. The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind Crops Prod. 2012;38:146–152. doi: 10.1016/j.indcrop.2012.01.023. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–319. doi: 10.1021/ja01269a023. [DOI] [Google Scholar]
- Brunner G. Gas extraction: an introduction to fundamentals of supercritical fluids and the application to separation processes. Heidelberg: Springer; 1994. [Google Scholar]
- Brunner G. Supercritical process technology related to energy and future directions—an introduction. J Supercrit Fluids. 2015;96:11–20. doi: 10.1016/j.supflu.2014.09.008. [DOI] [Google Scholar]
- Correa M, Mesomo M, Pianoski KE, Torres YR, Corazza ML. Extraction of inflorescences of Musa paradisiaca L. using supercritical CO2 and compressed propane. J Supercrit Fluids. 2016;113:128–135. doi: 10.1016/j.supflu.2016.03.016. [DOI] [Google Scholar]
- Dariva C, Rodrigues MRA, Caramao EB, Santos JG, Oliveira JV. The effects of temperature and pressure on the characteristics of the extracts from high-pressure CO2 extraction of Marjorana hortensis Moench. J Agric Food Chem. 2003;51:453–456. doi: 10.1021/jf020708s. [DOI] [PubMed] [Google Scholar]
- De Melo MMR, Silvestre AJD, Silva CM. Supercritical fluid extraction of vegetable matrices: applications, trends and future perspectives of a convincing green technology. J Supercrit Fluids. 2014;92:115–176. doi: 10.1016/j.supflu.2014.04.007. [DOI] [Google Scholar]
- Difonzo G, Russo A, Trani A, Paradiso VM, Ranieri M, Pasqualone A, Summo C, Tamma G, Silletti R, Caponio F. Green extracts from Coratina olive cultivar leaves: antioxidant characterization and biological activity. J Funct Foods. 2017;31:63–70. doi: 10.1016/j.jff.2017.01.039. [DOI] [Google Scholar]
- Duba KS, Fiori L. Supercritical CO2 extraction of grape seed oil: effect of process parameters on the extraction kinetics. J Supercrit Fluids. 2015;98:33–43. doi: 10.1016/j.supflu.2014.12.021. [DOI] [Google Scholar]
- Ferreira ICFR, Barros L, Soares ME, Bastos ML, Pereira JA. Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chem. 2007;103:188–195. doi: 10.1016/j.foodchem.2006.08.006. [DOI] [Google Scholar]
- Freitas LS, Oliveira JV, Dariva C, Jacques RA, Caramão EB. Extraction of grape seed oil using compressed carbon dioxide and propane: extraction yields and characterization of free glycerol compounds. J Agric Food Chem. 2008;56:2558–2564. doi: 10.1021/jf0732096. [DOI] [PubMed] [Google Scholar]
- Gil-Chávez JG, Villa JA, Ayala-Zavala JF, Basilio Heredia J, Sepulveda D, Yahia EM, González-Aguilar GA. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci Food Saf. 2013;12:5–23. doi: 10.1111/1541-4337.12005. [DOI] [Google Scholar]
- Goto M, Roy BC, Kodama A, Hirose T. Modelling supercritical fluid extraction process involving solute-solid interaction. J Chem Eng Jpn. 1998;31:171–177. doi: 10.1252/jcej.31.171. [DOI] [Google Scholar]
- Guinda Á, Castellano JM, Santos-Lozano JM, Delgado-Hervás T, Gutiérrez-Adánez P, Rada M. Determination of major bioactive compounds from olive leaf. LWT Food Sci Technol. 2015;64:431–438. doi: 10.1016/j.lwt.2015.05.001. [DOI] [Google Scholar]
- Jokić S, Horvat G, Aladić K. Design of SFE system using a holistic approach—problems and challenges. In: Lindy J, editor. Supercritical fluid extraction: technology, applications and limitations. New York: Nova Publishers; 2015. pp. 95–122. [Google Scholar]
- Khemakhem I, Gargouri OD, Dhouib A, Ayadi MA, Bouaziz M. Oleuropein rich extract from olive leaves by combining microfiltration, ultrafiltration and nanofiltration. Sep Purif Technol. 2017;172:310–317. doi: 10.1016/j.seppur.2016.08.003. [DOI] [Google Scholar]
- Krishnaiah D, Rosalam S, Rajesh N. Microencapsulation of Morinda citrifolia L. extract by spray drying. Chem Eng Res Des. 2012;90:622–632. doi: 10.1016/j.cherd.2011.09.003. [DOI] [Google Scholar]
- Lee OH, Lee BY, Lee J, Lee HB, Son JY, Park CS, Shetty K, Kim YC. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour Technol. 2009;100:6107–6113. doi: 10.1016/j.biortech.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Linstrom PJ, Mallard WG (2000) NIST chemistry web book, NIST Standard Reference Database Number 69
- Mazutti M, Mossi AJ, Cansian RL, Corazza ML, Dariva C, Oliveira JV. Chemical profile and antimicrobial activity of boldo (Peumus boldus Molina) extracts obtained by compressed carbon dioxide extraction. Braz J Chem Eng. 2008;25:427–434. doi: 10.1590/S0104-66322008000200020. [DOI] [Google Scholar]
- Mustafa A, Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal Chim Acta. 2011;703:8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Osorio-Tobón JF, Carvalho PIN, Rostagno MA, Petenate AJ, Meireles MAA. Extraction of curcuminoids from deflavored turmeric (Curcuma longa L.) using pressurized liquids: process integration and economic evaluation. J Supercrit Fluids. 2014;95:167–174. doi: 10.1016/j.supflu.2014.08.012. [DOI] [Google Scholar]
- Pardo-Castaño C, Velásquez M, Bolã G. Simple models for supercritical extraction of natural matter. J Supercrit Fluids. 2015;97:165–173. doi: 10.1016/j.supflu.2014.09.044. [DOI] [Google Scholar]
- Piva GS, Weschenfelder TA, Franceschi E, Cansian RL, Paroul N, Steffens C. Extraction and modeling of flaxseed (Linnum usitatissimum) oil using subcritical propane. J Food Eng. 2018;228:50–56. doi: 10.1016/j.jfoodeng.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian N, Jafari SM, Wani TA. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci Technol. 2015;42:150–172. doi: 10.1016/j.tifs.2014.12.009. [DOI] [Google Scholar]
- Rodrigues MRA, Krause LC, Santos JG, Dariva C, Oliveira JV, Caramao EB. Chemical composition and extraction yield of the extract of Origanum vulgare obtained from sub- and supercritical CO2. J Agric Food Chem. 2004;52:3042–3047. doi: 10.1021/jf030575q. [DOI] [PubMed] [Google Scholar]
- Setyaningsih W, Saputro IE, Barbero GF, Palma M, García Barroso C. Determination of melatonin in rice (Oryza sativa) grains by pressurized liquid extraction. J Agric Food Chem. 2015;63:1107–1115. doi: 10.1021/jf505106m. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Ravents RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1998;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sodeifian G, Ghorbandoost S, Sajadian SA, Saadati Ardestani N. Extraction of oil from Pistacia khinjuk using supercritical carbon dioxide: experimental and modeling. J Supercrit Fluids. 2016;110:265–274. doi: 10.1016/j.supflu.2015.12.004. [DOI] [Google Scholar]
- Sovová H. Rate of the vegetable oil extraction with supercritical CO2—I modeling of extraction curves. Chem Eng Sci. 1994;49:409–414. doi: 10.1016/0009-2509(94)87012-8. [DOI] [Google Scholar]
- Taamalli A, Arráez-Román D, Barrajón-Catalán E, Ruiz-Torres V, Pérez-Sánchez A, Herrero M, Ibañez E, Micol V, Zarrouk M, Segura-Carretero A, Fernández-Gutiérrez A. Use of advanced techniques for the extraction of phenolic compounds from Tunisian olive leaves: phenolic composition and cytotoxicity against human breast cancer cells. Food Chem Toxicol. 2012;50:1817–1825. doi: 10.1016/j.fct.2012.02.090. [DOI] [PubMed] [Google Scholar]
- Xie PJ, Huang LX, Zhang CH, You F, Zhang YL. Reduced pressure extraction of oleuropein from olive leaves (Olea europaea L.) with ultrasound assistance. Food Bioprod Process. 2015;93:29–38. doi: 10.1016/j.fbp.2013.10.004. [DOI] [Google Scholar]
- Xynos N, Papaefstathiou G, Psychis M, Argyropoulou A, Aligiannis N, Skaltsounis AL. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J Supercrit Fluids. 2012;67:89–93. doi: 10.1016/j.supflu.2012.03.014. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]