Abstract
Incorporation of Spirulina in milk as thermally dried powder has the disadvantages of non-uniform distribution with undesirable odor and flavor. Through homogenization (200 ± 10 bar), complete dispersion of fresh Spirulina biomass (7% w/w) in milk was achieved and thereafter a carotenoid enriched probiotic yogurt was developed. Confocal microscopy revealed porous Spirulina-milk protein matrix integrated with smaller fat globules in the yogurt. Spirulina led to a 29.56% increase in Lactobacillus acidophilus count, a 20% reduction in fermentation time and a total probiotic count of 1.2 × 107 CFU mL−1. The protein, total chlorophyll, total carotenoid and β-carotene content (on dry w/w basis) were 3.58 ± 0.08 g 100 g−1, 0.407 ± 0.018 mg g−1, 0.235 ± 0.016 mg g−1 and 13.28 ± 0.08 µg g−1, respectively. During storage (18 days at 6–8 °C), the L. acidophilus count reached 8.83 ± 0.11 log CFU mL−1 with 103.03% increase in the viability by day three and the yogurt retained 71.5% carotenoids. The probiotc Spirulina yogurt was found to be acceptable to consumers as evaluated by affective consumer test.
Keywords: Spirulina platensis, Homgenization, Fresh biomass, Probiotic, Fermentation, β-Carotene
Introduction
The increasing health awareness and preference for natural ingredients has led to an increased consumer base for natural foods with health benefits like probiotics. The fermented milk products like yogurt are considered as a preferred vehicle for probiotics. According to Food and Agriculture Organization (FAO) and World Health Organization (WHO) (2001), probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”. The probiotic products provide various clinically proven health benefits like modulation of host’s immune system, anti-carcinogenic and anti-mutagenic activity, alleviation of lactose intolerance etc. (Kailasapathy and Chin 2000). To achieve beneficial health effects and desirable functional properties from probiotics, sufficient number of probiotic lactic acid bacteria (LAB) at high viable rate must reach the intestine. In India, according to the regulatory body, Food Safety and Standards Authority of India (FSSAI), the standard requirement of lactic count in probiotic products is one million CFU g−1 (FSSAI 2011). It is essential to maintain a high level of probiotic cell count (> 106 CFU g−1) in probiotic products throughout their shelf life (Donkor et al. 2006). Spirulina sp., a photoautotrophic blue green microalga, is widely recognized as a food and dietary supplement owing to its excellent nutritive value. Spirulina contains about 63% protein, 18% carbohydrate and 4% fats. Spirulina is also a rich source of carotenoids as it contains (on dry w/w basis) about 0.5% total carotenoids including about 0.2% β-carotene, the pro-vitamin A (Gershwin and Belay 2007).
The bioavailability of β-carotene from Spirulina has been established both in animal and human studies (Annapurna et al. 1991a, b). In developing countries like India, the malnutrition and the vitamin A deficiency among pre-school children is still a major public health challenge (Arlappa 2011). Another major issue affecting the nutritional health of pre-school children has been identified as the frequent bouts of diarrhea in rural areas of India (Boudraa et al. 2001). Yogurt has been reported to be an important food product that can help in dealing with the occurrence of diarrhea among pre-school children (Boudraa et al. 2001). The Spirulina yogurt would offer a unique combination of probiotic benefits and enhanced carotenoid/β-carotene content to combat diarrhea and deficiency of vitamin A prevalent among the pre-school children, especially in the rural areas. The beneficial effect of addition of Spirulina biomass on microbiological viability and growth of lactic acid and probiotic bacteria in fermented milk and yogurt has been reported in several studies (De Caire et al. 2000; Varga et al. 2002; Beheshtipour et al. 2012). Beheshtipour et al. (2013) suggested that combination of probiotic bacteria with Spirulina platensis in fermented milk products enhances the functional and nutritional properties of the product. The Spirulina biomass could, therefore, serve as an important food ingredient for preparation of fermented milk/yogurt products to take benefit of its growth promoting effect on probiotic bacteria. However, such Spirulina containing milk products are not widely available in the market.
It is understood from the available literature that the studies on addition of Spirulina biomass in fermented milk and yogurt products, have been carried out with dry biomass. The spray dried Spirulina biomass poses major sensorial challenges in the form of a characteristic odor which descends into the final product and its insolubility in food formulations (Beheshtipour et al. 2013) thus limiting a wider consumer acceptance. Also, the use of dried Spirulina biomass puts a limitation on harnessing the maximum nutritional benefits of the microalga as thermal drying methods have considerable effect on its micronutrient contents, e.g., vitamins, β-carotene and amino acids (Dillon et al. 1995). The utilization of fresh biomass of Spirulina for product formulation may help in overcoming the sensorial perception associated with Spirulina while providing its inherent nutritional benefits. However, as evident from the literature, the potential benefits of fresh Spirulina biomass for food product development have remained unexplored.
The present study, therefore, aims at incorporation of fresh biomass of Spirulina for formulating a probiotic yogurt. It was hypothesized that the incorporation of fresh biomass of Spirulina would lead to a yogurt with an enhanced probiotic count, enriched carotenoids and acceptable sensory properties. The growth and viability of probiotic bacteria; and the nutritional, biochemical and physico-chemical properties were investigated along with affective consumer test based sensory evaluation.
Materials and methods
Fresh Spirulina platensis biomass and other ingredients
Spirulina platensis (strain SP6/CFTRI) was cultivated using Zarrouk’s medium in pilot scale outdoor open raceway pond (1000L capacity) at CSIR-CFTRI, Mysuru, India. The fresh wet biomass was harvested by passing the culture through nylon filter cloth, 400 micron mesh (Nylobolt®, Dishti Industries Pvt. Ltd., Maharashtra, India). The retained biomass was washed with deionized water. The term ‘Spirulina biomass’ wherever appears in the manuscript, implies ‘fresh wet biomass’. The other ingredients included toned milk (commercially available from Nandini Dairy, Karnataka Co-operative Milk Producer Federation (KMF) Ltd., India), skimmed milk powder (SMP) (Sagar, Amul, India) and sucrose (Parry White Label, India).
Preparation of yogurt starter and probiotic stock culture
Freeze dried commercial yogurt (FD-DVS YC-X16 Yo-Flex; Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus) and probiotic culture (FD-DVS nu-trish LA5, Lactobacillus acidophilus) were used (kind gift from CHR Hansen Pvt. Ltd., Mumbai, India). The stocks of both the starter and yogurt culture were freshly prepared separately under aseptic conditions by adding 5 g of Yo-Flex and 25 g of LA5 in 1 kg of sterile reconstituted skimmed milk (10% w/w) respectively, as per the instructions of the suppliers.
Formulation of Spirulina supplemented probiotic yogurt
The Spirulina biomass (85–90% moisture content) was added to toned milk at a concentration of 10% (w/w) to obtain Spirulina-milk slurry. The Spirulina-milk slurry was homogenized using a homogenizer (GEA NiroSoavi, Italy) at 200 ± 10 bar to obtain Spirulina-milk emulsion. The toned milk served as control and was also subjected to homogenizaton. The Spirulina-milk emulsion (10% w/w Spirulina biomass) was diluted using toned milk to obtain aliquots having 6, 7, 8 and 9% (w/w) Spirulina biomass respectively. To the control milk and Spirulina-milk emulsion aliquots, sugar (6% w/w) and skimmed milk powder (SMP, 1% w/w) were added followed by pasteurization at 90 °C for 10 min in a water bath. The pasteurized samples (100 mL each) were poured into pre-sterilized polystyrene cups and were allowed to cool down to 40 °C. The control milk and Spirulina-milk emulsion aliquots (6–10% w/w) were inoculated with 0.5% and 1.5% (v/w) of Yo-Flex and LA-5 stock cultures respectively. Fermentation method as described by Shah and Lankaputhra (1997) was followed. All the samples were covered tightly with aluminum foil, placed in sealed low density polyethylene (LDPE) bags and incubated at 42 °C. The samples were monitored hourly until the acidity and pH reached 0.8–0.9% and 4.6–4.7, respectively. The prepared yogurt samples were stored under refrigeration at 6–8 °C. The addition of food additives such as stabilizers, acidity regulators, flavors and colors was avoided as the focus of the study was on exploring the variation in physico-chemical properties of yogurt solely due to incorporation of fresh Spirulina biomass. The formulated yogurt products were referred to as; (a) Probiotic Control Yogurt (PCY) derived from toned milk subjected to homogenization and (b) Probiotic Spirulina Yogurt derived from Spirulina-milk emulsion (PSY, containing 6–10% w/w Spirulina biomass).
Microbiological analysis
Enumeration of L. acidophilus
The cell population of L. acidophilus was enumerated hourly, after inoculation, for pasteurized milk and Spirulina-milk emulsion, starting at time zero till the end of fermentation when the desired pH was achieved. The samples (1 mL aliquots) were diluted with 9 mL sterile peptone water (Hi-media, India) and subsequent serial dilutions were performed. The enumeration of L. acidophilus was done in accordance with ISO 20128 (2006) method using Lactobacillus MRS agar (Hi-Media, India). Stock solutions of Clindamycin hydrochloride and Ciprofloxacin hydrochloride (Hi-media, India) antibiotics were prepared by dissolving 2 mg and 20 mg of respective antibiotic in 10 mL of sterile water, and filtering through a sterile filter (0.22 microns pore size) (Hi-media, India). The Clindamycin and Ciprofloxacin antibiotic solutions were added to the sterile MRS agar medium at a concentration of 0.05 and 0.5% (v/v), respectively, to selectively allow the growth of L. acidophilus. Aliquots of serially diluted samples (100 µL) were spread on antibiotics containing MRS agar Petri plates and incubated under anaerobic condition at 37 °C for 72 h. The percentage increase/decrease in growth and viability of L. acidophilus during fermentation and storage respectively, was determined from final and initial cell population (CFU mL−1) as described by Beheshtipour et al. (2012).
Enumeration of other microorganisms
Total Plate Count agar (TPC, Hi-media, India) was used to enumerate total bacterial count in the products. Violet Red Bile agar (VRB, Hi-media, India) was used to determine the presence of Coliform bacteria and Rose Bengal Chloramphenicol agar (RBC, Hi-media, India) was used to determine the presence of yeast and mold in the products.
Physico-chemical analysis
pH and acidity
The aliquots of yogurt (1 mL) were mixed with distilled water in a ratio of 1:1 to measure the pH (Eutech pH Tutor, Singapore). The kinetic parameters described by Kristo et al. (2003), i.e., the maximum acidification rate (Vm, mUnit-pH min−1) and time taken to attain pH 4.6–4.7 (Te) were studied. The Vm was calculated from pH versus time (hours) curves using the equation . Total titrable acidity of the products expressed as percent lactic acid was determined through titration with 0.1 N sodium hydroxide (NaOH) solution using phenolphthalein indicator (ISO 11869 2012).
Viscosity measurement
The aliquots of 80 mL refrigerated yogurt were poured in 100 mL glass beakers and stirred with a glass rod (10 times clockwise and 10 times anticlockwise) to break the gel. The viscosity was measured by Brookfield RVDV-II pro viscometer (Brookfield, U.S.A.) using Helipath stand configuration (T-spindle, type D) at 2.5 rpm for 1 min at 8 °C (torque ranging between 10 and 90%). The mean of apparent viscosity values (in cP) was obtained from triplicate samples with a deviation of generally less than 5%.
Water holding capacity (WHC)
The water holding capacity was measured by weighing 20 g of yogurt into 50 mL tubes and the samples were centrifuged (Sorvall Legend X1R, Thermo Scientific, Germany) at 3000 g for 15 min at 4 °C. The supernatant was collected and weighed. Water holding capacity was calculated using the following equation;
where M2(g) is weight of whey after centrifugation and M1(g) is the initial weight of the yogurt.
Syneresis
The syneresis was estimated as described by Chawla and Balachandran (1994) using drainage method. The prepared yogurt samples stored under refrigeration for about 16 h were taken out and tempered at 25 °C for 2 h. The yogurt samples in polystyrene cups were loosened gently from the sides and emptied into the funnels (9 cm diameter) holding filter paper (Whatman, grade 1, 47 mm diameter) and the exudates were collected in measuring cylinders. The total volume (in mL) collected after 2 h of drainage was measured.
Texture profile analysis (TPA)
Texture profile analysis (TPA) was performed using texture analyzer (Universal Texture Machine, Model LR-5K, Lloyd Instrument Ltd., U.K.) using a cylinder probe (13 mm diameter). The prepared yogurt samples were filled (about 120 mL) up to 5 cm height in a glass beaker (10 cm height and 6 cm diameter) followed by tempering at 25 °C for 2 h prior to analysis. The probe was programmed to penetrate down to 10 mm (at 20% compression) depth into the set yogurt at a crosshead speed of 1 mm per second. The firmness of products was calculated from the obtained profile using Nexygen software (Lloyd material testing, West Sussex, U.K.).
Confocal laser scanning microscopy
The confocal laser scanning microscope (ZEISS LSM 700, Germany), operating in fluorescence mode, was used to study the microstructure of milk and prepared probiotic yogurt samples as described by Ciron et al. (2010). Acridine orange and Nile red fluorescent dyes (Sigma Aldrich, U.S.A.) were used to visualize protein and fat phases, respectively. The acridine orange dye was prepared in a concentration of 0.2% (w/w) in distilled water and Nile red dye was prepared by dissolving 10 mg in 100 mL of acetone. To 50 mL of toned milk, control milk (toned milk subjected to homogenization as described above), Spirulina-milk slurry, Spirulina-milk emulsion, probiotic control yogurt (PCY) and probiotic Spirulina yogurt (7% w/w, PSY) samples, 300 µL of acridine orange and 10 µL of Nile red dyes were added. The images were obtained using 63 × oil-immersion objective lens (numerical aperture = 1.4) at an excitation wavelength of 488 nm and 633 nm provided by argon and helium/neon lasers, respectively.
Biochemical analysis
Compositional analysis
The moisture content (%) of prepared yogurt samples was determined by oven drying. The total solids were expressed as the weight of the dried products. Total ash content was determined by calcination at 550 °C until constant weight. The ash was used for the estimation of mineral elements (sodium, potassium, calcium, magnesium, iron, zinc and copper) by procedure ISO 5984 (2002) using flame emission atomic absorption spectroscopy (AAS) (iCE 3000AA, Thermo Scientific, U.S.A.). The concentrations of elements were determined with respect to their respective standard solution (SRL, India) plots. Crude protein content was determined by calculating the percentage of nitrogen using Kjeldahl method and converted to crude protein by multiplying with a factor of 6.25 (Chronakis et al. 2000). Total carbohydrate content was estimated by phenol-sulfuric acid method (Singleton and Rossi 1965). Total fat content was estimated by Rose-Gottlieb method (AOAC 2000). All the estimations were carried out in triplicates. The total energy of the yogurt samples was calculated in accordance with the factor given by FAO for milk and milk products (FAO 2003).
Pigment analysis
The yogurt samples obtained after fermentation were freeze dried (Cool safe 55-4 pro, Scanvac, Denmark) and estimated for total chlorophyll (AOAC 1995) and carotenoids (Jensen 1978). To determine the concentration of β-carotene, HPLC analysis of the carotenoid extract of freeze dried yogurt was carried out as described by Ranga Rao et al. (2007). The extracts were dried by nitrogen evaporator (N-EVAP 111, Organomation Associates, U.S.A.) and 10 µL of pigment extract was injected into HPLC system (Shimadzu, LC-10A, Japan) equipped with C-18 column in isocratic mode and photo diode array detector (PDA). The mobile phase consisted of acetonitrile, dichloromethane and methanol (70:20:10 v/v/v) at a flow rate of 1 mL min−1. The pigments were detected by measuring the maximum absorbance over wavelength ranging from 370 to 800 nm. The chromatogram peak corresponding to β-carotene for the products was identified by comparing its retention time with respect to that of β-carotene standard solution (Sigma Aldrich, U.S.A.) chromatogram.
Sensory evaluation: affective (consumer) test
The sensory properties of freshly prepared probiotic control yogurt (PCY) and probiotic Spirulina yogurt (PSY, 6–10% w/w) stored under refrigeration at 6–8 °C were evaluated using the affective (consumer) test (Drake 2007). A total of 55 consumers (30 males and 25 females) between the ages of 22–50 years who were the regular consumers of yogurt products participated in the study. All the participants were served with an approximately 15 g of PCY and PSY 6–10% (w/w) placed in transparent polystyrene plastic cups covered with lids and labeled with random 3 digit codes. A 7-point degree of liking scale was used and consumers were instructed to cleanse their mouths with water between each of the samples tasted. Consumers were asked to rate their degree of like/dislike towards all six of the served samples in terms of; Like Extremely, Like Moderately, Like Slightly, Neither Like or Dislike, Dislike Slightly, Dislike Moderately and Dislike Extremely, respectively. The results were expressed in terms of percentage population distribution for each of the categories of likes and dislikes. The PSY sample with the majority liking was chosen for storage studies.
Storage studies
The prepared PSY samples were stored under refrigeration (6–8 °C) for 18 days. On every third day, the samples were analyzed for pH and titrable acidity, L. acidophilus count, total plate count, and total Coliform bacteria and, yeast and mold counts. The total chlorophyll and carotenoid content of the products was estimated on every sixth day. The affective (consumer) test for the sensory evaluation of the PSY was carried out by the same consumer panel as per the method described in the previous section on every third day.
Statistical analysis
All the microbiological, physico-chemical and biochemical determinations were performed in triplicates and the values were represented as mean ± S.D. The difference within the groups was statistically analyzed by using one way ANOVA followed by Tukey-Kramer multiple comparison test at significance level of P < 0.05. The difference between the groups was statistically analyzed by using two way ANOVA followed by Bonferroni post-tests to compare replicate means by row at significance levels of P < 0.001, P < 0.01 and P < 0.05 (GraphPad Prism 5.0, U.S.A.).
Results and discussion
Preparation of yogurt
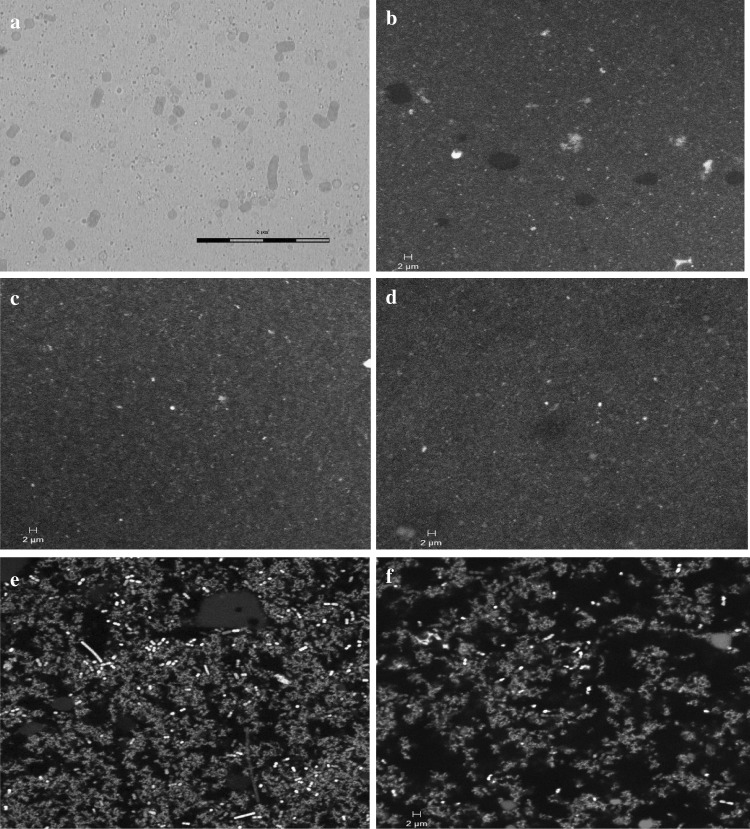
The homogenization of Spirulina-milk slurry was carried out to achieve disruption of Spirulina cells as well as for the uniform mixing and distribution of cellular contents of Spirulina into milk to obtain a Spirulina-milk emulsion. The fragile nature of S. platensis cell wall, composed mainly of murein and no cellulose, makes the disruption relatively easier. In the present study, a pressure value of 200 ± 10 bar was used for the homogenization of 10% (w/w) Spirulina biomass in milk and was found to be sufficient to achieve complete disruption of Spirulina cells. The microscopic observation of the Spirulina-milk emulsion showed disrupted cells and fragments of Spirulina (Fig. 2a).
Fig. 2.
a Photomicrograph of homogenized Spirulina-milk slurry showing disrupted cells and fragments of Spirulina platensis, Scale bar = 8 µm. Representative confocal micrographs, b toned milk, c control (toned milk subjected to homogenization), dSpirulina-milk emulsion, e probiotic control yogurt (PCY) and f probiotic Spirulina yogurt with 7% (w/w) fresh Spirulina biomass (PSY). The green region depicts protein matrix and the red region depicts fat globules, Scale bar = 2 µm (color figure online)
The physico-chemical properties of the yogurts prepared in the study, i.e., probiotic control yogurt (PCY) and probiotic Spirulina yogurt (PSY), are shown in Table 1. For probiotic Spirulina yogurts (PSY), a significant decrease in WHC (91.58 ± 0.07–79.42 ± 0.21%) and apparent viscosity (45,400 ± 270–40,200 ± 286 cP) was observed with an increase in biomass concentration (6–10% w/w). The firmness index declined significantly (0.59 ± 0.74–0.24 ± 0.37 N) with respect to control as the biomass concentration was increased beyond 7% (w/w). PCY was comparatively firmer (2.16 ± 0.12 N), more viscous (47,045 ± 326 cP) and possessed better water retention capacity (92.82 ± 0.08%).
Table 1.
Physico-chemical and biochemical characteristics; L. acidophilus and total plate count of probiotic control yogurt (PCY) and probiotic Spirulina yogurt (PSY, 6–10% w/w)
| Physico-chemical characteristics | Biochemical characteristics | Microbiological parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | WHC (%) | Syneresis (mL) | Viscosity (cP) | Firmness (N) | Total Chlorophyll Content (mg g−1 DW) | Total Carotenoid Content (mg g−1 DW) | L. acidophilus Count (log CFU mL−1) | Total Plate Count (log CFU mL−1) | |
| PCY | 92.82 ± 0.08a | 25.7 ± 0.02a | 47,045 ± 326a | 2.16 ± 0.12a | 0.048 ± 0.023a | 0.015 ± 0.014a | 7.96 ± 0.14a | 8.86 ± 0.14a | |
| PSY (6% w/w) | 91.58 ± 0.07b | 25.4 ± 0.01b | 45,400 ± 270b | 1.37 ± 0.34a,b | 0.360 ± 0.024b | 0.124 ± 0.018b | 8.35 ± 0.04b | 9.18 ± 0.08b | |
| PSY (7% w/w) | 88.74 ± 0.10c | 25.6 ± 0.02c | 43,067 ± 285c | 0.91 ± 0.22a,b | 0.407 ± 0.018b | 0.235 ± 0.016c | 8.32 ± 0.15b | 9.19 ± 0.11b | |
| PSY (8% w/w) | 85.18 ± 0.18d | 26.0 ± 0.01d | 42,000 ± 322d | 0.59 ± 0.74b | 0.488 ± 0.015c | 0.280 ± 0.003d | 8.36 ± 0.06b | 9.05 ± 0.13a,b | |
| PSY (9% w/w) | 83.42 ± 0.14e | 26.2 ± 0.02e | 41,400 ± 264d | 0.38 ± 0.86b | 0.520 ± 0.022c | 0.297 ± 0.020d,e | 8.47 ± 0.18b | 8.96 ± 0.10a,b | |
| PSY (10% w/w) | 79.42 ± 0.21f | 26.8 ± 0.03f | 40,200 ± 286e | 0.24 ± 0.37b | 0.636 ± 0.017d | 0.328 ± 0.016e | 8.90 ± 0.12c | 8.90 ± 0.06a,b | |
Data represents mean ± S.D. of three independent experiments. The superscripts (letters a–f) denotes significant differences. Mean values within the same column sharing the same superscript are statistically not significant (at P < 0.05) by one way ANOVA
Lopez-Fandino (2006) observed weaker and less firm gels for yogurts prepared from milk treated with high pressure (1000 bar) with added whey protein concentrate. The authors indicated high pressure induced denaturation of long β-lacto-globulin (Lg) bridges of milk proteins for this effect. In the present study, the pressure values used for homogenization were significantly lower (200 ± 10 bar) and weaker, less firm gels were observed only with Spirulina supplemented yogurt (PSY) and not with control yogurt (PCY). It was thus concluded that it’s not the homogenization pressure which affected the texture and firmness of yogurt, the effect might have been due to the incorporation of fresh Spirulina biomass.
Sensory evaluation
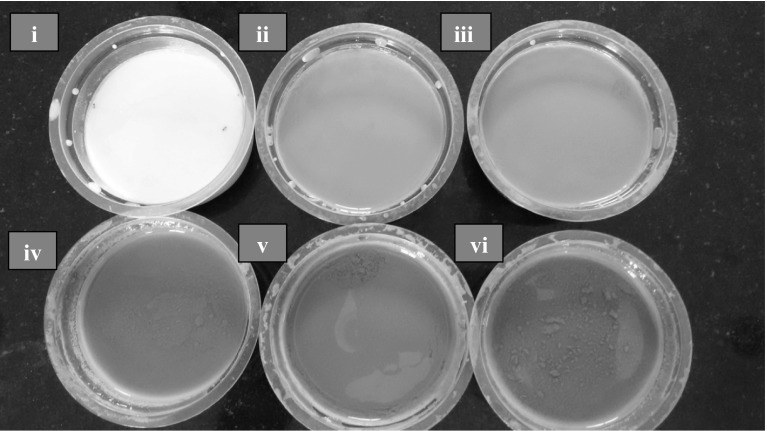
The photographs of the developed yogurts are presented in Fig. 1a. The PCY and PSY 6 and 7% (w/w) samples had a set consistency (Fig. 1i–iii) whereas with PSY 8% (w/w) onwards the yogurt showed a loose consistency (Fig. 1iv–vi). A rating of ‘Like Extremely’ was given to PCY by 82% of the consumers and to PSY (6 and 7% w/w) by 72 and 78% of the consumers, respectively. On the other hand, PSY 8% (w/w) was rated as ‘Dislike Moderately’ by 66% of the consumers. The Spirulina biomass concentration in PSY was maintained ≤ 10% (w/w) as concentrations higher than 10% (w/w) led to coagulation during pasteurization. PSY 9 and 10% (w/w) samples were ‘Extremely Disliked’ by the majority of consumers (80 and 92%, respectively) which might have been due to the after taste, enhanced greenish appearance and a watery kind of consistency. However, unlike dried biomass powder based Spirulina yogurt (Beheshtipour et al. 2012), it was observed that fresh biomass based Spirulina yogurts in the present study possessed a smooth texture with the absence of graininess at all the biomass concentrations. Based on the physico-chemical properties and affective (consumer) test, the PSY 7% (w/w) Spirulina biomass was chosen for storage studies.
Fig. 1.
Photograph of probiotic yogurt products developed (i) probiotic control yogurt (PCY); (ii)–(vi) probiotic Spirulina yogurt (PSY) with fresh Spirulina biomass 6, 7, 8, 9 and 10% (w/w) respectively (color figure online)
Confocal laser scanning microscopy
The confocal laser scanning microscopy was carried out for control (homogenized toned milk), Spirulina-milk slurry, Spirulina-milk emulsion, PCY and 7% (w/w) PSY. At least three images were obtained for each sample and the representative micrographs are presented in Fig. 2b–f. Conventional homogenization has been reported to provide a uniform distribution of milk constituents in low fat yogurts by reducing the size of fat globules (Ciron et al. 2010). In the present study, similar observations were made with the control milk and Spirulina-milk emulsion; both subjected to homogenization at 200 ± 10 bar. In both these samples (Fig. 2c and d), bigger fat globules were observed in control milk (Fig. 2b), were broken into smaller ones as a result of homogenization and the protein matrix appeared more porous. The similar effect of homogenization was observed for PCY and 7% (w/w) PSY (Fig. 2e and f).
Acidification kinetics during fermentation
During the process of yogurt development, the complete precipitation of caseinate particles occurs over a pH range of 4.6–4.7 (Rasic and Kurmann 1978). Hence, in the present study the end point of fermentation was set as time taken to attain pH 4.6–4.7 (Te). The acidification and pH profile of PCY and 7% (w/w) PSY presented in Fig. 3a shows an increase in acidity and decrease in pH during fermentation. It was observed that during the initial stage of fermentation (up to 1 h), the change in pH and acidity was minimal for both PCY and PSY. The pH decreased from 6.51 to 6.44 for PCY and from 6.56 to 6.35 for PSY; and increase in acidity was 0.21–0.22% for PCY and 0.24–0.25% for PSY. These initial changes could be attributed to relatively high buffering capacity of milk and lag phase of L. acidophilus growth (Beheshtipour et al. 2012). The maximum drop in pH for PCY was 0.38 during 5th to 6th hour while for PSY it was 0.47 during 4th to 5th hour. During these hours, maximum acidity increased in the same fashion for both PCY and PSY. The ‘Te’ to attain the optimal pH of 4.6–4.7 for the PCY and PSY was at the end of 6th and 5th hour of fermentation respectively. This showed that incorporation of Spirulina biomass decreased the fermentation time by 20%. It was also observed that fermentation in case of PSY had higher maximum acidification rate of 6.16 mUnit-pH min−1 while for PCY it was 5.16 mUnit-pH min−1. Oliveira et al. (2001) have reported a similar trend for milk supplemented with casein hydrolysate.
Fig. 3.
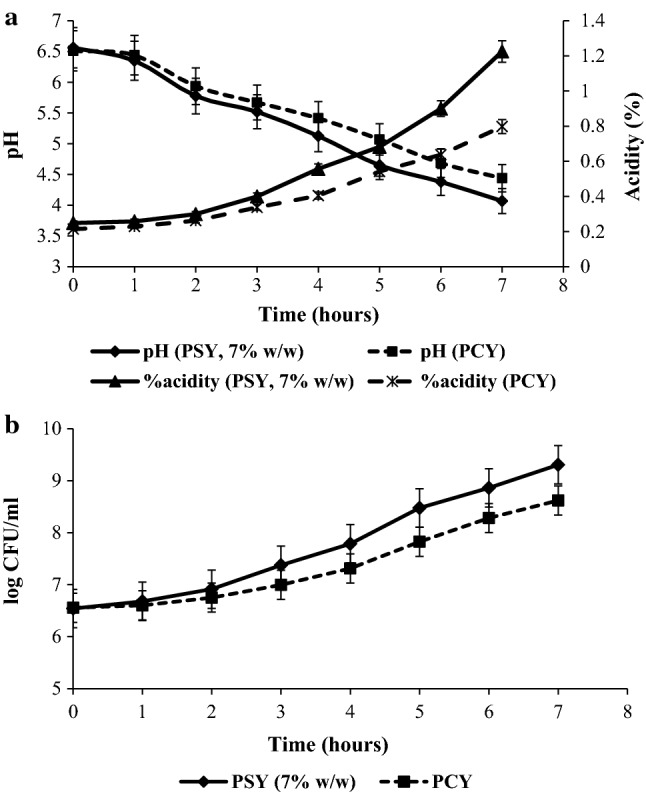
Probiotic control yogurt (PCY) and probiotic Spirulina yogurt (PSY, 7% w/w) during fermentation a pH and acidity profile b growth profile of L. acidophilus (error bars represent standard deviation)
Microbiological analysis
At the time of inoculation (0 h) the population of L. acidophilus was 6.55 log CFU mL−1 and the final count after fermentation differed significantly between PCY (7.96 ± 0.14 log CFU mL−1) and PSY 6–10% (w/w) which was higher than 8 log CFU mL−1 (8.35 ± 0.04–8.9 ± 0.12) (Table 1). The growth of L. acidophilus during fermentation for PCY and 7% (w/w) PSY up to 7 h of fermentation is shown in Fig. 3b. The initial increase in the growth of L. acidophilus (after 2 h of fermentation) was 5.7% for PSY and 2.97% for PCY respectively. After 4 h, the growth promotion was 19.05% for PSY and 11.53% for PCY. The desired pH value of 4.6–4.7 was attained after 5 h of fermentation in PSY and the growth promotion was 29.56% whereas in PCY the desired pH was achieved after 6 h of fermentation and the growth promotion was 26.31%. Microalgae have been reported to stimulate the growth of probiotic bacteria, increase their viability and acid production by providing essential compounds such as adenine, hypoxanthine and free amino acids (Beheshtipour et al. 2013). Both the PCY and PSY did not show the presence of yeast, mold and coliform bacteria. The present study, therefore, shows that incorporation of fresh biomass of Spirulina has a growth promoting effect on probiotic microorganisms.
Biochemical analysis
The Spirulina contains a rich profile of carotenoids comprising of β-carotene and xanthophylls like zeaxanthin and incorporation of fresh biomass of Spirulina led to a carotenoid enriched probiotic yogurt. The PCY prepared with milk alone showed a base level of total carotenoid content as 0.015 ± 0.014 mg g−1 (DW) and total chlorophyll content as 0.048 ± 0.023 mg g−1 (DW). For PSY, the incorporation of Spirulina biomass at 6–10% (w/w) significantly increased the carotenoid content of yogurt from 0.124 ± 0.018–0.328 ± 0.016 mg g−1 (DW), a 8–22 fold increase from base level; and total chlorophyll content from 0.36 ± 0.024–0.636 ± 0.017 mg g−1 (DW), a 7.5–13.3 fold increase from base level, respectively (Table 1). The consumer acceptable 7% (w/w) PSY was found to contain 13.28 ± 0.08 µg g−1 (DW) of β-carotene. The RDA of β-carotene for an adult Indian man and woman is 4800 µg day−1 and for children (4–6 years) is 3200 µg day−1 (ICMR 2010). The 100 g serving size of 7% (w/w) PSY would thus meet 27.30% and 40.93% of β-carotene RDA for Indian adults and children, respectively. The energy and proximate composition in terms of carbohydrate, ash and fat content of 7% (w/w) PSY was not significantly different from PCY except for a comparatively higher protein content of 3.58 ± 0.008% than PCY (3.01 ± 0.007%). The mineral content of PSY was comparable to PCY except for a slightly higher content of magnesium (24.62 ± 0.16 mg 100 g−1).
Storage studies
Total plate count (TPC), percentage viability and count of L. acidophilus, pH and acidity
The microbiological and physico-chemical profile of PCY and 7% (w/w) PSY during storage at 6–8 °C is presented in Table 2. The TPC count for PCY increased up to 9.09 ± 0.09 log CFU mL−1 (on day 6 of storage) which was significantly higher than in PSY. In case of PSY, the TPC count continued to decrease and near the end of storage period (day 15 and 18), it was significantly lower than that of PCY. This might be due to the growth promoting effect of supplementation of Spirulina biomass on probiotic microorganisms making their growth dominant over other bacteria. In PCY, the initial lag phase of the growth of probiotic L. acidophilus (Table 2) might have contributed towards the higher growth of the other bacteria. Also, during the entire storage period, both the PSY and PCY did not show any growth of yeast, mold and coliform organisms. It has been suggested that pathogenic and spoilage microorganisms are not able to survive in the fermented milks due to the high acidity and low levels of oxygen and production of antimicrobial compounds by the starter cultures in these products (Northolt 1983; Varga et al. 2002).
Table 2.
Changes in physico-chemical characteristics and microbiological parameters of probiotic control yogurt (PCY) and probiotic Spirulina yogurt (PSY, 7% w/w) during storage at 6–8 °C
| Day of storage | Physico-chemical characteristics | Microbiological parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | Acidity (%) | L. acidophilus count (log CFU mL−1) | Increase/decrease in viability of L. acidophilus (%)# | Total plate count (log CFU mL−1) | ||||||
| PCY | PSY (7% w/w) | PCY | PSY (7% w/w) | PCY | PSY (7% w/w) | PCY | PSY (7% w/w) | PCY | PSY (7% w/w) | |
| 0 | 4.55 ± 0.05 | 4.43 ± 0.02** | 0.76 ± 0.02 | 0.84 ± 0.02*** | 8.12 ± 0.11 | 8.57 ± 0.13* | 100.00 | 100.00 | 8.81 ± 0.16 | 8.93 ± 0.14 n.s. |
| 3 | 4.53 ± 0.03 | 4.34 ± 0.03*** | 0.78 ± 0.01 | 0.88 ± 0.03*** | 8.23 ± 0.14 | 8.83 ± 0.11*** | 101.35 | 103.03 | 8.96 ± 0.07 | 8.84 ± 0.09 n.s. |
| 6 | 4.50 ± 0.10 | 4.30 ± 0.01*** | 0.79 ± 0.02 | 0.90 ± 0.02*** | 8.11 ± 0.22 | 8.66 ± 0.16** | 99.88 | 101.05 | 9.09 ± 0.09 | 8.73 ± 0.16* |
| 9 | 4.48 ± 0.02 | 4.25 ± 0.01*** | 0.81 ± 0.01 | 1.07 ± 0.00*** | 7.86 ± 0.08 | 8.12 ± 0.21 n.s. | 96.80 | 94.75 | 9.02 ± 0.13 | 8.66 ± 0.18* |
| 12 | 4.45 ± 0.01 | 4.23 ± 0.01*** | 0.82 ± 0.00 | 1.12 ± 0.01*** | 7.71 ± 0.15 | 7.84 ± 0.24 n.s. | 94.95 | 91.48 | 8.92 ± 0.17 | 8.58 ± 0.14 n.s. |
| 15 | 4.43 ± 0.02 | 4.19 ± 0.02*** | 0.83 ± 0.01 | 1.17 ± 0.01*** | 7.43 ± 0.12 | 7.58 ± 0.16 n.s. | 91.50 | 88.45 | 8.83 ± 0.09 | 8.43 ± 0.21* |
| 18 | 4.41 ± 0.03 | 4.17 ± 0.01*** | 0.84 ± 0.02 | 1.22 ± 0.00*** | 6.98 ± 0.15 | 7.08 ± 0.19 n.s. | 85.96 | 82.61 | 8.65 ± 0.18 | 8.30 ± 0.16* |
Data represents mean ± S.D. of three independent experiments (except #). The mean values of PSY were compared against PCY by two way ANOVA, Bonferroni’s multiple comparison test; ***P < 0.001, **P < 0.01, *P < 0.05, n.s. (not significant) P > 0.05
In PSY, the percentage viability of L. acidophilus initially increased from 100 on day 0–103.03% on day 3 which was maintained up to 1 week. From day 9 onwards, it started to decline attaining the value of 82.6% by day 18 which was about 3% less than that of PCY (Table 2). A similar trend was observed by Varga et al. (2002) where the percentage viability of probiotic bacteria in yogurt supplemented with dry Spirulina platensis powder increased till day 3 and later declined. This decline in the viability of L. acidophilus in PSY is well correlated with the profile of L. acidophilus count. It was observed that till day 6 of storage the L. acidophilus count of PSY (8.66 ± 0.16 log CFU mL−1) remained significantly higher than PCY (8.11 ± 0.22 log CFU mL−1) and then registered a decline. In case of PSY, post acidification effect was observed as the acidity increased from 0.84 ± 0.02–1.22 ± 0.00% upon storage. The decline in viable L. acidophilus count in PSY during the later stages of storage as compared to PCY, could be the result of increasingly acidic environment (pH ≤ 4.3 from day 6 onwards) during storage. Beheshtipour et al. (2012) stated that pH is one of the most critical factors in fermented milks and the probiotic bacteria undergo a pH drop shock when there is a sudden decline in pH (Shafiee et al. 2010). In the present study, the rate of change in pH remained almost constant for the control PCY (0.02 ± 0.005 pH units) for every three days of storage period. On the other hand, PSY experienced an increased rate of change of pH during the first 3 days of storage (0.09 ± 0.001 pH units) before attaining the similar rate of pH change as that of PCY. The total probiotic count at the end of storage however, was 9.5 × 106 in PCY whereas in PSY it was 1.2 × 107 which shows that addition of fresh Spirulina biomass promoted growth and viability of probiotic bacteria.
Total chlorophyll and carotenoid content and sensory properties during storage
The 7% (w/w) PSY retained 91% and 71.5% of total chlorophyll and carotenoids respectively by the end of storage period (Fig. 4a). There was no significant change in β-carotene content during storage period (13.28 ± 0.08 µg g−1 (DW) on day zero and 13.10 ± 0.03 µg g−1 (DW) on day 18). The PSY received positive reviews from the consumer panelists during the first week of storage. On the days 0 and 3, the yogurt was rated as ‘Like Extremely’ by the panelists (94 and 86% consumers, respectively). On days 6 and 9, the yogurt was rated ‘Like Moderately’ and ‘Like Slightly’ by 74 and 80% of the consumers, respectively. Day 9 onwards, the consumers rated the yogurt as ‘Dislike Slightly’ on day 12 (72%) and ‘Dislike Moderately’ on days 15 (66%) and 18 (60%), respectively. The discussion with the consumers revealed that they experienced loose consistency in yogurt texture and after taste which only progressed as the storage period reached day 18. These changes in the consistency of PSY observed during the late stages of storage (after 12 days) can be attributed to the fact that no stabilizers or acidity regulators were used. Therefore, 7% (w/w) PSY obtained in the present study had the mandatory probiotic count, enriched carotenoid content, and; an appreciable shelf life. Such a product is important not only from a general health promoting point of view, but more so from the view point of targeted intervention to combat malnutrition among children.
Fig. 4.
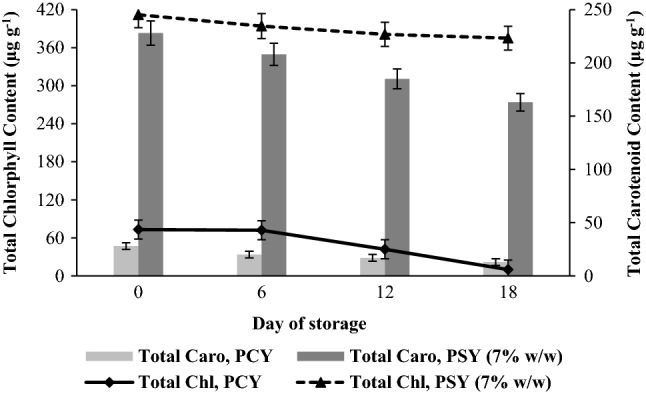
Total chlorophyll and total carotenoid content of probiotic control yogurt (PCY) and probiotic Spirulina yogurt (PSY, 7% w/w fresh biomass) during storage at 6–8 °C (error bars represent standard deviation)
Conclusion
The probiotic Spirulina yogurt (PSY) rich in carotenoids was prepared with fresh biomass of Spirulina platensis without using any food additives such as stabilizers, acidity regulators, etc. Deducing from the physico-chemical and affective (consumer) tests, the incorporation of 7% (w/w) biomass of S. platensis yielded a highly acceptable product. The PSY showed an accelerated growth of L. acidophilus during fermentation and maintained its viability upon storage. The study successfully established the feasibility of utilization of fresh biomass to develop Spirulina enriched food/dairy products. The effective cell disruption by homogenization at conventional pressure (200 ± 10 bar) implies the feasibility of scaling up of this process. Also, it has not escaped the notice of the authors that “Spirulina-milk emulsion” (fresh Spirulina biomass incorporated milk), a semi-processed raw material prepared in the present study, is an independent product in itself that can be used as a base for a variety of new product formulations.
Acknowledgements
Financial support from Department of Biotechnology (DBT), Government of India; Grant BT/PR6552/PBD/26/360/2012 is gratefully acknowledged. The authors thank Director, CSIR-CFTRI for his encouragement.
Abbreviations
- Chl
Chlorophyll
- Caro
Carotenoids
- PCY
Probiotic control yogurt
- PSY
Probiotic Spirulina yogurt
- Te
Time taken to attain pH 4.6–4.7
- SMP
Skimmed milk powder
- DW
Dry weight
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pravin Patel and Hitesh Jethani having equal contribution.
References
- Annapurna V, Shah N, Bhaskaram P, Bamji MS, Reddy V. Bioavailability of Spirulina carotenes in preschool children. J Clin Biochem Nutr. 1991;10(2):145–151. doi: 10.3164/jcbn.10.145. [DOI] [Google Scholar]
- Annapurna VV, Deosthale YG, Bamji MS. Spirulina as a source of vitamin A. Plant Food Hum Nutr. 1991;41(2):125–134. doi: 10.1007/BF02194081. [DOI] [PubMed] [Google Scholar]
- AOAC Official Method 940.03 (1995) Chlorophyll in plants photoelectric colorimetric method for total chlorophyll. In: Official methods of analysis of AOAC international, 16th edn. AOAC International, Arlington, USA
- AOAC Official Method 905.2 (2000) Fat in milk. Methods of test for dairy industry. In: Official methods of analysis of AOAC international, 17th edn. AOAC International, Gaithersburg, USA
- Arlappa N. Vitamin A deficiency is still a public health problem in India. Indian Paediatr. 2011;48(11):853–854. doi: 10.1007/s13312-011-0133-7. [DOI] [PubMed] [Google Scholar]
- Beheshtipour H, Mortazavian AM, Haratian P, Darani KK. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur Food Res Technol. 2012;235(4):719–728. doi: 10.1007/s00217-012-1798-4. [DOI] [Google Scholar]
- Beheshtipour H, Mortazavian AM, Mohammadi R, Sohrabvandi S, Khosravi-Darani K. Supplementation of Spirulina platensis and Chlorella vulgaris algae into probiotic fermented milks. Compr Rev Food Sci Food Saf. 2013;12(2):144–154. doi: 10.1111/1541-4337.12004. [DOI] [Google Scholar]
- Boudraa G, Benbouabdellah M, Hachelaf W, Boisset M, Desjeux JF, Touhami M. Effect of feeding yogurt versus milk in children with acute diarrhoea and carbohydrate malabsorption. J Pediatr Gastroenterol Nutr. 2001;33(3):307–313. doi: 10.1097/00005176-200109000-00015. [DOI] [PubMed] [Google Scholar]
- Chawla AK, Balachandran R. Studies on yogurt from buffalo milk: effect of different solids not fat content on chemical, rheological and sensory characteristics. Indian J Dairy Sci. 1994;47:762. [Google Scholar]
- Chronakis IS, Galatanu AN, Nylander T, Lindman B. The behaviour of protein preparations from blue-green algae (Spirulina platensis strain Pacifica) at the air/water interface. Colloids Surf, A. 2000;173(1–3):181–192. doi: 10.1016/S0927-7757(00)00548-3. [DOI] [Google Scholar]
- Ciron CIE, Gee VL, Kelly AL, Auty MAE. Comparison of the effect of high-pressure microfluidization and conventional homogenization of milk on particle size, water retention and texture of non-fat and low-fat yogurts. Int Dairy J. 2010;20(5):314–320. doi: 10.1016/j.idairyj.2009.11.018. [DOI] [Google Scholar]
- De Caire GZ, Parada JL, Zaccaro MC, De Cano MMS. Effect of Spirulina platensis biomass on the growth of lactic acid bacteria in milk. World J Microbiol Biotechnol. 2000;16(6):563–565. doi: 10.1023/A:1008928930174. [DOI] [Google Scholar]
- Dillon JC, Phuc AP, Dubacq JP. Nutritional value of the alga Spirulina. In: Simopoulos AP, editor. Plants in human nutrition. Switzerland: Karger; 1995. pp. 32–46. [DOI] [PubMed] [Google Scholar]
- Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Effect of acidification on the activity of probiotics in yoghurt during cold storage. Int Dairy J. 2006;16(10):1181–1189. doi: 10.1016/j.idairyj.2005.10.008. [DOI] [Google Scholar]
- Drake MA. Invited review: sensory analysis of dairy foods. J Dairy Sci. 2007;90(11):4925–4937. doi: 10.3168/jds.2007-0332. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO) (2003) Food energy: methods of analysis and conversion factors, p 87. Retrieved from http://www.fao.org/uploads/media/FAO_2003_Food_Energy_02.pdf
- Food, Agriculture Organization/World Health Organization (FAO/WHO) (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, p 2. Retrieved from http://www.fao.org/3/a-a0512e.pdf
- Food Safety and Standards Authority of India (FSSAI) (2011) Food safety and standards (food product standards and food additives) regulation, p 500. Retrieved from http://www.fssai.gov.in/home/fss-legislation/fss-regulations.html
- Gershwin ME, Belay A, editors. Spirulina in human nutrition and health. Boca Raton: CRC Press; 2007. [Google Scholar]
- Indian Council of Medical Research (ICMR) (2010) Nutrient requirements and recommended dietary allowances for Indians, p 298. National Institute of Nutrition (NIN), India. Retrieved from http://icmr.nic.in/final/rda-2010.pdf
- International Organization for Standardization (ISO) (2002) Feeding stuff-determination of crude ash (ISO5984-2002). Retrieved from https://www.iso.org/obp/ui/#iso:std:iso:5984:ed-2:v1:en
- International Organization for Standardization (ISO) (2006) Milk products: enumeration of presumptive L. acidophilus on selective medium—colony-count technique at 37 °C (ISO 20128/IDF 192:2006). Retrieved from https://www.iso.org/standard/35292.html
- International Organization for Standardization (ISO) (2012) Fermented milks—determination of titratable acidity (ISO/TS 11869/IDF/RM 150:2012). Retrieved from https://www.iso.org/standard/56875.html?browse=tc
- Jensen A. Chlorophylls and carotenoids. In: Hellebust JA, Craigie JS, editors. Handbook of phycological methods. Cambridge: Cambridge Press; 1978. pp. 59–70. [Google Scholar]
- Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organism with reference to Lactobacillus acidophilus and Bifidobacterium ssp. Immunol Cell Biol. 2000;78:80–88. doi: 10.1046/j.1440-1711.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- Kristo E, Biliaderis CG, Tzanetakis N. Modelling of rheological, microbiological and acidification properties of a fermented milk product containing a probiotic strain of Lactobacillus paracasei. Int Dairy J. 2003;13(7):517–528. doi: 10.1016/S0958-6946(03)00074-8. [DOI] [Google Scholar]
- Lopez-Fandino R. High pressure-induced changes in milk proteins and possible applications in dairy technology. Int Dairy J. 2006;16(10):1119–1131. doi: 10.1016/j.idairyj.2005.11.007. [DOI] [Google Scholar]
- Northolt MD. Pathogenic microorganisms in fermented dairy products. Neth Milk Dairy J. 1983;37:247–248. [Google Scholar]
- Oliveira MN, Sodini I, Remeuf F, Corrieu G. Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. Int Dairy J. 2001;11(11):935–942. doi: 10.1016/S0958-6946(01)00142-X. [DOI] [Google Scholar]
- Ranga Rao A, Dayananda C, Sarada R, Shamala TR, Ravishankar GA. Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol. 2007;98:560–564. doi: 10.1016/j.biortech.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Rasic JL, Kurmann JA. Yoghurt—scientific grounds, technology, manufacture and preparations. Copenhagen: Technical Dairy Publishing House; 1978. p. 466. [Google Scholar]
- Shafiee G, Mortazavian AM, Mohammadifar MA, Koushki MR, Mohammadi A, Mohammadi R. Combined effects of dry matter content, incubation temperature and final pH of fermentation on biochemical and microbiological characteristics of probiotic fermented milk. Afr J Microbiol Res. 2010;4(12):1265–1274. [Google Scholar]
- Shah NP, Lankaputhra WEV. Improving viability of Lactobacillus acidophilus and Bifidobacterium spp. in yogurt. Int Dairy J. 1997;7(5):349–356. doi: 10.1016/S0958-6946(97)00023-X. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. J Enol Vitic. 1965;16(3):144–158. [Google Scholar]
- Varga L, Szigeti J, Kovacs R, Foldes T, Buti S. Influence of Spirulina platensis biomass on the microflora of fermented ABT milks during storage (R1) J Dairy Sci. 2002;85(5):1031–1038. doi: 10.3168/jds.S0022-0302(02)74163-5. [DOI] [PubMed] [Google Scholar]