Abstract
Lben is one of the main dairy products in Morocco, which broadly plays a significant role in food industry due to its nutritional, taste, aroma and health features. Aroma is a main quality factor for this kind of fermented dairy products. In this study, aroma compounds were extracted by four different methods. According to the sensory analysis, solvent-assisted flavor evaporation exhibited the most representative and reproducible method of Lben matrices. In general, a total of 24 volatile compounds were found for the first time in Lben, including aldehyde, alcohols, acids, esters, and ketones. The Lben characteristic aroma was characterized by 15 odour-active compounds using the application of the aroma extract dilution analysis. On the basis of flavor dilution (FD) results, butanoic acid (FD = 1024, ripened cheese), acetoin (FD = 512, buttery-creamy), 2-heptanol (FD = 512, fatty) and hexanoic acid (FD = 512, cheesy-goat) were the most powerful key odorants in Lben.
Keywords: Moroccan Lben, Aroma, Key odorants, SAFE, GC–MS-olfactometry, AEDA
Introduction
Lben is a traditional fermented milk, which plays an important role in daily diet of Morocco and many other parts of the world, especially in Arabic countries (Samet-Bali et al. 2010). This product obtained traditionally by spontaneous fermentation of raw milk at room temperature, and nowadays produced industrially by adding selected starter cultures to the pasteurized milk, then packed and stored in safety and hygienic conditions. Lben, the acid and skim refreshing beverage, is widely consumed because of its nutritional values and organoleptic characters which is appreciated by consumers (Benkerroum and Tamime 2004; Samet-Bali et al. 2012). Manufacturing of this dairy product includes a multitude of complex enzymatic and chemical reactions having technological consequences mainly dictated by lactic acid bacteria (LAB) (Tamime 2003). The microbiological characterization of ‘Lben’ showed that mesophilic lactic acid bacteria are responsible for lactic fermentation and flavour development, whose the identified species are: Lactococcus lactis, Leuconostoc mesenteroides, Lactobaccilus plantarum, Enterococcus faecuim, Lactococcus garvieae and Enterococcus durans (Tantaoui-Elaraki et al. 1987; Benkerroum and Tamime 2004; Mangia et al. 2014). These LABs are able to coagulate milk, inhibit the growth of harmful microorganisms, ensure an extension of product shelf life by their acidifying power and they also contribute to improvements in nutritional and sensory properties such as freshness, texture, smoothness, and flavor (McSweeney and Sousa 2000) as well as the aromatization by the biosynthesis of the metabolites responsible for the typical aroma of the product (Van Kranenburg et al. 2002; Smit et al. 2004).
Aroma of milk and dairy products is considered as a key attribute in consumer acceptance, product selection and shelf life determination. Along with a series of biochemical processes, LABs by enzymatic reactions cause the formation of volatile compounds, especially key aromas, in fermented milk products (Van Kranenburg et al. 2002). The synthesis of aroma is mainly carried out according to three metabolic pathways: (1) glycolysis via citrate metabolism and conversion of pyruvate to various aromatic compounds, (2) lipolysis gives the free fatty acids which are precursors of different aroma compound groups such as ketones, lactones, esters, and etc., (3) proteolytic activity which can generate free amino acids by degradation of milk casein, which are precursors of various odorous compounds (McSweeney and Sousa 2000; Van Kranenburg et al. 2002; Smit et al. 2004). Although aromatic compounds in dairy products quantitatively and qualitatively can change by the types of LABs and technological processes, it may effect by aroma extraction methods.
Aroma characterization in dairy products possesses a major analytical challenge owing to the composition of its complex matrices (Urbach 1997), the nature, concentration and sensitivity of these aromatic compounds (Mariaca and Bosset 1997). Therefore, for providing a suitable extraction method close to the original product, the representativeness tests ought be a prerequisite to supplementary aroma analyses like gas chromatography–olfactometry (GC–O) (Schieberle et al., 1993). Despite that the extraction techniques of aromatic compounds are numerous; they are dictated by the nature of the matrix and the finality of the analysis. In the case of dairy products, the extraction methods that applied in order to obtain a representative aromatic extract are simultaneous distillation/extraction (SDE), dynamic head space (DHS), static head space (SHS), purge and trap extraction (PT), solid-phase microextraction (SPME), solvent-assisted flavor evaporation (SAFE) and solid phase extraction (SPE) (Mariaca and Bosset 1997; Ott et al. 1997; Siefarth and Buettner 2014).
The volatile profile of most foods contains vast number of compounds, but very limited part of these actually give characteristic aroma to the food. Therefore, a main duty in aroma investigations is to separate the strongly odour-active compounds from the less odorous or odorless compounds exist in foodstuffs (Schieberle et al. 1993). Screening techniques, like GC-O, and AEDA, have been progressed to discover key aroma compounds in a food extract. Aroma is a characteristic parameter as a main quality factor for dairy products; however, there is no study on the aroma profile and aroma-active compounds of Moroccan fermented milk “Lben” in the literature. The only work in the aromatic compounds of the Moroccan Lben that has been carried out by Boubekri et al. (1984) brings limited information. They studied the estimation of certain volatile substances considered aromatic, using static headspace method and other colorimetric method, and the following works until now are based on these results. Nevertheless, other volatile compounds may play an important role in the aroma of Lben, but remain to be identified and quantified.
Principal objective of the present study is to characterize aroma profile and key odorants of typical Moroccan Lben for the first time. The aim of this work was first to estimate a representativeness of Lben aromatic extract obtained from four diverse isolation techniques applying organoleptic analysis and, secondarily, the application of AEDA to determine the key odorants of Lben.
Materials and methods
Samples
A commercial Moroccan Lben, the most aromatic and similar to traditional Lben, was bought in 2017 from the local market and was carefully selected from several industrial products (six different company labels) according to a preliminary sensory analysis (Oliveira et al. 2002). It was industrially produced according to the traditional procedure, with some adjustments for increasing its safety and quality. Instead of the spontaneous fermentation and using of raw milk, industrial Lben produced by adding selected lactic acid bacteria (industrial starter cultures) to the pasteurized milk and then packed and stored at 4 °C according to Benkerroum and Tamime (2004). After the transition of the samples to the laboratory, they were kept at − 20 °C until further analysis.
Chemicals
Dichloromethane, diethyl ether, 4-nonanol, sodium chloride, and sodium sulfate were purchased from (Merck, Darmstadt, Germany). The water was purified by a Millipore-Q system (Millipore Corp., Saint-Quentin, France). Ethanol, ethyl lactate, 4-methyl-2-pentanol, 2-butanol, 2-pentanol, isoamyl lactate, acetoin, acetal, 2-heptanol, acetic acid, propanoic acid, 2,3-butanediol, 1,3-butanediol, butanoic acid, octanoic acid, hexanoic acid, 3-octanol, 2-decanol, (E)-3-hexanoic acid, and 2-butoxyethanol as the standard aroma compounds were obtained from the Sigma-Aldrich (Steinheim, Germany).
Isolation of aromatic extracts of Lben
The four different extraction methods, which have recently been used for the isolation of volatile compounds in the dairy products, were applied in order to extract aroma components: simultaneous distillation extraction (SDE) (Šípalová and Kráčmar 2011), solid-phase extraction (SPE) (Coulibaly and Jeon 1992), direct solvent extraction or liquid–liquid extraction (LLE) (Aubert et al. 2005) and solvent-assisted flavor evaporation (SAFE) (Engel et al. 1999). The objective of applying the different extraction techniques was to estimate a representative aromatic extract from Lben prior to the olfactometric analysis. Each extraction technique in Lben was performed in triplicate, and the extracts were kept at − 20 °C. The detail of each technique was well explained below.
Solid phase extraction method (SPE)
LiChrolut® RP-18 cartridge (Merck, Darmstadt, Germany) was preconditioned with 5 mL of methanol and 10 mL of distilled water. A 50 mL of Lben were passed through the cartridge at 3 mL min−1. Cartridge was washed with 10 mL of distilled water, then the columns was vacuum dried for 3 min, and washed with 3 mL of n-hexane and finally the compounds retained in the solid phase were eluted using 6 mL of diethyl ether at 3 mL/min. After the dehydration by anhydrous sodium sulfate, the pooled aromatic extract concentrated to 5 ml in Kuderna Danish (Sigma Aldrich, St. Louis, MO) equipped with Snyder column (Supelco, St. Quentin, France) and further concentrated to 200 µL under a gentle stream of purified nitrogen (Amanpour et al. 2015).
Solvent-assisted flavor evaporation method (SAFE)
The aromatic volatile compounds of Lben were extracted by the SAFE (Glasbläserei Bahr, Manching, Germany) under vacuum (10−3 Pa; Vacuubrand DCP 3000, Wertheim, Germany) (Engel et al. 1999). The isolation technique was differed from our earlier survey (Amanpour et al. 2015). Briefly, a volume of 40 mL of Lben containing 80 mL of diethyl ether as an extraction solvent were introduced into an Erlenmeyer with a 500 mL volume. The stir procedure was done on the mixtures over 45 min at 4 °C under pure nitrogen gas, then the centrifugation procedure was carried out on the content for 15 min at 4 °C and 5500 rpm. The organic phase recuperate was gently filled in the upper part of the transfer head. Mixture isolation happened when sample content was liberated drop wise into a round bottom flask as a distillation part that was partially submerged in warm water (40 °C). Under vacuum a less than (10−4 torr), the volatile part passed from the separation head into a receiving flask immersed in liquid nitrogen, which were condensed and frozen due to rapid decline in the degree of temperature. When the isolation of volatile compounds was accomplished, the receiving flask was picked up and let to thaw out at room temperature (Engel et al. 1999). The dehydration and concentration procedures described above according to the method of Amanpour et al. (2015).
Simultaneous distillation/extraction method (SDE)
The Likens-Nickerson apparatus (Neubert-Glas, Geschwenda, Germany) was used for the extraction of Lben aroma compounds by SDE. 50 mL of Lben, and 100 mL of saturated solution of NaCl were placed into a 500 mL flask. 40 mL of diethyl ether solvent were introduced into another 100 mL distillation flask. The temperatures of the sample mixture and the diethyl ether flasks were retained by a water bath at 70 and 40 °C, respectively, and the extraction was carried out for approximately 3 h. The volatile compounds were condensed and recovered in the diethyl ether flask. The dehydration and concentration procedures described above according to the method of Amanpour et al. (2015).
Liquid–liquid extraction method (LLE)
The aromatic volatile compounds of Moroccan Lben were also extracted using the LLE method (Aubert et al. 2005). Briefly, a volume of 40 mL of Lben and 80 mL of diethyl ether was placed into a 500 mL Erlenmeyer. The contents were agitated during 45 min at 4 °C under nitrogen gas and then the centrifugation of the mixture was realized for 15 min at 4 °C at 7000 rpm. The dehydration and concentration procedures described above according to the method of Amanpour et al. (2015).
Sensory analysis/representativeness test of the aromatic extract
Sample preparation and presentation
Sensory evaluation of Lben matrix and its aromatic extract were carried out by eight assessors (four females and four males between 28 and 50 years of age). The panelists were familiar with Lben and recruited to training session for the identification and recognition of Lben odours.
Various ways of estimating the representativeness of the aroma extracts of such studies can be applied. In the existing work, a cardboard sniffing strip (Granger-Veyron, Lyas, France) was used to investigate the represent properties of the aroma extracts provided by four different extraction methods. Cardboard strips have already used and evidenced reasonable outcomes for a representativeness of Iranian saffron (Amanpour et al. 2015). As a reference, Lben (10 mL) was placed in a brown coded flask (25 mL) for representativeness tests. The aroma extracts of samples obtained by using the four different techniques were adsorbed on the cardboard. After the vaporization of the solvent (approximate 1 min), the extremities of the strips were cut off and then placed in four various brown coded flasks and presented to the panel after 15 min (Amanpour et al. 2015).
Intensity and similarity tests
These two diverse tests were accomplished to estimate the closeness of the scent of Lben sample to aromatic extracts.
Similarity test The panelists compare the odour of different extracts with the reference sample (Lben). They were asked to sniffed and memorized the smells of aromatic extracts and answer how similar to Lben sample, placing on an unstructured scale of 100 mm, the rating scale opposes the characters “closest to the reference” on the right and “most different from the reference” on the left. The position of the sample on the unstructured scale was read as the distance from the side of very different from the reference.
Intensity test The panelists evaluated the intensity of odour of the extract by-report to the reference. An unstructured scale of 100 mm was used, anchored with “very strong odour” on the right and “no odour” on the left. The position of the sample on the unstructured scale was read as the distance in millimeters from the left side of no smell. Results of sensory tests were analyzed by analysis of variance by Stat graphics Plus software (Manugistic, Inc., Rockville, MD) (Selli and Kelebek 2011).
GC-FID and GC–MS-Olfactometric analyses of aroma compounds
A combined GC (Agilent 6890), FID (flame ionization detector), mass selective detector (Agilent 5973-MSD) and a sniffing (Gerstel ODP-2, MD, USA) system was used to perform aroma and aroma-active analysis.
Aroma compounds separated on a polar DB–Wax column (30 m length × 0.25 mm i.d. x 0.5 μm thickness, CA, USA). Helium, a carrier gas, was employed with a flow rate equal to 1.5 mL min−1. The temperature parameters of the method used in the present study was set up as follows; the initial temperature of oven was 40 °C for 4 min, the next increase rate was 3 °C min−1 up to 130 °C and that temperature was kept constant for 4 min. Afterwards, another temperature ramp was applied with a rate of 5 °C min−1 up to 240 °C. Finally, it was kept at this temperature for 8 min. The electron ionization mode: 70 eV and m/e series: 30–300 amu at scan rate of 2.0 scan s−1. All aroma compounds was identified by mass spectral database (NIST 98, Wiley 6, Flavor 2), retention index and chemical standards. After identification, calculation of each aroma compound concentration was performed by FID based on 4-nonanol (with a concentration of 41.5 mg/L) equivalents as the internal standard. In fact, the internal standard method was conducted to quantify the volatiles. 4-Nonanol was used as an internal standard in the extractions because it fulfilled all necessary criteria as an internal standard. A combination between experimental calibration by internal standards and FID response factors prediction was carried out. Therefore, quantification was calculated by below equation which was also used in our previous study:
where Ci: concentration of compound; Ai: peak area of compound; Astd: peak area of internal standard; Cstd: concentration of internal standard (5 mL/l00 mL); RF: response factor; and CF: calculation factor.
Aroma extract dilution analysis (AEDA)
AEDA is a quantitative GC-O technique used to detect the active aroma or key aroma having a relative contribution and sensory impact towards the total aroma quality of the products (Schieberle et al., 1993), which is defined on the basis of a dilution series of the original extract. The concentrated aromatic extract (200 µL) of Lben was stepwise diluted with diethyl ether as the solvent in the rates of 1:1, 1:2, 1:4, 1:8, 1:16,…, 1:1024 and so on. Three experienced panelists smelled aromatic extracts using olfactometric port in GC–MS-O devise. Dilutions and sniffing procedure was continued as far as there was not any odour smelled. During this procedure, each of odour perceived from the olfactometric port was represented as a flavor dilution (FD) factor. Regarding to the ratio mentioned above. It was meant that the greater the FD factor of a key aroma compound, the more effective on the profile of aroma (Rodriguez-Bencomo et al. 2015).
Descriptive analysis of Lben and its extracts
Nine descriptors composed of phenol, roasty, fatty, nutty, alcohol, cheesy, green, buttery, and earthy that provides their decisive aroma were determined by the expert panelists. Aroma profile analyses were performed by orthonasally scoring descriptors given above on a 100 mm scale anchored on both sides for the intensity of attributes by ‘none’ and ‘very strong’.
During training tests, the panelists developed vocabularies of nine descriptors (cheesy, creamy, buttery, fatty, yogurt, floral, whey, vinegary and medicine) depict the characteristic aroma of Lben. The aromatic extract obtained by four extraction methods and the reference sample were presented to the panel, which described the characteristic scent notes of samples, in order to give the orthonasally scoring above on a 100 mm scale anchored on both sides for the intensity of attributes by ‘none’ and ‘very strong’.
Statistical analysis
The results were expressed as the mean ± standard deviation (SD) of three measurements for the analytical determination. The findings of the sensorial data analysis such as descriptive analysis of Lben and its extracts as well as intensity and similarity tests were subjected to analysis of variance using the SPSS 22 software package (Chicago, USA), and Duncan’s multiple-comparison test was used to find significant differences among the different extraction methods. The significant differences in all results in the manuscript were expressed at a 95% confidence level (P ≤ 0.05).
Results and discussions
Sensory analysis
Representativeness test of Lben aromatic extracts
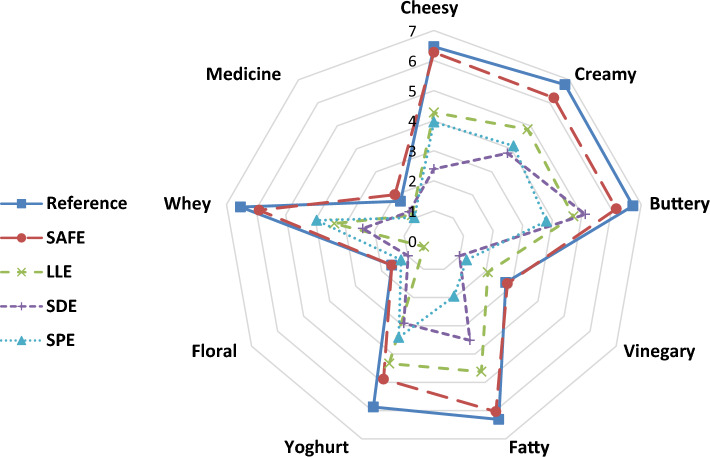
The isolated aromatic extracts by four diverse extraction methods (LLE, SPE, SAFE, SDE) were evaluated and compared to reference sample (Lben) by eight qualified panelists according to sensory tests in order to select and define the representative and reproducible method. The principle intensity assessing for the original product (Lben) and the extracts was exhibited on a spider graph via nine descriptors. As demonstrated in Fig. 1, odour descriptors of original product and its extracts were depicted as cheesy, creamy, buttery, vinegary, fatty, yogurt, floral, whey, and medicine. Among the descriptors, creamy, buttery, cheesy, whey, fatty and yogurt descriptors confirmed the greatest scores, while vinegary, medicine and floral attributes had the lowest scores.
Fig. 1.
Odor organoleptic profiles of the Moroccan Lben and its four diverse aromatic extracts
The Fig. 1 illustrates the differences between different tested techniques and shows that the organoleptic profile of the extract obtained by SAFE method is very close to that of the original product in comparison with other methods (SDE, SPE and LLE). Significantly, no statistical differences were detected between the SAFE method and Lben sample (original product) for descriptors under study.
Intensity and similarity evaluation of aromatic extract
Mean intensities and similarities of Moroccan Lben aroma discovered by the panelists for the extracts acquired by four diverse methods (SAFE, LLE, SDE and SPE) were presented in the Table 1.
Table 1.
Similarity and intensity scores in four diverse extraction methods
| Extraction technique | Similarity scaling (mm)* | Intensity scaling (mm)* |
|---|---|---|
| SAFE | 71.6a | 69.3a |
| SPE | 39.3d | 42.6c |
| SDE | 41.1c | 68.0a |
| LLE | 52.4b | 51.8b |
*Scalings with the same superscript letter were not statisticaly different at a level of 5%
The purpose of the intensity and similarity assessment tests is to the comparison of the representativeness of the odour of the aromatic extract with that of reference. From these extraction methods, SAFE was used to Lben for the first time, and gave the aromatic extract more representative than the other techniques. The similarity scores of the acquired aromatic extracts by the SAFE, SDE, SPE and LLE methods were detected to be 71.6, 41.1, 39.3 and 52.4 mm on strips of 100-mm unstructured scale, respectively and were statistically diverse compared to each other (p < 0.05). In comparison with other investigations made by SAFE in the case of dairy products, the results that we obtained were at an admissible rate. In this extraction method, the risk of formation of artifacts or degradation of sample during the distillation process is reduced. And, it gives an exhaustive and robust extraction of aromatic compounds, because it is carried out at low temperature. The SAFE unit can be more reliable for the identification of a broad spectrum of volatile aromatic compounds, especially compounds in low concentration and having a strong potential impact on the aroma of milk and dairy products (Havemose et al. 2007). Interesting results were obtained by SAFE technique in fresh milk produced by New Zealand cows using different diets, whose between 71 aroma compounds founded, 66 of them were identified (Bendall 2001). On the raw and treated goat’s milk study, Siefarth and Buettner (2014) detected 66 potent aroma compounds in heated goat milks (FD ≥ 8), by this method and identified 42 odor-active compounds which only 16 of the 42 key aromas were reported before in raw goat’s milk.
Also the application of SAFE method has shown better reproducibility for the quantitative analysis of wide volatile compounds in sweet cream butter in different conditions of storage and packaging material than other methods (Lozano et al. 2007). Additionally, in other different foodstuffs, SAFE method has already indicated its reliability for the extraction of aroma components in saffron, dill and savory spices (Amanpour et al. 2015, 2017) and orange juice (Beuttner and Schieberle 1999). The scores of 69.3, 68.0, 42.6 and 51.8 mm on strips of 100-mm unstructured scale exhibited the intensities of the acquired aromatic extracts by the SAFE, SDE, SPE and LLE methods, respectively. Therefore, odour intensity score of SAFE extract gave the highest value among the other extracts under study.
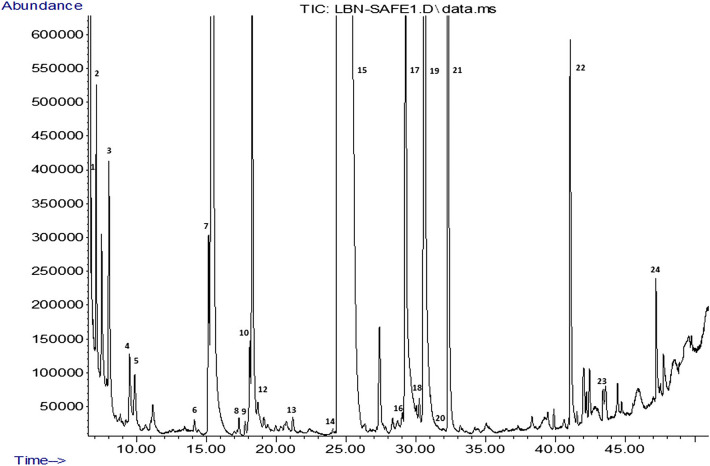
Volatile composition of Lben
Table 2 and Fig. 2 demonstrated the volatile compounds identified in the fermented milk Lben by SAFE method combined with GC–MS, the linear retention index values on DB-Wax column, the categories of these compounds, the mean values (µg/L) of the GC analyses of triplicate extractions and standard deviations. A total of 24 volatile compounds were identified and quantified in Lben for the first time. The total concentration of aroma compounds showed a value of 101,829 µg/L in Lben, comprising aldehydes (1), alcohols (13), acids (6), esters (2) and ketones (2). The characteristic aroma of Lben as a dairy product is generated mainly by various metabolism and catabolism processes result from microbial, enzymatic, and/or chemical transformations of lipids (lipolysis), proteins (proteolysis) and of lactose and citrate (glycolysis and pyruvate metabolism) (McSweeney and Sousa 2000; Van Kranenburg et al. 2002; Smit et al. 2004). Proteolysis is the most important biochemical process for odor formation in Lben and dairy products, which degrades caseins by the activities of rennet enzymes, and then LAB yields small peptides and free amino acids, subsequently converted into various volatile aroma compounds such as aldehydes, alcohols and carboxylic acids, the major aroma components (Van Kranenburg et al. 2002; Smit et al. 2004).
Table 2.
Aroma compounds identified in the Moroccan Lben using SAFE method
| No. | LRIa | Aroma compounds | Concentration (mean ± SD)b | Identificationc |
|---|---|---|---|---|
| 1 | 910 | Acetal | 3443.99 ± 39.79 | LRI, MS,Std |
| 2 | 931 | Ethanol | 12,972.52 ± 193.20 | LRI, MS,Std |
| 3 | 1019 | 2-Butanol | 2611.82 ± 25.56 | LRI, MS,Std |
| 4 | 1095 | 3-Methyl-2-hexanone | 1433.97 ± 20.79 | LRI, MS,tent |
| 5 | 1142 | 2-Pentanol | 623.27 ± 13.90 | LRI, MS,Std |
| 6 | 1160 | 1-Methoxy-2-propanol | 317.69 ± 3.26 | LRI, MS,tent |
| 7 | 1204 | 4-Methyl-2-pentanol | 678.31 ± 2.15 | LRI, MS,Std |
| 8 | 1220 | 2,4-dimethyl-3-pentanol | 266.55 ± 1.84 | LRI, MS,tent |
| 9 | 1264 | 1-Propoxy-2-propanol | 522.18 ± 11.27 | LRI, MS,tent |
| 10 | 1312 | Ethyl lactate | 9189.45 ± 10.69 | LRI, MS,Std |
| 11 | 1320 | Acetoin | 9606.33 ± 47.89 | LRI, MS,Std |
| 12 | 1332 | 2-Heptanol | 422.45 ± 1.26 | LRI, MS,Std |
| 13 | 1388 | 3-Octanol | 466.35 ± 5.55 | LRI, MS,Std |
| 14 | 1395 | 2-Butoxyethanol | 27,660 ± 1.63 | LRI, MS,Std |
| 15 | 1450 | Acetic acid | 41,064.73 ± 331.53 | LRI, MS,Std |
| 16 | 1538 | Propanoic acid | 275.63 ± 3.86 | LRI, MS,Std |
| 17 | 1545 | 2,3-Butandiol | 3732.48 ± 117.67 | LRI, MS,Std |
| 18 | 1570 | Isoamyl lactate | 562.36 ± 8.98 | LRI, MS,Std |
| 19 | 1578 | 1,3-Butanediol | 9570.14 ± 59.86 | LRI, MS,Std |
| 20 | 1592 | 2-Decanol | 330.66 ± 3.38 | LRI, MS,Std |
| 21 | 1628 | Butanoic acid | 2144.52 ± 32.87 | LRI, MS,Std |
| 22 | 1854 | Hexanoic acid | 666.40 ± 18.79 | LRI, MS,Std |
| 23 | 1954 | (E)-3-Hexanoic acid | 277.36 ± 2.65 | LRI, MS,Std |
| 24 | 2047 | Octanoic acid | 384.41 ± 25.60 | LRI, MS,Std |
| Total | 101,829.16 |
aLRI retention indices on DB-WAX column
bConcentration mean values based on three repetitions as µg/L
cIdentification: methods of identification; LRI (linear retention index), MS tent. (Tentatively identified by MS), Std (chemical standard); When only MS or LRI is available for the identification of a compounds, it must be considered as an attempt of identification
Fig. 2.
The total ion chromatogram (TIC) of Lben extract obtained by SAFE method
Among all 24 aromatic compounds identified in Lben, acids were present as a major chemical class, presenting 44% of the total volatiles (Table 2), followed by alcohols, ketones, esters and aldehyde. Acids are important components of the volatile compounds of many cheeses and may originate from lipolysis and also be derived from ketones, esters and aldehydes by oxidation (Curioni and Bosset 2002). Within acid compounds, acetic acid produced by lactic starter cultures was the main constituent (41,064 µg/L). Literature review showed that acetic acid were detected in milk (Bendall 2001), fermented milk (Li et al. 2011), cheese (Delgado et al. 2010), and butter (Lozano et al. 2007). This compound was accounted for the largest proportion (92%) of the total acid compounds. In addition to acetic acid, butanoic, hexanoic, octanoic, (E)-3-hexanoic and propanoic acids were detected. All these identified acids also were reported in milk, yoghurt and several dairy products (Ott et al. 1997).
Alcohols were the first predominant group in Lben. Among the 13 identified alcohol compounds, the four predominant compounds were ethanol (12,972 µg/L), 1,3-butanediol (9571 µg/L), 2,3-butanediol (3733 µg/L), and 2-butanol (2610 µg/L). Ethanol is a common terminal end product of glycolysis by the conversion of acetaldehyde by the enzyme alcohol dehydrogenase and catabolism of amino acids (Ott et al. 1997). It presents in Lben on high quantity, considered an important volatile compound contributed to characteristic aroma and flavor of the Lben (Benkerroum and Tamime 2004). Ethanol also reported as the principal alcohol and the major volatile compound in yoghurt (Urbach 1997).
Acetoin with 9606 µg/L was the main ketone quantitatively in Lben, representing 87% of the total ketones analyzed, followed by 3-methyl-2-hexanone (1432 µg/L). Acetoin is readily converted from diacetyl by the enzyme diacetyl reductase. Ester presents 10% of the total aroma compounds of Lben. Ethyl lactate relatively represents the higher amounts with 9189 µg/L than isoamyl lactate (562 µg/L). The only aldehyde identified in this study was acetal (3444 µg/L) that was not previously found in dairy product.
Key odorants of Moroccan Lben
The key odorants for the first time in Moroccan Lben using AEDA and GC–MS-O and also their odor activity values (OAV) were evaluated (Table 3). The OAV data are used for confirming the contribution of the key odorants to the overall aroma of Lben. The FD factors of the key aroma compounds detected in the range of 4 and 1024. A total of 15 various key odorants comprising alcohols (6), acids (4), ester (1), ketone (1), and unknown compounds (3) were detected. It can be observed the majority of the key odorants of Lben aroma have been previously disclosed as aroma-active compounds in other different dairy products such as cheese (Cornu et al. 2009; Zabaleta et al. 2016), fermented camel milk (Li et al. 2011) and butter (Lozano et al. 2007). The most aroma-active compounds in samples were predominantly alcohols, followed by carboxylic acids. Butanoic acid was the main aroma-active compound contributor to the overall aroma of Lben (FD = 1024) providing strong ripened cheese odour, followed by 2-heptanol, acetoin and hexanoic acid (FD = 512).
Table 3.
The most aroma-active compounds in the Moroccan Lben using SAFE method (FD ≥ 4)
| No. | LRIa | Compounds | Odour descriptionb | FDc | OT (ppb)d | OAVe |
|---|---|---|---|---|---|---|
| 1 | 701 | Unknown 1 | Fresh-green | 16 | – | – |
| 2 | 931 | Ethanol | Dust | 256 | 45101 | 3.7 |
| 3 | 981 | Unknown 2 | Yoghurt | 64 | – | – |
| 4 | 1019 | 2-Butanol | Medicine | 4 | 33001 | > 1 |
| 5 | 1312 | Ethyl lactate | Whey-creamy | 256 | nd | – |
| 6 | 1320 | Acetoin | Buttery-creamy | 512 | 8003 | 12.0 |
| 7 | 1332 | 2-Heptanol | Fatty | 512 | 412 | 10.3 |
| 8 | 1388 | 3-Octanol | Nutty-fatty | 256 | 782 | 6.0 |
| 9 | 1450 | Acetic acid | Vinegary | 128 | 22,0004 | 1.9 |
| 10 | 1538 | Propanoic acid | Fatty | 128 | 1005 | 2.7 |
| 11 | 1545 | 2,3-Butanediol | Creamy | 64 | 20,0002 | > 1 |
| 12 | 1578 | 1,3-Butanediol | Fatty-floral | 32 | 10,0002 | 1.0 |
| 13 | 1628 | Butanoic acid | Ripened cheese | 1024 | 1005 | 21.4 |
| 14 | 1734 | Unknown 3 | Buttery | 32 | – | – |
| 15 | 1854 | Hexanoic acid | Cheesy-goat | 512 | 35.65 | 18.7 |
aLRI retention indices on DB-WAX column
bOdour description: odour perceived by panelists during olfactometry
cFD factor is the highest dilution of the extract at which an odorant is determined by aroma extract dilution analysis
dOdour thresholds in water. These values obtained from the following references; 1Boonbumrung et al. (2001), 2Karl-Otto et al. (1988), 3Buttery et al. (1990), 4Buttery and Ling (1998), 5Larsen and Poll (1992)
eThe OAV obtained by dividing concentration of the compounds by their threshold
Alcohols were the predominant key odorants of Lben. A total of six different key alcohol compounds were identified, comprising ethanol (dust), 2-butanol (medicine), 2-heptanol (fatty), 3-octanol (nutty-oily) 2,3-butanediol (creamy) and 1,3-butanediol (fatty, floral). They are prevalent, which have already been found in other different dairy products (Cornu et al. 2009; Zabaleta et al. 2016). Among them, 2-heptanol (FD = 512; OAV = 10.3), 3-octanol (FD = 256; OAV = 6.0), and ethanol (FD = 256; OAV = 3.7) were the most powerful aroma-active alcohols in the studied sample. Hence, these most powerful odor-active alcohols might have the principle role for characterizing the Lben aroma constitution. These odorants provide the fatty, nutty, and dust aroma notes, which contributes the main fraction of global aroma of the Lben.
Although the number of the acid compounds were less than alcohols, they were the principal key odorants contributed in the overall aroma of Lben. A total of four different aroma-active carboxylic acids were identified, comprising acetic acid (vinegary), propanoic acid (fatty), butanoic acid (ripened cheese), and hexanoic acid (cheesy). Most of these active aroma compounds are widespread, which have just been found in many different dairy products (Lozano et al. 2007; Cornu et al. 2009; Li et al. 2011; Zabaleta et al. 2016). The odor threshold of carboxylic acids is lower than their concentrations in the current survey; thus, they have significant potential influences on the overall aroma of Lben. Butanoic acid was detected as a main aroma-active carbocylic acid with 1024 FD factor (OAV: 21.4), followed by hexanoic acid with 512 FD factor (OAV: 18.7). Butanoic acid, associated by sniffers with ripened cheese in Lben, has also been reported in potent odorant in Polish mold-ripened cheese (Lazur) by Majcher et al. (2017) providing cheesy odor. Although acetic acid was abundant compound with higher concentration (41,064 µg/L) than butanoic and hexanoic acids, it showed lower FD factor (FD = 128) than the others. Because, it has relatively high odor threshold value (22,000 ppb) (Buttery et al. 1988).
Acetoin was the only key ketone compound found in Lben extract and was the strong aroma (FD = 512; OAV = 2.0), providing buttery-creamy odor note. This compound exhibited the prevailing compound in aroma profile containing 9606 µg/L. Additionally, ethyl lactate (FD = 256, whey-creamy) was detected as an aroma-active ester. Moreover, tree unknown aromatic compounds may contribute to the overall aroma of Lben. Unknown 1 (LRI = 701, FD = 16) with a fresh-green odor notes, unknown 2 (LRI = 981, FD = 64) with a yoghurt odour note, and unknown 3 with a buttery odour note were found as unknown aroma-active compounds which were contributed in the characteristic aroma of Lben (Table 3).
In comparison with the result of sensory analysis (Fig. 1), the above-mentioned aroma-active compounds characterized by GC–MS-O showed similarity in the intense sensory description by panelists and had important potential effect on overall aroma of Lben.
Conclusion
This present study revealed important information for the first time on the volatile profile and key odorants of Moroccan Lben. Prior to GC–MS-O analysis, the extraction techniques (SPE, SAFE, LLE and SDE) were first checked using similarity and intensity tests. The extract obtained by SAFE of Lben seem more representative for GC–MS-Olfactometric analysis because the intensity and similarity properties were quite similar to the Lben compared to SPE, LLE and SDE. Application of the aroma extract dilution analysis revealed 15 aroma-active compounds, 13 of which could be identified in Lben aromatic extract. On the basis of FD factors, the strongest aroma-active compounds of Moroccan Lben were butanoic acid (FD = 1024), acetoin (FD = 512), 2-heptanol (FD = 512) and hexanoic acid (FD = 512).
Acknowledgements
Grateful acknowledgment is made for the laboratory of Agri-food and Food Safety, Dhar El Mahraz Faculty of Sciences, of Sidi Mohamed Ben Abdellah University, for the partial financial support of Salwa Tsouli Sarhir. We are also grateful to the members of the sensorial analysis panel, and to Research Assistant Gamze Guclu for her technical assistance. We thank also Dr. Aziz Amine for his valuable and careful revision of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amanpour A, Sonmezdag AS, Kelebek H, Selli S. GC–MS–olfactometric characterization of the most aroma-active components in a representative aromatic extract from Iranian saffron (Crocus sativus L.) Food Chem. 2015;182:251–256. doi: 10.1016/j.foodchem.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Amanpour A, Kelebek H, Selli S. Aroma constituents of shade-dried aerial parts of Iranian dill (Anethum graveolens L.) and savory (Satureja sahendica Bornm.) by solvent-assisted flavor evaporation technique. J Food Meas Charact. 2017;11:1430–1439. doi: 10.1007/s11694-017-9522-5. [DOI] [Google Scholar]
- Aubert C, Baumann S, Arguel H. Optimization of the analysis of flavor volatile compounds by liquid − liquid microextraction (LLME). Application to the aroma analysis of melons, peaches, grapes, strawberries, and tomatoes. J Agric Food Chem. 2005;53:8881–8895. doi: 10.1021/jf0510541. [DOI] [PubMed] [Google Scholar]
- Bendall JG. Aroma compounds of fresh milk from New Zealand cows fed different diets. J Agric Food Chem. 2001;49:4825–4832. doi: 10.1021/jf010334n. [DOI] [PubMed] [Google Scholar]
- Benkerroum N, Tamime AY. Technology transfer of some Moroccan traditional dairy products (lben, jben and smen) to small industrial scale. Food Microbiol. 2004;21:399–413. doi: 10.1016/j.fm.2003.08.006. [DOI] [Google Scholar]
- Beuttner A, Schieberle P (1999) Changes in the concentrations of key orange odorants induced by mastication of orange segments and orange juice. In: Abstracts of Papers of the American Chemical Society. Am Chem Soc 1155 16TH ST, NW, Washington, DC 20036 USA, pp 47–48
- Boonbumrung S, Tamura H, Mookdasanit J, et al. Characteristic aroma components of the volatile oil of yellow keaw mango fruits determined by limited odor unit method. Food Sci Technol Res. 2001;7:200–206. doi: 10.3136/fstr.7.200. [DOI] [Google Scholar]
- Boubekri C, Elaraki AT, Berrada M, Benkerroum N. Caractérisation physico-chimique du Iben marocain. Lait. 1984;64:436–447. doi: 10.1051/lait:1984643-64434. [DOI] [Google Scholar]
- Buttery RG, Ling LC. Additional studies on flavor components of corn tortilla chips. J Agric Food Chem. 1998;46:2764–2769. doi: 10.1021/jf980125b. [DOI] [Google Scholar]
- Buttery RG, Turnbaugh JG, Ling LC, et al. Contribution of volatiles to rice aroma. J Agric Food Chem. 1988;36:1006–1009. doi: 10.1021/jf00083a025. [DOI] [Google Scholar]
- Buttery RG, Teranishi R, Ling LC, Turnbaugh JG. Quantitative and sensory studies on tomato paste volatiles. J Agric Food Chem. 1990;38:336–340. doi: 10.1021/jf00091a074. [DOI] [Google Scholar]
- Cornu A, Rabiau N, Kondjoyan N, et al. Odour-active compound profiles in Cantal-type cheese: effect of cow diet, milk pasteurization and cheese ripening. Int Dairy J. 2009;19:588–594. doi: 10.1016/j.idairyj.2009.04.008. [DOI] [Google Scholar]
- Coulibaly K, Jeon IJ. Solid-phase extraction of less volatile flavor compounds from ultrahigh-temperature processed milk. J Agric Food Chem. 1992;40:612–616. doi: 10.1021/jf00016a017. [DOI] [Google Scholar]
- Curioni PMG, Bosset JO. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int Dairy J. 2002;12:959–984. doi: 10.1016/S0958-6946(02)00124-3. [DOI] [Google Scholar]
- Delgado FJ, González-Crespo J, Cava R, et al. Characterisation by SPME-GC-MS of the volatile profile of a Spanish soft cheese P.D.O. Torta del Casar during ripening. Food Chem. 2010;118:182–189. doi: 10.1016/j.foodchem.2009.04.081. [DOI] [Google Scholar]
- Engel W, Bahr W, Schieberle P. Solvent assisted flavour evaporation—a new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur Food Res Technol. 1999;209:237–241. doi: 10.1007/s002170050486. [DOI] [Google Scholar]
- Havemose MS, Justesen P, Bredie WLP, Nielsen JH. Measurement of volatile oxidation products from milk using solvent-assisted flavour evaporation and solid phase microextraction. Int Dairy J. 2007;17:746–752. doi: 10.1016/j.idairyj.2006.09.008. [DOI] [Google Scholar]
- Karl-Otto S, Hans-Dieter B, von Christof R, et al. Untersuchungen zur Struktur-Aktivitäts-Beziehung bei Geruchsstoffen 1. Mitteilung: Wahrnehmungsschwellenwerte und Geruchsqualitäten von gesättigten aliphatischen und alicyclischen Verbindungen mit Sauerstoff-FunktionInvestigations on the structure-activit. Zeitschrift für Leb und Forsch. 1988;187:215–223. doi: 10.1007/BF01043342. [DOI] [Google Scholar]
- Larsen M, Poll L. Odour thresholds of some important aroma compounds in strawberriesGeruchsschwellen einiger wichtiger Aromastoffe der Erdbeeren. Zeitschrift für Leb und Forsch. 1992;195:120–123. doi: 10.1007/BF01201770. [DOI] [Google Scholar]
- Li N, Zheng FP, Chen HT, et al. Identification of volatile components in Chinese Sinkiang fermented camel milk using SAFE, SDE, and HS-SPME-GC/MS. Food Chem. 2011;129:1242–1252. doi: 10.1016/j.foodchem.2011.03.115. [DOI] [PubMed] [Google Scholar]
- Lozano PR, Miracle ER, Krause AJ, et al. Effect of cold storage and packaging material on the major aroma components of sweet cream butter. J Agric Food Chem. 2007;55:7840–7846. doi: 10.1021/jf071075q. [DOI] [PubMed] [Google Scholar]
- Majcher MA, Myszka K, Gracka A, et al. Key odorants of Lazur, a Polish mold-ripened cheese. J Agric Food Chem. 2017;66:2443–2448. doi: 10.1021/acs.jafc.6b04911. [DOI] [PubMed] [Google Scholar]
- Mangia NP, Garau G, Murgia MA, et al. Influence of autochthonous lactic acid bacteria and enzymatic yeast extracts on the microbiological, biochemical and sensorial properties of Lben generic products. J Dairy Res. 2014;81:193–201. doi: 10.1017/S0022029914000119. [DOI] [PubMed] [Google Scholar]
- Mariaca R, Bosset JO. Instrumental analysis of volatile (flavour) compounds in milk and dairy products. Lait. 1997;77:13–40. doi: 10.1051/lait:199712. [DOI] [Google Scholar]
- McSweeney PLH, Sousa MJ. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait. 2000;80:293–324. doi: 10.1051/lait:2000127. [DOI] [Google Scholar]
- Oliveira CAF, Fernandes AM, Neto OCC, Fonseca LFL. Composition and sensory evaluation of whole yogurt produced from milk with different somatic cell counts. Aust J Dairy Technol. 2002;57:192. [Google Scholar]
- Ott A, Fay LB, Chaintreau A. Determination and origin of the aroma impact compounds of yogurt flavor. J Agric Food Chem. 1997;45:850–858. doi: 10.1021/jf960508e. [DOI] [Google Scholar]
- Rodriguez-Bencomo JJ, Kelebek H, Sonmezdag AS, Rodriguez-Alcala LM, Fontecha J, Selli S. Characterization of the aroma-active, phenolic, and lipid profiles of the pistachio (Pistacia vera L.) nut as affected by the single and double roasting process. J Agric Food Chem. 2015;63:7830–7839. doi: 10.1021/acs.jafc.5b02576. [DOI] [PubMed] [Google Scholar]
- Samet-Bali O, Bellila A, Ayadi MM, et al. A comparison of the physicochemical, microbiological and aromatic composition of Traditional and Industrial Leben in Tunisia. Int J Dairy Technol. 2010;63:98–104. doi: 10.1111/j.1471-0307.2009.00546.x. [DOI] [Google Scholar]
- Samet-Bali O, Ennouri M, Dhouib A, Attia H. Characterisation of typical Tunisian fermented milk: Leben. Afr J Microbiol Res. 2012;6:2169–2175. [Google Scholar]
- Schieberle P, Gassenmeier KF, Guth HU, et al. Character Impact Odour Compounds of Different Kinds of Butter. LWT Food Sci Technol. 1993;26:347–356. doi: 10.1006/fstl.1993.1070. [DOI] [Google Scholar]
- Selli S, Kelebek H. Aromatic profile and odour-activity value of blood orange juices obtained from Moro and Sanguinello (Citrus sinensis L. Osbeck) Ind Crops Prod. 2011;33:727–733. doi: 10.1016/j.indcrop.2011.01.016. [DOI] [Google Scholar]
- Siefarth C, Buettner A. The aroma of goat milk: Seasonal effects and changes through heat treatment. J Agric Food Chem. 2014;62:11805–11817. doi: 10.1021/jf5040724. [DOI] [PubMed] [Google Scholar]
- Šípalová M, Kráčmar S. Aroma active compounds in milk from goat fed basil (Ocimum basilicum) Acta Univ Agric Silvic Mendelianae Brun. 2011;59:171–177. doi: 10.11118/actaun201159030171. [DOI] [Google Scholar]
- Smit BA, Engels WJM, Wouters JTM, Smit G. Diversity of l-leucine catabolism in various microorganisms involved in dairy fermentations, and identification of the rate-controlling step in the formation of the potent flavour component 3-methylbutanal. Appl Microbiol Biotechnol. 2004;64:396–402. doi: 10.1007/s00253-003-1447-8. [DOI] [PubMed] [Google Scholar]
- Tamime AY. Fermented milks: a historical food with modern applications–a review. Eur J Clin Nutr. 2003;56:S2. doi: 10.1038/sj.ejcn.1601657. [DOI] [PubMed] [Google Scholar]
- Tantaoui-Elaraki A, El Marrakchi A, El Marrakchit A. Study of Moroccan dairy products: Iben and smen. MIRCEN J Appl Microbiol Biotechnol. 1987;3:211–220. doi: 10.1007/BF00933574. [DOI] [Google Scholar]
- Urbach G. The flavour of milk and dairy products: II. Cheese: contribution of volatile compounds. Int J Dairy Technol. 1997;50:79–89. doi: 10.1111/j.1471-0307.1997.tb01743.x. [DOI] [Google Scholar]
- Van Kranenburg R, Kleerebezem M, Van Hylckama Vlieg J, et al. Flavour formation from amino acids by lactic acid bacteria: Predictions from genome sequence analysis. Int Dairy J. 2002;12:111–121. doi: 10.1016/S0958-6946(01)00132-7. [DOI] [Google Scholar]
- Zabaleta L, Gourrat K, Barron LJR, et al. Identification of odour-active compounds in ewes’ raw milk commercial cheeses with sensory defects. Int Dairy J. 2016;58:23–30. doi: 10.1016/j.idairyj.2016.01.018. [DOI] [Google Scholar]