Abstract
The aim of this research is to investigate the effects of brown algae to solution ratio, feed rate, and pH on the multiple responses of Sargassum cristaefolium alginate extracted using twin screw extruder. Box–Behnken design was used to find out the optimum extrusion-assisted extraction parameters based on the responses of residence time distribution (RTD), yield, intrinsic viscosity, and molecular weight. The result showed that alginate extrusion-assisted extraction parameters affected on the movement of algae in the screw channel and physicochemical properties of S. cristaefolium alginate. The alginate extrusion-assisted extraction parameters have quadratic effect on the responses of RTD, yield, intrinsic viscosity and molecular weight. The predicted values at the optimum extrusion parameters as independent variables are the use of brown algae to solution ratio (3.11), feed rate (2.95 rpm), and pH 10.3. The M/G ratio of S. cristaefolium alginate based on fractions analysis is 0.29 (M/G ratio < 1), indicating that S. cristaefolium alginate contains guluronate fraction of 77.10% and manuronate fraction of 22.90%. Intrinsic viscosity of S. cristaefolium alginate in aqueous solution was determined and shown shear-thinning pseudoplastic.
Keywords: Alginate, Twin-screw extruder, M/G ratio, Rheology, Sargassum cristaefolium, Response surface method
Introduction
Alginate is the major structural polyshaccaride found in cell walls and intercellular matrix from brown algae tissue (Bertagnolli et al. 2014). Alginate in brown algae is present in the form of calcium, magnesium, and sodium salt, providing the strength and flexibility of the tissues (Sellimi et al. 2015). Alginate is widely used as cell immobilization, tissue engineering, microencapsulation nutraceutical, drugs delivery control, antitumor and antioxidant (Draget and Taylor 2011), in food application serves as thickener, stabilizer, and gelling agent (Gomez et al. 2009).
Alginate are linear copolymers constitute of two units of monomers is, β-(1,4)-d-manuronic acid (M) and α-(1,4)-l-guluronic acid (G). Alginate monomer has tree types sequences, i.e. d-manuronic acid blocks (MMM), l-guluronic acid blocks (GGG), and mixed guluronic acid and manuronic acid blocks (MGMGMG) with different proportion and sequences of monomers (Larsen et al. 2003). The M/G ratio and molecular weight affect on the functional and rheological characteristics of alginate (Andriamanantoanina and Rinaudo 2010).
Conventional alginate extraction is ineffective because it has some drawbacks, long time, requires a lot of reactants and solvents (Torres et al. 2007). Meanwhile extraction by using microwave assisted extraction, supercritical CO2 extraction and ultrasonic assisted extraction is difficult to apply in industries and expensive (Quitain et al. 2013). A more effective method by using twin screw extruder assisted extraction, short time, solvent and reactant reduced, little waste, safe and applicable for industries (Hernandez-Carmona 2013). This method was widely applied to oil extraction from sunflower and lignocellulose pretreatment for bioethanol production (Zheng and Rehmann 2014). Application of twin screw extruder on an alginate extraction process from brown algae has been reported Baron et al. (2010) who focused on the effect of screw speed on algae residence time distribution (RTD) in the screw channel. Hence, a previous research highlighted the interest of the effect of pretreatment on the RTD and physcochemical properties of alginate that was extracted by twin screw extruder assisted extraction (Sugiono et al. 2018). Meanwhile, the effect of extrusion-assisted extraction parameters such as brown algae to solution ratio, feed rate and pH on the physicochemical properties and rheological characteristic of alginate from Sargassum cristaefolium has not been examined.
Extraction parameters (solution ratio, feed rate, and pH) affected the properties of alginate (Fertah et al. 2014). The screw speed and feed rate during extrusion assisted extraction of alginate from Laminaria digitata affected on algae RTD in the screw channel (Baron et al. 2010). However, there is no study regarding the alginate extraction by using twin screw extruder from brown alga S. cristaefolium. Also, there is no reports on the optimatization of RTD, yield, intrinsic viscosity, and molecular weight, during alginate extraction from this alga. Therefore, it is important to study the role of extrusion conditions on residence time distribution of algae in the screw channel and physicochemical properties of extracted alginate. The aim of this research is to optimize extrusion-assisted extraction (EAE) parameters (brown algae: solution ratio, feed rate and pH) and characterization of alginate from brown alga S. cristaefolium.
Materials and methods
Materials
Brown alga S. cristaefolium were collected from Poteran Island, Sumenep, Madura, Indonesia, in April 2016. Fresh brown algae were washed with fresh water to remove all sand and epiphytes using tap water, soaked in 0.1% KOH for 1 h, and re-washed to remove impurities. The brown algae were then sun dried, ground, and sieved at 60 mesh. The brown algae were submerged in 0.1% formaldehyde overnight, washed, and dried at 50 °C for 6 h in cabinet dryer. All chemicals including KOH, formaldehyde, ethanol 96%, Na2CO3 were technical grade, and reagent for analysis such as hydrochloric acid (HCl), H2SO4, NaOH, were analytical grade.
Twin-screw extruder
The alginate extraction was performed using intermeshing co-rotating twin-screw extruder (Berto Industry BEX-DS-2256), with capacity of 7 kg/h. Three thermocouples were used to monitor the barrel temperatures during the extraction process of alginate in the barrel and the barrel temperature was displayed in the control panel. Diameter of die was 8 mm. The extruder was operated at screw speed of 0–180 rpm, and auger speed (feed rate) of 0–35 rpm. Barrel and screw profile of twin-screw extruder Berto Industry BEX-DS-2256 were shown in Fig. 1.
Fig. 1.
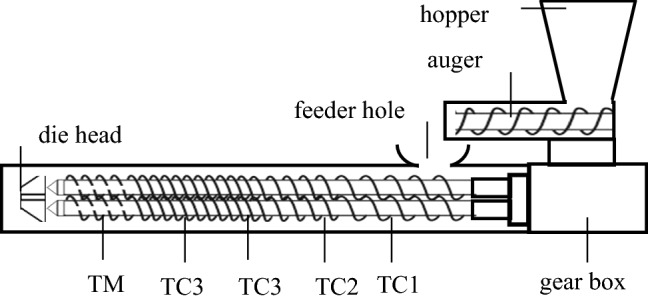
Scheme modular barrel and profile of twin screw extruder (Berto Industry BEX-DS-2256). TC = groove transfer direct pitch element (TC1 = 300 mm, TC2 = 220 mm, TC3 = 140 mm, TC4 = 120 mm), TM = groove mixing pitch element (80 mm), total long screw = 800 mm)
Alginate extraction using twin screw extruder
Brown alga S. cristaefolium was suspended in distilled water at ratio of 1:20 (w/v) and a 0.03 M HCl was added under strong stirring to achieve pH 3, and gently stirred at speed of 500 rpm for 64 min. The brown alga was rinsed by distilled water to eliminate excess of acid until neutral pH, and the remaining water was removed. Pre-treated brown algae is carried out by gradually adding Na2CO3 solution (pH 8–12) to various brown algae: solution ratio (w/v) (1–5), and then stirred and transferred into extruder’s hopper with feed rate of 20–35 rpm (Baron et al. 2010). Brown algae moved between screws channel and then was released as homogenous fluid in the opening die. The extrudate homogenous fluid was then dissolved in Na2CO3 solution (pH 8–12) with ratio of 1:10 (w/v), and then stirred. The filtrate was diluted with distilled water to ratio of 1:20 (b/v) based on the initial material weight, stirred and was centrifuged at 5000 rpm for 10 min. The sodium alginate precipitated with ethanol 96% in the ratio of 1:2 (v/v), kept for 1 h and then filtered. The precipitate was washed twice by ethanol 70% and 96%. Finally, the alginate was vacum-dried at 45 °C for 24 h.
RSM experimental design
A Box–Behnken Design was used to test the combined effect of three variables brown algae to solution ratio (x1), feed rate (x2), and pH (x3) on the multiple-response alginate (RTD, yield, intrinsic viscosity and molecular weight). The total run design had 15 combinations, randomly ordered with 3 replications at center point of each factor variables used (Montgomery 2005), are listed in Table 1. Relationship between independent variables and factors level as shown in Table 1.
Table 1.
Independent variables and factors level of Box–Behnken-design
| Independent variables | Factors level | ||
|---|---|---|---|
| −1 | 0 | + 1 | |
| Algae to solution ratio (X1) | 1:1 | 1:3 | 1:5 |
| Feed rate (rpm) (X2) | 20 | 27.5 | 35 |
| pH (X3) | 8 | 10 | 12 |
The center level of each independent variables, which is represented by denoting 0 number, were repeated 3 times. Whlist, the rest of factorials point involving 3 factors are represented by denoting − 1, 0 and + 1 numbers, it was repeated 12 times. The total treatments are 15 treatments.
Fitted the second-order polynomial model:
| 1 |
where Y is the predicted responses which are RTD, yield, intrinsic viscosity, molecular weight); β0 is the intercept coefficient; βi is the linear coefficient; βii is the quadratic coefficient; βij is the interaction coefficient of variables i and j; xi and xj are independent variables. The accuracy of polynomial model was analyzed using Design-Expert (Ver. 7) software to obtain correlation coefficient (R) and determination coefficient (R2) of each responses (RTD, yield, intrinsic viscosity, and molecular weight). The significance of R and R2 was statistically evaluated using F-test (P < 0.05). The validity of optimum condition between the prediction and the actual were evaluated using paired t test (P < 0.05) using Minitab (Ver. 16) software.
Residence time distribution (RTD)
In this study, we measured the residence time distribution (RTD). It is useful to characterize the flow pattern of algae under controlled operating conditions during extrusion. RTD was the time at which pre-treated brown algae injected into extruder’s hopper until the material is released from the opening die.
Yield
Yield was determined according to ratio of extracted alginate weight and initial weight of dry brown algae, then multiplied by 100%.
Intrinsic viscosity
Dynamic viscosity was assessed using a Ubbelohde glass viscometer with capillary diameter of 0.56 mm (Canon, USA) at 25 °C, immersed in a thermostated bath with a precision of ± 0.1 °C. Alginate (30 mg) was dissolved in 10 ml of distilled water, stirred for 3 h at 25 °C.The different concentrations (0.05–0.3 g/dL) of alginate were then made (Torres et al. 2007). The flow time of alginate solution (t) was relatively measured to distilled water (t0). Intrinsic viscosity was determined by extrapolating of ηsp/c concentration to zero.
| 2 |
| 3 |
| 4 |
| 5 |
Molecular weight
Determination of alginate molecular weight was based on correlation of viscosity average molecular weight. The viscometric-average molecular weight was determined from intrinsic viscosity in aqueous solution using Mark–Houwink equation (Eq 6). The formula for finding intrinsic viscosity of an aqueous solution is
| 6 |
where k and a are empirical coefficients dependent on the polymer and solvent-temperature systems. For alginate a value = 0.984 and k = 0.023 dl/g, as proposed by Clementi et al. (1998) quoted by Torres et al. (2007), Chee et al. (2011) and Fertah et al. (2014).
The [η] is intrinsic viscosity (dl/g), and Mw is molecular weight (kDa).
M/G ratio
Alginate (1 g) was hydrolyzed by dissolving in 25 ml of 0.25 M H2SO4 and stirred for 2 h. The mixture was hydrolyzed with reflux at temperature of 100 °C for 6 h. The hydrolyzed mixture was then cooled, and centrifuged at 5000 rpm for 5 min to separate precipitate (A) and supernatant (B). The supernatant (B) was neutralized with 1.0 M NaOH, 250 ml of 96% ethanol was added to precipitate poly-manuronic guluronic (PMG) and centrifuged at 5000 rpm 10 min, the precipitate as PMG dissolved in distilled water and dried by freeze drying (fraction 1). The precipitate (A) subsequently was dissolved in 0.1 M Na2CO3 and pH was adjusted 2.85 with 0.1 M HCl. After achieving the desired pH, mixture was centrifuged and the precipitate was expressed as poly-guluronic acid (PGA) (fraction 2). The filtrate is set to pH 1 with 0.1 M HCl and then centrifuged at 5000 rpm 10 min. The precipitate is poly-manuronic Acid (PMA) and then dried by freeze drying (fraction 3) (Chhatbar et al. 2009).
Rheological properties
The flow behaviour of alginate an aquoeus solution were measured using a Rheometer Brookfield, cone plate model HADV-1 + CP. Dynamic viscosity of alginate in aqueous solution at different concentration 2–3% (w/v) was measured at temperature of 25 °C with shear rate 0–600 s−1 (Torres et al. 2007).
Result and discussion
Residence time distribution
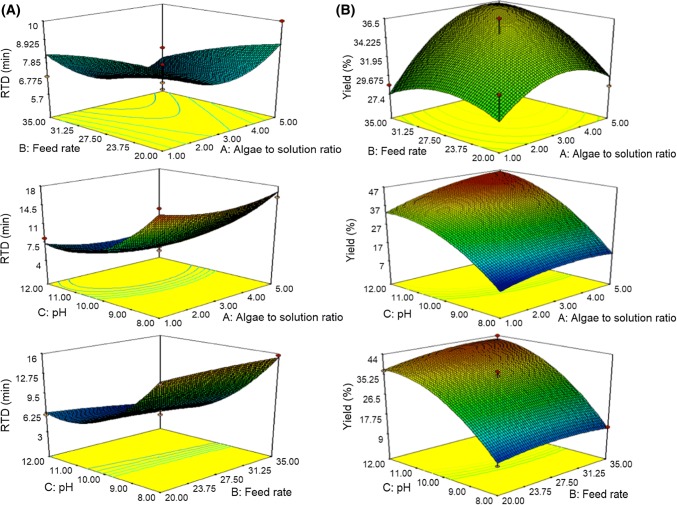
The effects of independent variables namely: feed rate and algae to solution ratio; pH and algae to solution ratio; pH and feed rate on RTD are shown in Fig. 2a The results showed that extrusion-assisted extraction parameters demonstrated a quadratic effect on the residence time distribution of algae in the screw channel. The residence time distribution increased with the increasing brown algae: solution ratio, feed rate and pH. This finding can be explained by some phenomena. Firstly, the increase of brown algae to solution ratio and pH causes the material to form viscoelastic fluid, low viscosity, and low die pressure; therefore, the materials move faster in the screw channel. Secondly, increasing feed rate causes shear and strong pressure on the material in the screw channel that make material move more quickly in the screw extruder. The result of this research is in accordance to that reported by Baron et al. (2010) who explained that algae move more quikly in the screw channel with increasing brown algae: water ratio and feed rate. Thirdly, the increase in pH level led to raise porosity of the cell wall and swelling properties of the cell, enhancing the interaction between cells and sodium carbonate solution to form viscoelastic fluid. This leads to low die pressure and algae move in the screw channel faster and is released from die more quickly (Kartika et al. 2005). The pH 8 and solution ratio of 1:5 showed the lowest alginate solubility. At this condition, the material does not form viscoelastic fluid; thus, the pressure and shear of the screw wall on the material cause the liquid to squeeze out of the die, increase die pressure, thereby movement and discard of algae from die are slower.
Fig. 2.
Response surface plots of the effect of extrusion parameters on the residence time distribution (a), and yield of alginate (b)
Yield
Extrution parameters evaluated in this study give quadratic effect on the yield of alginate extract. The highest alginate yield was at 45.54%, produced from brown algae to solution ratio (1:5), feed rate (27.5 rpm), and pH 12. In contrast, the lowest alginate yield was observed at 8.50%, resulting from brown algae to solution ratio (1:1), feed rate (27.5 rpm) and pH 8. The yield in this research is in accordance to the alginate yield reported by Vauchel et al. (2008) and Fertah et al. (2014).
Additionally, Fig. 2B showed that the alginate yield tended to increase with the increase of brown algae to solution ratio, feed rate and pH. The increase in ratio and pH produced more alginate contact area and interaction with viscoelastic fluid. There was an increase in alginate extractability at feed rate (27.5 rpm), but it decreased at feed rate (35 rpm) due to the reduction in SME effects. Increasing feed rate causes a decrease in shear rate, which led to decline of SME. At pH 12 and combination set of temperature, pressure and friction of the screw wall caused cell wall lignocellulose completely crumbles, thus alginate solubility in sodium carbonate is optimum (Sugiono et al. 2018), whereas at pH 8, alginate extractability is low. Tambunan and Rudiyansyah (2013) reported that alginate extraction with 8% sodium carbonate solution caused algae cell wall to expand and swell, thereby the alginate extractability was maximum.
Intrinsic viscosity
The intrinsic viscosity values of algae S. cristaefolium effected by the independent variables used in this experiments were in the range of 62.60–438.70 ml/g (Table 2). This finding was relatively similar to the data reported by Torres et al. (2007) that, alginate intrinsic viscosity of Sargassum vulgare was 410.0 ml/g. Furthermore the alginate intrinsic viscosity from L. digitata was 243.1 ml/g and from Cystoseira barbata was 283 ml/g as these data were reported by Fertah et al. (2014) and Sellimi et al. (2015). Meanwhile, alginate extract from EAE was lower than alginate extracts from L. digitata (810 ml/g) and Sargassum sp. (618 ml/g), as previously reported by Vauchel et al. (2008) and Rahelivao et al. (2013), respectively.
Table 2.
Box–Behnken design from RSM and multiple responses of alginate
| No. | Algae to solution ratio (w/v) | Feed rate (rpm) | pH | RTD (min) | Yield (%) | Intrinsic viscosity (ml/g) | Molecular weight (kDa) |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 20 | 8 | 15.00 | 9.56 | 131.54 | 61.08 |
| 2 | 1 | 27.5 | 8 | 18.00 | 8.50 | 62.60 | 28.72 |
| 3 | 5 | 20 | 10 | 10.00 | 28.47 | 317.60 | 19.61 |
| 4 | 3 | 27.5 | 10 | 7.50 | 34.55 | 438.70 | 207.74 |
| 5 | 1 | 20 | 10 | 8.00 | 30.90 | 178.50 | 83.29 |
| 6 | 1 | 35 | 10 | 6.80 | 28.57 | 344.80 | 162.64 |
| 7 | 5 | 27.5 | 8 | 16.00 | 13.25 | 139.90 | 65.03 |
| 8 | 3 | 27.5 | 10 | 6.00 | 36.50 | 401.45 | 189.83 |
| 9 | 5 | 35 | 10 | 7.20 | 33.34 | 262.83 | 123.43 |
| 10 | 3 | 35 | 8 | 15.70 | 12.25 | 225.80 | 105.78 |
| 11 | 1 | 27.5 | 12 | 8.30 | 31.74 | 170.99 | 79.74 |
| 12 | 3 | 27.5 | 10 | 6.70 | 33.68 | 416.16 | 196.89 |
| 13 | 5 | 27.5 | 12 | 5.00 | 45.54 | 107.50 | 49.75 |
| 14 | 3 | 35 | 12 | 4.00 | 43.95 | 130.36 | 60.52 |
| 15 | 3 | 20 | 12 | 6.00 | 36.84 | 175.88 | 82.06 |
| Pred. | 3.11 | 2.95 | 10.3 | 5.79 ± 1.58a | 37.54 ± 3.45b | 413.80 ± 30.19c | 195.77 ± 14.35d |
| Valid | 3.11 | 2.95 | 10.3 | 6.80 ± 0.089a | 34.96 ± 0.09b | 447.39 ± 18.15c | 211.93 ± 8.74d |
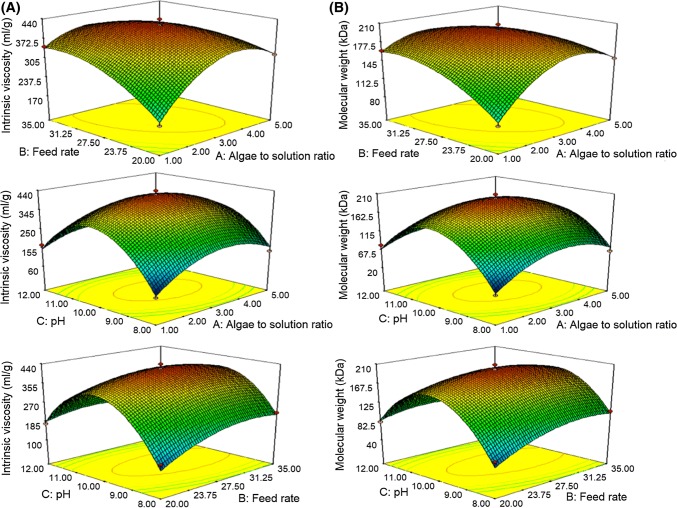
The effects of independent variables used on response surface plots of intrinsic viscosity are shown in Fig. 3a. Factors interaction between the independent variables depicted in Fig. 3a showed positive quadratic effects on alginate intrinsic viscosity. Intrinsic viscosity increased with increasing brown algae to solution ratio, feed rate and pH. The maximum value of intrinsic viscosity was achieved at brown algae to solution ratio (1:3), feed rate 27.5 rpm and pH 10. Laksmono et al. (2013) reported that high pH value could increase the alginate viscosity to reach the optimum value and then decreased as the effect of alginate depolymerization. The increase of brown algae to solution ratio and feed rate causes increasing contact area, as well as shear and stress in screw channel, therefore the extractability of alginate increases. Parameter interaction between brown algae to solution ratio with pH and combination of temperature, shear and stress in screw channel lead to porous and swollen lignocellulolitic materials in algae, thus high molecular weight of alginate can be extracted. At pH 8, the alginate intrinsic viscosity is lower because of the proton catalyzed alginate polymer hydrolysis. Meanwhile at pH 12, intrinsic viscosity is lower due to β-elimination reaction (Smidsrod et al. 1969; Haug et al. 1963).
Fig. 3.
Response surface plots of the effect of extrusion parameters on the intrinsic viscosity (a), and molecular weight of alginate (b)
Molecular weight
Treatment factors used in this experiments significantly affected the molecular weight of alginate from S. cristaefolium. Molecular weight values were in the range of 28.72–207.74 kDa (Table 2). This data is in accordance to alginate molecular weight reported by Torres et al. (2007), Vauchel et al. (2008) and Sellimi et al. (2015), but this finding data was lower than those reported by Rahelivao et al. (2013).
Response surface plots of molecular weight effected by factors interaction used at this experiments showed the quadratic effect (Fig. 3b). Molecular weight of alginate increases with the increasing brown algae to solution ratio, feed rate and pH. Maximum molecular weight (207.74 kDa) is obtained at brown algae to solution ratio (1:3), feed rate 27.5 rpm and pH 10. For treatment of brown algae to solution ratio (1:1), feed rate 20 rpm and pH 8 alginate molecular weight was lower than data reported by Sugiono et al. (2018), when they used specific mechanical energy (SME) extraction method. Kartika et al. (2010) and Huang and Ma (2016) reported that SME extraction method was inversely to the feed rate of alginate aqueous solution. The longer time needed for alginate stays inside the screw barrel, the stronger mechanical effect of SME, therefore the more degradation attack on the polymer of alginate, the higher the molecular weight of alginate produced. Haug et al. (1963) reported that alginate polymer hydrolysis occurred at pH 8 and produced lower alginate molecular weight. Whilst at pH 12 the alginate molecular weight is lower due to β-elimination reaction and alginate polymer chains degradation (Smidsrod et al. 1969).
Fitting model
Response surface methodology was used to evaluate and to optimize EAE variables based on multiple responses of alginate including RTD, yield, intrinsic viscosity, and molecular weight. Three evaluated variables are brown algae to solution ratio (1:1, 1:3, 1:5, w/v), feed rate (20, 27.5, 35 rpm), and pH (8, 10, 12) in Box Behnken Design with 3-replications in the center point as presented in Table 1. The center points were selected according to our previous experiment in preliminary stage. Regression multiple analysis was based on the experiment data. Prediction second check polynomial models of multiple responses of alginate were RTD, yield, intrinsic viscosity and molecular weight, respectively as presented in Table 3.
Table 3.
Quadratic models, significance codes and fitting models
| Coefficient | RTD (min) | Yield (%) | Intrinsic viscosity (ml/g) | Molecular weight (kDa) |
|---|---|---|---|---|
| Intercept | ||||
| β0 | + 6.73** | + 34.91** | + 418.77*** | + 198.15*** |
| Linear | ||||
| β1 | − 0.36ns | + 2.61* | + 8.87ns | + 4.18ns |
| β2 | − 0.66ns | + 1.54ns | + 20.03ns | + 9.54ns |
| β3 | − 5.18* | + 14.31** | + 3.11ns | + 1.43ns |
| Quadratic | ||||
| β11 | + 1.46ns | − 2.74ns | − 94.24** | − 44.98*** |
| β22 | − 0.19ns | − 1.85ns | − 48.60** | − 23.43* |
| β33 | + 3.63* | − 7.41ns | − 204.28** | − 97.36*** |
| Cross product | ||||
| β12 | − 0.40ns | + 1.80ns | − 55.27** | − 26.38** |
| β13 | − 0.32ns | + 2.26ns | − 35.20* | − 16.57** |
| β23 | − 0.67ns | + 1.11* | − 34.94* | − 16.56ns |
| Fitting model | ||||
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Lack of fit | 0.0584 | 0.7917 | 0.7876 | 0.3367 |
| R2 | 0.9984 | 0.9939 | 0.9123 | 0.9114 |
Equation of the type Y = β0+βx1+βx2 + βx3 + βx1x2 + βx1x3 + βx2x3 + βx1x1 + βx2x2 + βx3x3
Significance codes: ***P < 0.001; **0.001 < P < 0.01; *0.01 < P < 0.05; nsP > 0.05
Fitting model was evaluated as significant model, lack of fit and correlation coefficient which are presented in Table 3. Montgomery (2005) proposed that a good prediction model should have a significance level of P < 0.05, R2 ≥ 0.8 and lack of fit > 0.1. The independent and dependent variables were fitted to the second equation order model and examined for the goodness of fit. The results showed that the models in all the response variables were highly adequate because of P value in all the response variables is < P = 0.05. There is no significant lack of fit in all the response variables and R2 value in all the response variables are more than 80%. Therefore the response surface models developed were adequate.
Optimization and verification
Prediction of optimal parameters of extrusion assisted alginate extraction is brown algae to solution ratio 3.11, feed rate 2.95 rpm, pH 10.3 and the responses observed as follows: RTD of 5.79 min, yield of 37.54%, intrinsic viscosity of 413.80 ml/g, molecular weight of 195.77 kDa with desirability value of 0.881. The desirability value of predicted optimum process is in accordance to the desirability value reported by Sugiono and Soehono (2014). Desirability value ~ 1 means that prediction the optimal point has higher validity level (Lorbeer et al. 2015).
Verification of the optimal point was conducted by three replications and at this point, the responses RTD was 6.80 ± 0.089 min, yield of 34.96 ± 0.09%, intrinsic viscosity of 447.39 ± 18.15 ml/g, and molecular weight of 211.93 ± 8.74 kDa. The results of verification showed that the multiple responses of alginate were within the interval predicted (PI) range of 95% low and 95% high. The predicted values at the optimal conditions were in close agreement with experimental values (Table 2) and were found to be not significantly different at P > 0.05 using a paired t test. It is the evidence that validation experiment supports the optimum point of predictions and validity.
M/G ratio
M/G ratio affects on the alginate functional properties. The M/G ratio of S. cristaefolium alginate based on fractions analysis is 0.29 (M/G ratio < 1), guluronate fraction is 0.54 and manuronate is 0.16. This shows that the S. cristaefolium alginate contains guluronate fraction of 77.10% and manuronate fraction of 22.90%. The M/G ratio of S. cristaefolium alginate is relatively similar with Sargassum filipendulla (0.19), Sargassum muticum (0.31) and Sargassum polycystum (0.21) (Davis et al. 2003), but lower than the M/G ratio of S. vulgare (1.27/1.56) (Torres et al. 2007), Sargassum latifoilum (0.82) and Sargassum turbinarioides (0.94) (Larsen et al. 2003).
Variation in M/G ratio of alginate from brown algae is affected by species, seasonal variation, place of growth, type and age of algae tissue and extraction methods (Bertagnolli et al. 2014). The M/G ratio of alginate is lower than 1 (M/G ratio < 1) means that guluronate fraction is higher than manuronate fraction, and in this ratio, formation of gel strong and brittle. Conversely, if M/G ratio more than 1 (M/G ratio > 1), guluronate is lower than manuronate fraction and formation of gel is soft and elastic (Yang et al. 2013).
Steady-shear flow properties
Rheological properties S. cristaefolium alginate in aqueous solution at different range of concentration (2–3%, w/v) at temperature 25 °C were investigated by steady-shear flow test at shear rate (γ) 1–600 s−1. Power-law model was used to analyze rheological properties of sodium alginate (Eq. 7).
| 7 |
where σ is shear stress (Pa), k is consistency index (Pa sn), γ is shear rate (s−1), n is flow behavior index (dimensionless). Characteristic of liquid based on the value of flow behavior index (n) is Newtonian if flow behavior index n = 1. Liquid is pseudoplastic fluid if n < 1, and liquid is swelling plastic fluid if n > 1 (Rao et al. 2003). The different flow types are based on the curve of shear rate versus viscosity.
Flow behavior of S. cristaefolium alginate in aqueous solution at different range of concentration was shown in curve of shear rate versus shear stress (Fig. 4a). Figure 4a shows that shear stress increases at higher shear rate. Shear stress increases in higher alginate concentration due to alginate intermolecular interaction (Ma et al. 2014). Flow behavior of S. cristaefolium alginate in aqueous solution curve of shear rate versus shear stress was fitting by power-law flow model. S. cristaefolium alginate in aqueous solution has fluid shear-thinning pseudoplastic with flow behavior index less than 1 (n < 1). Higher alginate concentration causes a decrease in pseudoplasticity, and an increase in flow behavior index and consistency index, but power-law model is stable (data not shown). This finding is in accordance with the report of Yang et al. (2013). Cevoli et al. (2013) reported that power-law behavior of alginate in aqueos solution increased at concentration 1% and 1.5% (w/v). Meanwhile at concentration more than 1.5% power-law model not well, pseudoplasticity is decrease (n ~ 1). At alginate concentration > 1.5% alginate polymer chains forms a network entangled in solutions where alginate polymer chains are entangled each other (Mancini et al. 1996).
Fig. 4.
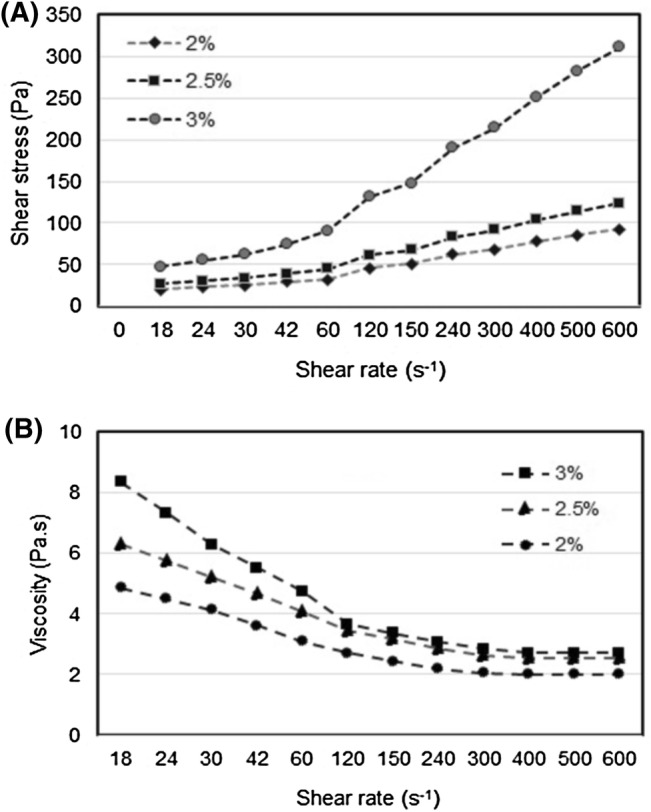
Flow behavior of Sargassum cristaefolium alginate in aqueous solution at different range concentration at temperature 25 °C (a) shear stress, (b) viscosity
Effect of alginate concentration on the flow behavior
Flow behavior properties of S. cristaefolium alginate in aqueous solution different range of concentration was plotted as curve shear rate versus viscosity (Fig. 4b). Figure 4b shows that S. cristaefolium alginate in aqueous solution exhibits shear-thinning pseudoplastic properties with flow behavior index n less than 1. Intrinsic viscosity decreases with increasing shear rate. Alginate viscosity is dynamic and is significantly affected by concentration, shear rate and temperature (Sellimi et al. 2015). Tunick (2011) reported that bonds-breaking and reformation of intermolecular bonds during frequency sweep might lead to structural change that affected on the rheological properties. At high frequency sweep test, inter and intramolecular bonds break and there is not enough time to reform or bonds-making (Ma et al. 2014). This phenomenon causes the permanent molecular change or disantanglement of alginate polymers chain, as the result is a decrease in viscosity. Flow fluid properties of S. cristaefolium alginate are in accordance with the report of by Sellimi et al. (2015) and Gomez et al. (2009), but different to the result of that reported by Torres et al. (2007).
The effect of different concentration of S. cristaefolium alginate on the dynamic viscosity (Fig. 4b) shows that alginate viscosity is higher in higher alginate concentration. At higher polymer concentration individual molecules start to overlap and form intermolecular junctions (Funami et al. 2009). It leads to limited arrangement and stretching of the alginate polymers chain. It should be noted that as the alginate concentration in a solution increases, the alginate viscosity also increases. Flow behavior of S. cristaefolium alginate in aqueous solution is pseudoplastic. Flow pseudoplastisity correlates well with increasing alginate concentration (Ma et al. 2014). Flow fluid pseudoplastic is characteristic of high structural polymers and molecular weight (Cevoli et al. 2013).
Conclusion
Extrusion parameters were evaluated in this study i.e. brown algae to solution ratio, feed rate and pH significantly affected on the multiple responses of S. cristaefolium alginate. The optimal extrusion parameters were brown algae to solution ratio 3.11, feed rate 2.95 rpm, and pH 10.3 with the response of RTD 6.80 ± 0.089 min, yield was 34.96 ± 0.09%, intrinsic viscosity 447.39 ± 18.15 ml/g, and molecular weight 211.93 ± 8.74 kDa. The M/G ratio of S. cristaefolium alginate was 0.29, with GG blocks 0.56 was higher than MM blocks 0.16, flow behavior of S. cristaefolium alginate in aqueous solution was shear-thinning pseudoplastic with flow behavior index n < 1. Twin Screw Extruder is a promising method to extract alginate from brown alga S. cristaefolium at the industrial scale.
Acknowledgements
Authors would like to thank Technopark, Bogor Agricultural University, for facilitating in using a twin-screw extruder. Part of the works were supported by Directorate of Research and Community Service, Directorate of Higher Education, Minister of Research and Technology, Higher Education, Rep. of Indonesia, under the Scheme Doctorate Dissertation Research 2016, Number DIPA-042.06.1.401516/2017.
Compliance with ethical standards
Conflict of interest
The authors declared that this study was absence of any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sugiono Sugiono, Email: yonosugiono78@yahoo.co.id.
Masruri Masruri, Email: masruri@ub.ac.id.
Teti Estiasih, Email: teties@yahoo.co.id.
Simon Bambang Widjanarko, Phone: +62-812-3388-005, Email: simonbw@ub.ac.id.
References
- Andriamanantoanina H, Rinaudo M. Alginate from five brown seaweed Madagascar. Carbohydr Polym. 2010;82:555–560. doi: 10.1016/j.carbpol.2010.05.002. [DOI] [Google Scholar]
- Baron R, Vauchel P, Kaas R, Arhaliass A, Legrand J. Dynamical modeling of a reactive extrusion process: focus on residence time distribution in a fully intermeshing co-rotating twin-screw extruder and application to an alginate extraction process. Chem Eng Sci. 2010;65:3313–3321. doi: 10.1016/j.ces.2010.02.019. [DOI] [Google Scholar]
- Bertagnolli C, Espindola APDM, Klienubing SJ, Tasic L, Silva MGCD. Sargassum felipendulla alginate from Brazil: Seasonal influence and characteristics. Charbohydr Polym. 2014;111:619–623. doi: 10.1016/j.carbpol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Cevoli C, Balestra F, Ragni L, Fabbri A. Rheological characterization of selected food hydrocolloids by traditional and simplified techniques. Food Hydrocoll. 2013;33:142–150. doi: 10.1016/j.foodhyd.2013.02.022. [DOI] [Google Scholar]
- Chee SY, Wong PK, Wong CL. Extraction and characterization of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J Appl Phycol. 2011;23:191–196. doi: 10.1007/s10811-010-9533-7. [DOI] [Google Scholar]
- Chhatbar M, Meena R, Prasad K, Siddhanta AK. Microwave assisted rapid method for hydrolysis of sodium alginate for M/G ratio determination. Carbohydr Polym. 2009;76:650–656. doi: 10.1016/j.carbpol.2008.11.033. [DOI] [Google Scholar]
- Clementi F, Mancini M, Moresi M. Rheology of alginate from Azotobacter vinelandii in aqueous dispersions. J Food Eng. 1998;36:51–62. doi: 10.1016/S0260-8774(98)00042-9. [DOI] [Google Scholar]
- Davis TA, Lanes F, Volesky B, Diaz-Pulido G, McCook L, Mucci A. 1H-NMR study of na-alginates extracted from Sargassum spp. in relation to metal biosorption. Appl Biochem Biotechnol. 2003;110:75–89. doi: 10.1385/ABAB:110:2:75. [DOI] [PubMed] [Google Scholar]
- Draget KI, Taylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011;25(2):251–256. doi: 10.1016/j.foodhyd.2009.10.007. [DOI] [Google Scholar]
- Fertah M, Belfkira A, Dahmane EM, Taurirte M, Brouillette A, Taurirte M. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arabian J Chem. 2014;5(3):1878–1888. [Google Scholar]
- Funami T, Fang Y, Noda S, Ishihara S, Nakauma M, Draget KI, Nishinari K, Phillips GO. Rheological properties of sodium alginate in an aqueous system during gelation in relation to supermolecular structures and Ca2+ binding. Food Hydrocoll. 2009;23:1746–1755. doi: 10.1016/j.foodhyd.2009.02.014. [DOI] [Google Scholar]
- Gomez CG, Lambrcht MVP, Lozano JE, Rinaudo M, Villar MA. Influence of the extraction-purification condition on final properties of alginates obtained from brown algae (Macrocystis pyrifera) Int J Biol Macromol. 2009;44:365–371. doi: 10.1016/j.ijbiomac.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Haug A, Larsen B, Smidsrod O. The degradation of alginates at different pH values. Acta Chem Scand. 1963;17(5):1466–1468. doi: 10.3891/acta.chem.scand.17-1466. [DOI] [Google Scholar]
- Hernandez-Carmona G. Conventional and alternative technologies for the extraction of algal polysaccharides. Mexico City: Woodhead Publishing Limited; 2013. pp. 472–514. [Google Scholar]
- Huang YL, Ma YS. The effect of extrusion processing on the physiochemical properties of extruded orange pomace. Food Chem. 2016;192:363–369. doi: 10.1016/j.foodchem.2015.07.039. [DOI] [PubMed] [Google Scholar]
- Kartika IA, Pontalier PY, Rigal L. Extraction of sunflower oil by twin-screw extruder: screw configuration and operating conditions effects. Bioresourc Technol. 2005;97:2302–2310. doi: 10.1016/j.biortech.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Kartika IA, Pontalier PY, Rigal L. Twin-screw extruder for oil processing of sunflower seeds: thermo-mechanical pressing and solvent extraction in a single step. Ind Crop Prod. 2010;32(3):297–304. doi: 10.1016/j.indcrop.2010.05.005. [DOI] [Google Scholar]
- Laksmono JA, Widjaya RR, Triajie F, Sastrosoeparno S. Parameters controlled on Indonesian Sargassum duplicatum extraction process to obtain a water-soluble alginate. Int J Basic Appl Sci IJBAS-IJENS. 2013;13(05):17–21. [Google Scholar]
- Larsen B, Salem DMSA, Sallam MAE, Mishrikey MM, Beltagy AI. Characterization of the alginates from algae harvested at the Egyptian Red Sea coast. Carbohydr Res. 2003;338:235–336. doi: 10.1016/s0008-6215(03)00378-1. [DOI] [PubMed] [Google Scholar]
- Lorbeer AJ, Lahnstein J, Bulone V, Nguyen T, Zhang W. Multiple-response optimization of the acidic treatment of the brown alga Ecklonia radiata for the sequential extraction of fucoidan and alginate. Biores Technol. 2015;197:302–309. doi: 10.1016/j.biortech.2015.08.103. [DOI] [PubMed] [Google Scholar]
- Ma J, Lin Y, Chen X, Zhao B, Zhang J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014;38:119–128. doi: 10.1016/j.foodhyd.2013.11.016. [DOI] [Google Scholar]
- Mancini M, Moresi M, Sappino F. Rheological behaviour of aqueous dispersions of algal sodium alginates. J Food Eng. 1996;28:283–295. doi: 10.1016/0260-8774(95)00068-2. [DOI] [Google Scholar]
- Montgomery DC. Response surface methods and designs. New York: Willy; 2005. [Google Scholar]
- Quitain AT, Kai T, Sasaki M, Goto M. Microwave–hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide deoiled Undaria pinnatifida. Ind Eng Chem Res. 2013;52(23):7940–7946. doi: 10.1021/ie400527b. [DOI] [Google Scholar]
- Rahelivao MP, Andriamanantoanina H, Heyraud A, Rinaudo M. Structure and properties of three alginates from madagascar seacoast algae. Food Hydrocoll. 2013;32(1):143–146. doi: 10.1016/j.foodhyd.2012.12.005. [DOI] [Google Scholar]
- Rao YM, Suresh AK, Suraishkumar GK. Free radical aspects of Xanthomonas campestris cultivation with liquid phase oxygen supply strategy. Proc Biochem. 2003;38:1301–1310. doi: 10.1016/S0032-9592(02)00328-X. [DOI] [Google Scholar]
- Sellimi S, Younes I, Ayed HB, Maalej H, Montero V, Rinaudo M, Dahia M, Mechichi T, Hajji M, Nasri M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int J Biol Macromol. 2015;72:358–1367. doi: 10.1016/j.ijbiomac.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Smidsrod O, Haug A, Larsen B. Degradation of alginate in the presence of reducing compounds. Acta Chem Scand. 1969;17(10):2628–2637. [Google Scholar]
- Sugiono Widjanarko SB, Soehono LA. Extraction optimization by response surface methodology and characterization of fucoidan from brown seaweed Sargassum polycystum. Int J Chemtech Res. 2014;6(1):195–205. [Google Scholar]
- Sugiono S, Masruri M, Estiasih T, Widjanarko SB. Multiple-response optimization of the acidic pre-treatment of the brown alga Sargassum cristaefolium for the alginate extraction using twin screw extruder. Biosci Res. 2018;15(2):683–693. [Google Scholar]
- Tambunan APM, Rudiyansyah Harlia. The effect concentration Na2CO3 on the yield of alginate Sargassum cristaefolium from Lemukutan. J Equat Chem. 2013;2(2):112–117. [Google Scholar]
- Torres MR, Saosa APA, Filho EATS, Melo DF, Feitosa JPA, Paula RCMD, Lima MGS. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr Res. 2007;342:2067–2074. doi: 10.1016/j.carres.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Tunick HM. Small-strain dynamic rheology of food protein networks. J Agric Food Chem. 2011;59:1481–1486. doi: 10.1021/jf1016237. [DOI] [PubMed] [Google Scholar]
- Vauchel P, Kaas R, Arhaliass A, Baron R, Legrand J. A new process for extracting alginates from Laminaria digitata: reactive extrusion. Food Bioprocess Technol. 2008;1(3):297–300. doi: 10.1007/s11947-008-0082-x. [DOI] [Google Scholar]
- Yang Y, Campanella OH, Hamaker BR, Zhang GGZ. Rheological investigation of alginate chain interactions induced by concentrating calcium cations. Food Hydrocoll. 2013;30(1):26–32. doi: 10.1016/j.foodhyd.2012.04.006. [DOI] [Google Scholar]
- Zheng J, Rehmann L. Extrusion pretreatment of lignocellulosic biomass: a review. Int J Mol Sci. 2014;15:18967–18984. doi: 10.3390/ijms151018967. [DOI] [PMC free article] [PubMed] [Google Scholar]