We study the pathogenesis of herpes simplex virus-mediated corneal disease. T cells play a critical role both in disease and in the maintenance of latency in neurons. Consequently, the focus of this study was to evaluate the role that T cell costimulation plays both in corneal disease and in controlling the ability of the virus to maintain a stable infection of the ganglia that innervate the cornea. We demonstrate that in the absence of costimulation with CD28, corneal disease does not take place. However, this costimulation does not prevent the ability of CD8+ T cells to develop and, thus, control latent infection of neurons. We conclude from these studies that CD28 costimulation is required for corneal destructive immune responses but that CD8+ T cells develop over time and help to maintain latency.
KEYWORDS: T cell costimulation, ocular immunology, viral pathogenesis
ABSTRACT
Corneal infection with herpes simplex virus 1 (HSV-1) leads to infection of trigeminal ganglia (TG), typically followed by the establishment of latency in the infected neurons. When latency is disrupted, the virus reactivates and migrates back to the cornea, where it restimulates the immune response, leading to lesions in a disease called herpetic stromal keratitis (HSK). HSK requires T cell activation, as in the absence of T cells there is no disease. We decided to determine if CD28 costimulation of T cells was required in HSK. The results indicated that C57BL/6 CD28−/− and BALB/c CD28−/− mice failed to develop recurrent HSK, while their wild-type counterparts did. In order to better understand the dynamics of TG infection in these mice, we evaluated the amount of virus in infected TG and the number of individual neurons harboring latent virus. The results indicated that CD28−/− mice possessed significantly increased genome levels in their TG but many fewer LAT-positive cells than wild-type mice from day 7 to day 30 but that after day 30 these differences became nonsignificant. We next evaluated total and antigen-specific CD8+ T cells in TG. The results indicated that there were significantly fewer CD8 T cells in TG from day 10 to day 25 but that after that the differences were not significant. Taken together, these data suggest that CD28 costimulation is required for HSK but that while initial infection of TG is greater in CD28−/− mice, this begins to normalize with time and this normalization is concurrent with the delayed development of antigen-specific CD8+ T cells.
IMPORTANCE We study the pathogenesis of herpes simplex virus-mediated corneal disease. T cells play a critical role both in disease and in the maintenance of latency in neurons. Consequently, the focus of this study was to evaluate the role that T cell costimulation plays both in corneal disease and in controlling the ability of the virus to maintain a stable infection of the ganglia that innervate the cornea. We demonstrate that in the absence of costimulation with CD28, corneal disease does not take place. However, this costimulation does not prevent the ability of CD8+ T cells to develop and, thus, control latent infection of neurons. We conclude from these studies that CD28 costimulation is required for corneal destructive immune responses but that CD8+ T cells develop over time and help to maintain latency.
INTRODUCTION
Corneal infection with herpes simplex virus 1 (HSV-1) typically follows a predictable course that involves proliferation first at the site of infection, followed by infection of the nerves found in the cornea and the subsequent retrograde transport of virus to the trigeminal ganglia (TG) (1–3). Once in the ganglia, the virus replicates there and some of these newly formed viruses return to the periphery (3), but within a week, infectious virus typically can no longer be detected in the TG of wild-type mice, as the virus establishes a latent infection of the neurons in the TG (2–6). During latency, the viral genome is present, but few active virions are detected in these infected neurons; however, they do express viral RNA, with latency-associated transcripts being one of the most abundant in these latently infected neurons (5, 6). This latent infection can be interrupted by various forms of stimuli, including immunosuppressive events, such as fever, menses, sunlight (UV), irradiation, stress, and trauma (7–11). Following reactivation, new viruses are formed, the virus travels via anterograde axonal transport back to the epithelial surface, and its replication and subsequent stimulation of a host immune response are responsible for the observed symptoms that define most cases of corneal keratitis (12–14). This disease is a leading cause of infectious blindness in the Western world, with one study determining a prevalence of HSV keratitis of 149/100,000 people (1).
In humans, primary disease is rare, occurring mostly in children and the immunosuppressed. Primary disease is most often clinically asymptomatic, although in 1 to 6% of cases it presents as blepharoconjunctivitis that heals without scarring (8). Primary disease typically begins by exposure through the corneal or oral epithelium. The virus replicates in these cells and then travels via retrograde axonal transport in sensory neurons to the sensory ganglia (most often the trigeminal ganglia), where it establishes latency. The dominant form of clinical disease is the result of reactivation of virus.
Recurrent disease in the cornea is an immunopathologic condition that is initiated by the renewed presence of virus in the cornea, which restimulates the immune response, leading to inflammation of the cornea and resulting in damage to the cornea. In humans, the inflammatory infiltrate in herpetic stromal keratitis (HSK) is characterized by the influx of a phenotypically diverse population of leukocytes consisting of lymphocytes, neutrophils, and mononuclear phagocytes (6, 7). Animal studies have shown that neutrophils are the cell type found in the greatest numbers in corneas displaying disease (15, 16). However, these studies have conclusively determined that HSK does not occur in the absence of T cells (17–20).
The activation of T cells requires not only the engagement of the T cell receptor with a complex of a major histocompatibility complex (MHC) class II antigen and a foreign peptide but also a costimulatory signal between antigen-presenting cells (APC) and the T cell (21, 22). For naive T cells, this interaction is typically between CD28 on the T cell and CD80 or CD86 on the APC (22). In the absence of this costimulatory interaction, T cells typically enter an inactive state of anergy in which they do not become effector cells. Very little has been reported on the role that the CD28 costimulatory pathway plays in primary HSK (23, 24), as the focus has been on the expression of CD80/86 and not directly on CD28. As for recurrent disease, nothing has been reported. Consequently, we set out to determine the consequences of ocular infection with HSV-1 on the development of both primary and recurrent HSK in mice, where this interaction does not occur. We present evidence demonstrating that neither primary nor recurrent HSK develops in mice, where the CD28 costimulatory pathway does not exist. We demonstrate that there is little to no corneal disease, as evidenced by a lack of opacity, neovascularization, or loss of corneal sensitivity, and that the cellular inflammation is significantly reduced following reactivation. In contrast, CD8 T cell immune responses in the trigeminal ganglia, while are initially impaired, do develop, leading to an imperfect control of the latent infection.
RESULTS
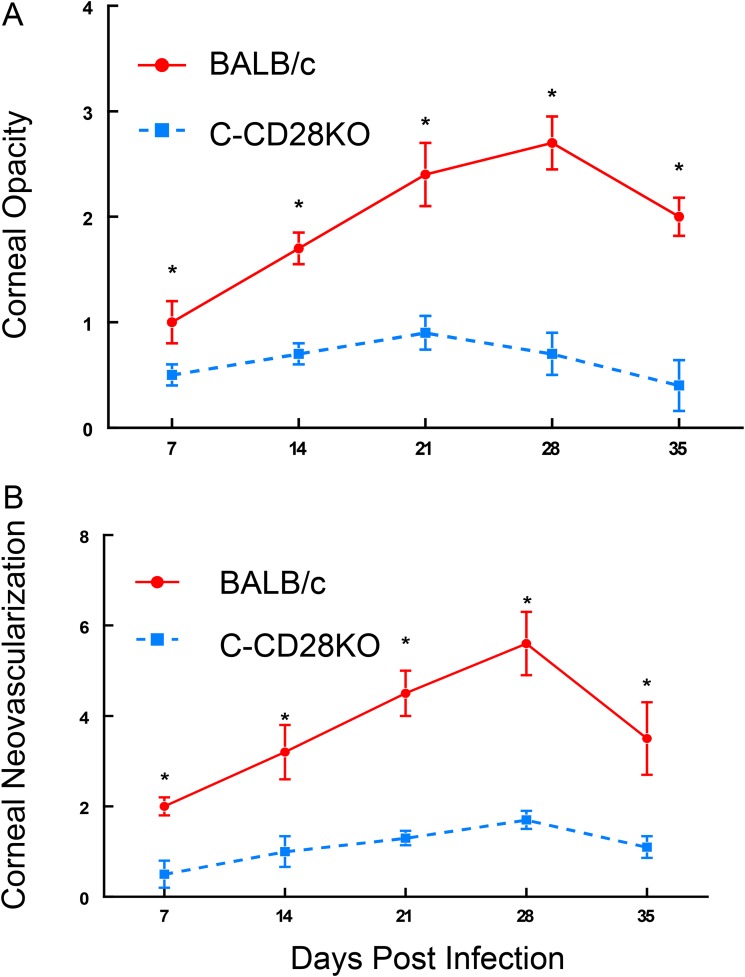
To determine whether CD28 costimulation is required for the development of corneal disease following infection with HSV-1, we infected both BALB/c and BALB/c CD28-knockout (C-CD28KO) mice with the KOS strain of HSV-1. As evidenced in Fig. 1, mice lacking CD28 displayed significantly reduced opacity and neovascularization compared to their wild-type counterparts. Since C57BL/6 (B6) mice do not routinely develop significant disease when infected with the KOS strain of HSV-1, we infected B6 and B6 CD28-knockout (B6-CD28KO) mice with the McKrae strain of HSV-1. Unfortunately, this infection did not prove to be successful, as even at 105 PFU/mouse, most of the B6-CD28KO mice succumbed to this infection (data not shown). Because of these observations, only BALB/c mice were used to evaluate the effects of CD28-mediated costimulation during primary disease.
FIG 1.
BALB/c CD28KO mice did not display significant acute HSK compared to wild-type BALB/mice when infected with 107 PFU of the HSV-1 KOS strain. BALB/c CD28KO mice (n = 25) were compared with wild-type BALB/c mice (n = 25) for corneal opacity (A) and corneal neovascularization (B). Wild-type mice displayed significant corneal opacity and neovascularization for all time points measured, while BALB/c CD28KO mice did not. *, P values ranged from <0.01 to 0.001.
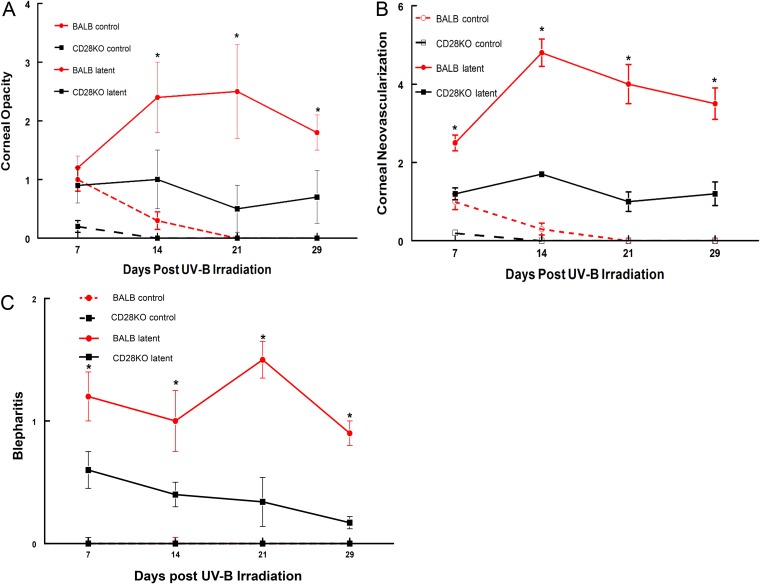
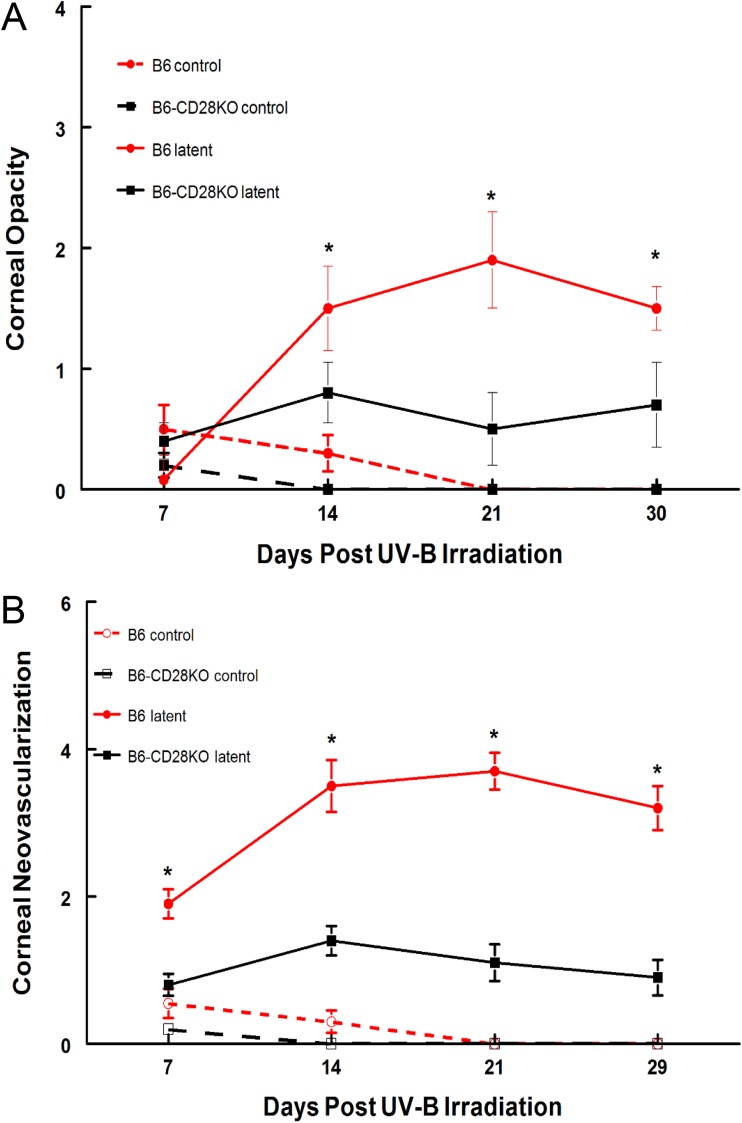
We next decided to test whether CD28 costimulation had any effects on recurrent HSK by establishing a latent infection in BALB/c and C-CD28KO mice with the McKrae strain of HSV-1. At 5 weeks postinfection, we treated these mice with UV-B to reactivate the virus. As shown in Fig. 2, the C-CD28KO mice displayed significantly reduced opacity, neovascularization, and blepharitis compared to wild-type BALB/c mice, once again demonstrating that CD28 costimulation is critical to developing a significant immune response that results in corneal disease. Interestingly, two remarkable observations were made when these mice were swabbed for infectious virus. The first was that over 10% of the C-CD28KO mice shed virus prior to UV-B reactivation. This is unusual, as we have observed this with only one other strain of mouse and that was a B6 gamma interferon (IFN-γ)-knockout (B6-IFN-γKO) strain (25), which demonstrated a 10% shedding rate prior to UV-B reactivation. Since wild-type as well as many other gene-targeted mice do not display viral shedding at 5 weeks postinfection, we considered this an indication that the responses in the TG were different in C-CD28KO mice than in BALB/c mice. The other observation was that C-CD28KO mice shed virus for much longer (up to day 12) than wild-type BALB/c mice, which did not shed virus for more than 6 days postreactivation (Table 1). Similar studies were also performed using B6 and B6-CD28KO mice. As expected, the B6-CD28KO mice display a significantly reduced disease profile compared to wild-type B6 mice (Fig. 3). These mice also demonstrated a pattern of viral shedding very similar to that of their BALB/c counterparts (Table 1). It should be noted that both B6-CD28KO and C-CD28KO mice displayed greater rates of reactivation (the percentage of mice with at least 1 day of measurable PFU), greater peak titers, and the persistence of viral shedding compared to their wild-type counterparts (Table 1). Consequently, these data do not support the notion that increased viral titers, the reactivation frequency, or the persistence of viral shedding is a good predictor of corneal disease, as the CD28KO mice did not demonstrate significant disease, despite showing greater values for these virological indices.
FIG 2.
BALB/c CD28KO mice did not display significant recurrent HSK compared to wild-type BALB/c mice. The eyes of the mice were infected with 106 PFU of the HSV-1 McKrae strain. At 6 weeks following infection, the mice were irradiated with UV-B to reactivate the latent infection. BALB/c CD28KO mice (n = 20) were compared with wild-type BALB/c mice (n = 20), along with UV-irradiated uninfected controls (n = 5 for both strains), for corneal opacity (A), corneal neovascularization (B), and blepharitis (C). *, P values for the comparison of the two strains ranged from 0.05 to 0.001.
TABLE 1.
Virological analysis of mice following UV-B reactivationa
| Mouse strain | % positive swabsb | Peak titerc | Total no. of shedding daysd | No. of days of shedding/mousee | Reactivation rate (%)f | Final day of sheddingg |
|---|---|---|---|---|---|---|
| B6 | 14 | 3.85 + 0.32 | 10 | 1.9 + 0.14 | 40 | 5 |
| B6-CD28KO | 33 | 4.79 + 0.34 | 32 | 3.7 + 0.3 | 65 | 12 |
| BALB/c | 11 | 3.65 + 0.38 | 8 | 1.6 + 0.11 | 35 | 6 |
| C-CD28KO | 28 | 4.25 + 0.42 | 28 | 3.3 + 0.18 | 55 | 12 |
Data are for 20 mice of each strain.
The percentage of virus-positive eye swabs (140 to 286 eye swab specimens per group) over the 12-day period following UV-B irradiation (P < 0.05 for CD28KO mice).
Peak viral titer, which occurred on day 3 postreactivation, expressed as the number of log10 PFU (P < 0.02 for CD28KO mice).
Total number of days that the mice were swab positive (P < 0.01 for CD28KO mice).
The number of days that a positive mouse shed virus (P < 0.05 for CD28KO mice).
The percentage of mice in which virus was reactivated (P < 0.02 for CD28KO mice).
The last day that a mouse was positive for a particular group.
FIG 3.
B6-CD28KO mice did not display significant recurrent HSK compared to wild-type B6 mice. The eyes of the mice were infected with 106 PFU of the HSV-1 McKrae strain. At 6 weeks following infection, the mice were irradiated with UV-B to reactivate the latent infection. B6 CD28KO mice (n = 30) were compared with wild-type B6 mice (n = 30), along with UV irradiated uninfected controls (n = 5 for both strains), for corneal opacity (A) and corneal neovascularization (B). *, P values for the comparison of the two strains ranged from 0.05 to 0.001.
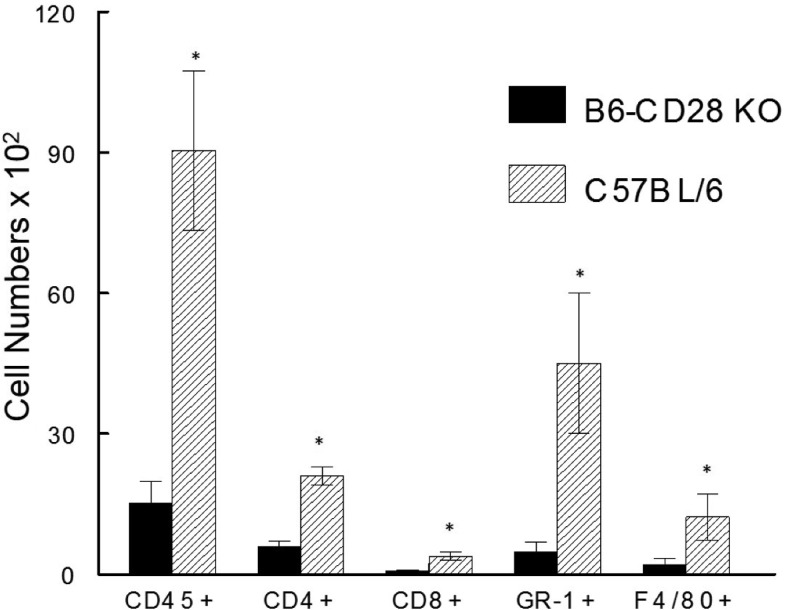
To better characterize the cellular infiltrate following reactivation, we isolated cells from the corneas of mice in which virus was reactivated at 17 days after UV-B-induced reactivation for flow cytometric analysis. These cells were initially gated on CD45+ cells to restrict our analysis to the inflammatory infiltrate and then evaluated for CD4, CD8, GR-1, F4/80, and CD11b expression. As seen in Fig. 4, significantly more CD45+ cells were isolated from B6 mice than from B6-CD28KO mice (8,874 ± 1,800 versus 1,746 ± 468, P < 0.01). Interestingly, while B6 mice displayed significantly greater numbers for all the surface molecules tested, the relative percentages of these cell types were quite similar when comparing B6 to B6-CD28KO mice (Fig. 4). Furthermore, functional analysis of CD8+ T cells isolated from the corneas of these mice did not reveal any significant differences in the percentage of cells expressing IFN-γ, tumor necrosis factor alpha (TNF-α), or CD107 (unpublished data).
FIG 4.
B6-CD28KO mice did not display significant numbers of inflammatory cells compared to wild-type B6 mice. Corneas were removed from latently infected B6 (n = 10) and B6-CD28KO (n = 10) mice at day 17 following reactivation. These corneas were disaggregated into single-cell suspensions and stained with antibodies against CD45, CD4, CD8α, GR-1, and F4/80. The cells were then analyzed by flow cytometry. The data represent the mean ± SEM for individually analyzed corneas from these groups. Then, the number of each type of cell was significantly greater in wild-type B6 corneas. *, P values ranged from 0.01 to 0.005.
As indicated earlier, B6-CD28KO mice, unlike their wild-type counterparts occasionally shed virus well beyond the normal day 7 postinfection (Table 1). This suggests that these mice do not have control over viral replication as tight as that seen with wild-type B6 mice and that latency might be similarly affected, as was evidenced by the fact that slightly more than 10% of mice without CD28 costimulation were shedding virus at the time of reactivation. We decided to address this observation in three different ways.
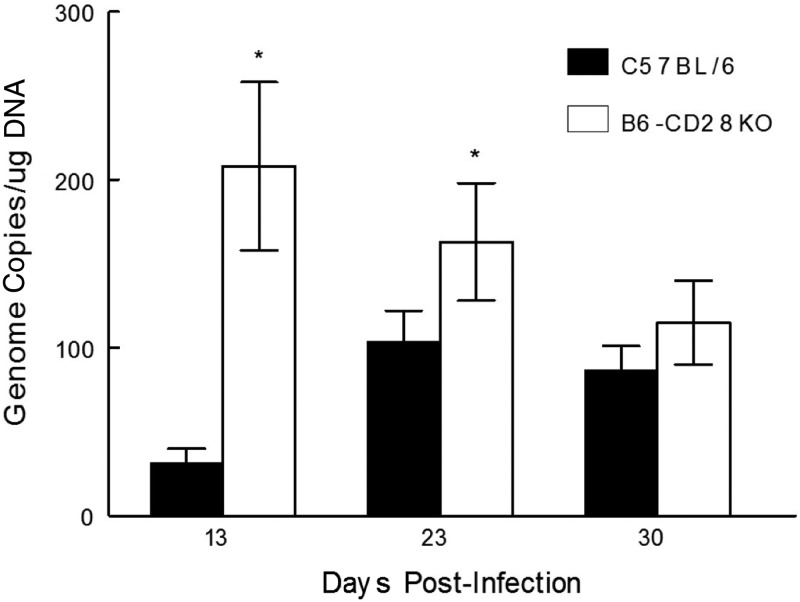
The first was to determine whether B6-CD28KO mice develop a greater infection of their TG than B6 mice. This analysis involved performing real-time PCR analysis to determine the relative amount of viral DNA in isolated trigeminal ganglia (TG). This analysis would allow us to determine the relative number of HSV-1 genome copies in these two strains of mice following infection. We had predicted that the B6-CD28KO mice would harbor significantly more viral DNA in their TG than B6 mice. This is precisely what we observed when the TG were analyzed at days 13 and 23 postinfection (Fig. 5). However, by day 30 the attrition of viral DNA in B6-CD28KO mice reduced their genome levels to where there were no significant differences between these two strains of mice. This suggests that mice without CD28 costimulation were still able, with time, to restrict the long-term infection of TG following infection.
FIG 5.
B6-CD28KO mice display significantly greater HSV-1 genome copy numbers than wild-type B6 mice at times of less than 30 days, but by day 30 these differences are not significant. Trigeminal ganglia were removed from latently infected B6 (n = 7-10) and B6-CD28KO (n = 7-10) mice at the indicated time points following infection. The data represent the mean ± SEM for individually analyzed trigeminal ganglia from these groups. *, statistical analysis indicated that P was <0.1 at day 13 and <0.05 at day 23.
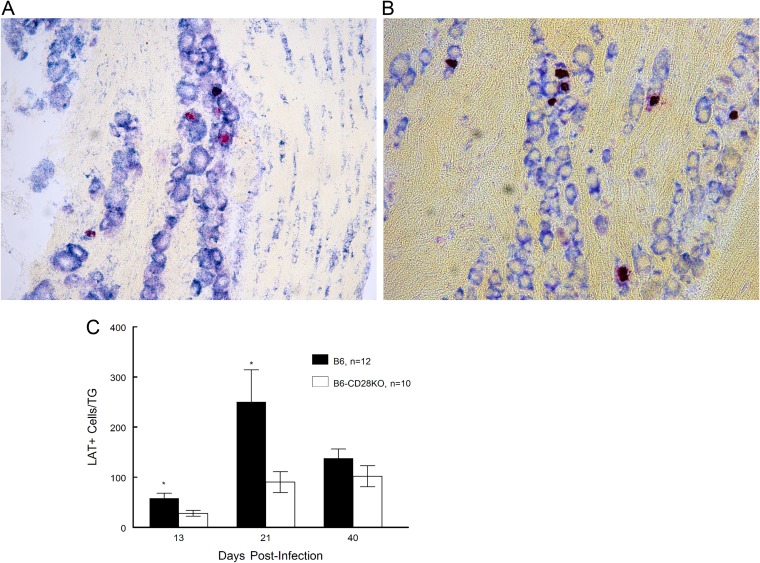
Since it was possible that differences in genome copy numbers could be the result of more genomes per neuron in B6-CD28KO, mice we also performed fluorescent in situ hybridization (FISH) analysis to quantitate the number of neurons expressing LAT as a means of quantifying latent infection with HSV-1 in infected TG. Previous studies examining HSV-1 latency have shown that using a similar methodology detects approximately 25% to 30% of latently infected ganglionic neurons (26–28). We anticipated that the results of that analysis would parallel the genome copy numbers, namely, that B6-CD28KO mice would display significantly greater numbers of latently infected cells. This, however, was not the case. Instead, B6 wild-type mice had significantly more LAT-positive (LAT+) cells than B6-CD28KO mice at early time points (days 13 and 21) following infection, but with time (day 40), as was observed for genome copy numbers, these differences became insignificant (Fig. 6).
FIG 6.
B6-CD28KO mice display significantly fewer LAT+ cells in the trigeminal ganglia than wild-type B6 mice at times of less than 40 days, but by day 40 these differences are not significant. Trigeminal ganglia were removed from latently infected B6 (n = 12) and B6-CD28KO (n = 10) mice at the indicated time points following infection. (A) A representative field for B6-CD28KO mouse trigeminal ganglia stained for LAT expression. Magnification, ×40. (B) A representative field for B6 mouse trigeminal ganglia stained for LAT expression. Magnification, ×40. (C) Data for LAT+ cells in the trigeminal ganglia in graphic form, wherein the data express the mean ± SEM for individually analyzed trigeminal ganglia from these groups. *, statistical analysis indicated that P was <0.1 at day 13 and <0.05 at day 21.
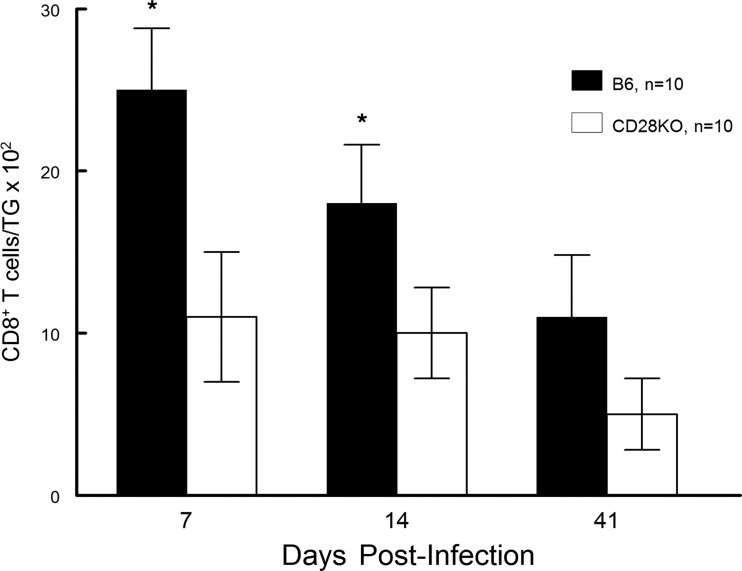
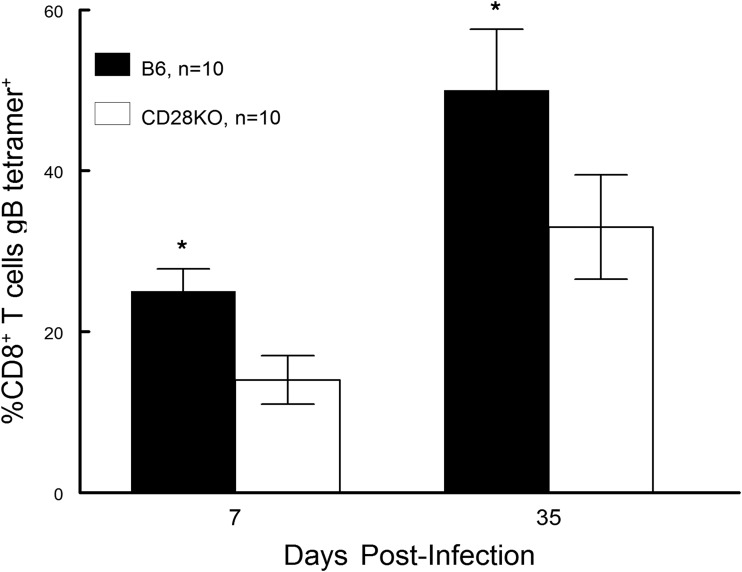
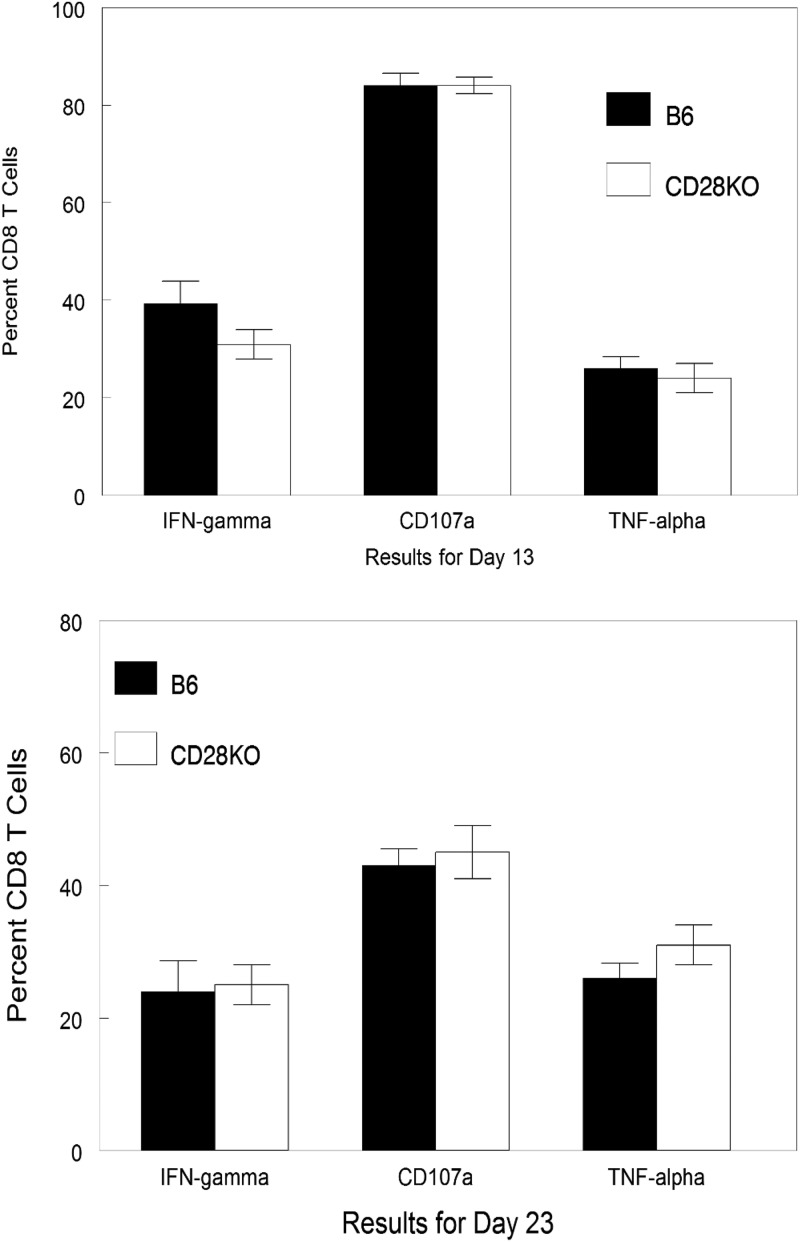
Finally, we decided to evaluate the cellular infiltrate found in the TG of infected mice. Previous studies have indicated that there is a significant influx of CD8+ T cells into the TG of infected mice (29, 30). Furthermore, in B6 mice, approximately 50% of these cells are gB tetramer positive, with the remainder of the CD8+ T cells being specific for other nondominant viral peptides (31). Results from our analysis were remarkably like those seen in Fig. 5 and 6. Namely, when evaluated at day 7 and day 14 postinfection, the CD8 T cell response in the TG of B6-CD28KO mice was significantly less than that seen with wild-type B6 mice; however, by day 41 there were no significant differences (Fig. 7). Interestingly, the percentage of gB-positive tetramer CD8+ T cells was consistently greater for wild-type B6 mice than for B6-CD28KO mice (Fig. 8). In contrast to differences in gB tetramer percentages, the overall functionality, as determined by the expression of CD107, TNF-α, and IFN-γ, of the CD8+ T cells found in the TG was the same between these two strains, as shown in Fig. 9. This led us to test whether there were any differences in the expression of exhaustion markers, such as PD-1 or TIM-3; however, such an analysis did not demonstrate any differences for CD8+ T cells found in B6 and B6-CD28KO mice (data not shown). Taken together, these results for CD8+ T cells isolated from TG indicate that the lack of CD28 costimulation does not prevent the development of a CD8 T cell response but does significantly delay it.
FIG 7.
B6-CD28KO mice display significantly fewer CD8+ T cells in latently infected trigeminal ganglia at days 7 and 14 but not at day 41. Trigeminal ganglia were removed from latently infected B6 (n = 10) and B6-CD28KO (n = 10) mice at the indicated time points, disaggregated into single-cell suspensions, and stained with antibodies against CD45 and CD8α. The cells were then analyzed by flow cytometry. The data represent the mean ± SEM for individually analyzed trigeminal ganglia from these groups. Then, the number of CD8 T cells was significantly greater in wild-type B6 mouse corneas (*, P < 0.02).
FIG 8.
B6-CD28KO mice display significantly fewer gB tetramer-expressing CD8+ T cells in latently infected trigeminal ganglia at days 7 and 35. Trigeminal ganglia were removed from latently infected B6 (n = 10) and B6-CD28KO (n = 10) mice at the indicated time points, disaggregated into single-cell suspensions, and stained with antibodies against CD45 and CD8α and with a gB-specific tetramer. Cells were then analyzed by flow cytometry. The data represent the mean ± SEM for individually analyzed trigeminal ganglia from these groups. Then, the number of CD8+ T cells expressing the gB tetramer was significantly greater in wild-type B6 corneas. *, P < 0.02 at day 7 and P < 0.05 at day 35.
FIG 9.
No differences in the percentages of functional CD8+ T cells were seen at either day 13 or day 23. Trigeminal ganglia were removed from latently infected B6 (n = 8) and B6-CD28KO (n = 8) mice at the indicated time points, disaggregated into single-cell suspensions, and stained with antibodies against CD45, CD8α, IFN-γ, CD107a, and TNF-α. Cells were then analyzed by flow cytometry. The data represent the mean ± SEM for individually analyzed trigeminal ganglia from these groups.
DISCUSSION
Recurrent HSV-1 leading to herpetic stromal keratitis (HSK) is the leading cause of infectious blindness in the Western world (1). Following primary corneal infection by HSV-1, latency is established and can be disrupted by various stressors, leading to reactivation. In humans, the primary disease is typically asymptomatic, while most cases demonstrating clinical disease are a result of reactivated HSV-1 (8, 12, 13, 32). Recurrent infection causes an immunopathologic condition that leads to corneal inflammation and chronic damage. Previous studies have demonstrated that HSK requires intact T cells (17–20) and an inflammatory infiltrate that consists of both neutrophils (15, 33) and macrophages (34, 35). Our present study extends those studies by indicating that CD28 costimulation of T cells is necessary for both primary and recurrent disease.
T cell activation requires both engagement between the T cells’ T cell receptor and the MHC class II antigen and foreign peptide, as well as various costimulatory signals. In the case of naive T cells, the costimulatory signal is typically CD28 on the T cell, which interacts with CD80/86 on the APC (21, 22). Previous studies from other groups have evaluated CD40/CD154 costimulation (36) and OX40/OX40L costimulation (37) in the context of acute infection with HSV-1. In both instances, these interactions did not appear to be directly involved in the effector phase of the disease, as blocking such responses did not alter the disease course (36, 37). We decided to focus on the CD28/CD80 or CD86 interaction, as it had not been sufficiently studied. Our studies demonstrate that mice lacking CD28 (BALB/c CD28KO mice) do not develop significant primary disease, suggesting that CD28 costimulation of CD4+ T cells, the cell type that mediates this disease (19, 38), is an essential factor in HSV-1 primary disease pathogenesis. In contrast, mice lacking CD80 and CD86, the binding partners for CD28, demonstrated a significantly increased pathology over that in wild-type mice in a vaginal model of HSV-2 infection (39). We further extended our observations to include recurrent HSK by measuring corneal disease in BALB/c CD28KO mice and B6-CD28KO mice in which virus was reactivated by treatment with UV-B. These mice displayed a significantly reduced pathology compared to their wild-type counterparts. This indicates that CD28-mediated costimulation is critical to the generation of an immune response that would result in corneal disease that could lead to permanent alterations of the cornea in the form of scarring.
Flow cytometric characterization of the cellular component that infiltrates the corneas of mice following reactivation revealed that the total number of inflammatory cells in wild-type mice significantly eclipsed that seen in their CD28KO counterparts. This observation was exactly as we predicted from the clinical scores and consistent with previous literature on this aspect of disease (3, 33–35). While B6-CD28KO mice did develop an inflammatory infiltrate consisting of CD4+ T cells, CD8+ T cells, neutrophils, and macrophages, their numbers were significantly less that that observed for wild-type B6 mice. Therefore, even though the numbers of inflammatory cells were less in B6-CD28KO mouse corneas, the percentages of each population were similar. This observation led us to conclude that clinical disease is directly related to the quantitative nature of the inflammatory infiltrate and not necessarily the qualitative nature of said infiltrate. A previous study investigating HSV-1 gB CD8+ memory responses indicated that they require CD28 costimulation for peak responses (40). While there were some fundamental differences in how those studies and our own were performed, such as expression of gB by a non-HSV-1 source and measurement of responses more than 60 days following initial infection, their results suggested that CD28 costimulation was responsible for the reduced expression of Bcl-2, which, in this context, resulted in less proliferation of CD8+ T cells. We did not investigate in our model whether mice lacking CD28 displayed differences in Bcl-2 expression. Nonetheless, CD28KO mice possessed fewer CD8 T cells in their corneas following reactivation of virus, but that was in the context of an overall reduction in total inflammatory infiltrate.
Interestingly, we also noted that approximately 10% of CD28KO mice demonstrated viral shedding prior to UV reactivation at day 30 postinfection. This is an unusual observation, as it is not seen in wild-type mice and is only rarely seen in other gene-targeted strains of mice (25). CD28KO mice also demonstrated a longer virus shedding period (up to 12 days) following UV reactivation. Taken together, these data suggest that the responses to HSV-1 that occur in the CD28KO mouse trigeminal ganglia (TG) differ from those that are seen in TG from wild-type mice. We took this to indicate that the CD28KO mice were less able to establish or control viral latency in their TG. As anticipated, real-time PCR analysis of B6-CD28KO mice showed significantly higher relative numbers of viral genome copies in these mice than in wild-type B6 mice at days 13 and 23 postinfection. However, by day 30 postinfection, B6-CD28KO and wild-type mice showed similar viral genome levels, suggesting that the CD28KO mice were still able to restrict long-term infection. That said, we believe that the initial increased presence of the viral genome in B6-CD28KO mice could very well be due to one of two primary mechanisms. The first and more likely primary mechanism would be the result of persistent viral replication in the cornea of B6-CD28KO mice that could continue the seeding of the TG during this phase of the infection. The other possibility could be that since the initial CD8 T cell response in the TG was impaired, this allowed for increased replication of virus in the TG, which would also lead to increased anterograde migration (3) of the virus back to the cornea, adding to the persistence of virus at the cornea. These two options are not mutually exclusive, and thus, both could be operational in these CD28KO mice.
We further suspected that the elevated viral genome copy numbers seen in CD28KO mice would also be reflected in a greater number of latently infected cells, but this theory was not borne out in practice. FISH analysis showed that wild-type B6 mice had significantly more LAT+ cells than CD28KO mice at early time points, with the difference then becoming insignificant at the later time points. While we were surprised by these data, it might not be so surprising when one thinks about the fact that LAT expression could be suppressed by more persistent seeding of the TG via retrograde transport of virus to the TG due to prolonged viral production in the periphery and that as peripheral viral production ends, this allows for the establishment of latency in TG neurons.
Previous studies have shown that there is a significant influx of CD8+ T cells in the TG of infected mice (26–28). When we analyzed CD8+ T cells isolated from infected TG, we found that CD28KO mice initially displayed significantly fewer CD8+ T cells at days 7 and 14 but that by day 41 there were no significant differences between CD28KO and wild-type mice. Similar responses were seen when cells were stained for the gB tetramer, which monitors HSV-1-specific CD8+ T cell responses. It is interesting to note that the percentages of cells expressing markers of functionality were not different, even though the absolute numbers were less in the CD28KO mice. Taken together, these data indicate that while the CD8 T cell response is delayed, it is not prevented by the lack of CD28 costimulation and that with time the generation of gB antigen-specific CD8+ T cells normalizes to that observed in wild-type mice. We did not directly address the CD8+ T cell response to the nondominant HSV-1 epitopes, which make up approximately half of the antigen-specific CD8+ T cell responses in the TG (41). However, the gB-specific CD8+ T cell component of the total CD8+ T cells that we isolated from infected TG in CD28KO mice was approximately 80% of the total, suggesting that subdominant HSV-1-specific CD8+ T cells may have an increased requirement for CD28 costimulation.
The studies described in this report clearly demonstrate that CD28 costimulation is an essential component of the pathogenesis of HSK, with CD28KO mice exhibiting decreased disease following primary infection as well as the recurrent HSK that occurs after UV-B reactivation. This suggests that CD4+ T cells are particularly sensitive to CD28 costimulation in the context of an HSV-1 infection and that those CD4+ T cells which might have the potential to cause significant corneal disease do not expand to the same levels seen in CD28-expressing mice. We reported similar findings when the migration of T cells was impaired by neutralizing chemokines CXCL10 and CXCL9, which significantly reduced corneal disease (42). In contrast, while CD28 costimulation compromises the animal’s initial ability to establish and control latent infection in the TG, this is a transient condition. With time, these CD28KO mice can establish levels of HSV-1 genomes, latently infected cells, and CD8+ T cells responses similar to those in CD28-expressing mice. While it remains possible that other costimulatory interactions could participate in activating T cell responses, there is a lack of evidence demonstrating that this is the case. We therefore conclude from these studies that CD8+ T cells can expand better than CD4+ T cells in the absence of CD28 costimulation.
MATERIALS AND METHODS
Mice.
Investigations with mice conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6 (B6) mice were purchased from NCI. The B6.129S2-Cd28tm1Mak/J (B6-CD28−/−) and C.129S2(B6)-Cd28tm1Mak/J (C-CD28−/−) mice were initially obtained from The Jackson Laboratory (Bar Harbor, ME) and were then maintained in our breeding colony.
Infection of mice.
For all experiments described here, we used age-matched male and female mice from the strains indicated above that were 6 to 10 weeks old. For primary disease, we infected the scarified corneas of BALB/c and C-CD28−/− mice with 107 PFU of the KOS strain of HSV-1 (38, 43). This strain was used for primary HSK because it does not have the mortality associated with other more neurovirulent strains of HSV-1 (43). Eye swab specimens were taken from these mice for 7 to 10 days following infection to monitor the persistence of viral shedding. For recurrent disease, mice were infected on the scarified cornea with 106 PFU of the HSV-1 McKrae strain as previously described (44). The McKrae strain of HSV-1 is used for reactivation studies because it was found to reactivate most reliably following UV-B irradiation (10). Each mouse received an intraperitoneal (i.p.) injection of 0.5 ml pooled human serum (50% effective dose for virus neutralization, 1:1,600; Sigma Chemicals, St. Louis, MO) concurrently with infection. Administration of anti-HSV antibodies at the time of ocular infection has been shown to protect mice from death and corneal disease during primary infection while allowing for the establishment of latency and subsequent reactivation of virus after corneal UV-B exposure. These antibodies are undetectable at the time of UV-B irradiation at 5 weeks after primary infection. To confirm infection, only mice displaying infectious virus obtained from eye swab specimens taken at 3 days postinfection were used for subsequent reactivation (20).
UV-B irradiation and virus reactivation.
Mice were reactivated from latency as previously described (43, 44). Briefly, the eyes of all latently infected mice were examined for corneal opacity before irradiation, and only animals with clear corneas were used. At least 5 weeks after primary infection, the eyes of latently infected and control mock-infected mice were exposed to 250 mJ/cm2 of UV-B light using a TM20 Chromato-Vu transilluminator (UVP, Inc., San Gabriel, CA), which emits UV-B at a peak wavelength of 302 nm. Irradiated mice were swabbed with sterile cotton applicators from day 0 to day 7, unless otherwise indicated. The swab material was cultured on Vero cells, as described above, to detect recurrent virus shedding from the cornea. Reactivation was defined as the finding of any HSV-positive eye swab on any day after UV-B exposure, with day 0 swabs serving as a control (44).
Clinical evaluation.
On the designated days after viral infection or UV-B reactivation, a masked observer, who is unaware of the experimental groups, examined the mouse eyes through a binocular dissecting microscope to score clinical disease. Stromal opacification was rated on a scale of from 0 to 4, where 0 indicates clear stroma, 1 indicates mild stromal opacification, 2 indicates moderate opacity with discernible iris features, 3 indicates dense opacity with the loss of defined iris detail except pupil margins, and 4 indicates total opacity with no posterior view. Corneal neovascularization was evaluated as described previously (43, 44) using a scale of from 0 to 8, where each of the four quadrants of the eye is evaluated for the density of vessels that have grown into them. Periocular disease was measured in a masked fashion on a semiquantitative scale as previously described (45). Note that uninfected, UV-B-irradiated control mice were used as a baseline for any effects due to UV-B irradiation.
Flow cytometric analysis.
Cells were isolated from the corneas and trigeminal ganglia as previously described (42). Briefly, corneas or trigeminal ganglia were excised at defined time points and incubated in phosphate-buffered saline–EDTA at 37°C for 15 min at 37°C. The corneal stroma was separated from the overlying epithelium and digested in 84 U collagenase type 1 (Sigma-Aldrich, St. Louis, MO) per cornea for 2 h at 37°C and then triturated to form a single-cell suspension. Suspensions were filtered through a 40-μm-pore-size cell strainer cap (BD Labware, Bedford, MA) and washed and then stained. Suspensions were initially stained with peridinin chlorophyll protein-conjugated anti-CD45 (clone 30-F11; BioLegend, San Diego, CA), and this was used to gate cells of bone marrow origin. For corneal suspension, cells were further stained with Alexa Fluor 700–GR-1 (clone RB6-8C5) and allophycocyanin-F4/80 (clone BM8) (BioLegend, San Diego, CA), fluorescein isothiocyanate-conjugated anti-CD4 (clone RM4-5) and phycoerythrin (PE)-conjugated anti-CD8α (clone 53-6.7) (Pharmingen), and PE-conjugated CD11b (clone M1/70; eBioscience, San Diego, CA). The strategy for analysis was to initially gate on live cells and then gate on the CD45+ cells. These CD45+ cells were further evaluated for T cell markers CD4 and CD8 or for macrophage markers F4/80+, CD11b+, and GR-1− or neutrophil markers GR-1+, CD11b+, and F4/80−. Cells isolated from the TG were initially stained with CD45 and CD8 as indicated above. Subsequently, they were stained with the HSV-1 gB tetramer to determine the number of antigen-specific T cells found in the TG. They were also stained with anti-IFN-γ (clone XMG1.2; BioLegend), CD107a (clone 1D4B; BD Pharmingen), TNF-α (clone MP6-XT22; BD Pharmingen), PD-1 (clone RMP1-30; BioLegend), and TIM-3 (clone 215008; R&D). Cells were then analyzed on a flow cytometer (a FACSAria flow cytometer with FACSDiva data analysis software; BD Biosciences).
qPCR for HSV-1 latent genomes of trigeminal ganglia.
Six- to 8-week-old B6 and B6-CD28KO mice were infected as described above. Trigeminal ganglia were harvested on the indicated days postinfection, and quantitative PCR (qPCR) was performed on these samples as described previously (25, 46). Initially, genomic DNA was extracted from the trigeminal ganglia using a DNeasy blood and tissue kit from Qiagen (catalog number 69506). The number of latent genomes per trigeminal ganglion was determined using primers to the thymidine kinase (tk) gene of HSV-1 designed to amplify a 70-bp fragment. The sequences of these primers are as follows: Tk (forward), 5′-CCAAAGAGGTGCGGGAGTTT-3′; Tk (reverse), 5′-CTTAACAGCTGTCAACAGCGTGCCG-3′. Purified HSV-1 infectious chromosomal DNA was diluted in the background of mouse DNA in 10-fold dilutions from 106 to 101 copies and used to prepare a standard curve for determination of the total genome copy number in latently infected trigeminal ganglia. The adipsin gene was used as internal reference as follows: the mouse adipsin forward primer was 5′-AGTGTGCGGGGATGCAGT-3′, and the reverse primer was 5′-ACGCGAGAGCCCCAGGTA-3′. Trigeminal ganglion DNA harvested from uninfected mice was used to generate mouse DNA standards ranging from 105 to 101 copies, from which the total copy number of the mouse adipsin gene in each of the infected trigeminal ganglion samples was determined. Each value for the tk gene copy number was normalized to the lowest value of the mouse adipsin gene copy number, and the number of copies of genome per trigeminal ganglion was then expressed on a log scale.
Sectioning of latently infected trigeminal ganglia.
Six- to 8-week-old B6 and B6-CD28KO mice were infected with 106 PFU of HSV-1 McKrae as described above to establish a latent infection. At days 13, 23, and 40 postinfection, mice were euthanized by CO2 inhalation and perfused with 0.1 M phosphate-buffered saline, followed by perfusion with 1% paraformaldehyde. TG were removed, kept on dry ice, batched in groups of 8, embedded in OCT compound (Miles, Inc., Torrance, CA), and snap-frozen in liquid nitrogen. Serial sections (5 μm) at the 20-μm intersection through each frozen block of tissue were mounted onto positively charged Fisher Scientific SuperFrost Plus slides (catalog number 12-550-15) and stored at −80°C.
Fluorescence in situ hybridization (FISH) of RNA.
Each QuantiGeneView (QGV; Affymetrix, Santa Clara, CA) mRNA oligonucleotide-based probe set consists of 20 oligonucleotide pairs that are complementary to different regions of the transcript. Each pair hybridizes to the target mRNA at adjacent sites and supports a branched DNA signal amplifier that is assembled via a series of sequential hybridization steps. The resulting signal comes from fluorophore-conjugated label probes and provides up to 8,000-fold signal amplification. In brief, an HSV-1 LAT probe set corresponding to bases 575 to 1837 of HSV-1 LAT M74421 (probe VF1-15519) was custom designed by Affymetrix; beta-actin was applied as a control probe to test the RNA quality (probe VB6-12823). Slides were processed strictly following the QuantiGene protocol. The main steps of pretreatment, protease digestion, sequential hybridization, and amplification were performed following the manufacturer's instructions by using a QG ViewRNA in situ hybridization tissue 2-plex assay kit (catalog number QVT0012; Affymetrix, CA). In brief, fixation was in 4% paraformaldehyde for 24 h at room temperature, followed by dehydration in an alcohol series. Prehybridization conditions were found to be optimal with 20 min of protease QF digestion. After hybridization, washing, preamplifier hybridization, amplifier hybridization, and the final amplification step for target mRNA utilized alkaline phosphatase-labeled probes in conjunction with the Fast Red chromogenic substrate for enzymatic amplification. Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) as described in the user manual before applying mounting medium. The target mRNA was visualized using a standard bright-field fluorescence microscope.
Statistics.
All statistical analyses were performed with the aid of Sigma Stat for Windows (version 2.0) software (Jandel, Corte Madera, CA). The log-rank test was used to compare disease scores. Student’s unpaired t test was used to compare virus titer data, genome copies, LAT+ cells, and flow cytometry numbers.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants EY16352 (to P.M.S.) and EY21247 (to P.M.S.) and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Saint Louis University.
REFERENCES
- 1.Tuli S, Sonal S. 2009. Herpes simplex keratitis In Yanoff JS, Duker M, Wiggs JL, Miller D, Azar DT, Goldstein MH, Rosen ES, Duker JS, Rao NA, Augsburger JJ, Sadum AA, Shuman JS, Diamond GR, Dutton JJ (ed), Ophthalmology, 3rd ed Elsevier Inc, Philadelphia, PA. [Google Scholar]
- 2.Preston CM. 2000. Repression of viral transcription during herpes simplex virus latency. J Gen Virol 81(Pt 1):1–19. doi: 10.1099/0022-1317-81-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Summers BC, Margolis TP, Leib DA. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J Virol 75:5069–5075. doi: 10.1128/JVI.75.11.5069-5075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A 99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham AL, Diefenbach RJ, Miranda‐Saksena M, Bosnjak L, Kim M, Jones C, Douglas MW. 2006. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis 194:S11–S18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 6.Knipe DM, Cliffe A. 2008. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol 6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 7.Smith JS, Robinson NJ. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 186:s3–s28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 8.Umene K, Sakaoka H. 1999. Evolution of herpes simplex virus type 1 under herpesvirus evolutionary processes. Arch Virol 144:637–656. doi: 10.1007/s007050050533. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Vogel JL, Narayanan A, Peng H, Kristie TM. 2009. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nat Med 15:1312–1317. doi: 10.1038/nm.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimeld C, Hill TJ, Blyth B, Easty D. 1989. An improved model of recurrent herpetic eye disease in mice. Curr Eye Res 8:1193–1205. doi: 10.3109/02713688909000044. [DOI] [PubMed] [Google Scholar]
- 11.Shimeld C, Hill TJ, Blyth WA, Easty DL. 1990. Passive immunization protects the mouse eye from damage after herpes simplex virus infection by limiting spread of virus in the nervous system. J Gen Virol 71:681–687. doi: 10.1099/0022-1317-71-3-681. [DOI] [PubMed] [Google Scholar]
- 12.Farooq AV, Shukla D. 2012. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol 57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azher TN, Yin XT, Tajfirouz D, Huang AJH, Stuart PM. 2017. Herpes simplex keratitis: challenges in diagnosis and clinical management. Clin Ophthalmol 11:185–191. doi: 10.2147/OPTH.S80475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. 2013. Herpes keratitis. Prog Retin Eye Res 32:88–101. doi: 10.1016/j.preteyeres.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Divito SJ, Hendricks RL. 2008. Activated inflammatory infiltrate in HSV-1-infected corneas without herpes stromal keratitis. Invest Ophthalmol Vis Sci 49:1488–1495. doi: 10.1167/iovs.07-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan R, Remeijer L, van Dun JM, Osterhaus AD, Verjans GM. 2007. Granulocyte macrophage colony-stimulating factor expression in human herpetic stromal keratitis: implications for the role of neutrophils in HSK. Invest Ophthalmol Vis Sci 48:277–284. doi: 10.1167/iovs.06-0053. [DOI] [PubMed] [Google Scholar]
- 17.Russell RG, Nasisse MP, Larsen HS, Rouse BT. 1984. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest Ophthalmol Vis Sci 25:938–944. [PubMed] [Google Scholar]
- 18.Newell CK, Martin S, Sendele D, Mercadal CM, Rouse BT. 1989. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol 63:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doymaz MZ, Rouse BT. 1992. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Invest Ophthalmol Vis Sci 33:2165–2173. [PubMed] [Google Scholar]
- 20.Keadle TL, Morris JL, Pepose JS, Stuart PM. 2002. CD4+ and CD8+ cells are key participants in the development of recurrent herpetic stromal keratitis in mice. Microb Pathog 32:255–262. doi: 10.1006/mpat.2002.0506. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins MK, Ashwell JD, Schwartz RH. 1988. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol 140:3324–3330. [PubMed] [Google Scholar]
- 22.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. 1991. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol 147:2461–2466. [PubMed] [Google Scholar]
- 23.Chen H, Hendricks RL. 1998. B7 costimulatory requirements of T cells at an inflammatory site. J Immunol 160:5045–5052. [PubMed] [Google Scholar]
- 24.Schrimpf JE, Tu EM, Wang H, Yee M, Wong YM, Morrison LA. 2011. B7 costimulation molecules encoded by replication-defective, vhs-deficient HSV-1 improve vaccine-induced protection against corneal disease. PLoS One 6:e22772. doi: 10.1371/journal.pone.0022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keadle TL, Alexander DE, Leib DA, Stuart PM. 2008. Interferon gamma is not required for recurrent herpetic stromal keratitis. Virology 380:46–51. doi: 10.1016/j.virol.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta A, Maggioncalda J, Bagasra O, Thikkavarapu S, Saikumari P, Valyi-Nagy T, Fraser NW, Block TM. 1995. In situ DNA PCR and RNA hybridization of herpes simplex virus sequences in trigeminal ganglia of latently infected mice. Virology 206:633–640. doi: 10.1016/S0042-6822(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 27.Chen XP, Mata M, Kelley M, Glorioso JC, Fink DJ. 2002. The relationship of herpes simplex virus latency associated transcript expression to genome copy number: a quantitative study using laser capture microdissection. J Neurovirol 8:204–210. doi: 10.1080/13550280290049642. [DOI] [PubMed] [Google Scholar]
- 28.Watson ZL, Washington SD, Phelan DM, Lewin AS, Tuli SS, Schultz GS, Neumann DM, Bloom DC. 2018. In vivo knockdown of the herpes simplex virus 1 latency-associated transcript reduces reactivation from latency. J Virol 92:e00812-18. doi: 10.1128/JVI.00812-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593–603. doi: 10.1016/S1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. 2011. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. J Immunol 186:3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinne JR, Abghar SZ, Stulting RD. 1992. The severity of herpes simplex viral keratitis in mice does not reflect the severity of disease in humans. Invest Ophthalmol Vis Sci 33:268–272. [PubMed] [Google Scholar]
- 33.West DM, Del Rosso CR, Yin XT, Stuart PM. 2014. CXCL1, but not IL-6 is required for recurrent herpetic stromal keratitis. J Immunol 192:1762–1767. doi: 10.4049/jimmunol.1302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller JK, Laycock KA, Nash NM, Pepose JS. 1993. Corneal Langerhans cell dynamics after herpes simplex virus reactivation. Invest Ophthalmol Vis Sci 34:2282–2290. [PubMed] [Google Scholar]
- 35.Chucair-Elliot AJ, Gurung HR, Carr MM, Carr D. 2017. Colony stimulating factor-1 receptor expressing cells infiltrating the cornea control corneal nerve degeneration in response to HSV-1 infection. Invest Ophthalmol Vis Sci 58:4670–4682. doi: 10.1167/iovs.17-22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu M, Lepisto AJ, Hendricks RL. 2004. CD154 signaling regulates the Th1 response to herpes simplex virus‐1 and inflammation in infected corneas. J Immunol 173:1232–1239. doi: 10.4049/jimmunol.173.2.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepisto AJ, Xu M, Yagita H, Weinberg AD, Hendricks RL. 2007. Expression and function of the OX40/OX40L costimulatory pair during herpes stromal keratitis. J Leukoc Biol 8:766–774. doi: 10.1189/jlb.0406293. [DOI] [PubMed] [Google Scholar]
- 38.Stuart PM, Summers B, Morris JE, Morrison LA, Leib DA. 2004. CD8+ T cells control corneal disease following ocular infection with HSV-1. J Gen Virol 85:2055–2063. doi: 10.1099/vir.0.80049-0. [DOI] [PubMed] [Google Scholar]
- 39.Thebeau LG, Morrison LA. 2002. B7 costimulation plays an important role in protection from herpes simplex virus type 2-mediated pathology. J Virol 76:2563–2566. doi: 10.1128/jvi.76.5.2563-2566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. 2007. Memory CD8+ T cells require CD28 costimulation. J Immunol 179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 41.St Leger AJ, Jeon S, Hendricks RL. 2013. Broadening the repertoire of functional herpes simplex virus type 1-specific CD8+ T cells reduces viral reactivation from latency in sensory ganglia. J Immunol 191:2258–2265. doi: 10.4049/jimmunol.1300585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tajfirouz D, West DM, Yin XT, Potter CA, Klein R, Stuart PM. 2017. CXCL9 compensates for the absence of CXCL10 during recurrent herpetic stromal keratitis. Virology 506:7–13. doi: 10.1016/j.virol.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris JE, Zobell S, Yin XT, Zaker H, Summers BC, Leib DA, Stuart PM. 2012. Mice with mutations in Fas and Fas ligand demonstrate increased herpetic stromal keratitis following corneal infection with HSV-1. J Immunol 188:793–799. doi: 10.4049/jimmunol.1102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keadle TL, Morrison LA, Morris JL, Pepose JS, Stuart PM. 2002. Therapeutic immunization with a virion host shutoff (vhs) defective, replication-incompetent HSV-1 strain limits recurrent herpetic ocular infection. J Virol 76:3615–3625. doi: 10.1128/jvi.76.8.3615-3625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith TJ, Ackland-Berglund CE, Leib DA. 2000. Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice. J Virol 74:3598–3604. doi: 10.1128/jvi.74.8.3598-3604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strand SS, Leib DA. 2004. Role of the VP16-binding domain of vhs in viral growth, host shutoff activity, and pathogenesis. J Virol 78:13562–13572. doi: 10.1128/JVI.78.24.13562-13572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]