Abstract
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer diagnosed in more than 200,000 women each year1 and is recalcitrant to targeted therapies2,3. Although TNBCs harbor multiple hyperactive receptor tyrosine kinases (RTKs)4–8, RTK inhibitors have been largely ineffective in TNBC patients thus far. We developed a broadly effective therapeutic strategy for TNBC that is based on combined inhibition of receptors that share the negative regulator PTPN12. Previously, we and others identified the tyrosine phosphatase PTPN12 as a tumor suppressor that is frequently inactivated in TNBC9,10. PTPN12 restrains several RTKs9,11–17, suggesting that PTPN12 deficiency leads to aberrant activation of multiple RTKs and a co-dependency on these receptors. This in turn leads to the therapeutic hypothesis that PTPN12-deficient TNBCs may be responsive to combined RTK inhibition. However, the repertoire of RTKs that are restrained by PTPN12 in human cells has not been systematically explored. By methodically identifying the suite of RTK substrates (MET, PDGFRb, EGFR, and others) inhibited by PTPN12, we rationalized a combination RTK-inhibitor therapy that induced potent tumor regression across heterogeneous models of TNBC. Orthogonal approaches revealed that PTPN12 was recruited to and inhibited these receptors after ligand stimulation, thereby serving as a feedback mechanism to limit receptor signaling. Cancer-associated mutation of PTPN12 or reduced PTPN12 protein levels diminished this feedback mechanism, leading to aberrant activity of these receptors. Restoring PTPN12 protein levels restrained signaling from RTKs, including PDGFRb and MET, and impaired TNBC survival. In contrast with single agents, combined inhibitors targeting the PDGFRb and MET receptors induced the apoptosis in TNBC cells in vitro and in vivo. This therapeutic strategy resulted in tumor regressions in chemo-refractory patient-derived TNBC models. Notably, response correlated with PTPN12 deficiency, suggesting that impaired receptor feedback may establish a combined addiction to these proto-oncogenic receptors. Taken together, our data provide a rationale for combining RTK inhibitors in TNBC and other malignancies that lack receptor-activating mutations.
Growth factor receptors are commonly hyperactivated in human TNBC, but the underlying mechanism(s) for this aberrant signaling remain elusive. The tyrosine phosphatase PTPN12 has been identified as a frequently inactivated tumor suppressor in TNBC9,10, and PTPN12 phosphatase function impairs the progression of primary and metastatic breast cancer9,18. Prior studies have also suggested that this phosphatase may restrain signaling from one or more growth factor receptors9,11–17,19, provoking the hypothesis that a loss of PTPN12 may unveil chronic RTK signaling in human cancers. However, the spectrum of PTPN12-regulated RTKs in TNBC has not been defined. To systematically identify PTPN12-regulated receptors, we individually expressed 47 RTKs (of the 58 RTKs in the human genome) in TNBC cells engineered with an inducible PTPN12 cDNA (referred to as SUM159ind-PTPN12 cells) (Supplementary Fig. 1a–c). Each FLAG-RTK was evaluated for its tyrosine phosphorylation (pTyr) with and without PTPN12 induction via pTyr immunoprecipitation (IP) and FLAG western blot (Fig. 1a,b and Supplementary Fig. 1a,b). Of the 19 RTKs for which pTyr was detected, PTPN12 induction reproducibly reduced tyrosine phosphorylation of 5 RTKs (MET, PDGFRβ, EGFR, HER2 and EPHA7; Fig. 1a,b). Likewise, reciprocal FLAG-IP and pTyr western blot indicated that tyrosine phosphorylation of these receptors was reduced by PTPN12-induction (Fig. 1b). Some of these RTKs have previously been reported as PTPN12 substrates (PDGFR β and HER2)9,11,14, suggesting that other RTKs identified herein (such as MET and EPHA7) may also be bona fide substrates of PTPN12. Although these data do not exclude the possibility that PTPN12 regulates additional RTKs in other contexts, these data suggest that PTPN12 restrains signaling from a select group of RTKs in TNBC cells (Fig. 1a,b). Consistent with this hypothesis, bimolecular fluorescence complementation (BiFC) revealed that four of the five RTKs also interacted with PTPN12 in independent TNBC cell lines (Fig. 1c and Supplementary Fig. 2), suggesting that PTPN12 regulates these receptors via binding and de-phosphorylation.
Figure 1.
The PTPN12 phosphatase inhibits mitogenic RTK signaling in TNBCs. (a,b) PTPN12 regulates the phosphorylation of a select set of proto-oncogenic RTKs. (a) Individual flag-tagged RTK cDNAs (n = 47) were expressed in TNBC cells engineered with a dox-inducible PTPN12-cDNA. RTK tyrosine-phosphorylation was assessed in the absence or presence of PTPN12-induction via phospho-tyrosine-immunoprecipitation (p-Tyr-IP) and FLAG-immunoblot. The difference in phospho-tyrosine in the PTPN12-induced and PTPN12-uninduced states is plotted for each RTK with detectable phospho-tyrosine. All of the RTKs exhibiting a reproducible decrease in tyrosine-phosphorylation after PTPN12-induction are marked in red (dotted line indicates greater than 30% decrease in PTPN12-induced state versus uninduced). (b) Representative p-Tyr-IP and FLAG immunoblots with cell lysates (top). The same cell lysates were analyzed via reciprocal FLAG immunoprecipitation and subsequent phospho-tyrosine-immunoblot or FLAG immunoblot (middle and bottom, respectively). (c) PTPN12 interacted with a subset of RTKs in TNBC cells. TNBC cells expressing PTPN12 fused to the n-terminus of YFP and indicated RTK cDNA fused to the c-terminus of YFP were assessed for interaction between PTPN12 and RTK proteins by YFP fluorescence (flow cytometry). Heat map indicates the percentage of YFP-positive cells for each interaction. Values are plotted for two technical replicates. (d) Expression of PTPN12-regulated RTKs in TNBC. The frequency distribution of expression values (RPKM) for each PTPN12-regulated RTK is plotted for 112 TNBCs from the TCGA data set. Median (white) and interquartile range (black bars) are shown. (e) PTPN12 reduced phosphorylation of endogenous HER2, EGFR, MET and PDGFRβ in TNBC cells. PTPN12 cDNA expression was induced in SUM159-ind-PTPN12 cells (±dox) and RTK phosphorylation was analyzed by western blotting with indicated antibodies. (f) Ectopic PTPN12 inhibited effectors of RTK signaling. SUM159-ind-PTPN12 cells in f were analyzed via western blotting with antibodies of the indicated proteins. Data in e and f are representative of two independent experiments. (g) The MET ligand HGF stimulated PTPN12-MET interaction. SUM159 cells expressing control-or eGFP-PTPN12 cDNAs were treated with HGF ligand. PTPN12-MET interaction was visualized via proximity ligation assay (PLA) assay with antibodies against MET and eGFP (eGFP-PTPN12). The percentage of positive cells was plotted in each condition (mean ± s.e.m., n = 4 technical replicates, P = 0.05 unpaired one-tailed Student’s t test). Representative images of PLA between MET and eGFP-PTPN12 in SUM159 cells expressing control-or eGFP-PTPN12 cDNAs. Red spots are regions of signal amplification. Nuclear stain (DAPI) is blue. Scale bars represent 10 μm. (h) The PDGFRβ ligand PDGF-BB stimulated PTPN12-PDGFRβ interaction. SUM159 cells engineered with a dox-inducible PTPN12-cDNA were stably transduced with lentiviruses expressing control- or PDGFRβ- FLAG cDNAs and subsequently treated with PDGF-BB ligand. PTPN12-PDGFRβ interaction was visualized and analyzed via PLA as in h with antibodies against PTPN12 and FLAG (PDGFRβ- FLAG) (mean ± s.e.m., n = 4 technical replicates ***P < 0.001 unpaired one-tailed Student’s t test).
Given that restoring PTPN12 decreases TNBC cell survival and tumor progression9, we hypothesized that one or more PTPN12-regulated RTKs might be involved in TNBC. To identify which RTKs might contribute to TNBC pathogenesis, we first assessed the expression of PTPN12-regulated RTKs in human TNBCs (TCGA cohort) as well as 14 TNBC patient-derived xenografts (PDXs). Consistent with prior studies4,6,7,20–22, four of five PTPN12-regulated RTKs (MET, PDGFRβ, EGFR and HER2) were broadly expressed in primary TNBCs and PDXs (Fig. 1d and Supplementary Fig. 3). Notably, restoring PTPN12 significantly downregulated phosphorylation of each of these endogenous RTKs as well as their downstream effectors in TNBC models (Fig. 1e,f and Supplementary Fig. 4), indicating that signaling from these RTKs is inhibited by PTPN12. PTPN12-MET and PTPN12-PDGFRβ interactions were rapidly enhanced following ligand stimulation (Fig. 1g,h), suggesting that PTPN12 may be recruited to active receptors to limit the duration or extent of RTK signaling. This interaction is also observed between endogenous PTPN12 and MET (Supplementary Fig. 5). This is consistent with recent proteomic data suggesting that PTPN12 is recruited to EGFR following ligand stimulation12. Collectively, these mechanistic data suggest that PTPN12 may function as a negative feedback inhibitor of several proto-oncogenic RTKs in TNBC.
To determine whether the ability of PTPN12 to restrain RTKs is abrogated in human cancer, we evaluated whether tumor-derived mutations in PTPN12 might impair its role as a negative regulator of RTK function. PTPN12 is frequently compromised by reduced protein levels in TNBC9,10. In addition, somatic mutations occur in the PTPN12 locus in breast cancer and other malignancies23,24 (TCGA Research Network, http://cancergenome.nih.gov/; COSMIC, http://cancer.sanger.ac.uk/cosmic). To assess which mutations, if any, are likely to affect PTPN12 function, we performed evolutionary action analysis on all PTPN12 somatic mutations from pan-cancer collections. Evolutionary action (EA) predicts the effect of genotype perturbations on protein fitness and function25. EA analysis revealed a strong enrichment of deleterious mutations (EA score > 75) in the phosphatase domain (Fig. 2a), consistent with prior reports that the catalytic activity of PTPN12 is required to suppress cellular transformation of mammary epithelial cells9. Notably, a single amino acid (H230) was targeted by recurrent mutations in TNBC and other malignancies. This amino acid is adjacent to the catalytic cysteine (C231) and resides in a highly conserved pocket of PTPN12 (Fig. 2b). Structural modeling suggests that tumor-derived mutations of this amino acid may alter spatial configuration of the catalytic cleft (including residues conserved in all PTPNs) (Fig. 2c) and are therefore likely to impair PTPN12 phosphatase function. Indeed, evaluation of purified wild-type and mutant PTPN12 revealed that the mutation of the H230 residue impaired enzymatic activity by >1,000-fold (Fig. 2d and Supplementary Fig. 6a). Consequently, we evaluated the effect of this mutation on the molecular and cellular effects of PTPN12. In TNBC cells, the PTPN12 H230Y variant was defective in restraining TNBC clonogenicity (Fig. 2e). Concordantly, the H230Y mutation also disrupted the ability of PTPN12 to reduce tyrosine phosphorylation of the RTKs MET and PDGFRβ (Fig. 2f and Supplementary Fig. 6b). Cumulatively, these data suggest that PTPN12 restrains several proto-oncogenic RTKs and that tumor-derived mutation of PTPN12 (or loss of PTPN12 expression) may lead to defective control of these RTKs in TNBCs.
Figure 2.
Tumor-derived PTPN12 mutations impair PTPN12-RTK regulation. (a) Deleterious tumor-derived PTPN12 mutations were enriched in the phosphatase domain. PTPN12 mutations (TCGA and COSMIC, pan-cancer) were analyzed by Evolutionary Action (EA)25. The EA score of each tumorderived mutation is designated by a black line. The red line demarcates EA scores for recurrent mutations at the H230 residue. (b) Evolutionary trace highlights the catalytic cleft of PTPN12. The three-dimensional crystallographic structure of the catalytic domain of human PTPN12 protein is depicted with surface residues colored according to evolutionary trace (ET) values for each amino acid. ET values are 0 (blue, low importance) to 100 (red, high importance). The catalytic residue C231 is depicted. (c) Recurrent cancer-derived mutations of the H230 amino acid are predicted to alter spatial configuration of the PTPN12 catalytic cleft. Ribbon plots of the PTPN12 catalytic cleft (top) with expanded views of the contacts between adjacent residues in the wild-type (WT, bottom left) and H230Y (bottom right) context. The dashed lines represent the minimum distance between H230 (orange), and the side chains of residues F117, M131 and Y146 (in yellow). The catalytic cysteine (C231, red) is indicated. (d) Mutation of the H230 residue resulted in decreased PTPN12 phosphatase activity. The phosphatase activities of PTPN12 wild type, PTPN12 H230Y and PTPN12 C231A (catalytic dead) mutants toward small artificial substrates pNPP and DiFMUP were assayed via in vitro phosphatase assays. Table displays the kcat (s−1), Km (μM) and kcat/Km for the indicated reactions. (e) Mutation of the H230 residue in PTPN12 was defective at suppressing growth of TNBC cells. SUM159 cells transduced with retroviruses encoding negative control, PTPN12 wild-type or mutant cDNA were analyzed for macroscopic colony formation. Quantification of colony number is plotted (mean ± s.e.m., n = 4 biological replicates ***P < 0.001 unpaired two-tailed Student’s t test). (f) Mutation of the H230 residue in PTPN12 impairs ability to constrain RTK signaling. SUM159 cells from e were analyzed for levels of the indicated proteins by western blotting. Data is representative of two independent experiments.
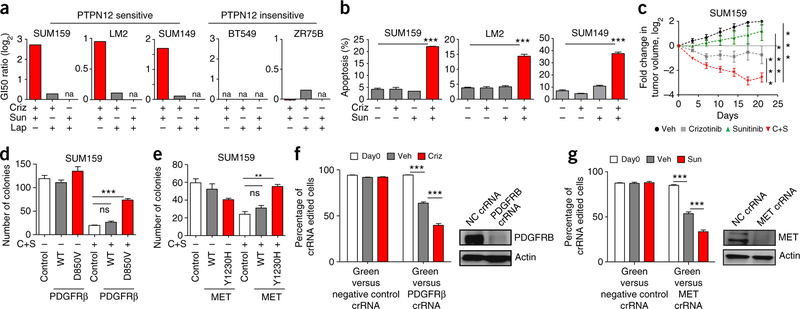
Given that restoring PTPN12 in TNBC cells suppresses oncogenic RTK signaling and impairs TNBC tumorigenic and metastatic potential9, we hypothesized that inhibiting combinations of PTPN12-regulated RTKs may also restrain TNBC survival and progression. In previous studies9, we began to evaluate this hypothesis, but could not develop an effective therapeutic combination because the spectrum of RTKs restrained by PTPN12 had not been defined. Based on the systematic identification of PTPN12-regulated RTKs in this study, we evaluated multiple combinations of tyrosine kinase inhibitors (TKis) that target PTPN12-regulated RTKs (MET, PDGFRβ, EGFR and HER2) for their effects on TNBC survival in several models that are sensitive or insensitive to PTPN12 function (Supplementary Fig. 7). GI50 (con- centration of drug that results in a 50% reduction in cell number) values for each single agent were calculated from drug response curves in three PTPN12-sensitive cell lines and two PTPN12-insensitive cell lines (Supplementary Fig. 8a,b). In addition, drug interaction scores for each combination were assessed by determining the GI50 of a TKi in the absence or presence of a second TKi targeting an independent RTK class. Drug interaction was measured as the ratio of predicted combination GI50 (assuming independent drug action) compared with the observed GI50 of the drug combination. Consistent with previous studies in vitro and clinical studies in breast cancer patients26–30, these TKis exhibited low or no single-agent activity across TNBC models (Supplementary Fig. 8a,b). Notably, the combination of Crizotinib and Sunitinib exhibited robust drug interaction in PTPN12-sensitive TNBC models but did not inhibit the growth of PTPN12-resistant TNBC cells (Fig. 3a and Supplementary Fig. 8a,b). In contrast, all of the other combinations exhibited no drug interaction (drug interaction score 0 in log2 scale) and modest to no effect on TNBC cell proliferation (Supplementary Fig. 8a,b). Crizotinib and Sunitinib induced apoptosis in combination, but not as single agents, in TNBC cells (Fig. 3b). Notably, the Crizotinib-Sunitinib combination elicited robust tumor regression of a TNBC cell line xenograft in vivo (Fig. 3c). The combination of Crizotinib-Sunitinib significantly decreased phosphorylation of the PTPN12-regulated RTKs PDGFRβ and MET (Supplementary Figs. 9a,b and 10), and gatekeeper mutants of these receptors PDGFRβ-D850V and MET-Y1230H31–33 restored TNBC clonogenicity (Fig. 3d,e) and receptor signaling (Supplementary Fig. 8c,d) in the presence of the drug combination. In addition, loss of function of MET or PDGFRβ (via CRISPR-Cas9) sensitized TNBC cells to single-agent Sunitinib or Crizotinib, respectively (Fig. 3f,g). These data suggest that inhibition of these receptors is, at least in part, responsible for the anti-TNBC effects of this TKi combination. These data are consistent with the hypothesis that PTPN12-sensitive TNBCs are dependent on PDGFRβ and MET signaling to support tumor cell survival.
Figure 3.
Combinatorial inhibition of PTPN12-regulated RTKs impairs TNBC cell survival. (a) TKis targeting the PTPN12-regulated receptors MET and PDGFRβ impair growth of PTPN12-sensitive TNBC cells, but not PTPN12-insensitive TNBC cells. The indicated TNBC cell lines were treated with a dose-matrix of the TKis Crizotinib (Criz), Sunitinib (Sun) and Lapatinib (Lap) alone or in combination. Cell numbers were assessed (n = 4 biological replicates, mean ± s.e.m.). The ratio of the predicted GI50 (assuming independent drug action) to the observed GI50 is reported as GI50 ratio. n.a. indicates that the GI50 was not reached. Associated drug curves and PTPN12-sensitivity data are provided in Supplementary Figures 7 and 8a,b. (b) The Crizotinib-Sunitinib combination induced apoptosis in PTPN12-sensitive TNBC. TNBC cell lines were treated with indicated agents for 48 h and apoptosis was assessed via Annexin V staining (mean ± s.e.m., n = 2 biological replicates, ***P < 0.001 one-way ANOVA, Tukey’s multiple-comparison test). (c) Crizotinib-Sunitinib combination suppressed primary tumor growth in TNBC cell line xenografts. SUM159 cells were transplanted in the mouse mammary gland, randomized following engraftment (200 mm3), and monitored for primary tumor growth. Log fold change in tumor volume is plotted over time in days (vehicle n = 8; Crizotinib/C n = 8; Sunitinib/S n = 8; C+S n = 10; mean ± s.e.m, **P < 0.001, ***P < 0.0001, two-way ANOVA, Tukey’s multiple comparison test). (d,e) Mutant PDGFRβ and MET conferred resistance to Crizotinib-Sunitinib therapy. SUM159 TNBC cells were transduced with retrovirus expressing RFP (control), PDGFRβ wild type or PDGFR β D850V mutant (d) or RFP (control), MET wild type or MET Y1230H mutant (e). Transduced cells were treated with C+S and analyzed for macroscopic colony formation (n = 3 biological replicates, mean ± s.e.m.; ***P < 0.001, unpaired two-tailed Student’s t test). (f) PDGFRβ loss of function sensitized TNBC cells to Crizotinib. eGFP-labeled SUM159 (GFP) cells were mixed at a 10:90 ratio with SUM159 cells edited with PDGFRβ or non-targeting control crRNA. Plates were treated with vehicle or 0.5 μM Crizotinib as indicated. At confluence, cells were passaged and processed for flow cytometry (n = 8 biological replicates mean ± s.e.m.; ***P < 0.001 unpaired two- tailed Student’s t test.) Inset, representative western blot for PDGFRβ protein in indicated cell lysates. (g) MET loss of function sensitized TNBC cells to Sunitinib. eGFP-labeled SUM159 (GFP) cells as described in f were mixed at a 10:90 ratio with SUM159 cells edited with MET or non-targeting control crRNA. Plates were treated with vehicle or 1 μM Sunitinib as indicated, passaged and analyzed as in described in f (n = 8 biological replicates mean ± s.e.m.; ***P < 0.001 unpaired two-tailed Student’s t test.). Inset, representative western blot for MET protein in indicated cell lysates.
TNBC is a heterogeneous class of breast cancer, with genetic and transcriptional profiles suggesting that TNBC is comprised of six to eight molecular subtypes4,34. To evaluate the efficacy of the Crizotinib- Sunitinib combination broadly across TNBCs, we tested this therapeutic strategy in PDXs from 14 independent TNBC patients (Fig. 4a) with largely chemo-refractory disease35. Consistent with prior analysis of primary breast cancers9,10,36, immunohistochemical analyses revealed that these PDXs expressed heterogeneous PTPN12 protein levels (Fig. 4b and Supplementary Fig. 11). To assess the effect of Crizotinib-Sunitinib combination in established tumors, we transplanted each PDX model and randomized them at 150–200 mm3 into vehicle or Crizotinib+Sunitinib treatment groups (Supplementary Table 1). Notably, in the PTPN12-deficient TNBC models PDX-1 and PDX-2, Crizotinib-Sunitinib treatment led to complete tumor regression, in contrast with the single-agent treatments (Fig. 4c,d and Supplementary Fig. 14).TumorregressionswereaccompaniedbydecreasedMET,PDGFRβ and downstream effector signaling (Supplementary Fig. 10), reduced proliferative index (Ki67; Fig. 4e) and a significant increase in apoptotic response (TUNEL; Fig. 4f). More broadly, across PDX models from 14 independent patients, Crizotinib-Sunitinib led to tumor regressions in 7 of 14 PDX models (as determined by RECIST criteria, 30% decrease in tumor volume, (Fig. 4g) without obvious toxicity (Supplementary Fig. 12). Notably, Crizotinib-Sunitinib efficacy correlated with reduced PTPN12 protein levels (P < 0.001; Fig. 4g and Supplementary Fig. 13), suggesting that impaired receptor feedback may establish a combined addiction to proto-oncogenic receptors such as MET and PDGFRβ. Crizotinib-Sunitinb elicited tumor regressions in PDXs harboring PIK3CA or TP53 alterations (Supplementary Table 2). Collectively, these pre-clinical studies provide a rationale for selecting TKi-combinations on the basis of tumor suppressor PTPN12 dysfunction and support clinical evaluation of Crizotinib-Sunitinib or other strategies to inhibit MET and PDGFR in chemorefractory TNBC patients.
Figure 4.
Crizotinib-Sunitinib combination therapy confers regression in PTPN12-deficient TNBC PDXs. (a) Schematic of pre-clinical PDX trial. PDXs established from 14 independent TNBC patients were evaluated for response to the Crizotinib-Sunitinib combination. (b) PTPN12 protein expression across TNBC PDX models is heterogeneous. Representative images of PTPN12 IHC are illustrated for PTPN12 protein scores ranging from 3 (max) to 0 (lowest). Scale bars represent 50 μm. (c) Crizotinib-Sunitinib treatment resulted in regression of PDX-1 (PTPN12-low) tumors. PDX-1, a PTPN12- deficient TNBC model, was randomized at 200 mm3 into four treatment groups (Vehicle, Crizotinib/C, Sunitinib/S and C+S) and assessed for changes in tumor volume over time. Tumor volume is plotted as log fold change in tumor volume from baseline over time (vehicle n = 6; C n = 6; S n = 6; C+S n = 11; mean ± s.e.m. ***P < 0.0001, two-way ANOVA, Tukey’s multiple comparison test). (d) Crizotinib-Sunitinib treatment resulted in regression of PDX-2 (PTPN12-low) tumors. PDX-2, a PTPN12-deficient TNBC model, was randomized, treated and tumor growth was analyzed as described in c (vehicle n = 8; C n = 5; S n = 6; C+S n = 7; mean ± s.e.m. ***P < 0.0001 two-way ANOVA, Tukey’s multiple comparison test). (e) Crizotinib-Sunitinib led to decreased proliferative index in PDX-1 tumors as assessed by Ki67 staining (vehicle n = 5; C+S n = 8; mean ± s.e.m. ***P < 0.001, at day 4 post treatment, unpaired two-tailed Student’s t test). (f) Crizotinib-Sunitinib led to increased apoptosis in PDX-1 tumors as assessed by TUNEL. Representative images from each treatment group are shown (right). Scale bars represent 50 μm (n = 6; mean ± s.e.m. ***P < 0.001 at day 4 post treatment, unpaired two-tailed Student’s t test). (g) Crizotinib-Sunitinib led to tumor regressions in PTPN12-deficient TNBC PDXs. For each PDX model, transplanted tumors were randomized into two treatment groups (vehicle or C+S) at 150–200 mm3 and monitored for tumor progression. The log fold change in tumor growth is plotted for the two treatment groups at the time point when vehicle-treated animals were euthanized due to tumor progression. PTPN12 IHC score (0–3) is indicated below the PDX model.
Inhibitors of oncogenic receptor tyrosine kinases comprise a substantial fraction of the targeted therapies in the cancer clinic37. The rational use of these inhibitors has largely been guided by receptor mutations (small nucleotide variants, amplification, and chromo-somal translocations) in human cancers. However, most cancers, such as TNBC, do not harbor frequent mutations in RTKs that can account for aberrant receptor signaling. Our results suggest that PTPN12 may serve as a negative-feedback regulator of proto-oncogenic RTKs. Our data posit that compromised PTPN12 function (through reduced protein dosage or mutation) may relieve feedback inhibition of these receptors in tumor cells. PTPN12 levels are often reduced in TNBC and other epithelial cancers10,36,38–42, raising the possibility that PTPN12-regulated receptors may be aberrantly hyperactive in many malignancies. Notably, we observed that treatment with a combination of inhibitors targeting PTPN12-regulated receptors (MET and PDGFRβ) led to significant regression in TNBC PDXs, and tumor response was correlated with PTPN12 deficiency. Collectively, these data are consistent with the hypothesis that the loss of RTK feedback (through inactivation of PTPN12 or other mechanisms) may be a common mechanism that unleashes oncogenic RTKs, providing a complementary rationale to leverage receptor inhibitors in patients with TNBC or other malignancies.
METHODS
Methods, including statements of data availability and any associated accession codes and references, are available in the online version of the paper.
ONLINE METHODS
Cell culture.
293T cells, MDA-MB-231, and MDA-MB-231 LM2 human breast cancer cells were cultured in DMEM (Gibco) supplemented with 10% FBS (FBS). SUM159 and SUM149 human breast cancer cells were cultured in F12 (Gibco) media supplemented with 5% FBS, 10 mM HEPES (Gibco), 5 µg/ml insulin (Invitrogen), and 1 µg/ml hydrocortisone. BT549 and ZR75-B human breast cancer cells were cultured in RPMI (ATCC) supplemented with 10% FBS. Cell lines were obtained from ATCC, and all cell lines are tested yearly for mycoplasma contamination. All cell lines were incubated at 37 °C and 5% CO2. Stable cell lines expressing cDNAs or shRNAs were generated by lentiviral or retroviral transduction in the presence of 8 µg/ml polybrene followed by selection with appropriate antibiotic resistance markers.
Cell proliferation assays.
Human breast cancer cells treated with inhibitors as indicated were seeded onto 96-well plates (Corning). At confluence, cells were fixed in 4% paraformaldehyde, and nuclei were stained with Hoeschst 3321 (1:1,000, Life Technologies). Nuclei were imaged and counted using the Celígo Imaging Cell Cytometer (Brooks).
Bimolecular fluorescence complementation.
PTPN12-wt cDNA was cloned into the pQCXIN-N-YFP fusion vector, in which N-terminal domain (residues 1–155) of Venus YFP was fused N-terminal to PTPN12 (bait). Human receptor tyrosine kinase [RTKs] cDNAs were obtained from one of several collections (human kinase open reading frame collection, Addgene, Invitrogen) or synthesized, fully-sequence verified, and individually recombined into pQCXIP-C-YFP fusion retroviral vector in which C-terminal Venus YFP (residues 156–239) was fused C-terminal to the RTKs (prey). SUM159 breast cancer cells or MDA-MB-231 cells (as indicated) were transduced with the bait retroviruses. Subsequently these cells were infected with the prey BiFC retroviruses expressing the RTKs, and cellular fluorescence was analyzed by flow cytometry in duplicate.
Immunoblotting.
Cells were lysed in 1× NP40 lysis buffer (50 mM Tris-HCl, pH7.6, 1% NP-40, 150 mM NaCl) with fresh addition of phosphatase (SIGMA) and EDTA-free protease (Roche) inhibitors. Pieces of tumor tissue were weighed and pulverized using a TissueLyser LT (Qiagen) in RIPA buffer (25 mM Tris-HCl, pH7.6, 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, protease inhibitor and phosphatase inhibitor cocktail) followed by centrifugation to clarify the lysates. Protein quantitation was then performed using Pierce BCA Protein assay kit (Thermo Scientific). SDS-PAGE was performed using standard methods and transferred to nitrocellulose membranes for western blotting. The following antibodies were used for western blotting: PTPN12 (Sigma HPA0070970), PTPN12 (Bethyl A301–302A), Vinculin (Sigma V9131), phosphotyrosine (4G10, Upstate 05–1050), p-Tyr-1000 (Cell Signaling 8954), phospho-HER2 (Y1248, Millipore 06–229); HER2 (Millipore 06–562), EGFR (Cell Signaling 2232); phospho-EGFR (Y1148, Cell Signaling 4404), Akt (Cell Signaling 2938), phospho-Akt (S473, Cell Signaling 9271), MAPK (Cell Signaling 9102), phospho-MAPK (T202/Y204, Cell Signaling 4377), P90RSK (Cell Signaling 9355), phospho-P90RSK (S380, Cell Signaling 9335), S6 (Cell Signaling 2217), phospho-S6 (S235/S236, Cell Signaling 2211), RSK½/3 (Cell Signaling 9355) phospho-P70 S6 Kinase (T421/S424) (Cell Signaling 9204); phospho-PDGFRβ (Y1021, Cell Signaling 2227); PDGFRB (Cell Signaling 3169); phospho-Met (Y1234/35 Cell Signaling 3077); MET (Cell Signaling 8198), EPHA7 (Santa Cruz sc-917), RAN (BD Biosciences-610340), FLAG (Sigma F1804). HRP-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. All antibodies were used at a dilution factor of 1:1,000 except Vinculin (1:3,000), Ran (1:5,000) and Actin (1:5,000).
Vectors and virus production.
For inducible cDNA experiments, PTPN12 cDNA were subcloned into the pINDUCER doxycycline-inducible lentiviral expression system43. Lentiviruses and retroviruses were produced by transiently transfecting shRNA or cDNA constructs using Mirus Bio TransIT transfection protocols into 293T cells and harvesting viral supernatants 48 h after transfection. PTPN12 mutant was generated by site-directed mutagenesis9. RTK cDNAs were also cloned into lentiviral vector pHAGE-PGK-cFLAG-HA-GAW-Puro. PDGFRB wt and PDGFRB D850V mutant [created by site-directed mutagenesis using QuikChange II Site-Directed Mutagenesis Kit] were cloned into retroviral vector pQCXIN-dest. MET wt and MET Y1230H mutant cDNAs33 were cloned into retroviral vector pQCXIN-dest.
Clonogenic assays.
For colony formation assays assessing PTPN12 sensitivity in breast cancer cell lines, cells were transduced with equivalent multiplicity of infection (moi) of retroviruses encoding eGFP (control) or PTPN12 cDNAs, seeded at a density of 500–6,000 cells per 6-cm plate (depending on cell line). 2 d after infection, cells were selected with neomycin and cultured until macroscopic colonies formed. All assays were performed in triplicate or quadruplicate.
For colony formation assays with RTK mutants, SUM159 cells were infected with viruses expressing pQCXIN-vector control, PDGFRβ wt or PDGFRβ D850V, MET wt or MET Y1230H cDNA at MOI = 1 appropriate to transduce all cells. Following drug selection with neomycin, transduced cells were seeded at low density (500 cells per 60 mm dishes) and treated with inhibitors. Three to four biological replicates were used per treatment group. Cells were re-fed every 3 d until macroscopic colonies were observed. Colonies were stained using Coomassie brilliant blue, and macroscopic colonies were quantified and normalized to vehicle-treated plates for each cell line where indicated.
Apoptosis assays.
The annexin V/dead cell assay was performed in a Muse cell analyzer (Merck Millipore) using a Muse annexin V and dead cell kit (MCH100105) to assess effects on apoptosis. In brief, MDA-MB-231 LM2, SUM159 or SUM149 breast cancer cells were seeded in 60 mm dishes and incubated in a humidified CO2 incubator for 24 h to allow cell attachment. The cells treated with specified inhibitors (SUM159– 1 µM Crizotinib and 2 µM Sunitinib; MDA-MB-231– 0.5 µM Crizotinib and 1 µM Sunitinib; SUM149– 1 µM Crizotinib and 2 µM Sunitinib) for 48 h. The cells were then harvested from the dish with trypsin to give single cell suspensions. Subsequently 100 µl of annexin V/dead reagent and 100 µl of a single cell suspension were mixed and incubated for 20 min at 20–25 °C in the dark. The cells were then analyzed using the Muse cell analyzer, and 5,000–10,000 cell events were collected for each sample. The assay utilizes annexin to detect PS on the membranes of apoptotic cells combined with a DNA dye, 7-aminoactinomycin D (7-AAD), for the exclusion of dead/nonviable cells.
Immunoprecipitation.
SUM159 cells were infected with pINDUCER doxycycline-inducible lentiviral expression system to inducibly express PTPN12 cDNA (SUM159ind-PTPN12 cells). SUM159ind-PTPN12 cells were then individually infected with lentiviruses expressing C-terminally FLAG tagged RTKs (pHAGE-PGK-Flag-RTK). SUM159ind-PTPN12 cells constitutively expressing a given RTK were treated ± dox treatment for PTPN12 cDNA induction for 2 d, followed by serum starvation ± dox for 24 h and then re-stimulation with media containing serum for 4 h. Lysates were collected using NP-40 lysis buffer and quantified using BCA Protein Assay Kit (Pierce #23225). For phosphotyrosine IP, lysates were processed according to manufacturer’s instructions (Catch and Release Phosphotyrosine, clone 4G10 Immunoprecipitation Kit #17–502). FLAG IPs were performed using the Catch and Release v2.0 Reversible immunoprecipitation system (#17–500) using 4µg M2 Flag antibody (Sigma) at 4C for each individual IP.
qRT-PCR.
RNA of SUM159ind-PTPN12 breast cancer cells transduced with lentiviruses expressing pHAGE-PGK-Flag tagged RTK was isolated using an RNAeasy kit (Qiagen). Subsequently cDNA was synthesized using the High Capacity RNA-to-cDNA kit (Applied Biosystems). Real-time RT-qPCR was performed in the triplicate on the samples using primers for Flag transcript (F-CGAGGACTACAAGGACGACG; R-TCGTAGGGGTATCCTCCAGC) and GAPDH. SUM159ind-PTPN12 breast cancer cells that were not transduced with pHAGE-PGK-Flag-RTK vector was used as negative control.
Molecular modeling of human PTPN12 protein.
The 3D structure of the human PTPN12 protein was based on the crystallographic structure with the Protein Data Bank identity of 5j8r, which represents the catalytic domain of the human protein tyrosine phosphatase non-receptor type 12 (ref. 16). The chain A of the q5j8r structure contained residues between the positions of 2 and 299. The relative evolutionary importance of PTPN12 residues was estimated with the evolutionary trace (ET) method44,45, using 62 homologous sequences aligned with MUSCLE46. The ET method computed importance rankings by systematically correlating residue variations with evolutionary divergences, in effect linking perturbations in sequence with perturbations in function as judged by phylogenetic distances. These rankings have been previously validated in large-scale retrospective analyses47 and from direct mutational studies that separated, rewired, or mimicked functional sites48–50. The impact of amino acid substitutions was estimated using the Evolutionary Action equation25. The protein structure figures were generated with the PyMol visualization software (The PyMOL Molecular Graphics System, Version 1.6 Schrödinger). The structure was colored according to the ET ranks by using PyETV51. The built-in functions of PyMol were used to substitute the amino acid H230 to Y, and to measure the distances between protein residues as the minimum atom-atom distances of the amino acid side chains.
Phosphatase assays with small artificial phospho-peptides.
The PET-15b encoding PTPN12–1-305 catalytic domain were subjected to quick change method to generate the plasmids encoding PTPN12-H230Y and PTPN12C231A mutants. The catalytic domains of PTPN12-wild type, PTPN12-H230Y and PTPN12-C231A were expressed in E. coli BL21 and purified by His-affinity column and the purity of the protein was examined by electrophoresis. The phosphatase activities of PTPN12-WT, PTPN12-H230Y and PTPN12-C231A mutants toward small artificial substrates pNPP, DiFMUP and OMFP were assayed as described before16. Briefly, all reactions were performed in 3,3-dimethylglutaric acid (DMG) buffer ([pH 7.0] 3,3-dimethyl glutarate 50 mM, DTT 2 mM,EDTA 1 mM, and the ionic strength was adjusted to 0.15 M) at 37 °C. For pNPP, 405-nm absorbance was used to monitor the reaction. For DiFMUP, the excitation wavelength was set up at 358 nm and the emission was detected at 455 nm for product measurement. For OMFP, the excitation wavelength was set up at 485 nm and emission was detected at 525 nm for product measurement. The data were gathered and fitted by Micheleas Menton equation using GraphPad Prism5 (GraphPad Software).
Immunofluorescence protocol.
Cells with and without antigen of interest were used for immunofluorescence protocol optimization, in which Flag (Sigma-Aldrich, F1804), PTPN12 (Bethyl, A301–302A), MET (Cell signaling, 8198P, AF276, 2.5 µg/ml), and GFP (Abcam, ab1218) antibodies were used. 48 h post seeding, cells were fixed by 4% paraformaldehyde for 5 min, permeabilized with 0.25% Triton X-100, blocked with 5% BSA, and incubated overnight with corresponding antibody. For PTPN12-IF validation SUM159 cells infected with viruses expressing PTPN12 shRNA (v2LHS_170948) were used.
PLA.
PLA was performed to visualize protein-protein interaction by microscopy52. For PDGFRβ-PTPN12 PLA, SUM159ind-PTPN12 cells with and without Flag-PDGFRβ expression (transduced ± pHAGE-PGK-Flag-PDGFRβ) were used. Cells were plated on poly-D-lysine coated 96-well plate for 48 h, followed by 24 h serum starvation, and stimulated with PDGF-BB (R&D, 220BB, 100 ng/ml) for 3 min. Cells were then stained with Flag antibody (1:500) in conjunction with PTPN12 antibody (1:100) using IF protocol described above. Cells exhibiting PLA spots greater than negative control thresholds were considered positive, and percent positive cells per condition was plotted. For MET-PTPN12 PLA, SUM159 parental or SUM159 cells transduced with MSCV-eGFP-PTPN12 virus was used. PLA protocol was performed as described above with HGF stimulation for 1min (R&D, 294-HG, 50 ng/ml). For staining GFP antibody (1:250) was used in conjunction with MET antibody (1:3,000). For endogenous MET and PTPN12 PLA, SUM159 cells transfected with negative control siRNA and MET siRNA (ThermoFisher, s8701) were stimulated with HGF for 3 min. MET antibody (AF276, 2.5 µg/ml) and PTPN12 antibody (Bethyl, 1:100) were used for staining. Cells exhibiting PLA spots greater than negative control thresholds were considered positive, and percent positive cells per condition was plotted. For all experiments, at least 60 cells were scored per condition. Proximity ligation reaction and signal amplification were performed according to the manufacturer’s protocol using the Duolink In situ Detection Reagents with PLA PLUS and MINUS probes for mouse and rabbit antibodies (DUO92101), and anti-goat MINUS probes (DUO92006) was used for goat antibody. Cells were then counterstained with NucBlue Fixed Cell Stain ReadyProbes reagent (R37606) to visualize nuclei. Images were collected by Nikon Ti-E inverted microscopy using 60× oil objective and 4 × 4 fields of view were stitched per condition per experiment. Maxintensity projection from 16-µm z-stacks with 1 µm each step were generated and used for PLA signal quantitation. PLA signal quantification was performed using a custom-made algorithm developed in MatLab. DAPI-stained nuclei were segmented using a watershed transform strategy. The centroid coordinates, eccentricity index and the orientation of the major axis relative to the longitudinal axis of the image for each nucleus were recorded. PLA dots were segmented by intensity thresholding, and their size, mean pixel intensity and centroid coordinates were measured. When appropriate, background speckles were removed by size and/or intensity filtering.
Assigning each PLA signal to a specific cell was performed by searching for the nearest nucleus. Because of the elongated shape of the cells, each cell was approximated to an elliptical shape and each PLA signal was allocated to a given cell when the function
is minimum, where (x,y) are the Cartesian coordinates of the PLA centroid relative to the nucleus centroid, and a and b are scaled factors: α = 60× c2 and , with c being the eccentricity index of the nucleus.
Generation of knockout clones using CRISPR-Cas9.
Edit-R synthetic crRNA and tracrRNA (Dharmacon, Cat #U-002005–20) for MET (Dharmacon, CR003156–02) or PDGFRβ (Dharmacon, CR-003163–02) or non-targeting control (Dharmacon, U-007501–00) were transfected into SUM159 cells stably expressing an inducible Cas9 cDNA. Briefly, 2 × 104 cells/well of a 24-well plate was seeded. The expression of Cas9 was induced with the addition of doxycycline solution (1 mg/ml) at least 24 h before transfection of the synthetic crRNA:tracrRNA complex. Cells were transfected to achieve 25 nM final concentration of the crRNA:tracrRNA complex. Cells were incubated at 37 °C in a humidified CO2 incubator for 48–72 h before proceeding with gene editing analysis. From this polyclonal edited population clonal cell isolation was performed by seeding cells in low density and picking individual clones, growing and expanding. Individual clones were assessed for evidence of gene editing via sequencing and westerns were performed to identify clones with the least target expression. These cells were subsequently used for functional assays.
In vitro competition assay.
SUM159 cells were transduced with virus expressing e-GFP recombined into a pHAGE-PGK vector to create a stable GFP labeled cell population. These cells were mixed at a 10:90 ratio with SUM159 breast cancer cells edited with MET or PDGFRβ or non-targeting control Edit-R synthetic crRNA (described above) and seeded into 96-well plates. Plates were treated with vehicle media or inhibitor (0.5 µM Crizotinib or 1 µM Sunitinib as indicated). At confluence, cells were passaged 1:10 and processed for flow cytometry. The in vitro competition assay was continued for four passages.
Tumorigenicity assays.
8 × 106 SUM159 breast cancer cells (per mouse) were injected with matrigel (8 million cells/50 µl media and mixed with 50 µl of matrigel) subcutaneously into the flank of female athymic nude Foxn1-nu mice (Harlan Labs). Tumor volume was measured using calipers, and once tumors achieved 150 mm3, mice were randomized onto different treatment arms including vehicle, 50 mg/kg/d of Crizotinib (Pfizer) and/or 20 mg/kg/d of Sunitinib (Pfizer). Mice were sacrificed once tumors reached 1,000 mm3, and tumor chunks were snap frozen for protein extraction and processed for paraffin embedding.
PDX pre-clinical trial.
All animal experiments were performed under IACUC approved protocols by Baylor College of Medicine. Fresh PDX tumor tissue fragments35 were transplanted in the cleared number four fat-pad (right abdominal) of 4-week-old SCID/Beige mice. Tumors were then allowed to reach a size of ~150–200 mm3 (length × width2)/2. Once treatment size was reached, tumors were randomized onto one of twotreatment arms: including vehicle or combination of 100 mg/kg/d Crizotinib (Pfizer) and 40 mg/kg/d Sunitinib (Pfizer). Mice were sacrificed once tumors reached 1,000 mm3, and tumor chunks were snap frozen for protein extraction and processed for paraffin embedding. Customized R Script derived from SummarySE from Rmisc package was used to summarize the log base 2 transformed tumor measurement data into mean of change, standard, deviation, and s.e.m. for each PDX at a specific time point. Data was not grouped by treatment variable.
Statistical analyses.
All experiments were performed on biological replicates unless otherwise specified. Sample size for each experimental group/condition is reported in the appropriate figure legends and methods. For cell culture experiments, sample size was not predetermined, and all samples were included in analyses. Statistically significant differences between control and experimental groups were determined using two-tailed/one-tailed unpaired Student’s t test, paired t test, one-way or two-way ANOVA with Tukey-Kramer multiple comparison test as indicated in the appropriate figure legend and methods text.
Immunohistochemistry.
A PTPN12 immuno-histochemical assay (Bethyl A301–302A) was optimized and validated on paraffin-embedded cell pellets from human mammary epithelial cells (HMECs) with or without PTPN12-shRNA expression (to confirm depletion of immuno-reactive signal) (Supplementary Fig. 11). PTPN12 protein expression was analyzed in a tissue microarray of constructed of 3 mm cores of PDX tumors and immuno-stained using protocols described before9. Briefly, antigen retrieval was performed by heating in 0.1MTris–HCl buffer (pH 9.0). Sections were incubated with PTPN12 primary antibody at a dilution of 1:100 20–25 °C for 1 h. Immunodetection was performed with the Envision+System (Dako). The enzyme was visualized after 15-min incubation with diaminobenzidine (DAB). Slides were counterstained with hematoxylin. Positive and negative controls were included in each staining. Cytoplasmic PTPN12 was evaluated based on a score that ranges from 0 (negative) to 3 (most intense staining) according to staining intensity. Ki67 (Dako) staining was performed using above described conditions with 1:200 antibody dilution. For TUNEL staining slides were incubated for 135 min with TUNEL reaction cocktail (Roche) at 37 °C. Subsequently incubation with horseradish peroxidase labeled streptavidin from Dako (LSA-HRP) at a 1:100 20–25 °C for 30 min was performed. Enzyme was visualized with DAB.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank members of T.F.W. laboratory for helpful comments. The authors also acknowledge the joint participation of the Adrienne Helis Melvin Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine for cancer research. The Dan L. Duncan Cancer Center Shared Resources was supported by the NCI P30CA125123 Center Grant and provided technical assistance including Cell-Based Assay Screening Service (D. Liu), Biostatistics & Informatics Shared Resource (S. Hilsenbeck), and Cytometry and Cell Sorting (J. Sederstrom; P30 AI036211 and S10 RR024574). M.T.L. was supported by the Susan G. Komen Foundation (KG120001), a NCI/NIH SPORE Supplement Award (P50 CA186784), and the Patient-derived Xenograft and Advance In vivo Models Core at Baylor College of Medicine and P30 Cancer Center Support Grant (NCI-CA125123). T.F.W. was supported by CPRIT (RP120583), the Susan G. Komen Foundation (KG090355), the NIH (1R01CA178039–01) and the DOD Breast Cancer Research Program (BC120604).
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
COMPETING INTERESTS
D.J.S. is an employee of Pfizer. J.G.C. is an employee of Mirati Therapeutics and former employee of Pfizer.
References
- 1.Anders C & Carey LA Understanding and treating triple-negative breast cancer. Oncology (Williston Park) 22, 1233–1239; discussion 1239–1240, 1243 (2008). [PMC free article] [PubMed] [Google Scholar]
- 2.Anders CK, Zagar TM & Carey LA The management of early-stage and metastatic triple-negative breast cancer: a review. Hematol. Oncol. Clin. North. Am 27, 737–749 viii (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchini G, Balko JM, Mayer IA, Sanders ME & Gianni L Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol 13, 674–690 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann BD et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest 121, 2750–2767 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hochgräfe F et al. Tyrosine phosphorylation profiling reveals the signaling network characteristics of Basal breast cancer cells. Cancer Res 70, 9391–9401 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Duncan JS et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell 149, 307–321 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen TO et al. Immunohistochemical and clinical characterization of the basal- like subtype of invasive breast carcinoma. Clin Cancer Res 10, 5367–5374 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Mertins P et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534, 55–62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun T et al. Activation of multiple proto-oncogenic tyrosine kinases in breast cancer via loss of the PTPN12 phosphatase. Cell 144, 703–718 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu MQ et al. Low expression of tyrosine-protein phosphatase nonreceptor type 12 is associated with lymph node metastasis and poor prognosis in operable triple- negative breast cancer. Asian Pac. J. Cancer Prev 14, 287–292 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Villa-Moruzzi E PTPN12 controls PTEN and the AKT signalling to FAK and HER2 in migrating ovarian cancer cells. Mol. Cell. Biochem 375, 151–157 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y et al. Temporal regulation of EGF signalling networks by the scaffold protein Shc1. Nature 499, 166–171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charest A, Wagner J, Kwan M & Tremblay ML Coupling of the murine protein tyrosine phosphatase PEST to the epidermal growth factor (EGF) receptor through a Src homology 3 (SH3) domain-mediated association with Grb2. Oncogene 14, 1643–1651 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Markova B, Herrlich P, Rönnstrand L & Böhmer FD Identification of protein tyrosine phosphatases associating with the PDGF receptor. Biochemistry 42, 2691–2699 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Ambjørn M et al. A loss-of-function screen for phosphatases that regulate neurite outgrowth identifies PTPN12 as a negative regulator of TrkB tyrosine phosphorylation. PLoS One 8, e65371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H et al. Crystal structure and substrate specificity of PTPN12. Cell Rep 15, 1345–1358 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Barr AJ et al. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell 136, 352–363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J et al. Loss of PTPN12 stimulates progression of ErbB2-dependent breast cancer by enhancing cell survival, migration, and epithelial-to-mesenchymal transition. Mol. Cell. Biol 35, 4069–4082 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao Z et al. A global analysis of the receptor tyrosine kinase-protein phosphatase interactome. Mol. Cell 65, 347–360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoadley KA et al. EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics 8, 258 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prat A et al. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 18, 123–133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zagouri F et al. High MET expression is an adverse prognostic factor in patients with triple-negative breast cancer. Br. J. Cancer 108, 1100–1105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas, N.. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forbes SA et al. The catalogue of somatic mutations in cancer (COSMIC). Curr. Protoc. Hum. Genet Chapter 10, Unit 10 11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsonis P & Lichtarge O A formal perturbation equation between genotype and phenotype determines the evolutionary action of protein-coding variations on fitness. Genome Res 24, 2050–2058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey LA et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J. Clin. Oncol 30, 2615–2623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller K et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med 357, 2666–2676 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Yardley DA et al. Phase I/II trial of neoadjuvant sunitinib administered with weekly paclitaxel/carboplatin in patients with locally advanced triple-negative breast cancer. Breast Cancer Res. Treat 152, 557–567 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Bianchi G et al. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs 20, 616–624 (2009). [PubMed] [Google Scholar]
- 30.Finn RS et al. Estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor expression and benefit from lapatinib in a randomized trial of paclitaxel with lapatinib or placebo as first-line treatment in HER2-negative or unknown metastatic breast cancer. J. Clin. Oncol 27, 3908–3915 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prenen H et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin. Cancer Res 12, 2622–2627 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Guida T et al. Sorafenib inhibits imatinib-resistant KIT and platelet-derived growth factor receptor beta gatekeeper mutants. Clin. Cancer Res 13, 3363–3369 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Qi J et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 71, 1081–1091 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sørlie T et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869–10874 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res 73, 4885–4897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xunyi Y et al. Clinicopathological significance of PTPN12 expression in human breast cancer. Braz. J. Med. Biol. Res 45, 1334–1340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu P, Nielsen TE & Clausen MH Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discov. Today 21, 5–10 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Su Z et al. PTPN12 inhibits oral squamous epithelial carcinoma cell proliferation and invasion and can be used as a prognostic marker. Med. Oncol 30, 618 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Cao X et al. Tyrosine-protein phosphatase non-receptor type 12 expression is a good prognostic factor in resectable non-small cell lung cancer. Oncotarget 6, 11704–11713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao X et al. Tyrosine-protein phosphatase nonreceptor type 12 is a novel prognostic biomarker for esophageal squamous cell carcinoma. Ann. Thorac. Surg 93, 1674–1680 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Luo RZ et al. Decreased expression of PTPN12 correlates with tumor recurrence and poor survival of patients with hepatocellular carcinoma. PLoS One 9, e85592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang XK et al. The prognostic significance of tyrosine-protein phosphatase nonreceptor type 12 expression in nasopharyngeal carcinoma. Tumour Biol 36, 5201–5208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meerbrey KL et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc. Natl. Acad. Sci. USA 108, 3665–3670 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichtarge O, Bourne HR & Cohen FE An evolutionary trace method defines binding surfaces common to protein families. J. Mol. Biol 257, 342–358 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Mihalek I, Res I & Lichtarge O A family of evolution-entropy hybrid methods for ranking protein residues by importance. J. Mol. Biol 336, 1265–1282 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Edgar RC MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mihalek I, Res I, Yao H & Lichtarge O Combining inference from evolution and geometric probability in protein structure evaluation. J. Mol. Biol 331, 263–279 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Baameur F et al. Role for the regulator of G-protein signaling homology domain of G protein-coupled receptor kinases 5 and 6 in beta 2-adrenergic receptor and rhodopsin phosphorylation. Mol. Pharmacol 77, 405–415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lichtarge O, Yamamoto KR & Cohen FE Identification of functional surfaces of the zinc binding domains of intracellular receptors. J. Mol. Biol 274, 325–337 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Ribes-Zamora A, Mihalek I, Lichtarge O & Bertuch AA Distinct faces of the Ku heterodimer mediate DNA repair and telomeric functions. Nat. Struct. Mol. Biol 14, 301–307 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Lua RC & Lichtarge O PyETV: a PyMOL evolutionary trace viewer to analyze functional site predictions in protein complexes. Bioinformatics 26, 2981–2982 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Söderberg O et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.