Abstract
Opuntia ficus indica by-products can be exploited as sources of high-value components for applications in food and other industries. The aim of the present work is to elucidate and optimize the mucilage extraction process from cladodes. The effect of five water-to-biomass ratios (1:1, 1:3, 1:5, 1:7, 1:9 w/v), pH range (2.0, 4.5, 7.0, 9.5, 12.0) and ionic strength (water supplemented with NaCl or CaCl2 at the concentration of 0.1, 1.0, 10.0 and 100.0 mM) were evaluated on mucilage yield. The analysis of the critical factors was done by the response surface methodology. Ultrasound and microwave assisted extractions were evaluated to improve the mucilage recovery and quality. In this work: (1) the development of a multivariate model to predict mucilage recovery on the basis of biomass/water ratio and time of extraction; (2) pH, ionic strength and temperature were found critical process variables by the application of Plackett–Burman design; (3) the optimal operating conditions obtained were found to be: 1:9 biomass/water ratio, pH 12.0, ionic strength 1.0 mM NaCl; (4) ultrasonic or microwave treatments are efficient tools to enhance the recovery of mucilage depending on its final uses. Within a multi-disciplinary approach, this work provides achievements for a more efficient extraction process of soluble polymers from cladodes. Further studies on green assisted extraction tools and their effects in terms of quality of extracts are required in order to obtain high added value bio-products.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03794-7) contains supplementary material, which is available to authorized users.
Keywords: Mucilage, Prickly pear, Cladodes, Green extraction processes, Response surface methodology
Introduction
New economic models based on efficient use of the resources in agriculture are strongly promoted by European Union policies with the twofold objectives of creating new productive chains recycling by-products and to reduce the cost of waste along the agro industrial processes. Opuntia ficus-indica is a species native to Mexico, commonly known as prickly pear, belonging to the family Cactaceae, well adapted to arid lands and to diversity of climates all over the world, including the Mediterranean basin, Middle East, South Africa, Australia and India (Griffith 2004; Stintzing and Carle 2005). In the Mediterranean areas, South Africa and South America this species is cultivated for its edible fruits (prickly pear), although in some countries different parts of the plant are utilized in food and cosmetic industry (Retamal et al. 1987; Saenz 2000; Sáenz 2002; Stintzing and Carle 2005; Cushman et al. 2015). The cactus pads (or cladodes) commonly known as nopales, when whole or nopalitos when cut into small pieces, are regularly consumed as vegetable or forage (16% or 22% of productive lands, respectively) in Mexico, since cactus pear is deeply embedded in local culture. Nevertheless, the last scientific studies demonstrated cladodes nutritional and functional value, hence there is a growing demand for O. ficus indica consumption as vegetable in US and Canada, where the product reaches sales equivalent to 31 and 2.86 million dollars, respectively (Ventura-Aguilar et al. 2017). The mucilage is a hetero-polysaccharide of high molecular weight (2.3 × 104 to 3 × 106 g mol−1) with ecological, biological, chemical and physical properties (Medina-Torres et al. 2000; Sáenz et al. 2004; León-Martínez et al. 2010); it constitutes approximately 14% of the dry weight of the cladodes and consists mainly of six sugars: arabinose, galactose, rhamnose, xylose, uronic acid and galacturonic acid (Trachtenberg and Mayer 1981). The economic importance of mucilage lies in its gelling properties with a multifunctional use in food, cosmetics and pharmaceutical industry (Del-Valle et al. 2005; Allegra et al. 2017). Medina-Torres et al. (2000) suggested viscosity values of mucilage solution at 10% comparable with a xanthan solution at 3%, with interesting implications for the economic and technical importance of xanthan gum. Furthermore, many studies based on clinical trials documented its antihyperlipidemic, antiobesity and hypocholesterolemic effects (Frati-Munari et al. 1983). Different authors discussed methods of extraction and purification of mucilage (Sáenz et al. 2004; Sepúlveda et al. 2007; Contreras-Padilla et al. 2016) or pectin (Bayar et al. 2017a, b) from Opuntia spp. However, in the last year, efforts to exploit extraction methods are carried out on industrial scale, in order to evaluate possible productive chains utilizing cladodes as by-products or as main commodity in dedicated crops. The use of water as extraction buffer is a traditional method in Mexico, which is effective for homemade applications, ease to apply. In the aqueous method, different factors such as temperature, mechanical processes (blending), extraction period and liquid/solid ratio can induce changes in the chemical and nutritional qualities of Opuntia cladodes, affecting the yield and quality of bio-product (Bayar et al. 2017a, b; De Santiago et al. 2018). The aim of the present research is to optimize the efficiency of mucilage extraction by water, reducing its cost and ensuring safe and high quality extract/product. The analysis of the critical factors affecting the extraction process was done by the response surface methodology which implicates the application of multivariate techniques on factors and responses relating to the process. Therefore, the specific objectives aimed to depict the most appropriate conditions for mucilage extraction from O. ficus-indica cladodes are the following: (1) to investigate the effects of dilution/ratio/time/biomass, pH and ionic strength on mucilage yield from cladode parenchyma, expressed as total sugar content; (2) to apply response surface methodology and Plackett–Burman design (Analytical Methods Committee 2013) to optimize the extraction procedure; (3) to compare the effects of ultrasonic and microwave assisted extraction (UAE and MAE, respectively) with respect to conventional method, also evaluating protein and uronic acids content in the extracts, in order to ameliorate the efficiency of the process.
Materials and methods
Plant materials
The cladodes were obtained from O. ficus-indica cv. Sanguigna plants grown in the region of Maccarese, Rome. The cladodes were collected early in the morning, in the summer–autumn months, from the beginning of June through October. The 2–3 years old cladodes were immediately used for the experimental trials; these were washed with tap water in order to remove impurities and spines, peeled from epidermis in both sides and then chopped into 1–2 cm pieces.
Extraction conditions
Biomass-to-water ratio (w/v), pH, ionic strength, temperature
We defined five biomass-to-water ratios: 1:1, 1:3, 1:5, 1:7, 1:9 (w/v); 200 g of fresh chopped cladodes were put in flasks with distilled water (200 mL in 1:1, 600 mL in 1:3, 1000 mL in 1:5, 1400 mL in 1:7 and 1800 mL in 1:9), then we left them soaking for 24 h at 25 °C, in dark condition without stirring. We filtered all the samples with a fine cloth (0.5 mm) until there was no mucilage dropping down. The mucilage was maintained at − 5 °C until the analysis. Each experiment was replicated three times.
Since the cladodes as experimental plant material have a buffering capacity (Corrales-García et al. 2004), we investigated a range of pH from 2 to 12 with and without buffer as a control. Fifty grams of fresh chopped cladodes in 250 mL of distilled water at pH 2.0, 4.5, 7.0, 9.5, 12.0 with and without a buffer (biomass-to-water ratio was 1:5) were replicated three times each. We used either hydrochloric acid or sodium hydroxide to adjust the pH in the samples without buffer and the McIlvaine’s tables (Sigma-Aldrich) to prepare the samples with buffer. The pH value was determined before and after 24 h of soaking.
In order to evaluate the effect of ionic strength, 50 g of fresh chopped cladodes were added to 250 mL of distilled water (biomass/water 1:5) supplemented with NaCl or CaCl2 at the concentration of 0.1, 1.0, 10.0 and 100.0 mM. A total of eight samples were compared to the control sample (50 g of plant material in 250 mL distilled water) after 24 h of soaking. Each experiment was carried out in triplicate. Mucilage was extracted from 50 g of fresh chopped cladodes in 250 mL of distilled water for 24 h at 20 or 80 °C. All solvents were purchased from Sigma–Aldrich (Milan, Italy).
Ultrasonic and microwave assisted extraction of mucilage
In order to increase the mucilage recovery, two sets of three times replicated samples (50 g of fresh chopped cladodes in 250 mL of distilled water, 1:5 w/v), have been subject to: (1) ultrasonic bathing (EMAG ZCC009, United States) at 40 kHz for 30 min; (2) microwaves (SMEG ME/203FX, Italy) at 500 W for 5 min. The percentage mucilage yield extracted from each samples was compared to the extraction obtained by conventional method (50 g of fresh chopped cladodes in 250 mL of distilled water at room temperature in the dark). Data were recorded a t0 (immediately after treatment); at t1 (after 16 h of maceration); at t2 (after 24 h of treatment).
Chemical analysis
Determination of total polysaccharides content
Forty mL of 96% ethanol was added to 10 mL of each water extract. Each sample (3 replicates per sample) was vortexed and left to form a precipitate for about 20 min. All the samples were then centrifuged at 9000 rpm for 5 min to let the mucilage precipitate on the bottom of the test tube. The supernatant was then eliminated and 5 mL of sulfuric acid 2 M was added in order to start the hydrolysis process. The tubes were left in a hot bath at 80 °C for 2 h. Then 1.4 g of CaCO3 was added in order to neutralize the excess of acid. The sample was centrifuged at 2.500 g for 15 min and the supernatant was separated and centrifuged again at the same conditions. Due to the reduced quantity of mucilage precipitate samples and the uncertainty of the error in the weighted quantity, we decided to proceed with total polysaccharides determination considered in proportion to 100 g of fresh biomass as a benchmark for determination of the mucilage yield (% yield). Similarly, Lefsih et al. (2016) report the % yield of the mucilage extraction according to the total polysaccharides content.
Determination of neutral sugar content
Total neutral sugar content was determined with the method of DuBois et al. (1956), which is based on the reaction among 98% sulphuric acid and the carbohydrate contained in the liquid samples as described above. All carbohydrates are transformed in furfurals (pentoses) and hydroxymethylfurfurals (hexose) which, in turn, react with phenol originating a golden yellow complex with a concentration, directly proportional to the amount of sugars in the sample. In a test tube 200 mL of sample or standard solution of known concentration of glucose or xylose + 250 μL 80% phenol + 5 mL 8% H2SO4 were added. After stirring for 1 min and waiting for 30 min to complete the reaction, the absorbance value was read at 490 nm (Varian Scary 50 Scan spectrophotometer). The results were obtained by interpolation of a calibration curve built from a series of points representing Abs vs known concentration of glucose or xylose. Total neutral sugar content was expressed as microgram equivalent of xylose per milligram of extracted soluble polymer x 100. All solvents were purchased from Sigma–Aldrich (Milan, Italy).
Determination of proteins and uronic acids content
The protein content was assessed using the method of Lowry et al. (1951), which combines two redox reactions: Cu2+ ions with the peptide bonds under alkaline conditions and the oxidation of aromatic protein residues (tryptophan and tyrosine) by Folin–Ciocalteau reagent. The Cu+2 ions produced in the first reaction react with the reduced Folin reagent producing an intense blue molecule known as heteropolymolybdenum blue. The concentration of the reduced Folin reagent (heteropolymolybdenum blue) was detected at 750 nm absorbance. The total concentration of protein in the sample can be deduced from the concentration of tryptophan and tyrosine residues that reduce the Folin–Ciocalteu reagent. Uronic acid contents were determined according to the method of Blumenkrantz and Asboe-Hansen (1973), 1.2 mL of sodium tetraborate 0.0125 M in 98% H2SO4 was added to 200 µL of sample at 4 °C. After cooling, the samples were shaken in a Vortex mixer and heated in a water bath at 100 °C for 5 min. After cooling in a water–ice bath, 20 µL of the 0.15% m-hydroxydiphenyl reagent in 0.5% NaOH was added. The tubes were shaken again and, within 5 min, absorbance measurements made at 520 nm (Varian Scary 50 Scan spectrophotometer). A blank sample was prepared adding 200 µL of distilled water + 1.2 mL of sodium tetraborate 0.0125 M in 98% H2SO4 + 0,5% NaOH. A calibration curve with this method was built using galacturonic acid as standard at six known concentrations from 0.4 to 0.05 mg/mL. The amount of uronic acids was expressed as % yield: grams of galacturonic acid equivalents/100 g of FW or DW of cladode.
Chemiometric statistical analysis
The analysis of variance was used in order to compare the means of mucilage yield (%) obtained at different conditions of dilution, time of process, pH or ionic strength of the buffer. Differences among the means were statistically significant depending on the random errors or the real effect of the operating changes in conditions. In addition, the process optimization by the quality by design (QbD) approach was applied, including the variation of two selected factors at one time in agreement with a defined design of experiment (DoE) and the multi-regression analysis of the experimental data. In detail, a full-factorial experimental design with two factors and three levels was performed in order to study the dependence of mucilage yield on the experimental process conditions, such as dilution and time. Nine experiments displayed geometrically as a face-edge centered square in agreement with the model matrix and the correlated experimental matrix were carried out. A nonlinear multiple regression to investigate the direct and indirect effects of each factor was applied to define the most suitable working conditions and develop predictive mathematical models.
Other independent experimental variables, such as pH, temperature and ionic strength can affect the mucilage extraction. In order to define which factors have the most significant effect, the experimental Plackett–Burmann design, according to the experimental conditions, was applied. Since this method with 4n experiments is suitable for studying 4n − 1 factors, the authors have performed a Plackett–Burmann design with eight experiments and seven factors, two of which are dummy factors without real meaning, only required for the mathematical elaboration.
Results and discussion
Full-factorial experimental design
Full-factorial experimental design was applied to evaluate the relationship between the predicted response and the independent variables. The following polynomial function (Eq. 1), was proposed to evaluate the mathematical regression:
| 1 |
where y is the response related to the mucilage percentage yield expressed as the grams of total sugars extracted from 100 grams of cladode; Xt and are dimensionless and normalized surrogate variables, respectively, defined on a coded scale from + 1 to − 1; they represent the true experimental factors from their lowest to highest level. Xt is related to the extraction time expressed in hours and refers to the dilution factor, expressed as the ratio between milliliters of water and the grams of cladode used in the experiment. Apart from b0, that is a constant with only a mathematical meaning, the b-coefficients represent the magnitude of direct and indirect effects: bt and are coefficients describing the importance of the direct effect of extraction time and dilution; is the coefficient for the indirect effect and the synergy of time and dilution; finally and are the coefficients describing the importance of the quadratic effect of the factors. The bar charts in Fig. 1 show the values of every b-coefficient obtained from the regression, highlighting both the standard deviation and the level of significance. The results indicate that both bt and are statistically significant with positive values to indicate that both factors have a direct positive effect on the extraction process. According to these data, significant differences were detected between the water/biomass ratios, with the % of mucilage yield at different dilutions largely dependent on the solid–liquid proportion. The efficiency of extraction process was very low at 1:1 ratio (0.95%) due to the rapid saturation of the solvent. By increasing progressively the ratio solid–liquid from 1:1 to 1:9, the increment of % yield was quadruplicate (3.8%). No plateau effect was detected, displaying additional potential mucilage to be extracted. This achievements agree with Sepúlveda et al. (2007) who reported the best mucilage yield was obtained with 1:7 pad/water ratio (at 40 °C), suggesting future research on greater quantity of water as solvent. Therefore the diffusivity of the solvent into mucilaginous cells increases in these conditions, enhancing the desorption of soluble polysaccharides from the tissue (Bayar et al. 2017a, b). The precision of the predicted response is very high, with the experimental error decreasing towards the inner zone due to the interpolation which allows the minimization of the leverage. Our results indicate the proposed model fits well the experimental data being appropriate for describing the relationship between independent variables and the response. Consequently, this model can be used to navigate the design space. In Fig. 2 the three-dimensional response surface (A) and the corresponding contour plot (B) indicate the trend of mucilage % yield as a function of both dilution and extraction-time conditions. In the level curves of the contour plot, the effect of ratio water/biomass on mucilage extraction, results more understandable. As expected from the full-factorial experimental design, the effect of dilution is more effective than maceration time. The coefficient is significant, showing an interaction between dilution and time factors. Our results showed a higher water/mass ratio promotes not only the efficiency of process by enhancing the dissolved mucilage threshold limit, but also the process rate by keeping high mucilage concentration gradients close to the water-cladode interface. For this reason, the time required for extraction decreases by increasing the water/mass ratio. Furthermore, the coefficient is statistically significant, showing that the dependence between extraction time and yield is linear only at the first step of the extraction process, later the aqueous solution becomes progressively saturated by mucilage solute and, consequently, the yield does not increase any more.
Fig. 1.
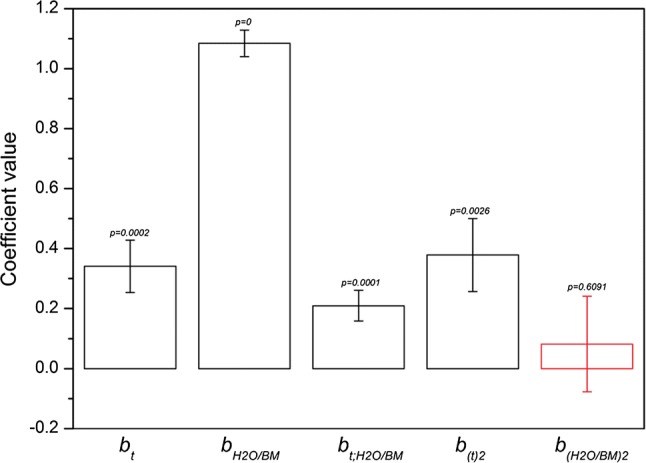
Bar charts related to the values of the b-coefficients of the mathematical motel describing the direct and indirect effects of both dilution and time on mucilage yield. In addition, it is showed the standard deviation and the statistical significance for every coefficient. The bars are not statistically significant
Fig. 2.
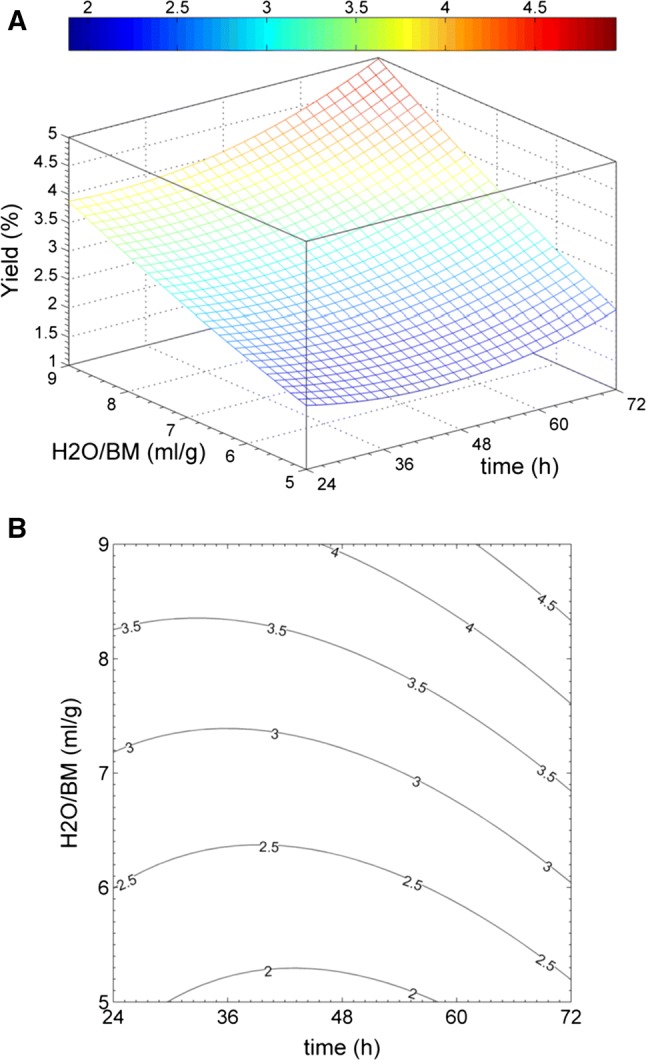
Multiple regression analysis related to the full-factorial experimental design where it is showed the dependence of mucilage yield % on both dilution H2O/BM (g/g) and time t(h): a 3-D plot with yield surface, b contour plot with yield line levels
Effect of pH, ionic strength and temperature on extraction efficiency
Among the external factors able to affect the extraction process, pH, ionic strength and temperature can be crucial in terms of mucilage removal and quality (Trachtenberg and Mayer 1981; Medina-Torres et al. 2000). As showed in Fig. 3, the pH showed a positive effect on mucilage extraction with statistically significant values. The optimum extraction condition was found at pH 12 with buffer (5.2%) followed by the treatment at pH 2 without buffer (4.3%). In the absence of buffer the percentage of mucilage extracted showed a reversed bell trend, due to pH-buffering capacity of Opuntia ficus-indica tissues rich in numerous small organic molecules typical of CAM plants, such as organic acids, which bear multiple acid–base groups able to act as buffer components in the physiological pH range (Corrales-García et al. 2004). In this regard, data on the effects of chemical factors such as pH or ionic strength on mucilage recovery are required in order to optimize the process extraction. High methoxyl pectin can form a gel under acidic conditions in the presence of high sugar concentrations at pH < 3.5 (Ngouémazong et al. 2012). From our results both acid and alkaline pH showed positive influence on mucilage yield (%) probably contributing to break mucilage cells structure letting them to free a major quantity of mucilage. Trachtenberg and Mayer (1981) demonstrated mucilage molecules shapes are clearly affected by pH, with viscosity loss at acidic conditions. We observed the same effect in the acid region, indeed the viscosity of mucilage varied according to the dissociation of acidic groups in polysaccharides and the repulsion between negative-charged, being more evident at increasing alkaline range, as reported by Medina-Torres et al. (2000). In this condition the polysaccharide solubility in water and viscosity was enhanced allowing a best yield extraction (Felkai-Haddache et al. 2015). Figure 4 presents the effect of ionic strength on mucilage yield (%) at cladode water ratio 1:5 using 0, 0.1, 1,10, and 100 mM NaCl or CaCl2. It is represented as box charts where box is determined by the 25th and 75th percentiles and the whiskers are determined by the 1st and 99th percentiles. The mean values are represented with white squares; while the medians are horizontal lines inside every box. The results showed significant differences among the treatments confirming a positive effect of cations concentration on mucilage recovery as expected for a polyelectrolyte. The presence of either NaCl or CaCL2 enhance the recovery of mucilage from Opuntia ficus indica cladodes; the most efficient extraction was observed at the concentrations of 1 mM NaCl or CaCl2, where the percentage yield values were ranging from 5.2% to 4.6%, respectively with an increment of + 35% or + 22% compared to the control (0 mM). However, as ionic strength increases, mucilage yield decreases. This behavior is more pronounced when using divalent ions, which are able to influence the physical parameters of mucilage such as the viscosity. In fact, the low-methoxyl pectin forms gel by interacting with divalent cations, particularly Ca2+ (Ngouémazong et al. 2012); therefore, the addition of high concentrations of Ca2+ promotes (Cárdenas et al. 2008; Lira-Ortiz et al. 2014) the formation of Ca-bridges with carboxyl groups (Han et al. 2017) in the wall cells of parenchyma tissue sample, decreasing the extraction and yield of mucilage. Different authors (Sepúlveda et al. 2007; Yoshida et al. 2010; Rodriguez-Felix and Cantwell 1988) showed an efficient Opuntia ficus-indica mucilage extraction from raw cladodes directly dependent on temperature; we included these parameter in our study, however our preliminary trials at 80 °C demonstrated that this factor could negatively influence the viscosity of extract by contributing to the hydrolysis of polysaccharides (Glibowski and Wasko 2008).
Fig. 3.
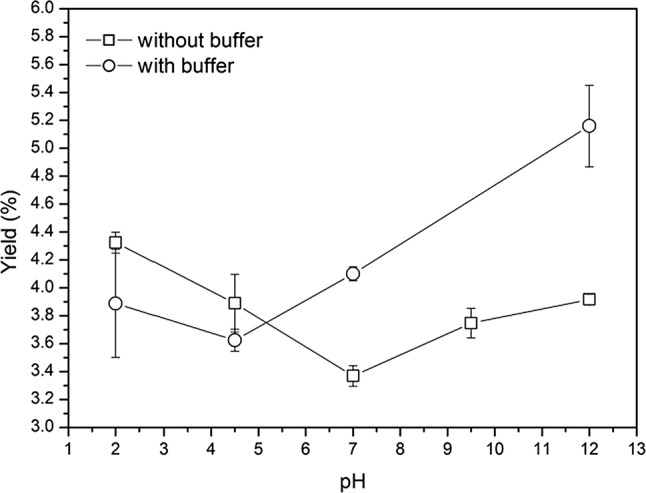
Effect of pH without or with buffer on mucilage yield at cladode–water ratio 1:5. The open squares and open circles represent the effect of pH without buffer (F = 8.91; P = 3.1E−05) or with buffer (F = 12.74; P = 1.9E−06) respectively. The points represent the mean of the yield % at the indicated pH, mean ± SD (n = 9)
Fig. 4.
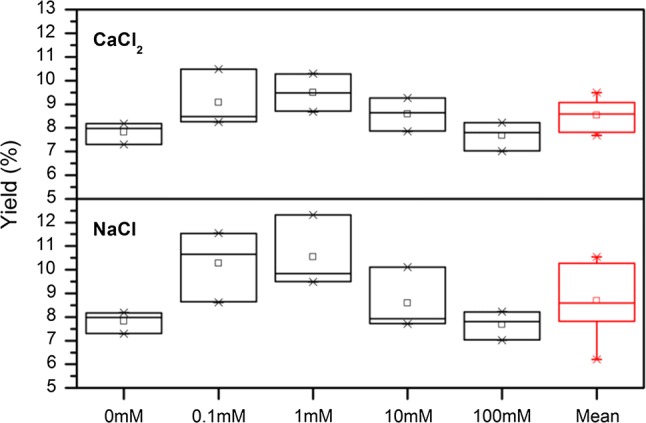
Effect of ionic strength on mucilage yield (%) at cladode water ratio 1:5 using different concentrations of NaCl (F = 11.43; P = 2.8E−6) or CaCl2 (F = 29.14; P = 2.2E−11). It is represented box charts where box is determined by the 25th and 75th percentiles and the whiskers are determined by the 1st and 99th percentiles. The mean values are represented with the squares; while the medians are horizontal lines inside every box
Plackett–Burmann design
A multi-regression analysis following the Plackett–Burmann design was performed by using a polynomial function with the linear terms only, Eq. 2.
| 2 |
where y is the mucilage yield, while Xt, , XpH, XT, Xµ are surrogate variables related to time, dilution, pH, temperature and ionic strength, while Xd1 and Xd2 are dummy factors. The b-coefficients related to the real factors are in the bar chart in Fig. 5, where both the standard deviation and the level of significance are showed. By comparing the b-coefficient values, it is possible to claim that biomass/water, temperature and ionic strength are the factors that have the most significant effect on mucilage yield.
Fig. 5.
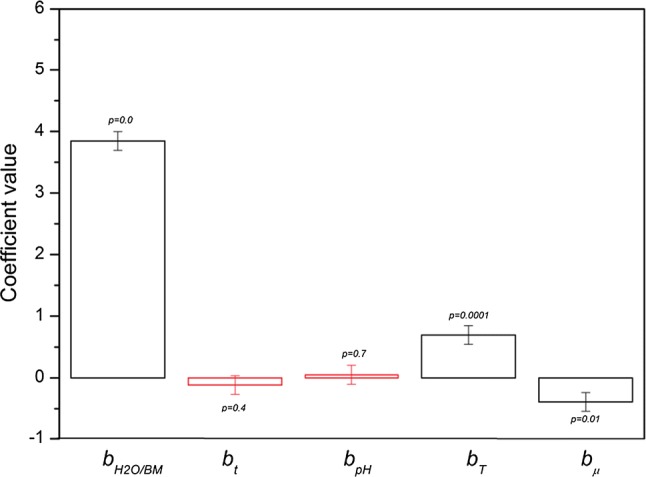
Bar chart related to the values of the b-coefficients of the mathematical motel obtained by following the Plackett–Burmann method, that describes the direct effects of dilution, time, pH, temperature and ionic strength on the extracted total sugar amounts. It is showed the standard deviation and the statistical significance for every coefficient. The bars are not statistically significant
Assisted mucilage extraction
Furthermore Felkai-Haddache et al. (2015) and Bayar et al. (2017a, b) reported an increment in mucilage yield from Opuntia ficus-indica peeled cladodes using microwave or ultrasonic assisted extractions. Studing Arabidopsis as plant model for mucilage extraction from seeds, Zhao et al. (2017) showed the ultrasonic treatment as an effective method to remove, in a very short time (20 s), the adherent hydrophobic mucilage compacted in seed coat and resistant to water extraction. Figure 6 presents the mean mucilage yield (%) after 0, 16 and 24 h (t0, t1, t2, respectively) from cladode–water ratio 1:5 comparing conventional, ultrasound or microwave assisted extraction methods. Significant differences were observed among the treatments and time of extraction. At t0 the microwave treatment resulted to be more efficient than the ultrasonic one, showing an increment in percentage yield of +83% compared to the control (2.2% and 1.2, respectively). However, after 16 h of extraction the mucilage yield (%), quadrupled in ultrasound-treated material (7.5%), remained at lower values in the other cases (about 5% after 24 h of extraction). Regarding the quality of extraction, we observed a loss of viscosity in extract from microwave instead of ultrasonic assisted extraction process. According to our results, MAE provided more proteins (0.19 ± 0.01 mg/mL) and uronic acids (0.19 ± 0.05 mg/mL) contents compared to UAE (0.01 ± 0.01 mg/mL; and 0.01 ± 0.01 mg/mL) or conventional methods (0.006 ± 0.01 mg/mL and 0.06 ± 0.01 mg/mL). Although our preliminary results showed UAE treatment to be more effective allowing the best of mucilage recovery in a shorter processing time, MAE provided more proteins and uronic acids than conventional and UAE extractions. These data are in agreement with Felkai-Haddache et al. (2015), who also reported the highest molecular weight in the polysaccharides extracted from MAE. In our experiments, we observed a loss of viscosity of mucilage extracted with microwave, as reported by Zheng et al. (2011), an excessive time-power exposure under the microwave fields leads to possible degradation of polysaccharide molecules. Thus, additional studies on extraction pre-treatments should be undertaken in order to depict the most appropriate conditions for the highest yield as well as for enhancing rheological mucilage properties depending on its final use.
Fig. 6.
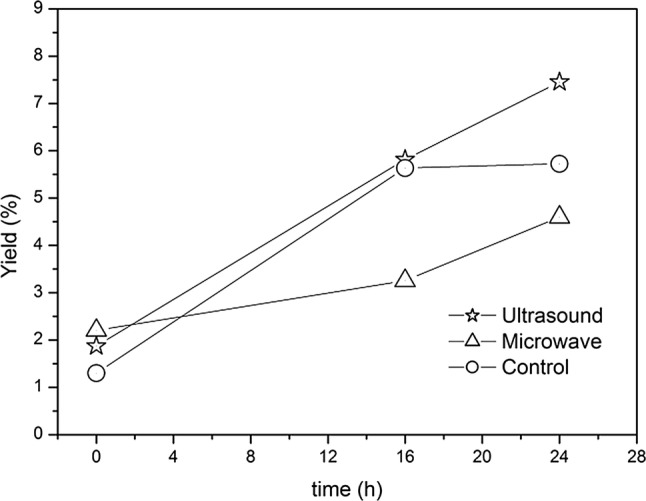
Mucilage yield (%) after 0, 16 and 24 h from cladode–water ratio 1:5 comparing conventional extraction and ultrasound or microwave pretreatment at t0. The figure displays mean values and standard errors from three replications (F = 132,288.8; P = 4.06E19)
Conclusion
Within a multi-disciplinary approach, the present work elucidated and optimized the water technological process to extract mucilage from cladodes. We propose a multivariate model to predict mucilage recovery at different levels of biomass/water ratio/extraction time, and we discussed a statistical methodology to identify the critical factors affecting the extraction procedure. Further studies on green assisted extraction tools and their effects in terms of quality of extracts are required in order to obtain high added value co-products. In particular research activities concerning the nutritional, functional and health benefits are recommended to make this by-product a potential thickening and stabilizing agent for food industry. Finally mucilage content could differ as a result of cultivar/genotypes, stage of development, geographical growth area; these data should be thorough as a part of a green strategy for a large scale utilization of Opuntia ficus indica agro-products that yields a range of target products from a given biomass.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
With the contribution of the Ministry of Foreign Affairs and International Cooperation, Directorate-General for the promotion of the Country System—Scientific and Technological Bilateral Cooperation Project Italy—Mexico. This scientific work is dedicated to our colleague and friend Tiziana Coccioletti, who died prematurely. Her enthusiasm and technical skills have enhanced and enriched the team work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allegra A, Sortino G, Inglese P, Settanni L, Todaro A, Gallotta A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of Dottato fig (Ficus carica L.) fruits. Food Packag Shelf Life. 2017;12:135–141. doi: 10.1016/j.fpsl.2017.04.010. [DOI] [Google Scholar]
- Analytical Methods Committee AMCTB Experimental design and optimisation (4): Plackett–Burman designs. Anal Methods. 2013;5:1901–1903. doi: 10.1039/c3ay90020g. [DOI] [PubMed] [Google Scholar]
- Bayar N, Bouallegue T, Achour M, Kriaa M, Bougatef A, Kammoun R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: optimization of experimental conditions and evaluation of chemical and functional proprieties. Food Chem. 2017;235:275–282. doi: 10.1016/j.foodchem.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Bayar N, Friji M, Kammoun R. Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chem. 2017;241:127–134. doi: 10.1016/j.foodchem.2017.08.051. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Cárdenas A, Goycoolea FM, Rinaudo M. On the gelling behavior of nopal (Opuntia ficus indica) low methoxyl pectin. Carbohydr Polym. 2008;73:212–222. doi: 10.1016/j.carbpol.2007.11.017. [DOI] [Google Scholar]
- Contreras-Padilla M, Rodriguez-Garcia M, Gutiérrez-Cortez E, Valderrama-Bravo MC, Rojas-Molina I, Rivera-Muñoz E. Physicochemical and rheological characterization of Opuntia ficus indica mucilage at three different maturity stages of cladode. Eur Polym J. 2016;78:226–234. doi: 10.1016/j.eurpolymj.2016.03.024. [DOI] [Google Scholar]
- Corrales-García J, Peña-Valdivia CB, Razo-Martínez Y, Sánchez-Hernández M. Acidity changes and pH-buffering capacity of nopalitos (Opuntia spp.) Postharvest Biol Technol. 2004;32:169–174. doi: 10.1016/j.postharvbio.2003.11.008. [DOI] [Google Scholar]
- Cushman JC, Davis SC, Yang X, Borland AM. Development and use of bioenergy feedstocks for semi-arid and arid lands. J Exp Bot. 2015;66(14):4177–4193. doi: 10.1093/jxb/erv087. [DOI] [PubMed] [Google Scholar]
- De Santiago E, Domínguez-Fernández M, Cid C, De Peña MP. Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia ficus-indica) Food Chem. 2018;240:1055–1062. doi: 10.1016/j.foodchem.2017.08.039. [DOI] [PubMed] [Google Scholar]
- Del-Valle V, Hernández-Muñoz P, Guarda A, Galotto MJ. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005;91(4):751–756. doi: 10.1016/j.foodchem.2004.07.002. [DOI] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Felkai-Haddache L, Remini H, Dulong V, Mamou-Belhabib K, Luc Picton, Madani K, Rihouey C. Conventional and microwave-assisted extraction of mucilage from Opuntia ficus-indica cladodes: physic-chemical and rheological properties. Food Bioprocess Technol. 2015;9(3):481–492. doi: 10.1007/s11947-015-1640-7. [DOI] [Google Scholar]
- Frati-Munari AC, Fernández-Harp JA, de la Riva H, Ariza-Andraca R, del Carmen-Torres M. Effect of nopal (Opuntia sp.) on serum lipids, glycemia and body weight. Arch Invest Med (Mex) 1983;14(2):117–125. [PubMed] [Google Scholar]
- Glibowski P, Wasko A. Effect of thermochemical treatment on the structure of inulin and its gelling properties. Int J Food Sci Technol. 2008;43:2075–2082. doi: 10.1111/j.1365-2621.2008.01825.x. [DOI] [Google Scholar]
- Griffith MP. The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): new molecular evidence. Am J Bot. 2004;91(11):1915–1921. doi: 10.3732/ajb.91.11.1915. [DOI] [PubMed] [Google Scholar]
- Han W, Meng Y, Hu C, Dong G, Qu Y, Deng H, Guo Y. Mathematical model of Ca2+ concentration, pH, pectin concentration and soluble solids (sucrose) on the gelation of low methoxyl pectin. Food Hydrocoll. 2017;66:37–48. doi: 10.1016/j.foodhyd.2016.12.011. [DOI] [Google Scholar]
- Lefsih K, Delattre C, Pierre G, Michaud P, Aminabhavi TM, Dahmoune F, Madani K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int J Biol Macromol. 2016;82:645–652. doi: 10.1016/j.ijbiomac.2015.10.046. [DOI] [PubMed] [Google Scholar]
- León-Martínez FM, Méndez-Lagunas LL, Rodríguez-Ramírez J. Spray drying of nopal mucilage (Opuntia ficus-indica): effects on powder properties and characterization. Carbohydr Polym. 2010;81(4):864–870. doi: 10.1016/j.carbpol.2010.03.061. [DOI] [Google Scholar]
- Lira-Ortiz AL, Reséndiz-Vega F, Ríos-Leal E, Contreras-Esquivel JC, Chavarría-Hernández N, Vargas-Torres A, Rodríguez-Hernández AI. Pectins from waste of prickly pear fruits (Opuntia albicarpa Scheinvar Reyna): chemical and rheological properties. Food Hydrocoll. 2014;37:93–99. doi: 10.1016/j.foodhyd.2013.10.018. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Medina-Torres L, Brito-De La Fuente E, Torrestiana-Sánchez B, Katthain R. Rheological properties of the mucilage gum (Opuntia ficus indica) Food Hydr. 2000;14:417–424. doi: 10.1016/S0268-005X(00)00015-1. [DOI] [Google Scholar]
- Ngouémazong DE, Tengweh FF, Fraeye I, Duvetter T, Cardinaels R, Van Loey A, Moldenaers P, Hendrickx M. Effect of de-methylesterification on network development and nature of Ca2+-pectin gels: towards understanding structure-function relations of pectin. Food Hydrocoll. 2012;26(1):89–98. doi: 10.1016/j.foodhyd.2011.04.002. [DOI] [Google Scholar]
- Retamal N, Duran JM, Fernandez J. Ethanol production by fermentation of fruits and cladodes of prickly pear cactus [Opuntia ficus-indica (L.) Miller] J Sci Food Agric. 1987;40:213–218. doi: 10.1002/jsfa.2740400304. [DOI] [Google Scholar]
- Rodriguez-Felix A, Cantwell M. Developmental changes in composition and quality of prickly perar cactus cladodes (nopalitos) Plant Food Hum Nutr. 1988;38:83–93. doi: 10.1007/BF01092314. [DOI] [PubMed] [Google Scholar]
- Saenz CH. Processing technologies: an alternative for cactus pear (Opuntia spp.) fruits and cladodes. J Arid Environ. 2000;46:209–225. doi: 10.1006/jare.2000.0676. [DOI] [Google Scholar]
- Sáenz CH. Cactus pear fruits and cladodes: a sources of functional components for foods. Acta Hort. 2002;581:253–263. doi: 10.17660/ActaHortic.2002.581.28. [DOI] [Google Scholar]
- Sáenz C, Sepúlveda E, Matsuhiro B. Opuntia spp. Mucilage’s: a functional component with industrial perspectives. J Arid Environ. 2004;57(3):275–290. doi: 10.1016/S0140-1963(03)00106-X. [DOI] [Google Scholar]
- Sepúlveda E, Sáenz C, Aliaga E, Aceituno C. Extraction and characterization of mucilage in Opuntia spp. J Arid Environ. 2007;68:534–545. doi: 10.1016/j.jaridenv.2006.08.001. [DOI] [Google Scholar]
- Stintzing FC, Carle R. Cactus stems (Opuntia spp.): a review on their chemistry, technology, and uses. Mol Nutr Food Res. 2005;49:175–194. doi: 10.1002/mnfr.200400071. [DOI] [PubMed] [Google Scholar]
- Trachtenberg SH, Mayer AM. Composition and properties of Opuntia ficus indica mucilage. Phytochemistry. 1981;20(12):2665–2668. doi: 10.1016/0031-9422(81)85263-6. [DOI] [Google Scholar]
- Ventura-Aguilar RI, Bosquez-Molina E, Bautista-Baños S, Rivera-Cabrera F. Cactus stem (Opuntia ficus-indica Mill): anatomy, physiology and chemical composition with emphasis on its biofunctional properties. J Sci Food Agric. 2017;97(15):5065–5073. doi: 10.1002/jsfa.8493. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Tsubaki S, Teramoto Y, Azuma J. Optimization of microwave-assisted extraction of carbohydrates from industrial waste of corn starch production using response surface methodology. Bioresour Technol. 2010;101(20):7820–7826. doi: 10.1016/j.biortech.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Zhao X, Quiao L, Wu AM. Effective extraction of Arabidopsis adherent seed mucilage by ultrasonic treatment. Sci Rep. 2017;7:40672. doi: 10.1038/srep40672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Yin F, Liu C, Xu X. Effects of process parameter of microwave assisted extraction (MAE) on polysaccharides yield from pumpkin. J Northeast Agric Univ. 2011;18(2):79–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


