Abstract
Introduction
The aim of this study was to investigate the rates of lobar atrophy in the asymptomatic microtubule-associated protein tau (MAPT) mutation carriers.
Methods
MAPT mutation carriers (n = 14; 10 asymptomatic, 4 converters from asymptomatic to symptomatic) and noncarriers (n = 13) underwent structural magnetic resonance imaging and were followed annually with a median of 9.2 years. Longitudinal changes in lobar atrophy were analyzed using the tensor-based morphometry with symmetric normalization algorithm.
Results
The rate of temporal lobe atrophy in asymptomatic MAPT mutation carriers was faster than that in noncarriers. Although the greatest rate of atrophy was observed in the temporal lobe in converters, they also had increased atrophy rates in the frontal and parietal lobes compared to noncarriers.
Discussion
Accelerated decline in temporal lobe volume occurs in asymptomatic MAPT mutation carriers followed by the frontal and parietal lobe in those who have become symptomatic. The findings have implications for monitoring the progression of neurodegeneration during clinical trials in asymptomatic MAPT mutation carriers.
Keywords: Frontotemporal dementia, Magnetic resonance image, MAPT, Asymptomatic, Longitudinal
Highlights
-
•
The trajectory change of brain atrophy on longitudinal magnetic resonance imaging collected over 10 years was investigated in asymptomatic microtubule-associated protein tau (MAPT) mutation carriers.
-
•
A sequential pattern of regional cortical atrophy rates involving the temporal lobe cortex early on, followed by the frontal and parietal lobe cortices in MAPT mutation carriers as they transition from asymptomatic stage to symptomatic stage, was described.
-
•
Our findings support the clinical utility of using longitudinal regional volumes for monitoring the neurodegenerative disease progression in MAPT mutation carriers starting from the asymptomatic stage.
1. Introduction
Frontotemporal lobar degeneration (FTLD) is a progressive clinical syndrome with several possible etiologies and with heterogeneous clinical features, including behavioral and language disorders, executive dysfunction, and impaired social cognition, while patients can also develop features of motor neuron disease, progressive supranuclear palsy or corticobasal syndrome [1]. About 30%-50% FTLD patients have an autosomal dominant pattern of inheritance [2], with many showing mutations in the microtubule-associated protein tau (MAPT) gene [3]. Mutations in the MAPT gene result in filamentous accumulation of hyperphosphorylated tau in neurons and glia leading to neurodegeneration, atrophy, white matter microstructure alterations, and metabolic changes years before the onset of clinical symptoms [3], [4], [5], [6], [7]. Families characterized by the presence of MAPT mutations can be investigated for early neurodegenerative changes and identifying biomarkers for tracking disease progression during potential disease-modifying therapies.
Structural magnetic resonance imaging (MRI) has captured disease-specific neurodegeneration in patients with mild cognitive impairment (MCI) [8], [9], Alzheimer's disease [10], [11], Lewy body dementia [12], and in familial and sporadic FTLD [5], [13], [14], [15], [16]. Some cross-sectional studies have suggested that cortical degeneration can be detected with MRI years before symptom onset in asymptomatic MAPT mutation carriers [5], [14], [17], [18], [19], but others have not shown any significant cortical volumetric differences between MAPT mutation carriers and controls [7], [20]. Recently, two longitudinal studies from a cohort of asymptomatic MAPT mutation carriers have found atrophy in the hippocampus but no cortical atrophy was reported in longitudinal analysis during up to 4 years of follow-up [21], [22]. Up to date, little is known about the trajectory of progression of atrophy on longitudinal MRI in asymptomatic MAPT mutation carriers.
In this study, we investigated the rates and trajectories of lobar atrophy on longitudinal MRI from asymptomatic stage to symptomatic stage in MAPT mutation carriers.
2. Methods
2.1. Participants
Participants in this study were enrolled in the Mayo Clinic Alzheimer's Disease Research Center and the Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS) studies at the Mayo Clinic site. LEFFTDS is a multisite study investigating the biomarkers of disease progression in familial FTLD mutation carriers. The present study included participants who screened positive for a mutation in MAPT and had been followed up between August 2006 and November 2018 at Mayo Clinic. Participants with MAPT mutations who had no clinical symptoms at baseline and underwent at least two follow-up evaluations were included (n = 14). Noncarriers from healthy first-degree relatives of the patients were included as the control group after DNA screening (n = 13). All participants were followed up prospectively with annual clinical examination at the time of MR examination, including a medical history review, mental status examination, a neurological examination by a clinician with FTLD expertise, and a neuropsychological examination. Participants were followed for a median of 9.2 years (range 2.0 to 11.7 years) and had at least 3 MRI scans, with a total of 166 MRI examinations. Of the 14 MAPT mutation carriers, 4 of them, all from a pallido-ponto-nigral degeneration (PPND) family with N279 K mutation [23], transitioned from asymptomatic stage to symptomatic stage during follow-up, which we refer to as converters. All converters were classified as MCI at the time of conversion, with two later developing features of mixed behavioral variant frontotemporal dementia and progressive supranuclear palsy (Richardson's syndrome), one developing behavioral variant frontotemporal dementia with parkinsonism and the other remaining MCI. The median age of symptom onset was 43.5 years with a range of 37–47 years, and the median follow-up interval was 9.7 years (range 8.7–11.7 years) for these four converters (typical for this PPND family). The remaining 10 MAPT mutation carriers (from 5 families) had no cognitive/behavioral/motor changes, which was corroborated by their informants and had an MMSE score of 30 throughout the median follow-up interval of 8.5 years (range 2.0–10.7 years), which we refer to as asymptomatic MAPT mutation carriers. The noncarriers did not differ from the asymptomatic MAPT mutation carriers and the converters on MMSE and DRS at baseline. The behavioral neurologist (B.F.B) who examined all the participants was blinded to the mutation status of all asymptomatic individuals regardless of mutation carrier status. None of the participants had structural lesions that could cause cognitive impairment or dementia, such as cortical infarction, subdural hematoma, or tumor, or had concurrent illness that would interfere with cognitive function other than FTLD on baseline and follow-up examinations.
All converters described in this report have undergone clinical genetic testing and therefore they and their proxies, as well as the examining neurologist, are aware of their mutation status. Most of the asymptomatic mutation carriers included in this report have not undergone clinical genetic testing and are therefore not aware of their mutation status. Details regarding each person have intentionally been excluded to maintain confidentiality.
Informed consent was obtained from all participants for participation in the studies, and after the three converters developed dementia, written assent was also obtained by their proxies. All procedures were approved by the Mayo Institutional Review Board.
2.2. MRI acquisition and process
All participants had volumetric MRI performed annually at 3T using an 8-channel phased array head coil (GE Healthcare, Milwaukee, WI) with a standard protocol. A 3D high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) was performed at each time point using following parameters: repetition time/echo time/inversion time = 2300/3/900 ms, flip angle 8°, in-plane resolution of 1.0 mm.
2.3. Region-level analysis
Baseline and annual changes in cortical volume were estimated for each participant. For the region-level analysis, we applied a fully automated in-house developed image-processing pipeline, using tensor-based morphometry with symmetric normalization (TBM-SyN) to compute the baseline lobar cortical volumes and changes over time in each participant [24]. For each participant, all their T1 structural MRI scans were iteratively coregistered to a mean image using a 6 degrees-of-freedom (6-DOF), followed by 9-DOF rigid body registration using SPM coregistration software [25] (www.fil.ion.ucl.ac.uk/spm). Then image intensity histograms across each participant's time series of images were normalized using an in-house developed differential bias correction algorithm [26]. The SyN diffeomorphic registration algorithm from ANTs software [26] was used to compute deformations between each pair of images, running the deformations explicitly in each direction, producing the Jacobian determinant images, and the “annualized” log of the Jacobian determinant from the deformation in each direction, to be parcellated into lobar regions of interest later. The voxel values of the Jacobian determinant image represent the expansion or contraction of each voxel over time, and those of the annualized log Jacobian determinant image can be thought of as analogous to an annualized percent change at each voxel. The SyN deformations were applied in each direction, respectively to the original bias corrected late and early images, to get the early image warped to the late, and the late image warped to the early image, and average them with their respective originals, resulting in a “synthetic late image” and a “synthetic early image.” We then segmented the synthetic early and late images into gray matter, white matter, and cerebrospinal fluid classes using SPM with our in-house developed template [27]. The discrete cosine deformations from the unified segmentation step were used to transform the in-house modified AAL atlas that included the frontal, temporal, parietal, and occipital region of interest labels from template space to the synthetic early and late image spaces, respectively [28].
2.4. Voxel-level analysis
To assess the rates of atrophy at the voxel level, we used only the first and last images for each participant and ran the TBM-SyN steps as detailed previously to produce an image of the annualized log of the Jacobian determinant over the largest possible time interval for each participant. These images were smoothed with a 6 mm full-width at half maximum Gaussian smoothing kernel.
2.5. Genetic analysis
Participants recruited into this study are part of known MAPT families. For each of the participants, we specifically sequence the exon harboring the known MAPT mutation observed in that family using previously published protocol [29]. Sequencing is also performed to detect variants in the following genes GRN, C9ORF72, TARDBP, PSEN1, PSEN2, and APP according to the LEFFTDS protocol. PCR amplicons were purified using the Multiscreen system (Millipore, Billerica, MA) and then sequenced in both directions using Big Dye chemistry following the manufacturer's protocol (Applied Biosystems, Foster City, CA). Sequence products were purified using the Montage system (Millipore) before being run on an Applied Biosystem 3730 DNA Analyzer. Sequence data were analyzed using either SeqScape (Applied Biosystem) or Sequencher software (Gene Codes, Ann Arbor, MI).
2.6. Statistical analysis
Baseline characteristics of converters with MAPT mutations were described with means, standard deviations, counts, and proportions. Baseline characteristics were compared among converters, asymptomatic MAPT mutation carriers and noncarriers using generalized linear mixed models (categorical variables), or linear mixed models (continuous variables), adjusting for age where appropriate and accounting for the family structure using random effects. Tukey contrasts were subsequently used for pairwise comparisons.
Assessments of voxel-level results were corrected for multiple comparisons by requiring the corrected false discovery rate (FDR) to be < 0.05 and the uncorrected P < .001.
We modeled the log of lobar cortical volumes to estimate rate of volume loss expressed as percentage per year using linear mixed-effect models with random intercepts to account for within-subject repeated-measures correlations nested in within-family correlations. This allowed for dependence in the repeated measures per subject and also dependence in family members. Families were assumed to be independent from each other. Because of the sample size restrictions, we were only able to use random intercepts in these models. Inclusion of random slopes and flex points would result in gross overfitting of the models and nonconvergence. We coded group (noncarriers, asymptomatic, or converter) using dummy variables with noncarrier as the reference and included two-way interactions for group × age at MRI scan. Because we were interested in specific individual lobar cortical volume change hypotheses and not a universal null hypothesis and did not want to inflate the probability of a type II error, we did not adjust for multiple comparisons in these analyses.
3. Results
The baseline characteristics of the converters, asymptomatic MAPT mutation carriers, and noncarriers are shown in Table 1. There were no differences between the 3 groups at baseline based on sex, education, age at initial MRI scan, and cognitive scores. The converters had more MRI scans compared to asymptomatic MAPT mutation carriers (P < .001) and noncarriers (P < .001), but there were no differences in follow-up time range among the 3 groups. At baseline, temporal and frontal lobe volumes were lower in converters compared to asymptomatic MAPT mutation carriers (P < .001) and noncarriers (P < .001). Similarly, parietal lobe volumes were also lower in converters compared to asymptomatic MAPT mutation carriers (P < .001) and noncarriers (P < .001). No significant difference in lobar volume was observed between asymptomatic MAPT mutation carriers and noncarriers at baseline.
Table 1.
Baseline characteristics of participants and lobar cortical volumes
| Noncarrier (N = 13) | Asymptomatic (N = 10) | Converter (N = 4) | P value | |
|---|---|---|---|---|
| No. female (%) | 7 (54) | 4 (40) | 2 (50) | .95 |
| Education, year | 16 (2) | 17 (3) | 15 (3) | .34 |
| Age at MRI scan, yr | 36 (12) | 31 (5) | 39 (3) | .21 |
| MMSE | 30 (1) | 30 (0) | 30 (0) | .17 |
| DRS total MOANS | 12 (2) | 11 (2) | 12 (1) | .90 |
| AVLT delay recalled MOANS | 12 (4) | 11 (2) | 14 (1) | .82 |
| No. of MRI scans | 5 (2) | 5 (2) | 11 (2) | <.001∗ |
| Follow-up, yr | 8 (3) | 7 (3) | 10 (1) | .49 |
| Cortical volume at baseline (cm3) | ||||
| Frontal | 138 (8) | 142 (18) | 106 (11) | .001∗ |
| Temporal | 117 (7) | 126 (12) | 85 (7) | <.001∗ |
| Parietal | 70 (7) | 71 (5) | 53 (2) | <.001∗ |
| Occipital | 64 (8) | 65 (6) | 53 (2) | .09 |
NOTE. Data shown are n (%) or mean (SD).
P values are from linear mixed models adjusting for age at MRI, also accounting for within-family correlations, followed by Tukey contrasts for pairwise comparisons.
Abbreviations: MRI, magnetic resonance imaging; MMSE, Mini–Mental State Examination; DRS, Dementia Rating Scale; MOANS, Mayo's Older Americans Normative Studies; AVLT, Auditory-Verbal Learning Test.
Converter is statistically different from other groups.
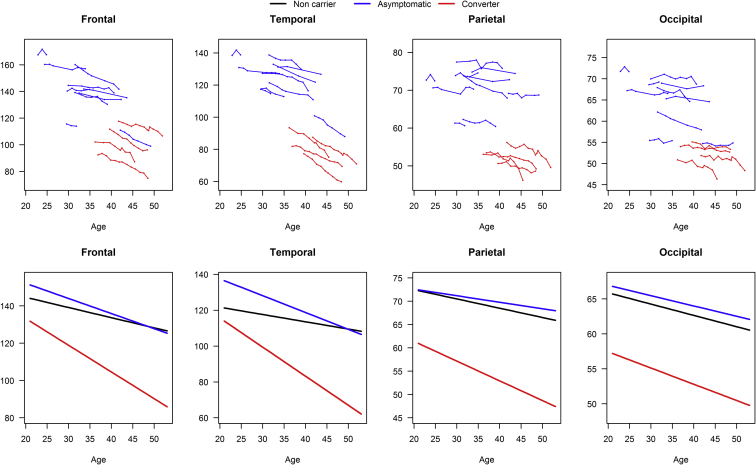
Fig. 1 shows the trajectories of change in lobar cortical volumes. The results of linear mixed-effects models are summarized in Table 2. Group × age at MRI scan interactions indicated that the converters had greater estimated annual rate of cortical atrophy in temporal (P < .001), frontal (P < .01), and parietal (P < .05) lobes compared to noncarriers. Similarly, the asymptomatic MAPT mutation carriers also had higher estimated annual rate of cortical atrophy in the temporal lobe than noncarriers (P < .01), but not in the frontal and parietal lobes. There was no difference in the estimated annual rate of occipital lobe cortical volume change among the 3 groups.
Fig. 1.
Lobar volume plotted against age at MRI. Data point trajectories for individual MAPT mutation carriers (top panel) and the predicted volumes (cm3) from the mixed models for each group (bottom panel) are shown. The blue line represents for the asymptomatic group who remained asymptomatic during the course of the study; the red line represents for the asymptomatic MAPT mutation carriers who converted; the black line represents the noncarriers. The noncarriers are not plotted in the top panel to show the data from the MAPT mutation carriers with clarity. Abbreviations: MAPT, microtubule-associated protein tau; MRI, magnetic resonance imaging.
Table 2.
Predicted annual change in lobar volumes from mixed-effects models with noncarriers as the reference group
| Frontal |
Temporal |
Parietal |
Occipital |
|
|---|---|---|---|---|
| Estimates (95% CI) | Estimates (95% CI) | Estimates (95% CI) | Estimates (95% CI) | |
| Intercept | 155.5 (144.3, 166.8)∗ | 129.8 (121.8, 137.8)∗ | 76.5 (71.5, 81.5)∗ | 69.1 (65.0, 73.2)∗ |
| Age at MRI scan | −0.55 (−0.82, −0.27)∗ | −0.41 (−0.62, −0.19)∗ | −0.20 (−0.29, −0.10)∗ | −0.16 (−0.24, −0.09)∗ |
| Asymptomatic | 12.8 (−5.23, 30.7) | 26.4 (13.6, 39.2)∗ | −1.10 (−8.62, 6.42) | 0.77 (−5.74, 7.29) |
| Converter | 6.37 (−17.0, 29.8) | 18.2 (1.66, 34.8)† | −6.63 (−17.0, 3.75) | −7.03 (−15.6, 1.54) |
| Asymptomatic × age at MRI scan | −0.26 (−0.69, 0.16) | −0.53 (−0.86, −0.20)‡ | 0.06 (−0.09, 0.21) | 0.01 (−0.10, 0.13) |
| Converter × age at MRI scan | −0.89 (−1.41, −0.36)‡ | −1.21 (−1.63, −0.80)∗ | −0.22 (−0.40, −0.05)† | −0.07 (−0.21, 0.07) |
NOTE. The noncarrier group regression equations contain the intercept and age at MRI terms. The asymptotic group equations add the “asymptotic” and “asymptotic × age at MRI scan” terms to the equation. The converter group equations instead add the “converter” and “converter × age at MRI scan” terms to the equation. These produce the predicted lines in Fig. 1. Because of the interactions in the models, age and group coefficients are not directly interpretable.
Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging.
P < .001.
P < .05.
P < .01.
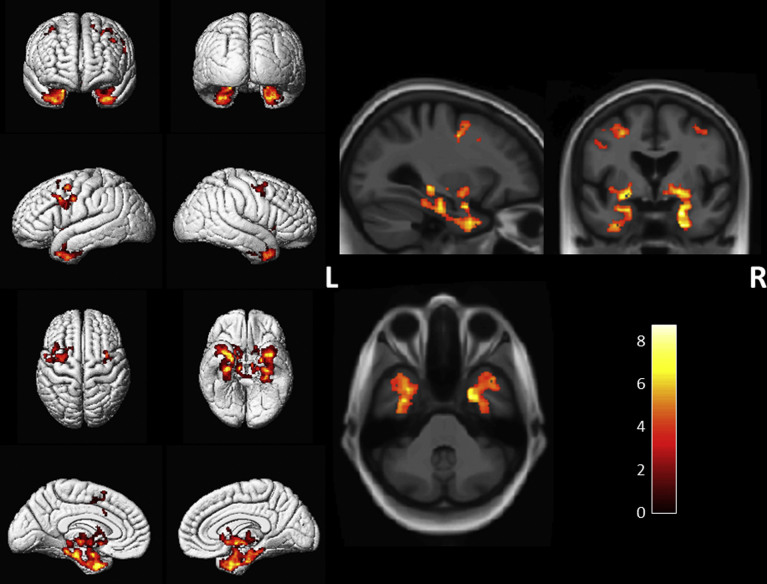
Voxel-based analysis showed that the annualized rate of atrophy was localized to the bilateral anterior and medial temporal lobe, inferior frontal lobe, limbic cortex, cingulate gyrus, precuneus, precentral gyrus, as well as the supplementary motor areas in all MAPT mutation carriers compared to noncarriers (corrected FDR <0.05) (Fig. 2). Because the MAPT mutation carriers also included four converters with N279 K mutation carriers from the same PPND family, we performed voxel-based analysis after removing the converters. Greater atrophy rates were found in the bilateral anterior and medial temporal lobe and orbitofrontal cortex in asymptomatic MAPT mutation carriers compared to noncarriers excluding the four converters (P < .001, not corrected for multiple comparisons).
Fig. 2.
Voxel-based analysis showed greater annualized rates of cortical atrophy in all MAPT mutation carriers compared to noncarriers. The results are shown at P < .05 after using the false discovery rate correction for multiple comparisons. Abbreviation: MAPT, microtubule-associated protein tau.
4. Discussion
In this longitudinal study using 166 serial MRI scans, we report regional rates of atrophy in MAPT mutation carriers from the asymptomatic stage, four of whom became symptomatic during follow-up. In converters who became symptomatic during follow-up, we found that cortical atrophy was already present at baseline in the temporal, frontal, and parietal lobes, although they were asymptomatic at that time. On the other hand, no differences in lobar volumes were observed in asymptomatic MAPT mutation carriers at baseline, who remained asymptomatic during follow-up compared to noncarriers. Our longitudinal data showed that the trajectories of lobar cortical atrophy were fastest in the temporal lobe, followed by frontal and parietal lobes in converters compared to noncarriers as they aged. The asymptomatic MAPT mutation carriers also showed a faster rate of temporal lobe cortical atrophy than noncarriers. Taken together, our cross-sectional and longitudinal findings demonstrated a sequential pattern of lobar atrophy in MAPT mutation carriers throughout the disease course, indicating that the temporal lobe cortical volume began declining during the asymptomatic stage, with an acceleration of the rates of atrophy during development of symptoms, followed by the involvement of the frontal and parietal lobes and sparing of the occipital lobe.
The temporal lobe cortical volumes declined in MAPT mutation carriers as early as the asymptomatic stage particularly in the bilateral medial temporal lobe on voxel-based analysis. This pattern is consistent with findings from previous cross-sectional MRI studies that demonstrate most prominent atrophy in the anteromedial temporal lobe and orbitofrontal cortex in asymptomatic MAPT mutation carriers [5], [14], [17]; however, others found no difference between asymptomatic MAPT mutation carriers and controls [7], [20]. Gray matter decline in the hippocampus in asymptomatic MAPT mutation carriers was found in a recent longitudinal voxel-based morphometry study during 2 years of follow-up [21]. Another study reported extensive gray matter volume loss mainly in temporal and frontal lobes in a converter group combining 5 MAPT mutation and 3 GRN mutation carriers but found no longitudinal gray matter volume loss in asymptomatic MAPT and GRN mutation carriers who did not convert compared with noncarriers [22]. In the present study, we found lower baseline temporal lobe cortical volumes in converters, who later became symptomatic during follow-up compared to noncarriers. On the contrary, in the asymptomatic MAPT mutation carriers who remained asymptomatic, the temporal lobe cortical volumes were not different form noncarriers at baseline on cross-sectional analysis. The discordance in the literature on temporal lobe atrophy in asymptomatic MAPT mutation carriers may be due to the differences in the proximity to the age of symptom onset in cross-sectional studies of different asymptomatic MAPT mutation carrier cohorts or the sensitivity of the image analysis algorithm to relatively small amount of change in gray matter volume.
Early and accelerated rates of atrophy in the temporal lobe is a consistent finding in symptomatic carriers of MAPT mutations [13], [16], suggesting that brain structural changes continue to occur in the same set of regions that are affected early in the disease. In keeping with that, the greatest atrophy rates were observed in the temporal lobe cortex in asymptomatic MAPT mutation carriers compared to noncarriers, which accelerated in those who became symptomatic. Given the increasing number of studies demonstrating early temporal atrophy in MAPT mutation carriers [13], [14], [15], [16], our findings confirm and extend those reports and demonstrate early involvement of temporal lobe cortical atrophy in a longitudinal study design.
In the present study, the finding that frontal lobes especially the orbitofrontal lobe cortex present starting from the asymptomatic stage only in converters compared to noncarriers and became more atrophic as the disease progressed is consistent with atrophy patterns reported in previous voxel-based morphometry studies of familial FTD [5]. The progressive involvement of the frontal lobes followed the involvement of the temporal lobes, and the motor cortices, including the primary and supplementary motor cortices, were also affected in the asymptomatic MAPT mutation carriers on voxel-based analysis. However, greater rates of atrophy in the primary and supplementary motor cortices in MAPT mutation carriers compared to noncarriers were no longer statistically significant after removing the converters with MAPT N279K mutations who became symptomatic during follow-up from the voxel-based analysis. Patients with MAPT N279K mutations have prominent motor symptoms early in the disease process [23], [30], [31], and therefore, involvement of the primary and supplementary motor cortices may be expected in this group, influencing the results of the voxel-based analysis [32], [33], [34], [35], [36]. In symptomatic MAPT mutation carriers, the frontal lobes were reported to have the fastest rates of atrophy, followed by the temporal and parietal lobes [37]. Taken together with our findings, the temporal progression of lobar atrophy may follow a sequential pattern in MAPT mutation carriers. Temporal lobe cortical atrophy occurs in the earliest stages during the asymptomatic stage. After the patients become symptomatic, the rates of atrophy may slow down in the temporal lobes but accelerates in the frontal lobes during the later stages of disease progression.
Our data showed that parietal lobe cortical atrophy, especially in the precuneus, was present starting from the asymptomatic stage only in converters compared to noncarriers, but with relatively slower rates of atrophy than the temporal and frontal lobes. However, the rates of cortical atrophy in the parietal lobe were not different from asymptomatic MAPT mutation carriers compared to noncarriers. Thus, the parietal lobe cortical atrophy occurred later than temporal lobe involvement, but also years before symptom onset when asymptomatic MAPT mutation carriers approaching closely to the symptom onset age, and progressed slower than temporal and frontal lobe cortical atrophy. This pattern of parietal cortical lobe atrophy is consistent with findings reported in symptomatic MAPT mutation carriers [15]. The early involvement of parietal lobe was also found in an 1H MR spectroscopy study that demonstrated early metabolite abnormalities in the posterior cingulate and precuneus cortex in asymptomatic MAPT mutation carriers [6]. Similarly, mild glucose hypometabolism in the parietal lobes on PET was found in N279K MAPT mutation carriers from the same PPND family, along with ballooned neurons and abundant tau inclusions in the parietal cortex at autopsy [33]. The early involvement of the parietal lobe cortex, particularly the paralimbic regions such as the precuneus, but a relatively slow progression of atrophy in this region may be associated with the gradual involvement of limbic pathways that connect the anteromedial temporal lobe to the medial parietal cortex in patients with MAPT mutations who are approaching the symptomatic stage [38], [39].
One strength of our study was the large number of serial MRIs collected over 10 years and tracking of the disease progression from asymptomatic to symptomatic stage; however, the relatively small number of participants in each group was still a limitation. Although one of our previous cross-sectional studies had shown similar patterns atrophy across different submutation types in MAPT mutation carriers [16], there may be variability in the rates of atrophy within the asymptomatic MAPT mutation carriers across different specific mutations. Subtypes of MAPT mutation have been shown to cause different types of tauopathies. These are mutations inside exon 10 (i.e., N279K, P301L, and S305N) that tend to form four tandem microtubule-binding domain repeat (4R-tau) pathology relative to 3 repeat (3R tau) pathology [40], and mutations outside exon 10 (i.e., V337M and R406W) that tend to form mixed 3 R/4R tau pathology. The 4 converters in the present study all had N279K MAPT mutations, and their trajectories of lobar atrophy, such as the supplemental motor cortex involvement, may be specific to the suspected 4R-tau-associated neurodegeneration in N279K kindreds. Further studies are needed to characterize the trajectories of rates of brain atrophy across the different MAPT mutations.
In summary, our data indicate a sequential pattern of regional cortical atrophy rates involving the temporal lobe cortex early on, followed by the frontal and parietal lobe cortices in MAPT mutation carriers as they transition from asymptomatic stage to symptomatic stage. Our findings support the clinical utility of using longitudinal regional volumes for monitoring the neurodegenerative disease progression in MAPT mutation carriers starting from the asymptomatic stage. Lobar cortical atrophy rates may also have a utility in clinical trials involving agents that potentially modify neurodegenerative disease pathology in asymptomatic MAPT mutation carriers.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations.
-
2.
Interpretation: Our findings provide details on a sequential pattern of regional cortical atrophy rates in microtubule-associated protein tau (MAPT) mutation carriers as they transition from asymptomatic stage to symptomatic stage.
-
3.
Future directions: The manuscript proposes a framework of using longitudinal regional volumes for monitoring the neurodegenerative disease progression in MAPT mutation carriers starting from the asymptomatic stage. Further studies are needed to characterize the trajectories of rates of brain atrophy across the different subtypes of MAPT mutation.
Acknowledgments
The authors extend their appreciation to the staff of all centers and particularly to their patients and their families for their participation in this protocol.
This work is supported by NIH grants U01 AG045390, U54 NS092089, U24 AG021886, U01 AG016976, and R01 AG 40042.
Footnotes
Boeve B. has served as an investigator for clinical trials sponsored by GE Healthcare and Axovant; he receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017); he serves on the Scientific Advisory Board of the Tau Consortium; and he receives research support from NIH, the Mayo Clinic Dorothy, and Harry T. Mangurian Jr. Lewy Body Dementia Program and the Little Family Foundation. Senjem M. owns medical-related stocks in the following companies, unrelated to the current work: Align Technology, Inc., CRISPR Therapeutics, Ionis Pharmaceuticals, Johnson & Johnson, LHC Group, Inc., Medtronic, Inc., Mesa Laboratories, Inc., Natus Medical Inc., and Varex Imaging Corporation; he has owned medical-related stocks within the past three years in the following companies, unrelated to the current work: Gilead Sciences, Inc., Globus Medical Inc., Inovio Biomedical Corp., Oncothyreon, Inc., Parexel International Corporation. Fields J. receives research support from NIH. Forsberg L. receives research support from NIH. Gavrilova R. receives research support from NIH. Graff-Radford J. for special issue on frontotemporal lobar degeneration, receives research support from the NIH. Graff-Radford N. receives royalties from UpToDate, has participated in multicenter therapy studies by sponsored by Biogen, TauRx, AbbVie, Novartis, and Lilly. He receives research support from NIH. Jack C.R. Jr. consults for Eli Lilly and serves on an independent data monitoring board for Roche but he receives no personal compensation from any commercial entity. He receives research support from the NIH. Jones D. receives research support from NIH and the Minnesota Partnership for Biotechnology and Medical Genomics. Knopman D. serves on the DSMB of the DIAN-TU study, is a site PI for clinical trials sponsored by Biogen, Lilly, and the University of Southern California, and is funded by NIH. Kremers K.W. receives research funding from AstraZeneca, Biogen, Roche, DOD, and NIH. Rademakers R. receives research funding from NIH and the Bluefield Project to Cure Frontotemporal Dementia. Boxer A. receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer's Drug Discovery Foundation, and the Alzheimer's Association; he has served as a consultant for Aeton, Abbvie, Alector, Amgen, Arkuda, Ionis, IPierian, Janssen, Merck, Novartis, Samumed, Toyama, and UCB and received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche, and TauRx. Rosen H. has received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from NIH. Wszolek Z. is partially supported by the NIH/NIA (primary) and NIH/NINDS (secondary) 1U01AG045390-01A1, Mayo Clinic Center for Regenerative Medicine, the gift from Carl Edward Bolch, Jr., and Susan Bass Bolch, The Sol Goldman Charitable Trust, and Donald G. and Jodi P. Heeringa, and he is an PI for Mayo Clinic Florida site on therapeutic Abbvie Inc. mediation trials. Kantarci K. serves on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc. and Data Monitoring Boards of Pfizer and Janssen Alzheimer Immunotherapy; she receives research support from the Avid Radiopharmaceuticals, Eli Lilly. She is funded by the Alzheimer's Drug Discovery Foundation and NIH. The other authors have nothing to disclose.
Contributor Information
Kejal Kantarci, Email: kantarci.kejal@mayo.edu.
LEFFTDS Consortium:
Giovanni Coppola, Bradford C. Dickerson, Susan Dickinson, Kelly Faber, Jamie Fong, Tatiana Foroud, Nupur Ghoshal, Jill Goldman MurrayGrossman, HilaryW. Heuer, John Hsiao, Ging-Yuek R. Hsiung, Edward Huey, David J. Irwin, Anna M. Karydas, John Kornak, Joel Kramer, Walter A. Kukull, Diane Lucente, Codrin Lungu, Ian R.A. Mackenzie, Scott McGinnis, Bruce L. Miller, Leonard Petrucelli, Madeline Potter, Eliana M. Ramos, Katherine P. Rankin, Katya Rascovsky, Leslie Shaw, Marg Sutherland, Nadine Tatton, Joanne Taylor, Arthur W. Toga, John Trojanowski, Sandra Weintraub, and Bonnie Wong
References
- 1.Bang J., Spina S., Miller B.L. Frontotemporal dementia. Lancet. 2015;386:1672–1682. doi: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohrer J.D., Warren J.D. Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol. 2011;24:542–549. doi: 10.1097/WCO.0b013e32834cd442. [DOI] [PubMed] [Google Scholar]
- 3.Ingram E.M., Spillantini M.G. Tau gene mutations: dissecting the pathogenesis of FTDP-17. Trends Mol Med. 2002;8:555–562. doi: 10.1016/s1471-4914(02)02440-1. [DOI] [PubMed] [Google Scholar]
- 4.Bunker J.M., Kamath K., Wilson L., Jordan M.A., Feinstein S.C. FTDP-17 mutations compromise the ability of tau to regulate microtubule dynamics in cells. J Biol Chem. 2006;281:11856–11863. doi: 10.1074/jbc.M509420200. [DOI] [PubMed] [Google Scholar]
- 5.Cash D.M., Bocchetta M., Thomas D.L., Dick K.M., van Swieten J.C., Borroni B. Patterns of gray matter atrophy in genetic frontotemporal dementia: results from the GENFI study. Neurobiol Aging. 2018;62:191–196. doi: 10.1016/j.neurobiolaging.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarci K., Boeve B.F., Wszolek Z.K., Rademakers R., Whitwell J.L., Baker M.C. MRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathology. Neurology. 2010;75:771–778. doi: 10.1212/WNL.0b013e3181f073c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dopper E.G., Rombouts S.A., Jiskoot L.C., den Heijer T., de Graaf J.R., de Koning I. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 2014;83:e19–e26. doi: 10.1212/WNL.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 8.Vemuri P., Weigand S.D., Knopman D.S., Kantarci K., Boeve B.F., Petersen R.C. Time-to-event voxel-based techniques to assess regional atrophy associated with MCI risk of progression to AD. Neuroimage. 2011;54:985–991. doi: 10.1016/j.neuroimage.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitwell J.L., Petersen R.C., Negash S., Weigand S.D., Kantarci K., Ivnik R.J. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 2007;64:1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risacher S.L., Anderson W.H., Charil A., Castelluccio P.F., Shcherbinin S., Saykin A.J. Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurology. 2017;89:2176–2186. doi: 10.1212/WNL.0000000000004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cash D.M., Ridgway G.R., Liang Y., Ryan N.S., Kinnunen K.M., Yeatman T. The pattern of atrophy in familial Alzheimer disease: volumetric MRI results from the DIAN study. Neurology. 2013;81:1425–1433. doi: 10.1212/WNL.0b013e3182a841c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray M.E., Ferman T.J., Boeve B.F., Przybelski S.A., Lesnick T.G., Liesinger A.M. MRI and pathology of REM sleep behavior disorder in dementia with Lewy bodies. Neurology. 2013;81:1681–1689. doi: 10.1212/01.wnl.0000435299.57153.f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohrer J.D., Ridgway G.R., Modat M., Ourselin S., Mead S., Fox N.C. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53:1070–1076. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fumagalli G.G., Basilico P., Arighi A., Bocchetta M., Dick K.M., Cash D.M. Distinct patterns of brain atrophy in Genetic Frontotemporal Dementia Initiative (GENFI) cohort revealed by visual rating scales. Alzheimers Res Ther. 2018;10:46. doi: 10.1186/s13195-018-0376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitwell J.L., Jack C.R., Jr., Boeve B.F., Senjem M.L., Baker M., Rademakers R. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology. 2009;72:813–820. doi: 10.1212/01.wnl.0000343851.46573.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitwell J.L., Jack C.R., Jr., Boeve B.F., Senjem M.L., Baker M., Ivnik R.J. Atrophy patterns in IVS10+16, IVS10+3, N279K, S305N, P301L, and V337M MAPT mutations. Neurology. 2009;73:1058–1065. doi: 10.1212/WNL.0b013e3181b9c8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohrer J.D., Nicholas J.M., Cash D.M., van Swieten J., Dopper E., Jiskoot L. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015;14:253–262. doi: 10.1016/S1474-4422(14)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spina S., Farlow M.R., Unverzagt F.W., Kareken D.A., Murrell J.R., Fraser G. The tauopathy associated with mutation +3 in intron 10 of Tau: characterization of the MSTD family. Brain. 2008;131:72–89. doi: 10.1093/brain/awm280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordes M., Wszolek Z., Calne D., Rodnitzky R.L., Pfeiffer R.F. Magnetic resonance imaging studies in rapidly progressive autosomal dominant parkinsonism and dementia with pallido-ponto-nigral degeneration. Neurodegeneration. 1992;1:217–224. doi: 10.1002/ana.410320303. [DOI] [PubMed] [Google Scholar]
- 20.Whitwell J.L., Josephs K.A., Avula R., Tosakulwong N., Weigand S.D., Senjem M.L. Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology. 2011;77:866–874. doi: 10.1212/WNL.0b013e31822c61f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panman J.L., Jiskoot L.C., Bouts M., Meeter L.H.H., van der Ende E.L., Poos J.M. Gray and white matter changes in presymptomatic genetic frontotemporal dementia: a longitudinal MRI study. Neurobiol Aging. 2019;76:115–124. doi: 10.1016/j.neurobiolaging.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Jiskoot L.C., Panman J.L., Meeter L.H., Dopper E.G.P., Donker Kaat L., Franzen S. Longitudinal multimodal MRI as prognostic and diagnostic biomarker in presymptomatic familial frontotemporal dementia. Brain. 2019;142:193–208. doi: 10.1093/brain/awy288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wszolek Z.K., Pfeiffer R.F., Bhatt M.H., Schelper R.L., Cordes M., Snow B.J. Rapidly progressive autosomal dominant parkinsonism and dementia with pallido-ponto-nigral degeneration. Ann Neurol. 1992;32:312–320. doi: 10.1002/ana.410320303. [DOI] [PubMed] [Google Scholar]
- 24.Cash D.M., Frost C., Iheme L.O., Unay D., Kandemir M., Fripp J. Assessing atrophy measurement techniques in dementia: Results from the MIRIAD atrophy challenge. Neuroimage. 2015;123:149–164. doi: 10.1016/j.neuroimage.2015.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vemuri P., Whitwell J.L., Kantarci K., Josephs K.A., Parisi J.E., Shiung M.S. Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage. 2008;42:559–567. doi: 10.1016/j.neuroimage.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 29.Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 30.Wszolek Z.K., Kardon R.H., Wolters E.C., Pfeiffer R.F. Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17): PPND family. A longitudinal videotape demonstration. Mov Disord. 2001;16:756–760. doi: 10.1002/mds.1131. [DOI] [PubMed] [Google Scholar]
- 31.Wszolek Z.K., Tsuboi Y., Uitti R.J., Reed L. Two brothers with frontotemporal dementia and parkinsonism with an N279K mutation of the tau gene. Neurology. 2000;55:1939. doi: 10.1212/wnl.55.12.1939. [DOI] [PubMed] [Google Scholar]
- 32.Arima K., Kowalska A., Hasegawa M., Mukoyama M., Watanabe R., Kawai M. Two brothers with frontotemporal dementia and parkinsonism with an N279K mutation of the tau gene. Neurology. 2000;54:1787–1795. doi: 10.1212/wnl.54.9.1787. [DOI] [PubMed] [Google Scholar]
- 33.Arvanitakis Z., Witte R.J., Dickson D.W., Tsuboi Y., Uitti R.J., Slowinski J. Clinical-pathologic study of biomarkers in FTDP-17 (PPND family with N279K tau mutation) Parkinsonism Relat Disord. 2007;13:230–239. doi: 10.1016/j.parkreldis.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Cheshire W.P., Tsuboi Y., Wszolek Z.K. Physiologic assessment of autonomic dysfunction in pallidopontonigral degeneration with N279K mutation in the tau gene on chromosome 17. Auton Neurosci. 2002;102:71–77. doi: 10.1016/s1566-0702(02)00205-9. [DOI] [PubMed] [Google Scholar]
- 35.Ferman T.J., McRae C.A., Arvanitakis Z., Tsuboi Y., Vo A., Wszolek Z.K. Early and pre-symptomatic neuropsychological dysfunction in the PPND family with the N279K tau mutation. Parkinsonism Relat Disord. 2003;9:265–270. doi: 10.1016/s1353-8020(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 36.Slowinski J., Dominik J., Uitti R.J., Ahmed Z., Dickson D.D., Wszolek Z.K. Frontotemporal dementia and Parkinsonism linked to chromosome 17 with the N279K tau mutation. Neuropathology. 2007;27:73–80. doi: 10.1111/j.1440-1789.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 37.Whitwell J.L., Boeve B.F., Weigand S.D., Senjem M.L., Gunter J.L., Baker M.C. Brain atrophy over time in genetic and sporadic frontotemporal dementia: a study of 198 serial magnetic resonance images. Eur J Neurol. 2015;22:745–752. doi: 10.1111/ene.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahoney C.J., Simpson I.J., Nicholas J.M., Fletcher P.D., Downey L.E., Golden H.L. Longitudinal diffusion tensor imaging in frontotemporal dementia. Ann Neurol. 2015;77:33–46. doi: 10.1002/ana.24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elahi F.M., Marx G., Cobigo Y., Staffaroni A.M., Kornak J., Tosun D. Longitudinal white matter change in frontotemporal dementia subtypes and sporadic late onset Alzheimer's disease. Neuroimage Clin. 2017;16:595–603. doi: 10.1016/j.nicl.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy A., Lonergan R., Olszewska D.A., O'Dowd S., Cummins G., Magennis B. Closing the tau loop: the missing tau mutation. Brain. 2015;138:3100–3109. doi: 10.1093/brain/awv234. [DOI] [PMC free article] [PubMed] [Google Scholar]