Abstract
Purpose
To report a case of hypertensive choroidopathy with bilateral bullous serous retinal detachments (SRDs), retinal pigment epithelial detachments (PEDs), and giant retinal pigment epithelial (RPE) tears.
Observations
A 68-year-old man with a history of hypertension and diabetes mellitus presented with bilateral visual loss of about 10 day's duration. He discontinued his oral medications for 2 months without the advice of a physician. At his first visit, the best-corrected visual acuities (BCVAs) were 0.02 in the right eye and 0.3 in the left eye (decimal notation), and the respective intraocular pressures were 15 and 13 mmHg. Bullous SRDs, large PEDs, and giant RPE tears were present bilaterally. Blot retinal hemorrhages and hard exudates were seen in the left eye. The systemic blood pressure was 231/77 mmHg, and bilateral lower leg edema was observed. Biochemical blood tests showed deteriorated renal function, hypoalbuminemia, and hyperglycemia. Ultra-wide-field fundus fluorescein angiography showed leakage at the areas of the SRDs and hyperfluorescent areas corresponding to the RPE tears bilaterally. Indocyanine green angiography showed hypofluorescent areas corresponding to the PEDs. Systemic computed tomography and magnetic resonance imaging were performed, and no malignancy was found. Based on these findings, hypertensive choroidopathy was diagnosed. A week after antihypertensive treatment, the SRDs and PEDs resolved bilaterally, and the BCVAs improved to 0.4 and 0.5 in the right and left eyes, respectively. The RPE tears remained in both eyes, although the SRDs and PEDs did not recur.
Conclusions and importance
Hypertensive choroidopathy must be considered in the differential diagnosis of SRDs and/or PEDs.
Keywords: Hypertensive choroidopathy, Retinal pigment epithelial detachment, Fluorescein/indocyanine green fundus angiography, Diabetic nephropathy
1. Introduction
Severe systemic hypertension can lead to multiple organ damage1 and often causes retinopathy, optic neuropathy, and choroidopathy.2
While hypertensive retinopathy and optic neuropathy are well known, hypertensive choroidopathy is far less common. Some cases of hypertensive choroidopathy have been reported in toxemia of pregnancy,3 renal failure,4 pheochromocytoma,5 and malignant hypertension.2,6 Rapid progression of hypertension causes obstruction of the choroidal arteries and choriocapillaries. Necrosis of the overlying retinal pigment epithelium (RPE) leading to choroidal ischemia may induce serous retinal detachments (SRDs) and RPE detachments (pigment epithelial detachments [PEDs]) as a result.1,3,6 However, few reports have described choroidopathy with a RPE tear.4
We report a case of choroidopathy with bilateral giant RPE tears and bullous SRDs and PEDs considered to have resulted from hypertension associated with diabetic nephropathy.
2. Case report
A 68-year-old man visited our hospital because he reported loss of visual perception. His chief complaint was visual disturbance in his left eye of 10 day's duration. Although he had been diagnosed with hypertension and diabetes mellitus and systemic medication was required, he discontinued his treatment for 2 months without the advice of a physician.
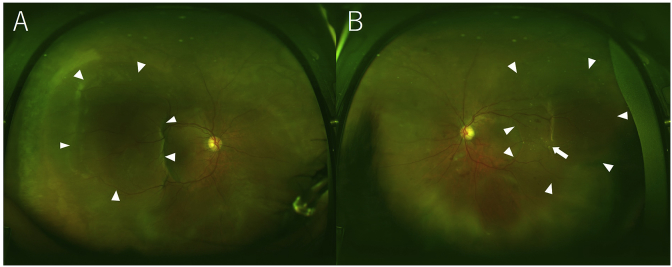
At the first examination, the best-corrected visual acuities (BCVAs) were 0.02 in the right eye and 0.3 in the left eye (decimal notation). The intraocular pressure values were 15 mmHg in the right eye and 13 mmHg in the left eye. The anterior segments were essentially normal except for moderate cataracts bilaterally. B-scan echography showed bilateral bullous exudative retinal detachments. The fundus examination showed SRDs and PEDs at the subfovea and peripheral regions, and bilateral giant RPE tears were seen in the mid-peripheral region (Fig. 1A and B). Blot retinal hemorrhages and hard exudates were identified in the left eye (Fig. 1B), and the retinal arteries and arterioles were narrow in both eyes.
Fig. 1.
Wide-field fundus images of the right eye (A) and left eye (B) at the initial presentation. Large retinal pigment epithelium detachments and retinal pigment epithelium tears are seen in both eyes (arrowheads). Blot retinal hemorrhages and hard exudates (arrow) are observed in the left eye.
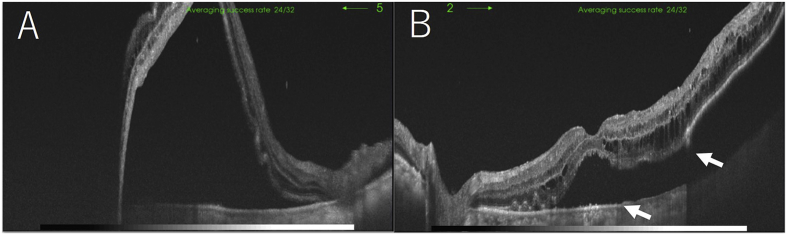
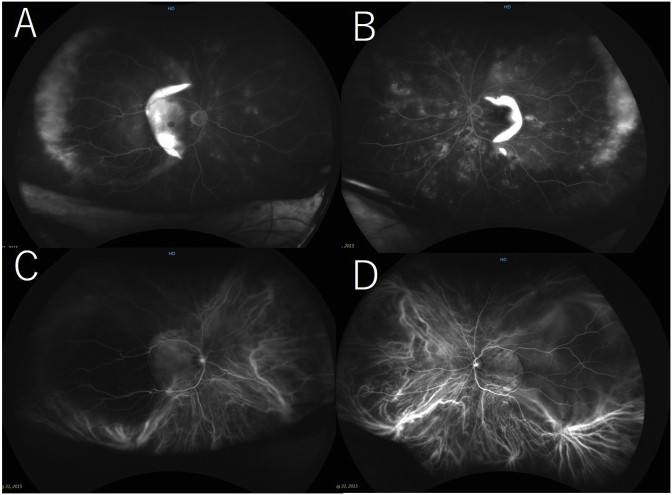
The systemic blood pressure was 231/77 mmHg, and bilateral lower leg edema was observed. Biochemical blood tests showed deteriorated renal function, hypoalbuminemia, and hyperglycemia. The serum creatinine level was 1.80 mg/dl (normal, 0.60–1.10 mg/dl), the serum albumin level was 2.0 g/dl (normal, 4.0–5.0 g/dl), and the blood sugar level was 295 mg/dl. The patient was admitted to our hospital for further evaluation of his general condition and an ophthalmologic examination. Optical coherence tomography (OCT) showed large SRDs in both eyes and a RPE rupture and PED in the left eye (Fig. 2A and B). Fundus wide-field fluorescein angiography (FA) showed mild leakage at the areas of the SRDs and hyperfluorescent areas by window defects corresponding to the RPE tears in both eyes (Fig. 3A and B). Fundus wide-field indocyanine green angiography (ICGA) showed hypofluorescent areas corresponding to the PEDs (Fig. 3C and D). Systemic computed tomography and magnetic resonance imaging did not detect any malignancies. The patient was diagnosed with hypertensive choroidopathy accompanied by retinopathy.
Fig. 2.
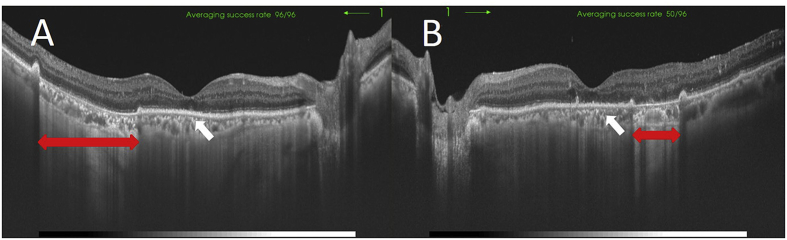
Optical coherence tomography images of the right eye (A) and left eye (B) at the initial presentation. Marked serous retinal detachments are seen in both eyes. A retinal pigment epithelium tear (arrows) and detachment are seen in the left eye.
Fig. 3.
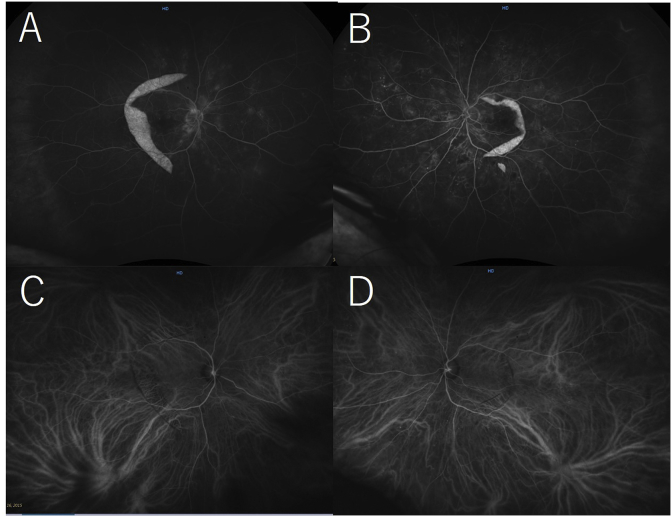
Wide-field fundus fluorescein angiography (FA) images of the right eye (A) and left eye (B) and an indocyanine green angiography (ICGA) image of the right eye (C) and left eye (D). On the FA images, leakage at the areas of the serous retinal detachments and hyperfluorescent areas corresponding to the retinal pigment epithelium tears (RPE) are seen in both eyes. On the ICGA images, hypofluorescent areas corresponding to the RPE detachments are seen in both eyes.
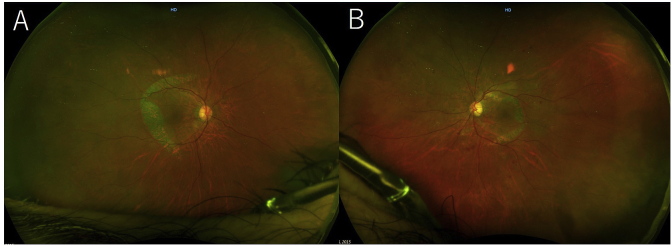
The patient then was referred to the internal medicine department for blood pressure and glycemic control. A week after starting treatment, the SRDs and PEDs resolved bilaterally (Fig. 4A and B), and the BCVAs improved to 0.4 and 0.5 in the right and left eyes, respectively. OCT showed absorption of the fluid under the SRDs and RPE defects in both eyes and the ellipsoid zone at macula was unclear (Fig. 5A and B). Fundus wide-field FA and ICGA performed 2 weeks later showed that the hyperfluorescence corresponding to the RPE tears remained on the FA images in both eyes (Fig. 6A and B). The hypofluorescent areas corresponding to the PEDs resolved on the ICGA images in both eyes (Fig. 6C and D). The patient was discharged from the hospital 18 days after admission.
Fig. 4.
Wide-field fundus images of the right eye (A) and the left eye (B) 1 week after admission. The serous retinal detachments and retinal pigment epithelial detachments have resolved in both eyes.
Fig. 5.
Optical coherence tomography images of the right eye (A) and left eye (B) 9 days after admission. The fluid under the serous retinal detachments has been absorbed and the retinal pigment epithelial defects (double arrows) remain in both eyes. The ellipsoid zone is unclear (arrows).
Fig. 6.
Wide-field fundus fluorescein angiography (FA) images of the right eye (A) and left eye (B) and indocyanine green angiography (ICGA) images of the right eye (C) and left eye (D) 2 weeks after admission to the hospital. The hyperfluorescent areas at the retinal pigment epithelium (RPE) tears are seen on the FA images in both eyes. The hypofluorescent areas corresponding to the RPE detachments have resolved on the ICGA images in both eyes.
3. Discussion
Hypertensive choroidopathy might be caused by choroidal ischemia due to occlusion of the choroidal arteries and choroidal capillaries. The choroidal arteries run a short course with less branching and flow vertically into the choriocapillaris; therefore, the rapid blood pressure increases might be affected directly by the choriocapillaris. Choroidal ischemia causes necrosis of the RPE, which may result in SRDs and PEDs.1
Treatment of hypertensive choroidopathy normalizes blood pressure,7 which in many cases normalizes the VA because the ellipsoid zone recovers along with remission of the SRD.3 However, in the current case, despite normalization of the blood pressure, the VA did not recover to the previous level but improved. In the current case, the reason why the VA did not improve sufficiently is considered to be that the outer segment layer was already damaged and the ellipsoid zone did not improve sufficiently. In many cases, normalization of blood pressure has improved the VA; however, cases have been reported in which the VA did not improve despite normalization of blood pressure.2,8
We experienced a case of hypertensive choroidopathy accompanied by SRDs, bullous PEDs, and giant RPE tears. These abnormal findings improved promptly when treatment resumed, and the VA also improved. In the current case, the patient's status deteriorated rapidly because he discontinued his antihypertensive and diabetes mellitus medications. The fact that the choroidopathy improved immediately after the systemic blood pressure was controlled suggests that the cause of the choroidopathy was the high hypertension. Bilateral bullous PEDs and concomitant giant RPE tears were noteworthy findings in the current case. Although the detailed mechanism of the PEDs and RPE tears is not fully known, possible factors include hypertension-induced increased hydrostatic pressure in the choroidal interstitium and decreased adhesion between the RPE and Bruch's membrane because of sub-RPE lipid deposition in aging eyes. Hydrostatic pressure in the choroidal interstitium may increase, possibly in association with disorders with or without vascular hyperpermeability. The former includes diabetic choroidopathy and uveitis, and the latter includes severe hypertension, choroidal venous obstruction (e.g., uveal effusion), and hypoalbuminemia or other disorders with decreased colloid osmotic pressure. Hypofluorescence under the PED may support this hypothesis. Lipid accumulation under the RPE starts in the fourth decade of life and progresses with age.9,10 This aging change may result in serous PEDs even in eyes without choroidal neovascularization as in early age-related macular degeneration (AMD), suggesting impairment of the adhesion of the RPE to Bruch's membrane. Taken together, severe hypertension-associated increased hydrostatic pressure in the choroidal interstitium may induce a bullous PED and subsequent giant RPE tear.
Hoskin et al.11 first reported RPE tears. They generally are complications associated with choroidal neovascularization in AMD, and tears can occur after laser photocoagulation, photodynamic therapy, and intravitreal injections of antivascular endothelial growth factor drugs.12, 13, 14, 15, 16 However, the precise mechanism has not been clarified. In addition, a few studies have reported patients with RPE tears who have hypertensive choroidal disease as in the current case.8 Even in this case, the pathogenic mechanism of the RPE tears was unknown, but we presumed that the onset of the PED occurred because of adherence to the RPE itself, and as a result, the subsequent RPE tears occurred.
In the current case, the SRDs and PEDs resolved with improvement of the hypertension, but the RPE tears remained. In general, the retinal tissue above the RPE defect progresses to atrophy, so the retina at the site of a residual RPE tear is thought to become atrophied in the future. However, because it is far from the macular region, progression of the retinal atrophy may have no major effect on the VA.
In conclusion, we describe a case of hypertensive choroidopathy accompanied by SRDs, bullous PEDs, and giant RPE tears. When SRDs and PEDs are observed, hypertensive choroidopathy also needs to be considered in the differential diagnosis. If poorly controlled hypertension is confirmed, it is necessary to promptly normalize the blood pressure with medical treatment.
Patient consent
Informed consent was obtained from the patient.
Funding/financial support
The authors have received no financial support related to this report.
Conflicts of interest
The authors declare that they have no conflicts of interest associated with this report.
Acknowledgement
The authors thank Medical International for professional English editing.
References
- 1.Ugarte M., Horgan S., Rassam S., Leong T., Kon C.H. Hypertensive choroidopathy: recognizing clinically significant end-organ damage. Acta Ophthalmol. 2008;86:227–228. doi: 10.1111/j.1600-0420.2007.01030.x. [DOI] [PubMed] [Google Scholar]
- 2.Bourke K., Patel M.R., Prisant L.M., Marcus D.M. Hypertensive choroidopathy. J Clin Hypertens. 2004;6:471–472. doi: 10.1111/j.1524-6175.2004.3749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song Y.S., Kinouchi R., Ishiko S., Fukui K., Yoshida A. Hypertensive choroidopathy with eclampsia viewed on spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013;251:2647–2650. doi: 10.1007/s00417-013-2462-9. [DOI] [PubMed] [Google Scholar]
- 4.Kameda Y., Hirose A., Iida T., Uchigata Y., Kitano S. Giant retinal pigment epithelial tear associated with fluid overload due to end-stage diabetic kidney disease. Am J Ophthalmol Case Reports. 2017;5:44–47. doi: 10.1016/j.ajoc.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tso M.O., Jampol L.M. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132–1145. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- 6.Hirano Y., Yasukawa T., Ogura Y. Bilateral serous retinal detachments associated with accelerated hypertensive choroidopathy. Int J Hypertens. 2010;2010:964513. doi: 10.4061/2010/964513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo B.P., Brown G.C. Update on the ocular manifestations of systemic arterial hypertension. Curr Opin Ophthalmol. 2004;15 doi: 10.1097/01.icu.0000124081.88389.14. 203-110. [DOI] [PubMed] [Google Scholar]
- 8.Tai Y.C., Huang J.C., Sun C.C., Yeung L. Bilateral retinal pigment epithelial rips in hypertensive choroidopathy. Taiwan J. Ophthalmol. 2016;6:150–154. doi: 10.1016/j.tjo.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J.D., Curcio C.A., Johnson M. Morphometric analysis of lipoprotein-like particle accumulation in aging human macular Bruch's membrane. Investig Ophthalmol Vis Sci. 2008;49:2721–2727. doi: 10.1167/iovs.07-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore D.J., Hussain A.A., Marshall J. Age-related variation in the hydraulic conductivity of Bruch's membrane. Investig Ophthalmol Vis Sci. 1995;36:1290–1297. [PubMed] [Google Scholar]
- 11.Hoskin A., Bird A.C., Sehmi K. Tears of detached retinal pigment epithelium. Br J Ophthalmol. 1981;65:417–422. doi: 10.1136/bjo.65.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gass J.D. Pathogenesis of tears of the retinal pigment epithelium. Br J Ophthalmol. 1984;68:513–519. doi: 10.1136/bjo.68.8.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendis R., Lois N. Fundus autofluorescence in patients with retinal pigment epithelial (RPE) tears: an in-vivo evaluation of RPE resurfacing. Graefes Arch Clin Exp Ophthalmol. 2014;252:1059–1063. doi: 10.1007/s00417-013-2549-3. [DOI] [PubMed] [Google Scholar]
- 14.Gass J.D. Retinal pigment epithelial rip during krypton red laser photocoagulation. Am J Ophthalmol. 1984;98:700–706. doi: 10.1016/0002-9394(84)90684-6. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein M., Heilweil G., Barak A., Loewenstein A. Retinal pigment epithelial tear following photodynamic therapy for choroidal neovascularization secondary to AMD. Eye (London) 2005;19:1315–1324. doi: 10.1038/sj.eye.6701765. [DOI] [PubMed] [Google Scholar]
- 16.Dhalla M.S., Blinder K.J., Tewari A., Hariprasad S.M., Apte R.S. Retinal pigment epithelial tear following intravitreal pegaptanib sodium. Am J Ophthalmol. 2006;141:752–754. doi: 10.1016/j.ajo.2005.10.053. [DOI] [PubMed] [Google Scholar]