Abstract
Background
Recent studies have indicated the significant association between non-alcoholic fatty liver disease (NAFLD) and depression. However, there is ongoing debate on whether the risk for depression is actually related with the presence and severity of NAFLD. Thus, this study was conducted to investigate the association between depression and NAFLD evaluated by diverse modalities.
Methods
A total of 112,797 participants from the Korean general population were enrolled. The study participants were categorized into three groups according to degree of NAFLD evaluated by ultrasonography, fatty liver index (FLI) and fibrosis-4 score (FIB-4). Depression was defined as a score of Center for Epidemiological Studies-Depression (CES-D) ≥ 16, and the odd ratios (ORs) and 95% confidence interval (CI) for depression (adjusted ORs [95% CI]) were assessed by multiple logistic regression analyses.
Results
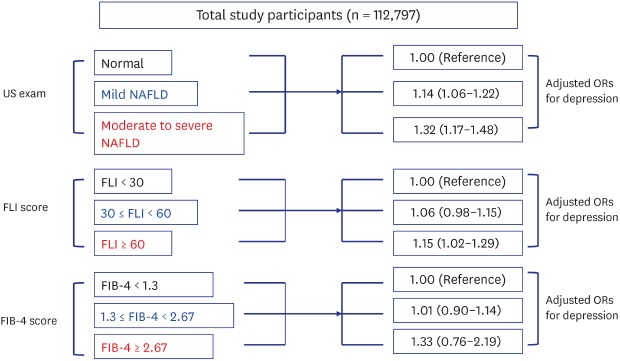
In the unadjusted model, the presence and severity of NAFLD was not significantly associated with depressive symptoms. However, in the fully adjusted model, ORs for depression increased in proportion to the degree of ultrasonographically detected NAFLD (mild fatty liver: 1.14 [1.06–1.22]; and moderate to severe fatty liver: 1.32 [1.17–1.48]). An association was also observed between depression and FLI (30 ≤ FLI < 60: 1.06 [0.98–1.15]; FLI ≥ 60: 1.15 [1.02–1.29]).
Conclusion
The presence and severity of NAFLD is significantly associated with depressive symptoms. In addition, this association was more distinct after adjusting for covariates including age, gender and insulin resistance. This finding indicates the necessity of further study evaluating the incidental relationship of depression with NAFLD.
Keywords: Depression, Non-Alcoholic Fatty Liver Disease, Ultrasonography, Fatty Liver Index
Graphical Abstract
INTRODUCTION
Major depression is a commonly occurring, serious, recurrent disorder linked to diminished role functioning and quality of life, medical morbidity, and mortality.1,2 Lifetime prevalence of depression varies widely from 3% in Japan to 17% in the ultrasonography (US),3,4 and the number of people who suffer from depression during their lives falls within an 8%–12% range in most countries.5,6 Considering clinical significance and prevalence of depression, identifying medical conditions associated with depression would be an important public health achievement in the depression pandemic.
NAFLD is the most common cause of chronic liver disease in Western countries, and is expected to be the most frequent indication for liver transplant by 2030.7 Also in Asian countries, NAFLD is a rapidly increasing chronic liver disease corresponding to rising incidence of obesity. Large population based surveys in Asian countries indicated that the prevalence of NAFLD varied from 10% to 29% in population subgroups, depending on age, gender and ethnicity.8
Over the last decade, it has been reported that the clinical burden of NAFLD is not only confined to liver-related morbidity and mortality, but there is growing evidence that NAFLD is a multisystem disease, affecting extra-hepatic organs and regulatory pathways.9 Previous studies have reported that NAFLD linked to a substantial increase in risk for metabolic complications such as type 2 diabetes mellitus and cardiovascular disease independent of conventional risk factors.10,11,12 In particular, recent studies suggested potential positive relationship between NAFLD and major depressive disorder (MDD).13,14,15 These studies indicated that the presence and histologic severity of NAFLD was significantly associated with depression.13,14,15 However, there were population based studies displaying no significant relationship between depression and NAFLD.16,17
This debate among observational studies confers the necessity of a large-scale study applying diverse methodologies to diagnosing NAFLD. Thus, the present study enrolled a large cohort of adults from the Korean population and investigated the association between NAFLD and depression.
METHODS
Study design and participants
The study data were provided by the Kangbuk Samsung Health Study (KSHS) cohort. The majority of study participants consisted of Korean men and women undergoing a medical health check-up program at the Health Promotion Center of Kangbuk Samsung Hospital, Sungkyunkwan University, Seoul, Korea.
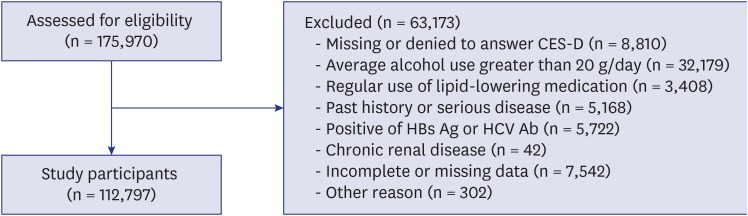
A total 175,970 men and women, aged from 18 to 91 years old, who had received the regular health check-up program between January 2014 and December 2014 participated in the KSHS. Among the 175,970 participants, 8,810 did not answer the Center for Epidemiological Studies-Depression (CES-D) questionnaire. Among the 167,160 participants, 53,913 were excluded for various reasons: 32,179 were excluded because of average alcohol use greater than 20 g per day; 3,408 were receiving medication for lipid-lowering agents; 5,168 had a past history of severe disease such as cancer, stroke or angina; 5,534 had a positive serologic marker for hepatitis B surface antigen (HBsAg); 188 had a positive serologic marker for hepatitis C virus antibody (HCVAb); 42 had chronic renal disease (Creatinine > 2), 7,542 were excluded for missing data in serologic markers like hepatitis C antibody and hepatitis B antigen or alcohol consumption, and 302 were excluded for another reason such as cirrhosis. The total number of eligible participants was 112,797 (Fig. 1).
Fig. 1. Flow chart of enrolled study participants.
Clinical and laboratory measurement
Study data consisted of medical information based on a self-administered questionnaire, anthropometric and laboratory measurements. All of the subjects were required to fulfill the self-administered questionnaire that includes past medical history, sociodemographic information and health-related behaviors like alcohol consumption, smoking and physical activity. The detailed methods for self-administered questionnaire, anthropometric and laboratory measurements are described in an article of our group.18 Diabetes mellitus (DM) was defined in individuals with as follows; fasting glucose ≥ 126 mg/dL, HbA1c ≥ 6.5%, currently administering anti-diabetic medication, and presence of previous history of DM. Presence of hypertension was determined in individuals with current use of anti-hypertensive medication, past history of hypertension or measured blood pressure (BP) ≥ 140/90 mmHg.
Diagnosis of depression and NAFLD
Depressive symptoms were assessed using the Korean versions of CES-D scale.19 The CES-D is a self-report questionnaire designed to assess the current prevalence of depressive symptoms in the general population.20 We used 4-factor 20-item CES-D scale, which rated from 0 to 3, with 0 indicating that the depressive symptom was experienced rarely and 3 indicating that the depressive symptom was experienced most of the time. Individuals with depressive symptoms was defined as CES-D score is more than 16.
The presence and degree of fatty liver were defined according to the results of abdominal US with a 3.5-MHz transducer (Logic Q700 MR; GE, Milwaukee, WI, USA). Examiners did not have any information for the aim of the study without knowledge of laboratory values. Abdominal US was performed by standard criteria for diagnosing fatty liver based on parenchymal brightness, liver-to-kidney contrast, deep beam attenuation, and bright vessel walls.21,22 Intra- and inter-observer reliability of ultrasound diagnosis of fatty liver were measured to minimize observer dependent diagnostic error. The inter-observer reliability and intra-observer reliability for fatty liver diagnosis were substantial (kappa static of 0.74) and excellent (kappa static of 0.94), respectively.
In non-invasive scoring systems, Fatty Liver Index (FLI) and Fibrosis-4 (FIB-4) score were calculated by the following formula.23,24
| FLI = e0.953 × ln triglyceride + 0.139 × BMI + 0.718 × ln GGT + 0.053 × waist circumference – 15.745/(1 + e0.953 × ln triglyceride + 0.139 × BMI + 0.718 × ln GGT + 0.053 × waist circumference – 15.745) × 100 |
FIB-4: age (years) × AST [U/L]/(platelets [109/L] × (ALT [U/L])1/2)
The cutoff points of scoring systems are 30 and 60 in FLI, and 1.3 and 2.67 in FIB-4, respectively.25
Statistical analysis
Data are described by means ± standard deviation in continuous variables and by proportions (or percentage) in categorical variables, respectively. All study participants were stratified into three groups according to US finding (absent fatty liver, mild fatty liver, moderate to severe fatty liver). ANOVA for continuous variables and χ2 test for categorical variables were used in comparing clinical characteristics and parameters among groups
Using logistic regression analysis, we investigated the odds ratios (ORs) and 95% confidence interval (CI) of fatty liver assessed by abdominal US, FLI, and FIB-4. First, we analyzed the association between depression and fatty liver defined by abdominal US, FLI, and FIB-4 alone. Then, the association between the fatty liver and depression was adjusted for three models including potential confounders (Model 1: age, gender, family income per month, marriage state, job (unemployment), average alcohol use (gram per day), smoking, systolic blood pressure, HDL cholesterol, and diabetes; Model 2: Model 1 + BMI and physical activity (IPAQ); Model 3: Model 2 + HOMA-IR). All adjusted models checked for the presence of multicollinearity and over-dispersion, and then fitting models were selected. We also analyzed the ORs in gender subgroup because of the high proportion of men in NAFLD group and high prevalence of depression in women participants. Statistical significance was considered as P values < 0.05. All statistical analyses were performed using R 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Kangbuk Samsung Hospital (Seoul, Korea) (IRB No. KBSMC 2014-12-023-003). The informed consent requirement was waived by IRB because data was only retrospectively accessed for analytical purpose.
RESULTS
Clinical characteristic of the study population
The total number of study participants was 112,797 (54,753 men and 58,044 women). The mean age was 40.09 ± 7.73 years, and the proportion of participants with depressive symptoms was 10.6% (n = 11,903). Main demographic and clinical characteristics of study participants according to the categories of fatty liver were shown in Table 1. The prevalence of fatty liver is higher in men than women, whereas the prevalence of depressive symptoms was higher in women than men. This disproportional prevalence of fatty liver and depressive symptoms lead to the higher proportion of depression (CES-D ≥ 16) and higher CES-D score in participants without fatty liver than participants with fatty liver. The proportion of FLI ≥ 60 indicated a positive relationship with the degree of fatty liver assessed by abdominal US. There were rare participants with advanced fibrosis assessed by FIB-4.
Table 1. Main clinical characteristics.
| NAFLD | Normal (n = 81,162) | Mild (n = 25,181) | Moderate to severe (n = 6,454) | P value | |
|---|---|---|---|---|---|
| Gender | |||||
| Women | 50,887 (62.7) | 6,295 (25.0) | 862 (13.4) | < 0.001 | |
| Men | 30,275 (37.3) | 18,886 (75.0) | 5,592 (86.6) | ||
| Glucose, mg/dL | 91.9 ± 9.5 | 98.6 ± 16.7 | 104.3 ± 24.0 | < 0.001 | |
| AST, U/L | 19.2 ± 9.6 | 23.2 ± 13.8 | 32.5 ± 21.9 | < 0.001 | |
| ALT, U/L | 16.7 ± 10.2 | 28.8 ± 18.8 | 52.0 ± 31.8 | < 0.001 | |
| GGT, U/L | 20.1 ± 19.4 | 36.6 ± 35.0 | 54.4 ± 52.1 | < 0.001 | |
| Serum creatinine, mg/dL | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | < 0.001 | |
| Total cholesterol, mg/dL | 188.8 ± 31.5 | 205.7 ± 34.2 | 209.3 ± 36.0 | < 0.001 | |
| HDL-cholesterol, mg/dL | 63.6 ± 15.1 | 50.2 ± 11.8 | 45.3 ± 10.0 | < 0.001 | |
| LDL-cholesterol, mg/dL | 114.7 ± 29.2 | 134.2 ± 30.7 | 140.9 ± 32.2 | < 0.001 | |
| HbA1c, % | 5.5 ± 0.3 | 5.7 ± 0.5 | 5.9 ± 0.8 | < 0.001 | |
| Height, cm | 165.3 ± 8.1 | 169.4 ± 8.0 | 171.5 ± 7.5 | < 0.001 | |
| Weight, kg | 60.2 ± 10.3 | 72.9 ± 10.3 | 82.8 ± 12.0 | < 0.001 | |
| Waist circumference, cm | 77.7 ± 7.8 | 87.8 ± 7.0 | 95.2 ± 7.9 | < 0.001 | |
| SBP, mmHg | 104.5 ± 11.3 | 112.9 ± 12.0 | 117.8 ± 11.8 | < 0.001 | |
| DBP, mmHg | 67.4 ± 8.9 | 73.7 ± 9.6 | 77.0 ± 9.5 | < 0.001 | |
| Age, yr | 39.4 ± 7.7 | 42.2 ± 7.6 | 40.3 ± 6.7 | < 0.001 | |
| HOMA-IR | 1.2 ± 0.7 | 2.0 ± 1.3 | 3.2 ± 2.0 | < 0.001 | |
| BMI, kg/m2 | 21.9 ± 2.6 | 25.4 ± 2.7 | 28.1 ± 3.3 | < 0.001 | |
| Average alcohol use, g/day | 4.7 ± 4.6 | 6.1 ± 5.0 | 6.4 ± 5.0 | < 0.001 | |
| Hypertension, % | 5.3 | 15.3 | 23.2 | < 0.001 | |
| Diabetes, % | 1.2 | 5.9 | 13.3 | < 0.001 | |
| HEPA (IPAQ), % | 13.3 | 11.9 | 9.5 | < 0.001 | |
| Married, % | 85.5 | 89.2 | 87.0 | < 0.001 | |
| Family income per month, % | |||||
| < 2 million won | 2.2 | 1.6 | 1.4 | < 0.001 | |
| 2–4 million won | 17.5 | 14.2 | 17.3 | ||
| 4–6 million won | 32.5 | 32.0 | 34.6 | ||
| ≥ 6 million won | 47.8 | 52.1 | 46.7 | ||
| Current smoker, % | 11.3 | 23.1 | 24.8 | < 0.001 | |
| Unemployment, % | 34.5 | 22.7 | 17.9 | < 0.001 | |
| CES-D ≥ 16 | 9,033 (11.1) | 2,242 (8.9) | 628 (9.7) | < 0.001 | |
| Women | 6,943 (13.6) | 864 (13.7) | 147 (17.1) | < 0.001 | |
| Men | 2,090 (6.9) | 1,378 (7.3) | 481 (8.6) | < 0.001 | |
| CES-D score | 6.8 ± 7.3 | 6.3 ± 6.7 | 6.3 ± 7.0 | < 0.001 | |
| Women | 7.6 ± 7.7 | 7.8 ± 7.7 | 8.3 ± 8.6 | < 0.001 | |
| Men | 5.6 ± 6.2 | 5.7 ± 6.2 | 6.0 ± 6.6 | < 0.001 | |
| FLI ≥ 60 | 1,588 (2.0) | 5,402 (21.5) | 4,073 (63.1) | < 0.001 | |
| FIB-4 ≥ 2.67 | 166 (0.20) | 45 (0.18) | 14 (0.22) | < 0.001 | |
Continuous variables are expressed as mean ± standard deviation or number (%)
NAFLD = non-alcoholic fatty liver disease, AST = aspartate aminotransferase, ALT = alanine aminotransferase, GGT = gamma-glutamyltransferase, HDL = high density lipoprotein cholesterol, LDL = low density lipoprotein cholesterol, SBP =systolic blood pressure, DBP= diastolic blood pressure, HOMA-IR = homeostasis model assessment of insulin resistance, BMI = body mass index, HEPA = health-enhancing physically active, IPAQ = international physical activity questionnaire, CES-D = center for epidemiological studies-depression, FLI = fatty liver index, FIB-4 = fibrosis-4.
Relationship between depression and NAFLD
Table 2 showed the association of depression with ultrasonographically detected fatty liver, FLI and FIB-4 score. The univariate ORs indicated the negative association of depression with the degree of ultrasonographically detected fatty liver, FLI and FIB-4 score. However, adjustment for covariates patterned the dose-dependent relationship between depression and ultrasonographically detected fatty liver, FLI and FIB-4 score, despite the statistical insignificance in some cases.
Table 2. The OR of depression (CES-D ≥ 16) according to NAFLD diagnosed by US, hepatic steatosis FLI, and FIB-4.
| Variables | OR (95% CI) | ||||
|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | ||
| Abdominal US | |||||
| Normal | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| Mild fatty liver | 0.78 (0.74–0.82) | 0.78 (0.74–0.82) | 1.14 (1.06–1.22) | 1.14 (1.06–1.22) | |
| Moderate to severe fatty liver | 0.86 (0.79–0.94) | 0.86 (0.79–0.94) | 1.39 (1.25–1.55) | 1.32 (1.17–1.48) | |
| FLI | |||||
| FLI < 30 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| 30 ≤ FLI < 60 | 0.73 (0.69–0.77) | 0.73 (0.69–0.77) | 1.11 (1.03–1.19) | 1.06 (0.98–1.15) | |
| FLI ≥ 60 | 0.77 (0.72–0.83) | 0.77 (0.72–0.83) | 1.24 (1.13–1.37) | 1.15 (1.02–1.29) | |
| FIB-4 | |||||
| FIB-4 < 1.3 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| 1.3 ≤ FIB-4 < 2.67 | 0.88 (0.81–0.96) | 0.88 (0.81–0.96) | 0.97 (0.86–1.09) | 1.01 (0.90–1.14) | |
| FIB-4 ≥ 2.67 | 1.01 (0.64–1.50) | 1.01 (0.64–1.50) | 1.42 (0.84–2.26) | 1.33 (0.76–2.19) | |
Model 1 covariates: age, sex, family income per month, marriage state, job (unemployment), average alcohol use (gram per day), smoking, SBP, HDL cholesterol, and diabetes. Model 2 covariates: Model 1 + BMI and physical activity (IPAQ). Model 3 covariates: Model 2 + HOMA-IR.
OR = odds ratio, CES-D = center for epidemiological studies-depression, NAFLD = non-alcoholic fatty liver disease, US = ultrasonography, FLI = fatty liver index, CI = confidence interval, FIB-4 = fibrosis-4, SBP =systolic blood pressure, HDL = high density lipoprotein cholesterol, BMI = body mass index, IPAQ = international physical activity questionnaire, HOMA-IR = homeostasis model assessment of insulin resistance.
Table 3 and Table 4 presents the association of depression with ultrasonographically detected fatty liver, FLI and FIB-4 score in men and women, respectively.
Table 3. The OR of depression (CES-D ≥ 16) according to NAFLD diagnosed by US, hepatic steatosis FLI, and FIB-4 in men.
| Variables | OR (95% CI) | ||||
|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | ||
| Abdominal US | |||||
| Normal | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| Mild fatty liver | 1.06 (0.99–1.14) | 1.21 (1.12–1.32) | 1.21 (1.11–1.33) | 1.24 (1.13–1.36) | |
| Moderate to severe fatty liver | 1.27 (1.14–1.41) | 1.44 (1.27–1.63) | 1.47 (1.27–1.69) | 1.49 (1.29–1.73) | |
| FLI | |||||
| FLI < 30 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| 30 ≤ FLI < 60 | 0.97 (0.90–1.05) | 1.05 (0.96–1.15) | 1.06 (0.95–1.17) | 1.06 (0.95–1.17) | |
| FLI ≥ 60 | 1.07 (0.98–1.17) | 1.20 (1.07–1.35) | 1.22 (1.05–1.42) | 1.22 (1.05–1.42) | |
| FIB-4 | |||||
| FIB-4 < 1.3 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| 1.3 ≤ FIB-4 < 2.67 | 0.90 (0.78–1.03) | 0.96 (0.80–1.15) | 1.01 (0.84–1.20) | 1.00 (0.83–1.20) | |
| FIB-4 ≥ 2.67 | 1.21 (0.65–2.05) | 1.32 (0.64–2.41) | 1.23 (0.62–2.44) | 1.34 (0.62–2.53) | |
Model 1 covariates: age, family income per month, marriage state, job (unemployment), average alcohol use (gram per day), smoking, SBP, HDL cholesterol, and diabetes. Model 2 covariates: Model 1 + BMI and physical activity (IPAQ). Model 3 covariates: Model 2 + HOMA-IR.
OR = odds ratio, CES-D = center for epidemiological studies-depression, NAFLD = non-alcoholic fatty liver disease, US = ultrasonography, FLI = fatty liver index, FIB-4 = fibrosis-4, CI = confidence interval, SBP =systolic blood pressure, HDL = high density lipoprotein cholesterol, BMI = body mass index, IPAQ = international physical activity questionnaire, HOMA-IR = homeostasis model assessment of insulin resistance.
Table 4. The OR of depression (CES-D ≥ 16) according to NAFLD diagnosed by US, hepatic steatosis FLI, and FIB-4 in female.
| Variables | OR (95% CI) | ||||
|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | ||
| Abdominal US | |||||
| Normal | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| Mild fatty liver | 1.01 (0.93–1.09) | 1.09 (0.98–1.21) | 1.03 (0.92–1.15) | 1.02 (0.92–1.14) | |
| Moderate to severe fatty liver | 1.30 (1.08–1.55) | 1.43 (1.12–1.80) | 1.19 (0.92–1.54) | 1.19 (0.91–1.53) | |
| FLI | |||||
| FLI < 30 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| 30 ≤ FLI < 60 | 1.17 (1.06–1.29) | 1.25 (1.10–1.42) | 1.18 (1.02–1.36) | 1.17 (1.02–1.35) | |
| FLI ≥ 60 | 1.51 (1.31–1.74) | 1.55 (1.27–1.88) | 1.36 (1.08–1.72) | 1.37 (1.08–1.72) | |
| FIB-4 | |||||
| FIB-4 < 1.3 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |
| 1.3 ≤ FIB-4 < 2.67 | 0.93 (0.83–1.04) | 0.98 (0.83–1.14) | 1.03 (0.88–1.21) | 1.02 (0.87–1.20) | |
| FIB-4 ≥ 2.67 | 1.10 (0.55–1.99) | 1.57 (0.70–3.17) | 1.27 (0.56–2.89) | 1.33 (0.54–2.86) | |
Model 1 covariates: age, family income per month, marriage state, job (unemployment), average alcohol use (gram per day), smoking, SBP, HDL cholesterol, and diabetes. Model 2 covariates: Model 1 + BMI and physical activity (IPAQ). Model 3 covariates: Model 2 + HOMA-IR.
OR = odds ratio, CES-D = center for epidemiological studies-depression, NAFLD = non-alcoholic fatty liver disease, US = ultrasonography, FLI = fatty liver index, FIB-4 = fibrosis-4, CI= confidence interval, SBP =systolic blood pressure, HDL = high density lipoprotein cholesterol, BMI = body mass index, IPAQ = international physical activity questionnaire, HOMA-IR = homeostasis model assessment of insulin resistance.
In men, the ORs for depression steadily increased through adjustment for covariates in model 1, model 2 and model 3 (Table 3). Dose dependent pattern of relationship was found between the adjusted ORs for depression and the degree of ultrasonographically detected fatty liver, FLI and FIB-4 score. However, statistical significance in adjusted ORs was found in groups with ultrasonographically detected fatty liver and group with FLI ≥ 60.
This pattern of relationship was similarly observed in women, but statistical significance was different from that in the men group (Table 4). Statistical significance was identified only in group stratified by FLI even after adjustment for covariates, and groups with ultrasonographically detected fatty liver and FIB-4 did not show the statistical significance.
DISCUSSION
In the present study, the presence of ultrasonographically detected NAFLD was more significantly associated with depressive symptoms, compared with non-NAFLD group, even after adjusting for confounding factors. Additionally, this association was re-confirmed between depression and FLI. These findings are in line with that of previous studies showing the significant association between NAFLD and depression.13,14,15 However, in a study analyzing data from National Health and Nutrition Examination Survey, depression was significantly associated only with hepatitis C out of chronic liver disease.17This discrepancy necessitates the more generalized results from a large number of study participants from the general population using various methodology in diagnosing fatty liver. As our findings were obtained from 112,797 Koreans evaluated by ultrasonography and FLI, our results may be epidemiologic evidence showing the association between NAFLD and depression.
Our study implies that advanced NAFLD may be more associated with depression. This finding may be supported by previous studies that reported a significant relationship between histologic severity of NAFLD and MDD.13,14 In 258 Japanese patients with biopsy-proven NAFLD, the comorbid state of major depressive disorder was associated with more severe histological liver steatosis and worse treatment outcomes.13Studies of NAFLD in the United States also indicated that depression was significantly associated with more severe hepatocyte ballooning.14 These findings empirically support our hypothesis that the likelihood for depression increases under the more advanced NAFLD. However, when study participants were stratified by FIB-4 score and evaluated for the relationship of FIB-4 with depression, we did not observe a statistical significance. This finding can be explained by clinical characteristics of our study participants. Our study participants mostly comprised of apparently healthy Korean adults who received a medical health check-up, and people with chronic liver diseases were initially excluded from the study participants. Thus, the portion of people with advanced fibrosis or hepatitis might be low. Actually, the rate of advanced fibrosis evaluated by FIB-4 score was 0.2%, which is not readily able to show the statistical significance between FIB-4 and depression.
Recent studies have recognized reciprocal relationship between depression and metabolic diseases such as diabetes, obesity and metabolic syndrome.26,27,28 In particular, there is an increasing interest in whether casual relationships are involved between insulin resistance and depression. The proposed link is consistent with reports that depression is associated with metabolic diseases via insulin resistance. Insulin resistance is known to play a pivotal role in the development and progression of NAFLD. Thus, it can be speculated that insulin resistance in individuals with NAFLD may trigger pathologic process associated with depression. Interestingly, while the general prevalence of depression and average CES-D score were generally higher in non-NAFLD group than NAFLD group, ORs for depression more significantly increased in NAFLD groups than the non-NAFLD group after adjusting for metabolic factors including gender. This finding might be explained by gender difference in the prevalence of depression and NAFLD. It is known that there is gender difference in the prevalence of depressive disorders and NAFLD. While depression is more common in women,6,29 NAFLD is more prevalent in men.7 Also in our study, women had a prevalence of depression (13.6%) up to twice that of men (6.9%), whereas men had the higher prevalence of NAFLD (44.7%) than women (12.3%). Thus, the rate of individuals having both depression and NAFLD increased after adjusting for gender, which might contribute to increased association between NAFLD and depression after adjusting for gender.
The pathophysiological basis accounting for our findings is likely to be interaction among metabolic derangements related to insulin resistance, depression and NAFLD. It was hypothesized that psychological problems are associated with metabolic disorders via visceral fat accumulation. Psychological factors including depression can activate hypothalamic- pituitary-adrenal (HPA) axis, producing hypersecretion of corticotrophin-releasing hormone and cortisol.30 Such a dysregulation of HPA axis promotes deposition of visceral adipose tissue,30 resulting in the release of inflammatory cytokines such as interleukin (IL)-1 and IL-6 and tumor necrosis factor (TNF)- α.31,32 Both IL-6 and TNF-α have been implicated in insulin resistance, which is considered to be the key factor in mediating metabolic abnormalities.33,34 These metabolic abnormalities play a significant role in the development and progression of NAFLD. Thus, it can be postulated that association between depression and NAFLD is mediated by metabolic condition of individuals according to their susceptibility.
Merits of our study are its large sample size and reliable medical data of laboratory and imaging exams, which enabled us to precisely evaluate the association between depression and NAFLD by diverse modalities.
Nonetheless, there were several limitations in the study. First, NAFLD was assessed only by non-invasive modalities. Although non-invasive modalities including ultrasonography have satisfactorily evaluated fat deposition in the liver, it is subjective to diagnose NAFLD only by non-invasive modalities. More reliable results could be obtained from histologic proof or other imaging exams like elastography, but it was inappropriate to conduct these exams in a population-based epidemiological study. Nonetheless, this issue should be acknowledged as a major limitation of the study.
Second, our data is not enough to investigate the history of anti-depressant medication and diagnosed MDD. This limit is attributable to the feature of our health check-up data that our study was based on. As most of the study subjects were employees who were working in Korean companies, they tended to feel a sense of burden in revealing their psychiatric issues. Thus, we could not obtain the precise information for their history of anti-depressant medication and MDD.
Third, our results cannot suggest the potential mechanism for our findings. In particular, we could not provide any evidence for the role of insulin resistance mediating the association between depression and fatty liver. Although our adjusting covariates included HOMA-IR, the degree of association did not markedly decrease after adjustment for HOMA-IR. This finding may be attributable to the gender difference in the prevalence of depression and NAFLD, and the limited role of HOMA-IR in reflecting insulin resistance. Future studies should be conducted to identify the underlying mechanism for association between NAFLD and depression.
In conclusion, in the large Korean population, we showed the significant association between NAFLD and depression. The depression was more significantly associated with more advanced condition of NAFLD. These findings suggest the increased risk of depression in NAFLD, which warrants further studies to clarify their incidental relationship.
ACKNOWLEDGMENTS
This study was based on medical data collected and arranged by Kangbuk Samsung Cohort Study (KSCS). Therefore, this study could only be done by virtue of the labor of all staffs working in KSCS and Total Healthcare Center, Kangbuk Samsung Hospital. We are especially appreciative of Ms. JiinAhn and prof. Yoosoo Chang in Kangbuk Samsung Cohort team.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jung JY, Ryoo JH.
- Data curation: Jung JY, Park SK, Ryoo JH.
- Formal analysis: Jung JY, Park SK, Ryoo JH.
- Investigation: Jung JY, Chung PW, Park SK, Oh CM, Chung PW, Ryoo JH.
- Methodology: Jung JY, Park SK, Oh CM, Ryoo JH.
- Validation: Jung JY, Park SK, Oh CM, Chung PW, Ryoo JH.
- Writing - original draft: Jung JY, Park SK, Chung PW.
- Writing - review & editing: Jung JY.
References
- 1.Spijker J, Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Functional disability and depression in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand. 2004;110(3):208–214. doi: 10.1111/j.1600-0447.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- 2.Ustün TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184(5):386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 3.Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 6.Murphy JM, Laird NM, Monson RR, Sobol AM, Leighton AH. A 40-year perspective on the prevalence of depression: the Stirling County Study. Arch Gen Psychiatry. 2000;57(3):209–215. doi: 10.1001/archpsyc.57.3.209. [DOI] [PubMed] [Google Scholar]
- 7.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1) Suppl:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Chitturi S, Farrell GC, Hashimoto E, Saibara T, Lau GK, Sollano JD, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22(6):778–787. doi: 10.1111/j.1440-1746.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174–1197. doi: 10.1002/hep.26717. [DOI] [PubMed] [Google Scholar]
- 10.Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57(4):1378–1383. doi: 10.1002/hep.26183. [DOI] [PubMed] [Google Scholar]
- 11.Lizardi-Cervera J, Aguilar-Zapata D. Nonalcoholic fatty liver disease and its association with cardiovascular disease. Ann Hepatol. 2009;8(Suppl 1):S40–S43. [PubMed] [Google Scholar]
- 12.Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2013;58(10):3017–3023. doi: 10.1007/s10620-013-2743-5. [DOI] [PubMed] [Google Scholar]
- 13.Tomeno W, Kawashima K, Yoneda M, Saito S, Ogawa Y, Honda Y, et al. Non-alcoholic fatty liver disease comorbid with major depressive disorder: the pathological features and poor therapeutic efficacy. J Gastroenterol Hepatol. 2015;30(6):1009–1014. doi: 10.1111/jgh.12897. [DOI] [PubMed] [Google Scholar]
- 14.Youssef NA, Abdelmalek MF, Binks M, Guy CD, Omenetti A, Smith AD, et al. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013;33(7):1062–1070. doi: 10.1111/liv.12165. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein AA, Kallman Price J, Stepanova M, Poms LW, Fang Y, Moon J, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52(2):127–132. doi: 10.1016/j.psym.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Surdea-Blaga T, Dumitraşcu DL. Depression and anxiety in nonalcoholic steatohepatitis: is there any association? Rom J Intern Med. 2011;49(4):273–280. [PubMed] [Google Scholar]
- 17.Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of chronic liver disease with depression: a population-based study. Psychosomatics. 2013;54(1):52–59. doi: 10.1016/j.psym.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Jung JY, Park SK, Ryoo JH, Oh CM, Kang JG, Lee JH, et al. Effect of non-alcoholic fatty liver disease on left ventricular diastolic function and geometry in the Korean general population. Hepatol Res. 2017;47(6):522–532. doi: 10.1111/hepr.12770. [DOI] [PubMed] [Google Scholar]
- 19.Cho MJ, Kim KH. Use of the center for epidemiologic studies depression (CES-D) scale in Korea. J Nerv Ment Dis. 1998;186(5):304–310. doi: 10.1097/00005053-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 21.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 22.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54(3):1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6(1):33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 25.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunbar JA, Reddy P, Davis-Lameloise N, Philpot B, Laatikainen T, Kilkkinen A, et al. Depression: an important comorbidity with metabolic syndrome in a general population. Diabetes Care. 2008;31(12):2368–2373. doi: 10.2337/dc08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications. 2005;19(2):113–122. doi: 10.1016/j.jdiacomp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 29.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 30.Björntorp P. Visceral fat accumulation: the missing link between psychosocial factors and cardiovascular disease? J Intern Med. 1991;230(3):195–201. doi: 10.1111/j.1365-2796.1991.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 31.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 32.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 34.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]