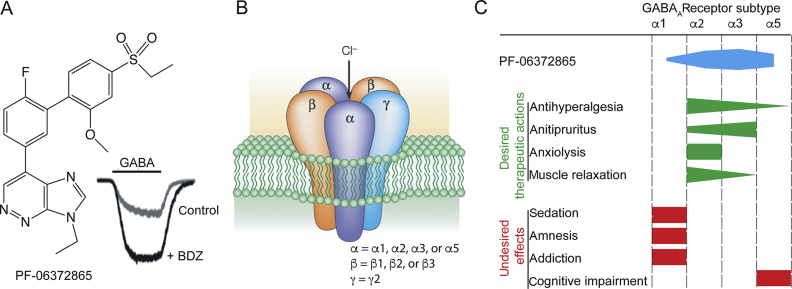
γ-Aminobutyric acid type A (GABAA) receptors, ligand-gated ion channel receptors for the inhibitory neurotransmitter GABA, mediate neuronal inhibition in the CNS, including the spinal cord. Based on their subunit compositions (more specifically the α subunit isoform included in the pentameric receptor complex), they can be subdivided into six major subtypes (from α1 to α6). GABAA receptors containing α1, α2, α3, or α5 subunits are sensitive to modulation by classical benzodiazepines, whereas those containing α4 or α6 subunits instead of α1, α2, α3, or α5 subunits are insensitive. The compound PF-06372865, the subject of a study by Nickolls and colleagues1 in a recent issue of the British Journal of Anaesthesia, was developed by Pfizer Inc (Cambridge, UK) as a partial agonist at the benzodiazepine binding sites of receptors containing α2, α3, or α5 subunits.1 It is mainly this lack of activity at α1 GABAA receptors that distinguishes PF-06372865 from classical benzodiazepines. Why is this important, and why should avoiding activity at α1 GABAA receptors convey analgesic efficacy to benzodiazepine site ligands?
Let us first consider the neurophysiological basis of GABAergic analgesia. This concept is rooted in the gate control theory of pain, in which Melzack and Wall2 proposed that inhibitory (mainly GABAergic) neurones of the spinal dorsal horn gate incoming nociceptive input and prevent the activation of ‘pain’ signalling projections by non-painful sensory input. A large body of evidence indicates that the efficacy of this inhibitory control is compromised in chronic pain states through several mechanisms.3 Accordingly, drugs that facilitate GABAergic inhibition should in principle be able to correct this deficit. Indeed, local spinal administration of drugs that enhance synaptic inhibition reverse heightened pathological pain sensitivity.4, 5 Work in mice expressing genetically engineered GABAA receptor α subunits has shown that α2 (and α3) GABAA receptors are the most relevant GABAA receptor subtypes for spinal analgesia, and that α1 and α5 receptors contribute either nothing (α1) or very little (α5) to this process.5, 6 This profile fits well with the enriched expression of α2 and α3 GABAA receptors within the spinal dorsal horn, particularly the superficial layers where incoming nociceptive fibres terminate.7 The dispensability of α1 GABAA receptors for analgesia was a crucial finding, as receptors of this subtype cause the great majority of the unwanted effects of classical benzodiazepine agonists including sedation, memory impairment, tolerance (loss of efficacy during chronic treatment), and addiction8 (Fig. 1). This segregation provides a hint as to why sparing activity at α1 receptors would confer analgesic activity.
Fig 1.
(a) Chemical structure of PF-06372865, a subtype-selective partial agonist at the benzodiazepine-binding site of α2, α3, and α5 γ-aminobutyric acid A (GABAA) receptors. The inset illustrates chloride currents through GABAA receptor channels recorded in the absence (control) and presence of a benzodiazepine (BDZ). (b) Schematic illustration and subunit composition of heteropentameric GABAA receptors. (c) Comparison of the subtype-selectivity of PF-06372865 (blue) with the contribution of the different GABAA receptor subtypes to desired (green) and undesired (red) in vivo actions of benzodiazepines.
The clinically tolerated doses of benzodiazepines (for indications other than anaesthesia) are limited by undesired sedation. In fact, typical clinically used doses of classical benzodiazepines yield receptor occupancies <30%.9, 10, 11, 12 Preclinical work has shown that this is too low to yield significant relevant analgesia, explaining why classical non-selective benzodiazepines are basically devoid of analgesic efficacy. Only when activity at α1 GABAA receptors is sufficiently reduced can the higher doses needed for analgesic efficacy be reached without putting a patient (or an animal) to sleep (see the work of Zeilhofer and colleagues13). Inspired by these results, several groups have tested subtype-selective benzodiazepine site agonists in various rodent pain models. Originally, these compounds were developed by groups working in pharma companies, and in academia in the quest for non-sedative anxiolytics. The major outcome of these studies was that such compounds reverse pathological hyperalgesia in most neuropathic and inflammatory pain models and also in postoperative pain, provided that the compounds possessed sufficiently high modulatory activity and were used at sufficiently high doses. Such antihyperalgesic activity does not occur with classical (non-selective) benzodiazepines given systemically. In light of these encouraging preclinical data, scientists have eagerly awaited clinical studies.
The first clinical study of potential analgesic actions of a subtype-selective benzodiazepine site ligand (PF-06372865, the same compound studied by Nickolls and colleagues1) did not assess efficacy in experimental human pain, as expected, but rather was a phase II clinical trial in patients with chronic low back pain.14 This trial yielded clearly negative results. We speculated15 about the possible reason(s) for this failure such as species differences in target receptor expression and function between rodents and humans, the low predictive value of preclinical read-outs (both very fashionable critiques nowadays), an inappropriate patient population (patients with a neuropathic pain component were actually excluded), and insufficient drug dosing.
The new study provides new insights and helps to further narrow the reasons of the failed low back pain trial. PF-06372865 was tested in a battery of experimental pain models in healthy volunteers, and its efficacy was compared with that of pregabalin, the current gold standard in neuropathic pain treatment. PF-06372865 proved efficacious in several models with effect sizes comparable with those of pregabalin. Notably, these positive results suggest that selective α2 and α3 GABAA receptor modulators exert analgesia not only in rodents but also in man, thereby largely ruling out species differences or low predictive values of read-outs in rodent experiments as underlying causes of the failure in the low back pain trial. Instead, the new study supports that insufficient drug dosing was the main problem in the low back pain trial. Nickolls and colleagues1 tested single doses of 15 and 65 mg that led to estimated receptor occupancy of 50% and 80%, respectively, whereas the low back pain trial used smaller doses of 2.5 and 7.5 mg given twice per day, with estimated peak steady-state α2 GABAA receptor occupancies of 30% and 50%.14 Preclinical studies in mice indicated that ∼70% of α2 GABAA receptors need to be drug-bound (even when a high intrinsic activity ligand is used) to achieve a significant reduction in pain thresholds.6 Therefore, the new study supports a lack of sufficient drug dosing as the most probable reason for failure of PF-06372865 in the low back pain trial. This is further supported by another recently published clinical trial on PF-06372865 that used the same dose regimens as the low back pain trial and failed to demonstrate efficacy against generalised anxiety disorders.16
Although the results of Nickolls and colleagues1 generally support previous preclinical findings in mouse pain models, there are also interesting discrepancies. The most obvious in our opinion is that PF-06372865 showed efficacy against acute nociceptive pain, specifically in the mechanical pressure pain, cold pressor pain, and electrically evoked pain tests in humans. No significant efficacy was detected in acute models of hyperalgesia (the sunburn model) and against heat-evoked pain. PF-06372865 was therefore mostly effective against pain modalities that appeared resistant to GABAA receptor modulation in mouse experiments.17 The reasons for these differences are not entirely clear. However, it is tempting to speculate that differences in the read-out measures are relevant. In the human study, PF-06372865 mainly affected pain tolerance thresholds but less so pain detection thresholds, which were the main readouts in the mouse pain models. Taking these differences into account, the study results are consistent with what could have been predicted from previous preclinical experiments.
Although the new study is far from providing a definitive answer to the ongoing question of translatability in drug research and development, this was never its intended purpose. Importantly, it puts the previous negative result of the low back pain trial into perspective. If insufficient drug dosing was the reason for the failure of the low back pain trial, the good news is that clinical studies published so far have reported excellent tolerability1, 14, 16 and suggest that there is significant space for dose augmentation before unwanted effects become dose limiting.
In the film Back to the Future, the main character Marty McFly successfully returns home from accidentally being sent 30 yr into the past to find his family in much better circumstances than before he left. This concept, rooted in science fiction, holds some allure in the real-life world of drug research and development. If only we could go back in time and tweak our experimental designs. The film ends with their car converted into a hovercraft flying at the camera and the words ‘To be continued…’ flashing on the screen. We hope that this is also the case for drugs targeting specific GABAA receptor subtypes in chronic pain.
Authors' contributions
All authors have contributed equally and approve this editorial.
Declarations of interest
The authors have no conflicts to declare.
References
- 1.Nickolls S.A., Gurrell R., van Amerongen G. Pharmacology in translation: the preclinical and early clinical profile of the novel α2/3 functionally selective GABAA receptor positive allosteric modulator PF-06372865. Br J Pharmacol. 2018;175:708–725. doi: 10.1111/bph.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 3.Zeilhofer H.U., Benke D., Yévenes G.E. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol. 2012;52:111–133. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- 4.Huang X., Shaffer P.L., Ayube S. Crystal structures of human glycine receptor α3 bound to a novel class of analgesic potentiators. Nat Struct Mol Biol. 2017;24:108–113. doi: 10.1038/nsmb.3329. [DOI] [PubMed] [Google Scholar]
- 5.Knabl J., Witschi R., Hösl K. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 6.Ralvenius W.T., Benke D., Acuña M.A., Rudolph U., Zeilhofer H.U. Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat Commun. 2015;6:6803. doi: 10.1038/ncomms7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul J., Zeilhofer H.U., Fritschy J.M. Selective distribution of GABAA receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J Comp Neurol. 2012;520:3895–3911. doi: 10.1002/cne.23129. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph U., Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinotoh H., Iyo M., Yamada T. Detection of benzodiazepine receptor occupancy in the human brain by positron emission tomography. Psychopharmacology (Berl) 1989;99:202–207. doi: 10.1007/BF00442808. [DOI] [PubMed] [Google Scholar]
- 10.Pauli S., Farde L., Halldin C., Sedvall G. Occupancy of the central benzodiazepine receptors during benzodiazepine treatment determined by PET. Eur Neuropsychopharmacol. 1991;1:229–231. [Google Scholar]
- 11.Malizia A., Forse G., Haida A. A new human (psycho)pharmacology tool: the multiple organs coincidences counter (MOCC) J Psychopharmacol. 1995;9:294–306. doi: 10.1177/026988119500900402. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M., Woods S.W., Verhoeff N.P. Changes of benzodiazepine receptors during chronic benzodiazepine administration in humans. Eur J Pharmacol. 1999;368:161–172. doi: 10.1016/s0014-2999(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 13.Zeilhofer H.U., Ralvenius W.T., Acuna M.A. Restoring the spinal pain gate: GABAA receptors as targets for novel analgesics. Adv Pharmacol. 2015;73:71–96. doi: 10.1016/bs.apha.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Gurrell R., Dua P., Feng G. A randomised, placebo-controlled clinical trial with the α2/3/5 subunit selective GABA-A positive allosteric modulator PF-06372865 in patients with chronic low back pain. Pain. 2018;159:1742–1751. doi: 10.1097/j.pain.0000000000001267. [DOI] [PubMed] [Google Scholar]
- 15.Zeilhofer H.U., Neumann E., Munro G. Spinal GABAA receptors for pain control-lost in translation? Pain. 2018;159:1675–1676. doi: 10.1097/j.pain.0000000000001268. [DOI] [PubMed] [Google Scholar]
- 16.Simen A., Whitlock M., Qiu R. An 8-week, randomized, phase 2, double-blind, sequential parallel-group comparison study of two dose levels of the GABAA positive allosteric modulator PF-06372865 compared with placebo as an adjunctive treatment in outpatients with inadequate response to standard of care for generalized anxiety disorder. J Clin Psychopharmacol. 2019;39:20–27. doi: 10.1097/JCP.0000000000000997. [DOI] [PubMed] [Google Scholar]
- 17.Ralvenius W.T., Neumann E., Pagani M. Itch suppression in mice and dogs by modulation of spinal α2 and α3GABAA receptors. Nat Commun. 2018;9:3230. doi: 10.1038/s41467-018-05709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]