Summary
The study of rare families with inherited pain insensitivity can identify new human-validated analgesic drug targets. Here, a 66-yr-old female presented with nil requirement for postoperative analgesia after a normally painful orthopaedic hand surgery (trapeziectomy). Further investigations revealed a lifelong history of painless injuries, such as frequent cuts and burns, which were observed to heal quickly. We report the causative mutations for this new pain insensitivity disorder: the co-inheritance of (i) a microdeletion in dorsal root ganglia and brain-expressed pseudogene, FAAH-OUT, which we cloned from the fatty-acid amide hydrolase (FAAH) chromosomal region; and (ii) a common functional single-nucleotide polymorphism in FAAH conferring reduced expression and activity. Circulating concentrations of anandamide and related fatty-acid amides (palmitoylethanolamide and oleoylethanolamine) that are all normally degraded by FAAH were significantly elevated in peripheral blood compared with normal control carriers of the hypomorphic single-nucleotide polymorphism. The genetic findings and elevated circulating fatty-acid amides are consistent with a phenotype resulting from enhanced endocannabinoid signalling and a loss of function of FAAH. Our results highlight previously unknown complexity at the FAAH genomic locus involving the expression of FAAH-OUT, a novel pseudogene and long non-coding RNA. These data suggest new routes to develop FAAH-based analgesia by targeting of FAAH-OUT, which could significantly improve the treatment of postoperative pain and potentially chronic pain and anxiety disorders.
Keywords: anandamide, anxiolytic, endocannabinoids, pain insensitivity, postoperative analgesia
Fatty-acid amide hydrolase (FAAH) is the major catabolic enzyme for a range of bioactive lipids called fatty-acid amides (FAAs).1, 2 These FAAs include N-acyl ethanolamines, such as anandamide (AEA), that act as endogenous ligands for cannabinoid receptors (i.e. endocannabinoids). Other substrates of FAAH include palmitoylethanolamide (PEA), oleoylethanolamine (OEA), and N-acyl-taurines. 2-Arachidonoylglycerol (2-AG) is another related endocannabinoid and FAA, but is metabolised mostly by monoacylglycerol lipase (MAGL). AEA has roles in nociception, fear-extinction memory, anxiety, and depression.3, 4 FAAH knockout mice have elevated brain concentrations of AEA, display an analgesic phenotype in response to acute thermal stimuli, and show reduced pain in formalin and carrageenan inflammatory models.5, 6 FAAH is therefore an attractive drug target for treating pain, anxiety, and depression, although recent clinical trials with FAAH inhibitors were unsuccessful.7, 8
The human FAAH gene contains a commonly carried hypomorphic single-nucleotide polymorphism (SNP) (C385A; rs324420; C allele frequency 74%, A 26%) that significantly reduces the activity of the FAAH enzyme.9 Genetic association studies have investigated the link between this and other FAAH SNPs and pain sensitivity.10, 11, 12 Notably, homozygous carriers of the hypomorphic SNP (A allele) in a cohort of women undergoing breast cancer surgery were less sensitive to cold pain and had a reduced need for postoperative analgesia.10 Furthermore, a mouse knock-in model of the human SNP showed that both the mouse and human SNP carriers display enhanced fear-extinction learning and decreased anxiety-linked behaviours.13 Here, we describe a pain-insensitive patient with a non-anxious disposition presenting with a novel genetic disorder associated with loss of function of FAAH.
Case report
A 66-yr-old Caucasian female presented to Raigmore Hospital in Inverness, Scotland for orthopaedic surgery, specifically a trapeziectomy with ligament reconstruction and tendon interposition and extensor pollicis longus realignment after a diagnosis of bilateral pantrapezial osteoarthritis. There was significant deformity and deterioration in the use of the right thumb, which was reported as painless before operation. The pre-assessment note classed her as ASA physical status 1, but highlighted that she had a history of vomiting after intake of morphine.
For the surgery, she received general anaesthesia with an ultrasound-guided axillary nerve block. She received fentanyl 50 μg i.v., propofol 200 mg i.v., ondansetron 4 mg i.v. intraoperatively, and levobupivacaine 0.25% (20 ml) for the axillary nerve block. After operation, her pain intensity score was 0/10 until the next day when she was discharged home. The only postoperative analgesic she received in hospital was paracetamol 1 g i.v. in the PACU on the day of her surgery. She also received cyclizine 50 mg i.v. twice. Extraordinarily, she required no postoperative analgesics other than paracetamol for this known painful surgery (trapeziectomy), even after the axillary nerve block had worn off. She showed no pain from pinching or from peripheral i.v. cannula manipulation, which led to further investigations.
The patient had been diagnosed with osteoarthritis of the hip, which she reported as painless, which was not consistent with the severe degree of joint degeneration. At 65 yr of age, she had undergone a hip replacement and was administered only paracetamol 2 g orally on Postoperative days 1 and 2, reporting that she was encouraged to take the paracetamol, but that she did not ask for any analgesics. She was also administered a single dose of morphine sulphate 10 mg orally on the first postoperative evening that caused severe nausea and vomiting for 2 days. After operation, her pain intensity scores were 0/10 throughout except for one score of 1/10 on the first postoperative evening. Her past surgical history was notable for multiple varicose vein and dental procedures for which she has never required analgesia. She also reported a long history of painless injuries (e.g. suturing of a laceration and left wrist fracture) for which she did not use analgesics. She reported numerous burns and cuts without pain (Supplementary Fig. S1), often smelling her burning flesh before noticing any injury, and that these wounds healed quickly with little or no residual scar. She reported eating Scotch bonnet chilli peppers without any discomfort, but a short-lasting ‘pleasant glow’ in her mouth. She described sweating normally in warm conditions.
The patient lives with her husband, and has a daughter and a son from her previous marriage. Her family history is unremarkable for neuropathy or painful conditions. Her mother and daughter appear to perceive pain normally. Her father (now deceased) had little requirement for pain killers. Her son also reports of having some degree of pain insensitivity, but not to the same extent as her. She does not take any medication at present, and is fit and active with no medical conditions apart from arthritis (Supplementary Fig. S2). She is talkative and happy with an optimistic outlook. On the Generalized Anxiety Disorder-7 anxiety questionnaire taken at age 70, she scored 0/21, classified as mild (the lowest category).14 Likewise, on the Patient Health Questionnaire-9 for depression, she scored 0/29, classified as mild.15 She reported long-standing memory lapses (e.g. frequently forgetting words mid-sentence and placement of keys). She also reported never panicking, not even in dangerous or fearful situations, such as in a recent road traffic accident.
After the painless trapeziectomy surgery and a history of ‘painless operations’, she was referred to and further investigated by pain genetics teams from University College London and the University of Oxford at age 67 yr. Ethical approval was granted from both institutions, and written consent taken from the patient, her two children, and mother. On clinical examination, she had multiple scars around the arms and on the back of her hands. Quantitative sensory testing (Supplementary Fig. S3) demonstrated hyposensitivity to noxious heat both in the hands and feet (see Supplementary data for further clinical details).
Genetic tests identify a microdeletion downstream of FAAH
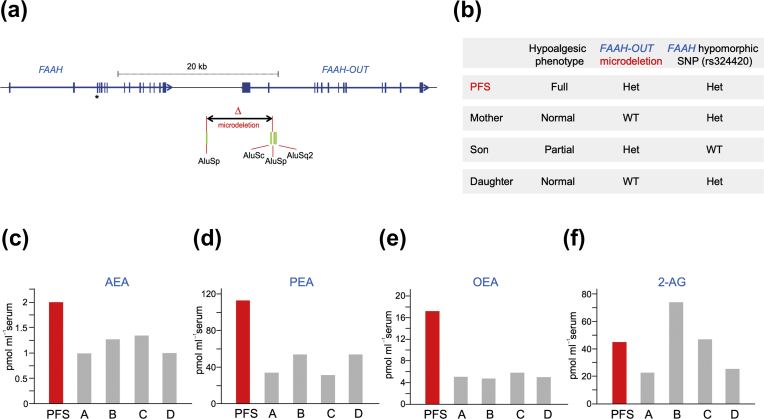
Genomic DNA was isolated from the patient, her two children, and her mother for exome sequencing. After filtering of variants, four candidate mutations in the patient and her son were identified, but none were considered likely to be causal for the phenotype (see Supplementary data). We broadened our genetic analyses and searched for cytogenetic copy number changes across the genome using the CytoScan™ HD Array (Thermo Fisher Scientific, UK). This identified an ∼8 kb heterozygous microdeletion on Chromosome 1 that began ∼4.7 kb downstream from the 3′ end of FAAH (Fig 1a; Supplementary Fig. S4). Polymerase chain reaction and sequencing analyses confirmed that the patient co-inherited the microdeletion and FAAH hypomorphic SNP allele (rs324420) (Fig 1b). Her unaffected mother and daughter did not carry the microdeletion, but her son, who also has some pain-sensitivity deficits, was heterozygous for the microdeletion (Supplementary Fig. S5), but did not carry the hypomorphic SNP allele. One Colombian male (HG01353) (pain phenotype unknown) out of 5008 alleles screened in the 1000 Genomes Project also carries a similar-sized microdeletion (esv3585936 in Supplementary Table S1), but is homozygous wild type for FAAH SNP rs324420.
Fig 1.
(a) Genomic map showing FAAH, FAAH-OUT, and microdeletion. Human chromosome 1 showing the gene footprints of FAAH and FAAH-OUT. Exons are denoted by blue boxes and the direction of transcription shown by arrows. FAAH single-nucleotide polymorphism (SNP) rs324420 maps to Exon 3 (indicated by an asterisk). The 8131 bp microdeletion detected in the patient is shown flanked by Alu repeated sequences (green boxes), which likely predispose the genomic region to rearrangements. The promoter region and Exons 1 and 2 of FAAH-OUT map to the deleted sequence. (b) Genotype summary. The proband carries both the FAAH-OUT microdeletion and the hypomorphic FAAH SNP, and displayed a full hypoalgesic phenotype. Her son carries the FAAH-OUT microdeletion and had a partial hypoalgesic phenotype. Neither the unaffected mother nor daughter carries the microdeletion. (c–f) Circulating anandamide (AEA), palmitoylethanolamide (PEA), oleoylethanolamine (OEA), and 2-arachidonoylglycerol (2-AG) concentrations. Concentrations of AEA, PEA, OEA, and 2-AG were measured by mass spectrometry from blood samples obtained from the patient and four unrelated normal controls. AEA, PEA, and OEA are substrates for FAAH; 2-AG is not. Controls A and B are homozygous wild type for the hypomorphic SNP; Controls C and D are heterozygous carriers. Average values for the controls were AEA (1.2 pmol ml−1), PEA (43.4 pmol ml−1), OEA (5.1 pmol ml−1), and 2-AG (42.2 pmol ml−1), which is consistent with previous data using a similar measurement protocol.16 Average values for the patient (two measurements) were AEA (2.0 pmol ml−1), PEA (113.1 pmol ml−1), OEA (17.3 pmol ml−1), and 2-AG (45 pmol ml−1).
Given the extraordinary phenotype in the patient and the vicinity of the microdeletion to FAAH, we investigated how the microdeletion could be pathogenic. Molecular cloning experiments (see Supplementary data) identified novel 5′exons of an expressed FAAH pseudogene, herein called FAAH-OUT (2.845 kb cDNA; KU950306), that mapped to within the microdeletion (Fig 1a). Tissue expression analyses showed FAAH-OUT to be expressed in a wide range of tissues, including fetal and adult brain, and in dorsal root ganglia (DRG; Supplementary Fig. S6). FAAH-OUT likely encodes a long non-coding RNA (Supplementary Fig. S7). We considered that the microdeletion may negate the normal function of FAAH through a reduction in neural expression of FAAH-OUT or through loss of a critical genomic regulatory element for FAAH, and hence, obtained blood samples to measure FAAH-regulated lipids.
Elevated FAA concentrations in blood
To determine the effects of carrying both the microdeletion in FAAH-OUT and the hypomorphic FAAH SNP, we measured the circulating FAA concentrations from blood samples from the patient and four controls, two of which were heterozygous carriers of the SNP. Circulating concentrations of AEA were increased by 70% and of OEA and PEA approximately tripled compared with controls (Fig. 1c–e). The concentrations of 2-AG, another endocannabinoid that is mainly degraded by MAGL and not FAAH, were largely unaltered (Fig 1f). These results are consistent with FAAH having a significant loss of function in the patient.
Discussion
The endocannabinoid system is an important physiological system that performs a wide array of homeostatic functions and is important for pain perception.17 FAAH is a critical enzyme for the breakdown of a range of bioactive lipids (including the endocannabinoid AEA and related FAAs and N-acyl-taurines) with diverse physiological roles. Mouse modelling of FAAH loss of function mutations and pharmacological inhibition studies have shown a range of phenotypes, including hypoalgesia, accelerated skin wound healing, enhanced fear-extinction memory, reduced anxiety, and short-term memory deficits.6, 13, 18, 19, 20, 21 Furthermore, human hypomorphic FAAH SNPs are associated with a reduced need for postoperative analgesia, increased postoperative nausea and vomiting induced by opioids, and decreased anxiety-linked behaviours.10, 13, 16, 22, 23, 24
Here, we report a new human genetic disorder in a patient with hypoalgesia, altered fear and memory symptoms, and a non-anxious disposition. This disorder is attributable to co-inheritance of a microdeletion in a novel pseudogene and a known FAAH hypomorphic SNP. The microdeletion is flanked by repeated sequences that likely predispose the region to genomic rearrangements, as seen in other genomic disorders.25 Consequently, there are likely to be additional similar individuals in the general population. The likelihood that this disorder has been under-reported is highlighted by the fact that the patient was diagnosed at age 66 yr despite a recurrent history of painless injuries. Lipid profiling in peripheral blood showed significant increases in AEA, OEA, and PEA, which could be further exaggerated in the brain and DRG. Further work is needed to understand which FAA is the major contributor to the painless phenotype.
The microdeletion removes the promoter and first two exons of FAAH-OUT, but how this disrupts the function of FAAH is still to be elucidated. A hypothesis is that the FAAH-OUT transcript normally functions as a decoy for microRNAs as a result of the high sequence homology, and protects FAAH mRNA from degradation (Supplementary Fig. S7).26 Alternatively, FAAH-OUT may have an epigenetic role in regulating FAAH transcription, or the deletion removes a critical transcriptional regulatory element.25, 27 Future work will help us to understand whether targeting FAAH-OUT by viral shRNA or gene editing techniques is an effective analgesic/anxiolytic drug development strategy.
This patient provides new insights into the role of the endocannabinoid system in analgesia and more specifically on the FAAH genomic locus, and highlights the importance of the adjacent, previously uncharacterised FAAH-OUT gene to pain sensation. Given the previous failure of FAAH-inhibitor analgesic drug trials, this report has significance, as it provides a new route to developing FAAH-related analgesia through targeting of FAAH-OUT.
Authors' contributions
Clinical work: HH, JDR, DLHB, DS.
Molecular genetics: AMH, ALO, MCL, SL, SJG, JJC.
Exome sequencing data analyses: JTB.
Bioinformatics: AMH, ALO, JTB, MCL, JJC.
Blood preparation and anandamide analyses: MNH, MvD, MM.
Research design: DS, JJC.
Wrote the manuscript with help from all authors: JJC.
Approved the final manuscript: all authors.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Acknowledgements
The authors would like to thank the family for participation in this study, and the volunteers for donating blood samples for analyses. The authors thank John N. Wood for helpful discussions and advice throughout this project. The authors also thank Patrick Fox, Hamish Hay, and Louise Reid (Raigmore Hospital, Inverness, UK); Iain Jones (Southern General Hospital, Glasgow, Scotland); and Judith Singleton (Leith Walk Surgery, Edinburgh, UK) for help with blood sampling, and Sylvie Rose (formerly Addenbrooke's Hospital, Cambridge, UK) for help and advice regarding the cytogenetics analyses. The authors thank the Southern Alberta Mass Spectrometry Centre, located in and supported by the Cumming School of Medicine, University of Calgary, for their services in targeted liquid chromatography–tandem mass spectrometry.
Handling editor: H.C. Hemmings Jr
Editorial decision: 22 February 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.02.019.
Contributor Information
Devjit Srivastava, Email: dev.srivastava@nhs.net.
James J. Cox, Email: j.j.cox@ucl.ac.uk.
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
Medical Research Council (Career Development Award G1100340 to JJC); Wellcome Trust (200183/Z/15/Z to JJC, 095698Z/11/Z and 202747/Z/16/Z to DLHB); Alzheimer's Society (research fellowship to JTB), University of Cambridge Academic Foundation Programme (to MCL); Molecular Nociception Group (to MCL); National Institutes of Health (Bethesda, MD, USA) Ruth L. Kirschstein Institutional National Research Service Award (to MCL); Wellcome Trust funded London Pain Consortium (to JDR); Colciencias through a Francisco Jose de Caldas Scholarship (LASPAU, Harvard University) (to JDR); Canadian Institutes of Health Research (CIHR; to MNH); CIHR (postdoctoral funding to MM).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Deutsch D.G., Chin S.A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 2.Cravatt B.F., Giang D.K., Mayfield S.P., Boger D.L., Lerner R.A., Gilula N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 3.Woodhams S.G., Sagar D.R., Burston J.J., Chapman V. The role of the endocannabinoid system in pain. Handb Exp Pharmacol. 2015;227:119–143. doi: 10.1007/978-3-662-46450-2_7. [DOI] [PubMed] [Google Scholar]
- 4.Mechoulam R., Parker L.A. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 5.Cravatt B.F., Demarest K., Patricelli M.P. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtman A.H., Shelton C.C., Advani T., Cravatt B.F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Huggins J.P., Smart T.S., Langman S., Taylor L., Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–1846. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Kerbrat A., Ferre J.C., Fillatre P. Acute neurologic disorder from an inhibitor of fatty acid amide hydrolase. N Engl J Med. 2016;375:1717–1725. doi: 10.1056/NEJMoa1604221. [DOI] [PubMed] [Google Scholar]
- 9.Chiang K.P., Gerber A.L., Sipe J.C., Cravatt B.F. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 10.Cajanus K., Holmstrom E.J., Wessman M., Anttila V., Kaunisto M.A., Kalso E. Effect of endocannabinoid degradation on pain: role of FAAH polymorphisms in experimental and postoperative pain in women treated for breast cancer. Pain. 2016;157:361–369. doi: 10.1097/j.pain.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 11.Kim H., Mittal D.P., Iadarola M.J., Dionne R.A. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Med Genet. 2006;43:e40. doi: 10.1136/jmg.2005.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenbaum L., Tegeder I., Barhum Y., Melamed E., Roditi Y., Djaldetti R. Contribution of genetic variants to pain susceptibility in Parkinson disease. Eur J Pain. 2012;16:1243–1250. doi: 10.1002/j.1532-2149.2012.00134.x. [DOI] [PubMed] [Google Scholar]
- 13.Dincheva I., Drysdale A.T., Hartley C.A. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun. 2015;6:6395. doi: 10.1038/ncomms7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowe B., Decker O., Muller S. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. 2008;46:266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spagnolo P.A., Ramchandani V.A., Schwandt M.L. FAAH gene variation moderates stress response and symptom severity in patients with posttraumatic stress disorder and comorbid alcohol dependence. Alcohol Clin Exp Res. 2016;40:2426–2434. doi: 10.1111/acer.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aizpurua-Olaizola O., Elezgarai I., Rico-Barrio I., Zarandona I., Etxebarria N., Usobiaga A. Targeting the endocannabinoid system: future therapeutic strategies. Drug Discov Today. 2017;22:105–110. doi: 10.1016/j.drudis.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Sasso O., Pontis S., Armirotti A. Endogenous N-acyl taurines regulate skin wound healing. Proc Natl Acad Sci USA. 2016;113:E4397–E4406. doi: 10.1073/pnas.1605578113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goonawardena A.V., Sesay J., Sexton C.A., Riedel G., Hampson R.E. Pharmacological elevation of anandamide impairs short-term memory by altering the neurophysiology in the hippocampus. Neuropharmacology. 2011;61:1016–1025. doi: 10.1016/j.neuropharm.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clapper J.R., Moreno-Sanz G., Russo R. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kathuria S., Gaetani S., Fegley D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 22.Sadhasivam S., Zhang X., Chidambaran V. Novel associations between FAAH genetic variants and postoperative central opioid-related adverse effects. Pharmacogenomic. J. 2015;15:436–442. doi: 10.1038/tpj.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hariri A.R., Gorka A., Hyde L.W. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunduz-Cinar O., MacPherson K.P., Cinar R. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry. 2013;18:813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harel T., Lupski J.R. Genomic disorders 20 years on—mechanisms for clinical manifestations. Clin Genet. 2018;93:439–449. doi: 10.1111/cge.13146. [DOI] [PubMed] [Google Scholar]
- 26.Ebert M.S., Sharp P.A. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20:R858–R861. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elling R., Robinson E.K., Shapleigh B. Genetic models reveal cis and trans immune-regulatory activities for lincRNA-Cox2. Cell Rep. 2018;25 doi: 10.1016/j.celrep.2018.10.027. 1511.e6-1524.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.