Abstract
Objectives
The present study explored the changes in spontaneous regional activity in post-traumatic stress disorder (PTSD) patients, who experienced severe traffic accidents.
Methods
20 drug-naive PTSD patients and 18 healthy control subjects were imaged using resting-state functional magnetic resonance imaging (rs-fMRI) and analyzed by the algorithm of regional homogeneity (ReHo).
Results
Compared to the healthy control group, the PTSD group showed decreased ReHo values in the right angular gyrus. In addition, a negative correlation was found between the activity level of the angular gyrus and the CAPS score.
Conclusion
The dysfunctions were found in the memory- and emotion-related areas, suggested a possible mechanism of memory dysregulation that might be related to the intrusive memory symptoms of PTSD. These results provided imaging evidence that might provide an in-depth understanding of the intrinsic functional architecture of PTSD.
Keywords: PTSD, Angular gyrus, fMRI, Traffic accidents
Highlights
-
•
This research focus on the PTSD patients without comorbidities, so that the confounding factors could be avoided.
-
•
This research using regional homogeneity measured the temporal synchronization of the intrinsic spontaneous brain activity of the voxels. ReHo is a reliable and efficient index that has been successfully applied in many conditions.
-
•
This study explored the changes in spontaneous regional activity in post-traumatic stress disorder patients, who experienced severe traffic accidents.
-
•
Compared to the normal control group, the PTSD group showed lower ReHo values in the right angular. These results provided imaging evidence that might provide an in-depth understanding of the intrinsic functional architecture of PTSD.
1. Introduction
Post-traumatic stress disorder (PTSD) is a trauma- and stress-related disorder that develops in the aftermath of the exposure to severe traumatic events, including severe traffic accidents, natural disasters, and combat (Association, A.P., 2000). PTSD occurs in 5–10% of the general population (Yehuda et al., 2015), which is primarily caused by severe traffic accidents (Sun et al., 2015). Following the progression of PTSD, some patients suffer from comorbidities (Gupta, 2013) and self-harm behaviors (Kessler and Wang, 2008; Sareen et al., 2007) that could be severely damaging to the health of the patients. The primary symptoms of PTSD include intrusive memory, negative cognition, hyper-vigilance, and avoidance; among these, the intrusive memory is the hallmark symptom of PTSD (Graham et al., 2003). Due to the high rate of dissociative symptoms and cognitive deficits, an in-depth understanding of the neurobiological mechanisms of PTSD is essential.
Resting-state functional magnetic resonance imaging (rs-fMRI) serves as an effective method to reveal the intrinsic brain functional alterations underlying PTSD. Notably, the inconsistent findings have been reported in the literature, putatively due to disparate experimental approaches, different trauma types, and various control groups (Patel et al., 2012). Some rs-fMRI studies focused on the remote functional connectivity (FC). Using the long-range FC analysis, some aberrant changes have been found in the emotion processing and memory-related areas, including precuneus, middle temporal gyrus, insula, amygdala, and parahippocampal gyrus (Jin et al., 2013; Lei et al., 2015). Some inconsistent findings have been reported for the amygdala, precuneus, hippocampus, and the middle prefrontal cortex (Bluhm et al., 2009; Phan, 2011; Sripada et al., 2012). However, these studies focused on the long-range alterations in the human brain; if these long-range patterns of connectivity were discovered by a seed-based method, a bias might result from the specifically selected seed region.
As a progressive approach to analyze the functional specialization, regional homogeneity (ReHo) measured the temporal synchronization of a given voxel with its nearest neighbors (Zang et al., 2004). ReHo is a reliable and efficient index (Zuo et al., 2013) that has been successfully applied in several conditions, including primary insomnia (Wang et al., 2016a, Wang et al., 2016b), diabetes (Peng et al., 2016), Parkinson's disease (Wu et al., 2009), and nicotine addiction (Chen and Mo, 2017). A previous PTSD study compared the ReHo of 54 PTSD patients (earthquake survivors) and 74 traumatized controls. The results showed increased ReHo values in the left inferior parietal lobe and right superior frontal gyrus and decreased ReHo values in the right middle temporal gyrus and right lingual gyrus in PTSD patients (Yin et al., 2012). Another study combined the ReHo with long-range FC to study the intrinsic brain alterations in 27 PTSD patients (typhoon survivors), 33 traumatized controls, and 30 healthy controls. The PTSD patients showed increased ReHo values in the insula, parahippocampal gyrus, middle frontal gyrus, precuneus, and middle cingulate gyrus and decreased ReHo values in the lingual gyrus, amygdala, and bilateral frontal cortex (Ke et al., 2016). Furthermore, the study also focused on the natural disaster survivors, who experienced severe mental and physical injuries, due to which, they might develop multiple mental disorders simultaneously (Zongtang et al., 2017), and these comorbidities could be confounding factors (Koch et al., 2016). Therefore, the current study focused on comparing the PTSD patients (without comorbidities) with the normal individuals.
In the present study, we illustrated the ReHo maps for the PTSD patients and normal controls and detected significant differences in spontaneous brain activities. Next, we performed correlation analysis with clinical indicators (CAPS, SAS, SDS, MMSE) and mean ReHo value from clusters with significant group differences. Based on the previous studies, we hypothesized that the PTSD patients would exhibit aberrant ReHo values in regions that might be related to memory and emotion, such as the hippocampus/parahippocampal gyrus, amygdala, and precuneus.
2. Materials and methods
2.1. Participants
Twenty drug-naive PTSD patients (7 males and 13 females; mean age: 38.7 ± 8.2 years; range: 25–52 years) and 18 healthy control subjects (7 males and 11 females; mean age: 42.17 ± 8.38 years; range 24–52 years), matched demographically, were enrolled at Guangdong Second Provincial General Hospital, China, in this study. From January 2016 to October 2017, 25 patients, who had experienced severe traffic accidents received first-aid in the emergency center of the Guangdong Second Provincial General hospital. Subsequently, 20 were diagnosed with PTSD, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Version-IV (known as DSM-IV) and CAPS by an experienced psychiatrist (H.R.W, 25 years of clinical experience). In addition, 18 healthy participants recruited from the local community constituted the healthy control group, and each was matched in terms of gender, hand dominance, age, and education level.
The inclusion criteria for the PTSD patients were as follows: (i) Personal experience of a severe traffic accident, witness of death, or severe physical injury; (ii) Age > 18 years; (iii) Fulfilling the criteria of DSM-IV; (iv) Right hand dominance. The PTSD patients were excluded when: (i) The criteria of DSM-IV was not fulfilled or CAPS score < 40; (ii) Patients with brain injury or other abnormality was detected on MRI; (iii) Contraindication in MRI; (iv) Preexisting mental disorders; (v) Pregnancy, nursing, or menstruation; (vi) Drug abuse, alcohol addiction, or recent medication that might affect the brain function.
Healthy control subjects were enrolled according to following criteria: (i) Age > 18 years; (ii) No brain lesions; (iii) No contraindication in MRI; (iv) No mental disorder and drug abuse history; (v) Females were not pregnant, nursing, or menstruating.
The image datasets were evaluated by two experienced radiologists who were blinded to whether the images from the PTSD patients or the healthy control. The clinical and demographic data were analyzed by SPSS version 23.
The research ethics committee of the Institute at Guangdong Second Provincial General Hospital approved this study. Each participant was provided a precise information table, and written consent was obtained prior to the MR scanning.
2.2. Methods
2.2.1. Assessment of PTSD and mental status
Before resting-state MRI, all participants were required to undergo CAPS, Minimum Mental State Examination (MMSE), Self-rating Depression Scale (SDS), and Self-Rating Anxiety Scale (SAS), under the supervision of a psychiatrist, in order to ensure that the mental status of all the participants was fully understood and appropriately classified.
2.2.2. MRI data acquisition
All the participants underwent resting-state MRI in a 3.0-T MR imager (Ingenia; Philips, Best, The Netherlands) at the Department of Medical Imaging at Guangdong Second Provincial General Hospital. The sequence consisted of gradient echo-planar imaging (EPI) with the following parameters: repetition time (TR)/echo time (TE) = 2000 ms/30 ms; matrix = 64 × 64; field-of-view = 230 mm × 230 mm; flip angle = 90°; slice thickness = 3.6 mm, 0.6 mm gap; interleaved scanning; 38 transverse slices covering the whole brain at all 240 volumes were acquired for each participant within 480 s. During rs-fMRI scanning, subjects were instructed to close their eyes and lie still but avoid falling asleep or thinking of anything in particular. Additionally, the T1-weighted images and T2-FLAIR images were obtained in order to detect the brain lesions. The T1-weighted images were obtained using a fast field echo (FFE) pulse sequence with repetition time (TR) = 25 ms, echo time (TE) = 4.1 ms, flip angle (FA) = 30°, acquisition matrix = 256 × 256, field of view (FOV) = 230 mm2, slice thickness = 1.0 mm, and 160 sagittal slices. Subsequently, some questions were asked to evaluate the cooperation status of the subjects.
2.2.3. Data processing and ReHo calculations
All EPI data were processed using DPARSF 3.0 Advanced Edition (http://rfmri.org/DPARSF). The first 10 time points were discarded to allowed for stabilization of the magnetic field and participants adapting to the fMRI noise. The remaining rs-fMRI datasets were corrected for intra-volume acquisition time delay, inter-volume head motion and co-registered with anatomical scan. The co-registered anatomical images were segmented into gray matter, white matter and cerebrospinal fluid. These, functional images were then normalized into the Montreal Neurologic Institute(MNI) space with an isotropic voxel size 3 × 3 × 3 mm3. We took the mean framewise displacement(FD) Jenkinson as head motion reference standard. We eliminated the subjects with motion (mean FD Jenkinson) >2 × SD above the group mean motion (Jenkinson et al., 2002; Yan et al., 2013). No participant was eliminated in this step. Furthermore, a regression analysis was conducted to minimize the influence on head motion (Friston 24 model), cerebrospinal fluid, and white matter. Following which, the high-frequency physiological noise and low-frequency drift were filtered (0.01–0.08 Hz).
ReHo was calculated based on the assumption that voxels within the functional brain region were characterized by the synchronization of the Blood oxygen level dependency (BOLD) time series. Kendall's coefficient of concordance(KCC) was used to measure ReHo of the time series of a given voxel with those of its nearest 26 neighbors in voxel-wise way. The ReHo value indicated the degree of regionally temporal synchronization of the cluster with a given voxel in the center. For optimization, each ReHo map was divided by its average KCC of the brain. Then, the standardized ReHo map was spatially smoothed with an 8-mm full-width at half- maximum (FWHM) Gaussian kernel.
2.3. Statistical analysis
A two-sample t-test was performed to evaluate the differences on smoothed ReHo maps between the PTSD patient group and the healthy control group. Due to the small sample size of our study, nonparametric permutation tests were performed based on 5000 times random permutations. The clusters with Threshold-Free Cluster Enhancement(TFCE) corrected p-value <0.05 were reported in the Table 2 and Fig. 1. Additionally, we also performed multiple comparisons correction according to the Monte Carlo method (Alphasim, https://afni.nimh.nih.gov) with a voxel-level threshold of p < 0.001 and the cluster-level threshold of p < 0.05 (see in Supplement Fig. 1). A two-sample t-test was performed to evaluate the differences in CAPS, MMSE, SAS, and SDS scores (p < 0.05) as well as age and education level between PTSD patients and healthy controls. A chi-square test was used to evaluate the difference in gender composition between the PTSD group and healthy control group. The statistical analysis was performed using SPSS version 23.
Table 2.
Brain regions with abnormal ReHo in PTSD patients.
| Brain area | MNI |
||||
|---|---|---|---|---|---|
| Voxel size | x | y | z | T value | |
| Angular R | 30 | 60 | −57 | 33 | −3.1771 |
L: left; R: Right; MNI: Montreal Neurological Institute; ReHo, regional homogeneity.
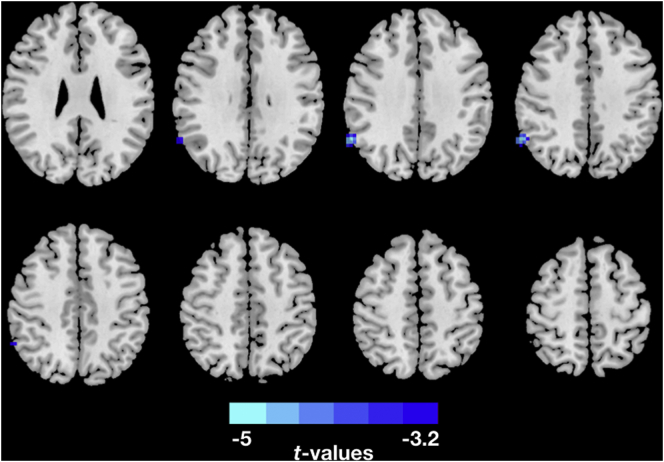
Fig. 1.
Two-sample t-test was performed to test the difference in ReHo maps between PTSD group and Healthy controls at each voxel. Nonparametric permutation tests were performed based on 5000 times random permutations for multiple comparison correction. The clusters with Threshold-Free Cluster Enhancement(TFCE) corrected p-value < 0.05. The PTSD patients showed decreased ReHo in the right Angular.
In order to explore the associations between ReHo map alterations and CAPS, MMSE, SAS, and SDS scores, the mean ReHo values of significant areas were extracted independently using REST (resting-state fMRI data analysis toolkit; http://resting-fmri.sourceforge.net). Due to the small sample size of our study, Spearman's rank correlation analysis was conducted between the test scores and average ReHo values in significantly different areas by SPSS 23.0 (p < 0.05).
3. Results
3.1. Demographic and clinical tests
Demographic and clinical data are summarized in Table 1. No significant differences were observed in between-group difference with respect to gender (p = 0.64), age (p = 0.21), MMSE (p = 0.20), SAS (p = 0.40), SDS (p = 0.82) and education level (p = 0.67). The CAPS scores of the PTSD patients differed significantly from those of the healthy controls (Table 1).
Table 1.
Demographic and clinical characteristics of PTSD patients and controls.
| Characteristic | PTSD (N = 20) | HC(N = 18) | t Value | P value |
|---|---|---|---|---|
| Age(y) | 38.7 ± 8.2 | 42.2 ± 8.4 | −1.286 | 0.207 |
| Sex* | 0.062 | |||
| Male | 7 | 7 | ||
| Female | 13 | 11 | ||
| Head movement | 0.155 ± 0.413 | 0.160 ± 0.421 | −0.024 | 0.837 |
| Education | 11.2 ± 2.7 | 9.5 ± 3.7 | 1.599 | 0.119 |
| CAPS | 54.9 ± 9.1 | 10.1 ± 4.6 | 18.799 | 0.0001 |
| SAS | 45.9 ± 10.0 | 48.2 ± 6.5 | −0.852 | 0.400 |
| SDS | 48.3 ± 12.0 | 47.4 ± 9.6 | 0.257 | 0.799 |
Unless otherwise indicated, data are mean ± standard deviation. *For gender composition, X2 = 0.062 and v = 1; CAPS: Clinician-Administered PTSD Scale for DSM-IV. SAS: Self-rating Anxiety Scale. SDS: Self-rating Depression Scale.
3.2. Altered ReHo in PTSD patients
Compared to the healthy control group, the PTSD group showed decreased ReHo values in right angular gyrus (Fig. 1 and Table 2). The results corrected for multiple comparisons with nonparametric permutation tests showed a significant decreased ReHo values in the right angular. Additionally, we also found decreased ReHo values in the right angular gyrus, right supramarginal gyrus and increased ReHo values in the right parahippocampal gyrus, right precuneus, and right postcentral gyrus by using Monte Carlo method for multiple comparison correction (Supplement Fig. 1).
3.3. Correlations between ReHo values of abnormal regions and CAPS score
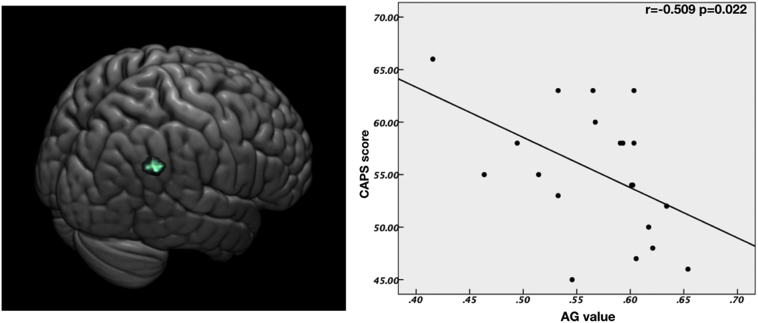
Due to the limited effect size of our study, we performed Spearman's rank correlations between the clinical indicator and mean ReHo values. (see in Fig. 2). Significant significant negative correlations were found between the angular gyrus ReHo and the CAPS score (r = −0.509, p = 0.022).
Fig. 2.
Correlation between right angular gyrus and CAPS scores. The ReHo values of right angular gyrus was negatively correlated with the CAPS scores in the CAPS group (r = 0.509, p = 0.022). The brain slice on the left showed the right angular gyrus with significant group difference. AG, angular gyrus.
4. Discussion
In the present study, we used the resting state fMRI and the ReHo approach to measure the abnormal neuronal activity in the local brain areas. Compared to the normal control group, the PTSD group showed lower ReHo values in the right angular gyrus. The decreased ReHo values also showed significant negative correlation with the CAPS score.
The angular gyrus is an important hub integrating multisensory, sensorimotor function and cognition (Ke et al., 2018). It is also associated with the dissociation symptom (Blanke and Arzy, 2016). In this study, we found decreased ReHo in the right angular gyrus, this may indicate a decreased activity in this area. Previous positron emission tomography study also found a decreased glucose absorption in the angular gyrus (Chung et al., 2006; Molina et al., 2010). In this study, the decreased ReHo value in the right angular was negatively correlated with the symptom severity of PTSD (CAPS score). In our previous study of dynamic functional connectivity of PTSD, we found a decreased dynamic functional connectivity in the angular gyrus (Fu et al., 2019). Previous diffusion tensor imaging also found a diminished subcortical integrity in angular gyrus (Schuff et al., 2011). Therefore, we suspected that the alteration of angular gyrus is universal in PTSD. A previous voxel-based morphometry (VBM) study showed that the severity of dissociative symptoms was positively correlated with the volume of the angular gyrus in PTSD patients (Nardo et al., 2013). Our result was converse to that from the VBM study, potentially due to the different methods used or the compensatory responses in the brain cortex (Bansal et al., 2018). Therefore, we speculated that the angular gyrus may be a unique region linked to dissociative symptoms of PTSD. Additionally, the angular gyrus is a key region of the DMN (Bluhm et al., 2008). The aberrant FC within brain structures within the DMN was consistently found in the resting-state and task-related studies (Liu et al., 2013; Birn et al., 2014; Kennis et al., 2015). Previous FC studies used precuneus (Lanius et al., 2009) as the seed point and found increased connectivity between the precuneus and left angular gyrus, which indicated DMN disturbances in PTSD patients. The current result is also supported by a previous study, which revealed aberrant changes within the DMN in PTSD patients using independent component analysis (Zhang et al., 2015). Previous study indicated gene variants was associated with the alteration between the precuneus and the angular gyrus in PTSD (Miller et al., 2016). To date, abnormality in the angular gyrus is not fully understood, but may be an important neurobiological change for PTSD.
In the present study, we reported the group differences using the TFCE method for multiple comparison. When using the Monte Carlo method for correction, we found increased ReHo in the right parahippocampal gyrus, right middle temporal gyrus, right postcentral gyrus and right precuneus, while decreased ReHo in the angular gyrus, inferior frontal gyrus, left middle fontal gyrus and right supramarginal gyrus (see in Supplement Fig. 1). In neuroimaging studies, different multiple comparison correction method may lead to different results (Poline et al., 1997). However, Monte Carlo method was widely used multiple comparison correction method in neuroimaging studies. (Ho et al., 2014; Kaiser et al., 2015; Wang et al., 2016a, Wang et al., 2016b). Although some alterations of ReHo value in our study might not survive after stringent multiple comparison correction. It seems clear that PTSD may be associated with functional or structural alteration in these areas. Therefore, it is meaningful to discuss these results.
The precuneus was identified to be active in PTSD as compared to healthy controls in explicit memory tasks (Jackowski et al., 2008). A previous study of the cortical volume of PTSD showed increased cortical volume in the left precuneus and that PTSD severity was positively correlated with the precuneus cortical volume; the study also suggested that these changes in precuneus may be related to intrusive memory symptoms (Li et al., 2016).
The parahippocampal gyrus is vulnerable in the patients with PTSD, and the abnormal activity of parahippocampal gyrus is associated with dissociative symptoms (Meng et al., 2014; Sakamoto et al., 2005). Francati et al. (Francati et al., 2007) suggested that the increased activity in the parahippocampal gyrus may trigger flashbacks and intrusive thoughts. Prior PTSD studies consistently reported the aberrant functional alterations in the parahippocampal gyrus (Patel et al., 2012). Furthermore, a significant positive correlation was established between the increased ReHo values in the parahippocampal gyrus and the CAPS scores. This clinically relevant finding was consistent with that of a previous resting-state ReHo study in natural disaster PTSD patients (Ke et al., 2017).
The abnormality of the supramarginal gyrus was found consistently in neuroimaging studies that focused on different imaging techniques, including task-based fMRI, rs-fMRI, and VBM (McDermott et al., 2016; Woodward et al., 2009; Yang et al., 2004; Zhang et al., 2016a, Zhang et al., 2016b). These changes may not be negative, and traumatic events may have positive impacts on PTSD patients (Tedeschi and Calhoun, 1996). One of the benefits associated with the post-traumatic event is “relating to others” (Taku et al., 2007). The supramarginal gyrus and angular gyrus are the key components of the temporoparietal junction area that is crucial in mentalizing (Biswal et al., 1995). A previous study of post-traumatic patients with positive outcomes suggested that the aberrant changes in temporoparietal junction area might be associated with improved mentalizing ability or empathy, such that the patients might develop improved interpersonal relationships in post-traumatic life (Fujisawa et al., 2015).
In a task-state fMRI study (Morey et al., 2008), the severity of PTSD was correlated with the activation of combat-related stimuli in the middle frontal gyrus, thereby suggesting that emotional processing usurped the executive regions. A study utilized fMRI and DTI (Sun et al., 2015) and reported abnormal functional homogeneity and anatomical connectivity efficiency in the bilateral middle frontal gyrus. Furthermore, in those who developed PTSD, this abnormality was identified within 2 days post-trauma, suggesting that the bilateral middle frontal gyrus might serve as a potential predictive marker. Recently, a longitudinal study (Ke et al., 2016) of acute PTSD indicated that the decreased activation in the middle frontal gyrus, precuneus, and cerebellum might be associated with clinical recovery.
Yin et al. (Yin et al., 2012) found decreased ReHo values in the right middle temporal gyrus in patients with PTSD than in traumatized subjects. Bluhm et al. (Bluhm et al., 2009) found decreased connectivity between the posterior cingulate and right middle temporal gyrus. The middle temporal gyrus can restrain the function of the amygdala, thereby moderating the fear condition in animal models (Jarrell et al., 1987; Romanski and LeDoux, 1993). Therefore, the increased activity in the right middle temporal gyrus in this study might be associated with an increased inhibition of the function of the amygdala.
Nevertheless, the present study had several limitations. First, although we used stringent inclusion and exclusion criteria for the selection of the subjects, the small sample size might have affected the results, and hence, future studies could benefit from larger samples. Second, we speculated that the angular plays a critical role in intrusive memory symptoms under resting conditions; thus, verifying this aberrant activity in the region in task-based conditions is essential.
5. Conclusion
The current study was based on rs-fMRI data using a ReHo method to explore the correlation of PTSD with brain regions that provided the imaging evidence for the abnormalities in the PTSD brain differentiation. Our findings included the memory- and emotion-related areas. A correlation was established between the brain region showed significant group difference and the symptom severity (CAPS). The dysfunction in suggested a putative mechanism of memory and emotional dysregulation in PTSD patients.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Number: 81471639, 81771807, 81701111) and the Natural Science Foundation of Guangdong Province (Grant Number:2015A030313723, 2016A020215125, 2017A020215077). Guangzhou Science and Technology Program key projects (Grant Number: 201607010056).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101951.
Contributor Information
Junzhang Tian, Email: Jz.tian@163.com.
Guihua Jiang, Email: jiangguihua1970@163.com.
Appendix A. Supplementary data
Supplementary material
References
- Association, A.P . 2000. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. (Washington) [Google Scholar]
- Bansal R., Hellerstein D.J., Peterson B.S. Evidence for neuroplastic compensation in the cerebral cortex of persons with depressive illness. Mol. Psychiatr. 2018;23:375–383. doi: 10.1038/mp.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Patriat R., Phillips M.L., Germain A., Herringa R.J. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress. Anxiety. 2014;31:880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blanke O., Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. Neuroscientist. 2016;11:16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Osuch E.A., Lanius R.A., Boksman K., Neufeld R.W.J., Théberge J., Williamson P. Default mode network connectivity: effects of age, sex, and analytic approach. Neuroreport. 2008;19:887–891. doi: 10.1097/WNR.0b013e328300ebbf. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E.A., Frewen P.A., Stevens T.K., Boksman K., Neufeld R.W.J., Théberge J., Lanius R.A. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatr. Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Chen H., Mo S. Regional homogeneity changes in nicotine addicts by resting-state fMRI. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0170143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.A., Kim S.H., Chung S.K., Chae J.-H., Yang D.W., Sohn H.S., Jeong J. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin. Neurophysiol. 2006;117:637–642. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Francati V., Vermetten E., Bremner J.D. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress. Anxiety. 2007;24:202–218. doi: 10.1002/da.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S., Ma X., Wu Y., Bai Z., Yi Y., Liu M., Lan Z., Hua K., Huang S., Li M., Jiang G. Altered local and large-scale dynamic functional connectivity variability in posttraumatic stress disorder: a resting-state fMRI study. Front. Psychiatr. 2019;10:15057–15058. doi: 10.3389/fpsyt.2019.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T.X., Jung M., Kojima M., Saito D.N., Kosaka H., Tomoda A. Neural basis of psychological growth following adverse experiences: a resting-state functional MRI study. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0136427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K.S., Lee A.C.H., Brett M., Patterson K. The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cogn. Affect. Behav. Neurosci. 2003;3:234–254. doi: 10.3758/cabn.3.3.234. [DOI] [PubMed] [Google Scholar]
- Gupta M.A. Review of somatic symptoms in post-traumatic stress disorder. Int. Rev. Psychiatr. 2013;25:86–99. doi: 10.3109/09540261.2012.736367. [DOI] [PubMed] [Google Scholar]
- Ho T.C., Connolly C.G., Blom E.H., LeWinn K.Z., Strigo I.A., Paulus M.P., Frank G., Max J.E., Wu J., Chan M., Tapert S.F., Simmons A.N., Yang T.T. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol. Psychiatr. 2014:1–13. doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski A.P., Douglas-Palumberi H., Jackowski M., Win L., Schultz R.T., Staib L.W., Krystal J.H., Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatr. Res. 2008;162:256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell T.W., Gentile C.G., Romanski L.M., McCabe P.M., Schneiderman N. Involvement of cortical and thalamic auditory regions in retention of differential bradycardiac conditioning to acoustic conditioned stimuli in rabbits. Brain Res. 1987;412:285–294. doi: 10.1016/0006-8993(87)91135-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jin C., Qi R., Yin Y., Hu X., Duan L., Xu Q., Zhang Z., Zhong Y., Feng B., Xiang H., Gong Q., Liu Y., Lu G., Li L. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol. Med. 2013;44:1927–1936. doi: 10.1017/S003329171300250X. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder. JAMA Psychiatry. 2015;72:603–619. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J., Zhang L., Qi R., Li W., Hou C., Zhong Y., He Z., Li L., Lu G. A longitudinal fMRI investigation in acute post-traumatic stress disorder (PTSD) Acta Radiol. 2016;57:1387–1395. doi: 10.1177/0284185115585848. [DOI] [PubMed] [Google Scholar]
- Ke J., Chen F., Qi R., Xu Q., Zhong Y., Chen L., Li J., Zhang L., Lu G. Post-traumatic stress influences local and remote functional connectivity: a resting-state functional magnetic resonance imaging study. Brain Imaging Behav. 2017:1–10. doi: 10.1007/s11682-016-9622-6. [DOI] [PubMed] [Google Scholar]
- Ke J., Zhang L., Qi R., Xu Q., Zhong Y., Liu T., Li J., Lu G., Chen F. Typhoon-related post-traumatic stress disorder and trauma might lead to functional integration abnormalities in intra- and inter-resting state networks: a resting-state fmri independent component analysis. Cell. Physiol. Biochem. 2018;48:99–110. doi: 10.1159/000491666. [DOI] [PubMed] [Google Scholar]
- Kennis M., Rademaker A.R., van Rooij S.J.H., Kahn R.S., Geuze E. Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum. Brain Mapp. 2015;36:99–109. doi: 10.1002/hbm.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Wang P.S. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu. Rev. Public Health. 2008;29:115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety. 2016;33:592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Coupland N.J., Hegadoren K.M., Rowe B., Thà berge J., Neufeld R.W.J., Williamson P.C., Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr. Scand. 2009;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lei D., Li K., Li L., Chen F., Huang X., Lui S., Li J., Bi F., Gong Q. Disrupted functional brain connectome in patients with posttraumatic stress disorder. Radiology. 2015;276:818–827. doi: 10.1148/radiol.15141700. [DOI] [PubMed] [Google Scholar]
- Li S., Huang X., Li L., Du F., Li J., Bi F., Lui S., Turner J.A., Sweeney J.A., Gong Q. Posttraumatic stress disorder: structural characterization with 3-T MR imaging. Radiology. 2016;280:537–544. doi: 10.1148/radiol.2016150477. [DOI] [PubMed] [Google Scholar]
- Liu F., Guo W., Fouche J.-P., Wang Y., Wang W., Ding J., Zeng L., Qiu C., Gong Q., Zhang W., Chen H. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct. Funct. 2013;220:101–115. doi: 10.1007/s00429-013-0641-4. [DOI] [PubMed] [Google Scholar]
- McDermott T.J., Badura-Brack A.S., Becker K.M., Ryan T.J., Bar-Haim Y., Pine D.S., Khanna M.M., Heinrichs-Graham E., Wilson T.W. Attention training improves aberrant neural dynamics during working memory processing in veterans with PTSD. Cogn. Affect. Behav. Neurosci. 2016:1–10. doi: 10.3758/s13415-016-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Qiu C., Zhu H., Lama S., Lui S., Gong Q., Zhang W. Anatomical deficits in adult posttraumatic stress disorder: A meta-analysis of voxel-based morphometry studies. Behav. Brain Res. 2014;270:307–315. doi: 10.1016/j.bbr.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Miller M.W., Sperbeck E., Robinson M.E., Sadeh N., Wolf E.J., Hayes J.P., Logue M., Schichman S.A., Stone A., Milberg W., McGlinchey R. 5-HT2A gene variants moderate the association between PTSD and reduced default mode network connectivity. Front. Neurosci. 2016;10 doi: 10.3389/fnins.2016.00299. (56–11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M.E., Isoardi R., Prado M.N., Bentolila S. Basal cerebral glucose distribution in long-term post-traumatic stress disorder. World J. Biol. Psychiatr. 2010;11:493–501. doi: 10.3109/15622970701472094. [DOI] [PubMed] [Google Scholar]
- Morey R.A., Petty C.M., Cooper D.A., LaBar K.S., McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatr. Res. 2008;162:59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardo D., Högberg G., Lanius R.A., Jacobsson H., Jonsson C., Hällström T., Pagani M. Gray matter volume alterations related to trait dissociation in PTSD and traumatized controls. Acta Psychiatr. Scand. 2013;128:222–233. doi: 10.1111/acps.12026. [DOI] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.A. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Peng J., Qu H., Peng J., Luo T.-Y., Lv F.-J., chen L., Wang Z.-N., Ouyang Y., Cheng Q.-F. Abnormal spontaneous brain activity in type 2 diabetes with and without microangiopathy revealed by regional homogeneity. Eur. J. Radiol. 2016;85:607–615. doi: 10.1016/j.ejrad.2015.12.024. [DOI] [PubMed] [Google Scholar]
- Phan K.L. 2011. Altered Amygdala Resting-State Functional Connectivity in Post-Traumatic Stress Disorder 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline J.B., Worsley K.J., Evans A.C., Friston K.J. Combining spatial extent and peak intensity to test for activations in functional imaging. NeuroImage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Romanski L.M., LeDoux J.E. information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb. Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Fukuda R., Okuaki T., Rogers M., Kasai K., Machida T., Shirouzu I., Yamasue H., Akiyama T., Kato N. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: A functional MRI study. NeuroImage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sareen J., Cox B.J., Stein M.B., Afifi T.O., Fleet C., Asmundson G.J.G. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom. Med. 2007;69:242–248. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- Schuff N., Zhang Y., Zhan W., Lenoci M., Ching C., Boreta L., Mueller S.G., Wang Z., Marmar C.R., Weiner M.W., Neylan T.C. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. NeuroImage. 2011;54:S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R., King A., Garfinkel S., Wang X., Sripada C., Welsh R., Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J. Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.-W., Hu H., Wang Y., Ding W.-N., Chen X., Wan J.-Q., Zhou Y., Wang Z., Xu J.-R. Inter-hemispheric functional and anatomical connectivity abnormalities in traffic accident-induced PTSD: a study combining fMRI and DTI. J. Affect. Disord. 2015;188:80–88. doi: 10.1016/j.jad.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Taku K., Calhoun L.G., Tedeschi R.G., Gil-Rivas V., Kilmer R.P., Cann A. Examining posttraumatic growth among Japanese university students. Anxiety Stress Coping. 2007;20:353–367. doi: 10.1080/10615800701295007. [DOI] [PubMed] [Google Scholar]
- Tedeschi R.G., Calhoun L.G. The posttraumatic growth inventory: measuring the positive legacy of trauma. J. Trauma. Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- Wang T., Li S., Jiang G., Lin C., Li M., Ma X., Zhan W., Fang J., Li L., Li C., Tian J. Regional homogeneity changes in patients with primary insomnia. Eur. Radiol. 2016;26:1292–1300. doi: 10.1007/s00330-015-3960-4. [DOI] [PubMed] [Google Scholar]
- Wang T., Liu J., Zhang J., Zhan W., Li L., Wu M., Huang H., Zhu H., Kemp G.J., Gong Q. Altered resting-state functional activity in posttraumatic stress disorder: A quantitative meta- analysis. Nat. Publ. Group. 2016:1–14. doi: 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward S.H., Schaer M., Kaloupek D.G., Cediel L., Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch. Gen. Psychiatry. 2009;66:1373–1382. doi: 10.1001/archgenpsychiatry.2009.160. [DOI] [PubMed] [Google Scholar]
- Wu T., Long X., Zang Y., Wang L., Hallett M., Li K., Chan P. Regional homogeneity changes in patients with Parkinson's disease. Hum. Brain Mapp. 2009;30:1502–1510. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.-G., Craddock R.C., Zuo X.-N., Zang Y.-F., Milham M.P. Standardizing the intrinsic brain: Towards robust measurement of inter-individual variation in 1000 functional connectomes. NeuroImage. 2013:1–17. doi: 10.1016/j.neuroimage.2013.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Wu M.-T., Hsu C.-C., Ker J.-H. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci. Lett. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Hoge C.W., McFarlane A.C., Vermetten E., Lanius R.A., Nievergelt C.M., Hobfoll S.E., Koenen K.C., Neylan T.C., Hyman S.E. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- Yin Y., Jin C., Eyler L.T., Jin H., Hu X., Duan L., Zheng H., Feng B., Huang X., Shan B., Gong Q., Li L. Altered regional homogeneity in post-traumatic stress disorder: a restingstate functional magnetic resonance imaging study. Neurosci. Bull. 2012;28:541–549. doi: 10.1007/s12264-012-1261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y., Jiang T., Lu Y., He Y., Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu F., Chen H., Li M., Duan X., Xie B., Chen H. Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. J. Affect. Disord. 2015;187:114–121. doi: 10.1016/j.jad.2015.08.043. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wu Q., Zhu H., He L., Huang H., Zhang J., Zhang W. Multimodal MRI-based classification of trauma survivors with and without post-traumatic stress disorder. Front. Neurosci. 2016;10:785–789. doi: 10.3389/fnins.2016.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xie B., Chen H., Li M., Liu F., Chen H. Abnormal functional connectivity density in post-traumatic stress disorder. Brain Topogr. 2016;29:1–7. doi: 10.1007/s10548-016-0472-8. [DOI] [PubMed] [Google Scholar]
- Zongtang X., Jiuping X., Zhibin W. Mental health problems among survivors in hard- hit areas of the 5.12 Wenchuan and 4.20 Lushan earthquakes. J. Ment. Health. 2017;0:1–8. doi: 10.1080/09638237.2016.1276525. [DOI] [PubMed] [Google Scholar]
- Zuo X.-N., Xu T., Jiang L., Yang Z., Cao X.-Y., He Y., Zang Y.-F., Castellanos F.X., Milham M.P. Toward reliable characterization of functional homogeneity in the human brain: Preprocessing, scan duration, imaging resolution and computational space. NeuroImage. 2013;65:374–386. doi: 10.1016/j.neuroimage.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material