Abstract
Background
Previous studies have found widespread pain processing alterations in the brain in chronic low back pain (cLBP) patients. We aimed to (1) identify brain regions showing altered amplitude of low-frequency fluctuations (ALFF) using MRI and use these regions to discriminate cLBP patients from healthy controls (HCs) and (2) identify brain regions that are sensitive to cLBP pain intensity changes.
Methods
We compared ALFF differences by MRI between cLBP subjects (90) and HCs (74), conducted a discriminative analysis to validate the results, and explored structural changes in key brain regions of cLBP. We also compared ALFF changes in cLBP patients after pain-exacerbating manoeuvres.
Results
ALFF was increased in the post-/precentral gyrus (PoG/PrG), paracentral lobule (PCL)/supplementary motor area (SMA), and anterior cingulate cortex (ACC), and grey matter volume was increased in the left ACC in cLBP patients. PCL/SMA ALFF reliably discriminated cLBP patients from HCs in an independent cohort. cLBP patients showed increased ALFF in the insula, amygdala, hippocampal/parahippocampal gyrus, and thalamus and decreased ALFF in the default mode network (DMN) when their spontaneous low back pain intensity increased after the pain-exacerbating manoeuvre.
Conclusions
Brain low-frequency oscillations in the PCL, SMA, PoG, PrG, and ACC may be associated with the neuropathology of cLBP. Low-frequency oscillations in the insula, amygdala, hippocampal/parahippocampal gyrus, thalamus, and DMN are sensitive to manoeuvre-induced spontaneous back pain intensity changes.
Keywords: anterior cingulate cortex, chronic low back pain, MRI, pain, paracentral lobule, support vector machine
Editor's key points.
-
•
Brain pain processing alterations in chronic low back pain (cLBP) patients may be useful for diagnosis and guiding therapy.
-
•
Amplitude of low-frequency fluctuations (ALFF) and structural data were measured by magnetic resonance imaging (MRI) in cLBP patients and healthy controls.
-
•
Subjects with cLBP showed selective regional increases in ALFF and grey matter volume increases in the anterior cingulate cortex.
-
•
ALFF was sensitive to manoeuvre-induced spontaneous low back pain intensity changes in cLBP.
-
•
These changes may be useful for classifying chronic low back pain and monitoring symptom changes clinically.
Low back pain (LBP) is a leading cause of disability worldwide.1 However, the current treatment methods for chronic LBP (cLBP) are far from satisfactory, and markedly increased rates of opioid overdose and addiction amongst cLBP patients highlight the urgent need to better understand the pathophysiology of the disorder and develop new therapeutic options.2
Accumulating evidence suggests that cLBP is associated with brain functional and structural changes in widespread brain regions, including the primary and secondary somatosensory/motor cortex, paracentral lobule (PCL), supplementary motor area (SMA), anterior cingulate cortex (ACC), amygdala, thalamus, and insula.1, 3, 4, 5 However, the results of cLBP brain-imaging studies vary widely,4 calling for studies with larger sample sizes to validate previous findings. Determining the physiological significance of these alterations and applying these findings to clinical practice remain challenging.6 Brain regions that show structural/functional differences in cLBP patients compared with healthy controls (HCs) may be useful as biomarkers to distinguish cLBP patients from HCs.7 Brain regions that are sensitive to cLBP pain intensity changes may have potential for cLBP severity monitoring and measurement of treatment response.8
Recently, amplitude of low-frequency fluctuations (ALFF), an index of low-frequency oscillations, has gained increased attention. Although still under investigation, studies have linked ALFF with cerebral blood flow9 and task-evoked activation10 in human subjects. An animal study using an acute stroke model in rats found that the signal intensity of abnormal ALFF increased and migrated from the core of ischaemic lesion areas to the periphery.11 One advantage of ALFF is that it can focus on the neural processes of key regions rather than correlations among regions. In addition, ALFF has the best balance between test–retest reliability and replicability among commonly used resting state (RS)-functional MRI (fMRI) metrics.12
We aimed to identify brain regions showing functional changes (as measured by ALFF) and structural changes in cLBP patients, apply a support vector machine (SVM) classifier13 to explore the most promising brain regions for discriminating cLBP patients from HCs based on ALFF results, and identify brain regions sensitive to cLBP pain intensity changes.
Methods
Participants
Two cohorts of non-specific cLBP patients and HCs were used in this study. The first cohort included 90 cLBP patients aged 22–50 yr and 74 age- and gender-matched HCs. Only patients whose pain duration was longer than 6 months and whose pain intensity was 4 or higher on a 0–10 VAS during screening were included. The second cohort, used to validate the machine learning results obtained from the first cohort, included 18 cLBP patients and 18 age- and gender-matched HCs. Characteristics and pain-related parameters for cLBP and HC subjects are presented in Table 1. The study was approved by the Institutional Review Board at Massachusetts General Hospital (Boston, MA, USA), and all subjects signed informed consent forms. See Supplementary material for the inclusion/exclusion criteria for cLBP subjects.
Table 1.
Demographic subject characteristics and behavioural statistics data of chronic low back pain (cLBP) and healthy control (HC) subjects. The values presented are ‘mean (minimum–maximum)’ for age and ‘mean (standard deviation)’ for others.
| Characteristics | Cohort 1 |
Cohort 2 |
||
|---|---|---|---|---|
| cLBP (n=90) | HC (n=74) | cLBP (n=18) | HC (n=18) | |
| Age (yr) | 34.46 (20–50) | 32.44 (23–50) | 36.11 (23–49) | 37.11 (23–50) |
| Gender (male/female) | 38/52 | 31/43 | 7/11 | 6/12 |
| Pain duration (yr) | 6.94 (6.21) | NA | 5.27 (3.66) | NA |
| Pain bothersomeness∗ | 5.06 (1.88) | NA | NA | NA |
| BDI | 6.12 (6.00) | NA | NA | NA |
| Pain intensity (low/high pain condition)† | 31.34 (18.70)/55.23 (19.56) | NA | NA | NA |
Pain bothersomeness: low back pain bothersomeness during the past week.
Pain intensity of 76 cLBP subjects whose pain intensity increased in the post-maneuver scan; the pain intensity of patients in the high pain condition was significantly higher than that of patients in the low pain condition (P<0.001). BDI, Beck Depression Inventory.
Experimental procedures
All cLBP patients underwent two MRI scan sessions. The first MRI session included a three-dimensional structural T1-weighted MRI and an RS-fMRI scan. Subjects were required to rate their pain intensity on a 0–100 continuous VAS before and after the RS-fMRI scan. Then, subjects stepped out of the scanner and performed pain-exacerbating manoeuvres to increase their LBP by ∼30%. The manoeuvres were tailored to each subject based on what the subject reported would exacerbate their LBP, such as lumbar flexion, extension, or rotation.14 After the manoeuvres, which took 10–15 min, subjects entered the scanner for an identical RS-fMRI scan. Manoeuvres were not performed if subjects were reluctant to increase their pain or if their pain was too strong (>70 on a 0–100 VAS). HCs did not perform manoeuvres and underwent only one MRI scan session. We also assessed cLBP for the past week using the Pain Bothersomeness Scale, a tool that has demonstrated substantial construct validity in measuring the severity of LBP,15, 16 and we had participants complete the Beck Depression Inventory (BDI) before the MRI scan. See Supplementary material for details of the experimental procedures and MRI data acquisition.
Functional analysis
Functional data preprocessing and statistical analysis were performed in Data Processing Assistant for Resting-State fMRI (DPABI) version 2.3 (Institute of Psychology, Chinese Academy of Sciences, Beijing, China), Statistical Parametric Mapping 12 (SPM12, Wellcome Trust Centre for Neuroimaging, University College London, London, UK), and FMRIB Software Library (FSL) version 5.0 (Analysis Group, FMRIB, Oxford, UK). After preprocessing and a fast Fourier transform, ALFF was calculated as the mean of amplitudes within 0.01–0.10 Hz.17 The ALFF map was transformed to z-scores for analysis. Further ALFF analysis was performed based on a 90% group mask (meaning 90% of subjects have this voxel) generated in DPABI. Subjects whose head motion exceeded the group mean (mean relative root mean square [RMS] displacement)+2×Group standard deviation (SD) (mean relative RMS displacement) were excluded.18 See Supplementary material for details on data preprocessing and ALFF calculation.
We conducted a two-sample t-test to compare whole-brain ALFF between cLBP subjects (pre-manoeuvre) and HCs (including age and gender as covariates). A paired t-test was conducted to compare whole-brain ALFF between subjects in the high pain condition and subjects in the low pain condition (including age and gender as covariates). Similar to a previous study,12 we utilised permutation testing with threshold-free cluster enhancement (TFCE) to threshold our results at P<0.05 corrected. The TFCE was conducted in the Permutation Analysis of Linear Models package19 in DPABI with 5000 permutations and cluster-forming threshold z>2.3. Partial correlations controlling for age and gender were applied in SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) to identify ALFF z-values related to cLBP duration, cLBP bothersomeness, and pain intensity. All results were corrected for multiple comparisons using a false discovery rate (FDR) threshold of P<0.05.
Structural analysis
Given the importance of the ACC in pain modulation,20 consistent alterations in its function/structure in chronic pain,21 and its significant ALFF increase and correlation with pain bothersomeness in cLBP subjects observed in this study, we also investigated the brain morphometry changes of the ACC. Morphometry data analysis was performed using FreeSurfer version 6.0 (Laboratory for Computational Neuroimaging, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, USA).22 The recon-all command was applied for automatic surface reconstruction and local gyrification index calculation. The process included skull stripping, volumetric labelling, intensity normalisation, white matter (WM) segmentation, surface atlas registration, surface extraction, and gyral labelling. Cortical thickness, surface area, and cortical volume of the bilateral ACC (defined from the Destrieux atlas in FreeSurfer23) were extracted for further analysis.
Statistical analysis of morphological measures was performed in SPSS version 24.0 (SPSS Inc.). We used a general linear model approach to calculate the group difference between cLBP subjects and HCs for each morphological measure, with gender and age as covariates. Cortical thickness, surface area, and cortical volume were also corrected for the mean cortical thickness, total surface area, and intracranial volume, respectively.24
Discriminative analysis
Brain areas that showed significant differences between cLBP subjects (pre-manoeuvre) and HCs in the ALFF analysis were used as regions of interest (ROIs) for the discriminative analyses with two objectives: (1) classify cLBP subjects and HCs using ALFF z-values and (2) assess the generalisability of the identified ROIs using an independent dataset (the second cohort).
In the first step, machine learning models were trained to classify cLBP subjects (pre-manoeuvre) and HCs using ALFF z-values within ROIs. An SVM13 classifier was used, and the implementation of SVM was based on a library for SVM (LIBSVM).25 To reduce the risk of overfitting, the analysis was based on leave-one-out cross validation.26 To quantify the performance of the SVM classifier, classification accuracy, sensitivity, and specificity were calculated and assessed by permutation testing. In the second step, the machine learning models trained in the first cohort of subjects were directly applied to an independent cohort of patients (second cohort), without any model fitting, to further assess the generalisability of the identified ROIs. See Supplementary material for details on the classification.
Results
Ninety cLBP subjects and 74 age- and gender-matched HCs were included in the first cohort (Table 1). Eighteen cLBP subjects and 18 age- and gender-matched HCs were included in the second cohort. In the first cohort, RS-fMRI was applied before and after a pain-exacerbating manoeuvre. Pain intensity ratings were recorded before and after each RS-fMRI scan. The average of pre- and post-scan pain ratings was calculated to represent pain intensity for the particular RS-fMRI scan. The pain intensity remained the same in six subjects and decreased in eight subjects during the second resting-state fMRI scan because: (1) subjects were reluctant to perform the manoeuvre or (2) pain intensity decreased during the second scan, although it increased right after the manoeuvre. We excluded these 14 subjects in the comparison between subjects in the high pain condition and subjects in the low pain condition (Table 1). The BDI scores were 6.12 (SD 6.00), indicating minimal depressive symptoms in the cLBP subjects.
Comparison between chronic low back pain and healthy control subjects
Two cLBP subjects and three HCs were excluded because of head motion; 88 cLBP subjects and 71 HCs were included in the final analysis. Head motion evaluated by mean relative RMS displacement was not significantly different (P=0.1) between cLBP subjects (0.078, SD 0.034) and HCs (0.069, SD 0.032).
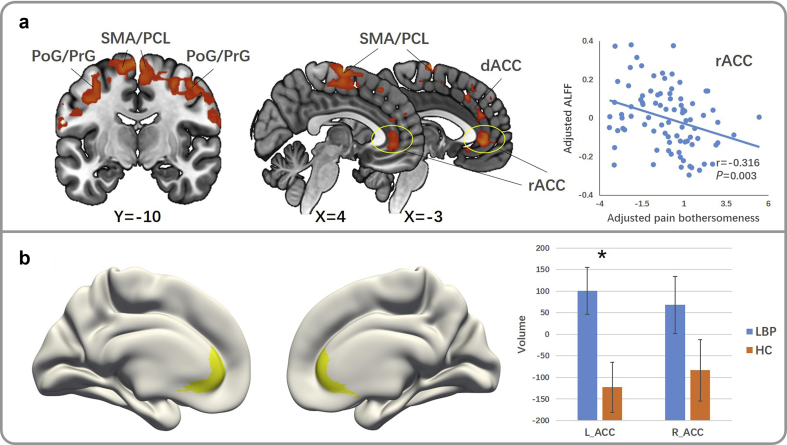
The comparison between cLBP subjects in the first MRI scan (pre-manoeuvre) and HCs showed increased ALFF in the bilateral post-/precentral gyrus (PoG/PrG), PCL/SMA, rostral ACC (rACC), and left dorsal ACC (dACC) in cLBP subjects (Fig. 1a and Table 2). There was no significantly decreased ALFF in cLBP subjects compared with HCs. The comparison between cLBP subjects in the second scan (post-manoeuvre) and HCs showed similar, but less robust increases in bilateral PoG/PrG, PCL/SMA, rACC, and left dACC in cLBP subjects. Partial correlation analysis revealed a significant negative correlation between left rACC ALFF z-values and pain bothersomeness in the past week in cLBP subjects (pre-manoeuvre) (P=0.003, FDR P=0.018, r=−0.316) (Fig. 1a).
Figure 1.
Group comparison between pre-manoeuvre chronic low back pain (cLBP) and healthy control (HC) subjects. (a) Results of whole-brain amplitude of low-frequency fluctuations (ALFF) group comparison (cLBP>HC). There is a negative correlation between cLBP bothersomeness and ALFF z-value in the left rACC of cLBP patients [P=0.003, false discovery rate (FDR) P=0.018, r=−0.316, adjusted for age and gender]. (b) Group comparisons of neuroanatomic measures in the ACC. The volume (P=0.006, FDR P=0.036, adjusted for age, gender, and intracranial volume) of the left ACC in cLBP patients is significantly larger than in HC subjects. ACC, anterior cingulate cortex; dACC, dorsal anterior cingulate cortex; L, left; LBP, low back pain; PCL, paracentral lobule; PoG, postcentral gyrus; PrG, precentral gyrus; R, right; rACC, rostral anterior cingulate cortex; SMA, supplementary motor area.
Table 2.
Results of amplitude of low-frequency fluctuations (ALFF) analysis. Results reported for whole brain ALFF analysis with age and gender as covariates. Amy, amygdala; cLBP, chronic low back pain; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; HC, healthy control; Hip, hippocampal gyrus; L, left; MNI, Montreal Neurological Institute; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; PCL, paracentral lobule; pHip, parahippocampal gyrus; PoG, postcentral gyrus; PrG, precentral gyrus; R, right; rACC, rostral anterior cingulate cortex; SMA, supplementary motor area; TPJ, temporoparietal junction.
| Contrast | Regions | Peak MNI |
Peak z-value | Voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| cLBP>HC | dACC_L | −6 | 36 | 27 | 3.97 | 1812 |
| PoG/PrG_L | −42 | −6 | 35 | 3.90 | ||
| PoG/PrG_R (upper) | 31 | −6 | 52 | 3.73 | ||
| SMA/PCL | 2 | −9 | 70 | 3.34 | ||
| PoG/PrG_R (lower) | 57 | 6 | 21 | 3.92 | 238 | |
| rACC | 0 | 42 | −3 | 3.56 | 221 | |
| High pain>low pain | Insula_R | 42 | 21 | −9 | 5.11 | 599 |
| Hip/pHip/Amy_R | 27 | −6 | −36 | 4.71 | ||
| Insula_L | −36 | 10 | −14 | 4.33 | 54 | |
| Hip/pHip_L | −27 | −3 | −27 | 3.97 | 36 | |
| Thalamus_L | −6 | −12 | 15 | 3.34 | 43 | |
| Low pain>high pain | TPJ_L | −39 | −60 | 30 | 6.74 | 41 |
| mPFC/rACC | 12 | 51 | 30 | 6.14 | 5366 | |
| dlPFC_L | −36 | 33 | 27 | 5.02 | ||
| dlPFC_R | 27 | 42 | 27 | 5.18 | ||
| Precuneus/PCC | −12 | −57 | 60 | 4.59 | ||
| TPJ_R | 54 | −33 | 48 | 3.87 | ||
In brain morphometry analysis, cLBP subjects had increased volume in the left ACC (uncorrected P=0.006, FDR corrected P=0.036, Fig. 1b and Table 3) compared with HCs. There was no significant difference between cLBP and HC subjects in cortical thickness or surface area of the ACC.
Table 3.
Results of structural analysis. Group comparison of morphological measures in anterior cingulate cortex between chronic low back pain (cLBP) and healthy control subjects.
| Measures | Hemisphere | F | P-value | FDR P-value | ↑ or ↓ in cLBP |
|---|---|---|---|---|---|
| Thickness | L | 0.490 | 0.485 | 0.485 | – |
| R | 2.772 | 0.098 | 0.183 | – | |
| Area | L | 3.855 | 0.051 | 0.153 | – |
| R | 1.313 | 0.254 | 0.305 | – | |
| Volume | L | 7.748 | 0.006∗ | 0.036† | ↑ |
| R | 2.416 | 0.122 | 0.183 | – |
Results that are significant in two sample t-test (P<0.05) without P-value correction.
Results that are significant after false discovery rate (FDR) correction (FDR P<0.05). L, left; R, right.
Discriminative analysis
Brain areas that showed significant differences between cLBP subjects and HCs in the ALFF analysis were chosen as ROIs for discriminative analysis. The ROIs included the rACC, dACC, SMA/PCL, left PoG/PrG, right PoG/PrG (lower part), and right PoG/PrG (upper part). All ROIs were created by taking the overlap of the Automated Anatomical Labeling (AAL) regions and the binary masks of clusters survived in the whole brain ALFF comparison between cLBP subjects (pre-manoeuvre) and HCs. Peak Montreal Neurological Institute (MNI) coordinates of the clusters are presented in Table 2 and voxel size of the ROIs in Table 4. Independent models were applied for each ROI to test the contribution of a single brain region. The PCL/SMA, left dACC, left PoG/PrG, right PoG/PrG (upper), right PoG/PrG (lower), and rACC obtained accuracies of 71.1% (P=0.001), 63.5% (P=0.022), 59.7% (P=0.036), 61.6% (P=0.008), 65.4% (P=0.002), and 64.8% (P=0.002), respectively (Table 4). When we applied machine learning models trained in the first cohort of subjects directly to the second cohort, accuracies for different brain regions were 66.7% for the PCL/SMA (P=0.006), 61.1% for the left dACC (P=0.099), 58.3% for the left PoG/PrG (P=0.268), 52.8% for the right PoG/PrG (upper) (P=0.362), 61.1% for the right PoG/PrG (lower) (P=0.145), and 61.1% for the rACC (P=0.158) (Table 4).
Table 4.
Results of discriminative analysis.
| Regions | Voxels | Training in cohort 1 |
Testing in cohort 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy | P-value | Sensitivity | Specificity | Accuracy | P-value | Sensitivity | Specificity | ||
| dACC_L | 49 | 63.5 | 0.022∗ | 70.5 | 54.9 | 61.1 | 0.099 | 55.6 | 66.7 |
| PoG/PrG_L | 471 | 59.7 | 0.036∗ | 68.4 | 46.6 | 58.3 | 0.268 | 67.8 | 48.9 |
| PoG/PrG_R (upper) | 227 | 61.6 | 0.008∗ | 71.8 | 46.6 | 52.8 | 0.362 | 50 | 55.6 |
| PCL/SMA | 272 | 71.1 | 0.001∗ | 78.4 | 62.0 | 66.7 | 0.006∗ | 72.2 | 61.1 |
| PoG/PrG_R (lower) | 238 | 65.4 | 0.002∗ | 69.3 | 60.6 | 61.1 | 0.145 | 61.1 | 61.1 |
| rACC | 221 | 64.8 | 0.002∗ | 75 | 52.1 | 61.1 | 0.158 | 66.7 | 55.6 |
Regions performing well in the discriminative analysis (P<0.05). dACC, dorsal anterior cingulate cortex; L, left; PCL, paracentral lobule; PoG, postcentral gyrus; PrG, precentral gyrus; R, right; rACC, rostral anterior cingulate cortex; SMA, supplementary motor area.
Comparison between patients with high pain and patients with low pain
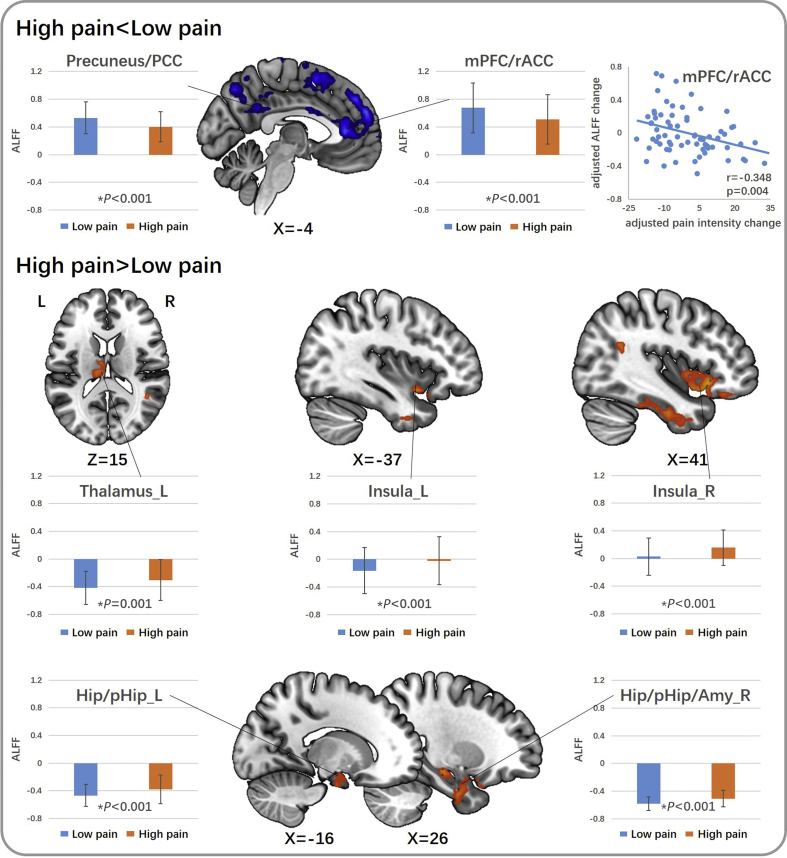
Seven cLBP subjects were excluded because of excessive head motion; 69 subjects were included in the final analysis. Head motion evaluated by mean relative RMS displacement was not significantly different (P=0.499) between subjects with high pain (0.078, SD 0.030) and subjects with low pain (0.075, SD 0.034). The comparison between the high and low pain conditions showed that higher pain is associated with increased ALFF in the bilateral anterior insula, hippocampal gyrus, parahippocampal gyrus, right amygdala, and left thalamus, and decreased ALFF in the bilateral medial prefrontal cortex (mPFC), rACC, precuneus gyrus, posterior cingulate cortex (PCC), temporoparietal junction (TPJ), and dorsolateral prefrontal cortex (Fig. 2 and Table 2). mPFC/rACC ALFF change was negatively correlated with pain intensity change in cLBP subjects (P=0.004, FDR P=0.028, r=−0.348) (Fig. 2).
Figure 2.
Group comparison between chronic low back pain (cLBP) patients with high and low pain. Brain regions showing decreased amplitude of low-frequency fluctuations (ALFF) when low back pain intensity increased in cLBP are presented on the top. Brain regions showing increased ALFF when low back pain intensity increased in cLBP are presented on the bottom. Each brain region is presented with a plot showing ALFF z-value (mean [standard deviation]) of cLBP patients in low pain condition and high pain condition. There is a negative correlation between manoeuvre-induced pain intensity change and ALFF change in the mPFC/rACC in cLBP patients (P=0.004, false discovery rate P=0.028, r=−0.348, adjusted for age and gender). Amy, amygdala; Hip, hippocampal gyrus; L, left; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; pHip, parahippocampal gyrus; R, right; rACC, rostral anterior cingulate cortex. *Results that are significant by paired t-test.
Discussion
We investigated ALFF and ACC morphometry differences between cLBP subjects and HCs and ALFF changes after back pain increasing manoeuvres. We found increased ALFF in the bilateral PCL, SMA, PoG, PrG, rACC, and left dACC and increased left ACC grey matter volume in cLBP subjects compared with HCs. The ALFF in the rACC was negatively correlated with cLBP bothersomeness in the past week. Discriminative analysis showed that PCL/SMA ALFF can significantly discriminate cLBP subjects from HCs. We also found that when LBP increased, cLBP subjects showed increased ALFF in limbic areas (such as the anterior insula, hippocampus, parahippocampus, amygdala, and thalamus) and decreased ALFF in regions of the default mode network (including the mPFC, rACC, precuneus, PCC, and TPJ). mPFC/rACC ALFF change was negatively correlated with pain intensity change in cLBP subjects.
Brain functional and structural changes associated with chronic low back pain
We found greater ALFF in the PCL, SMA, PoG, PrG, rACC, and dACC in cLBP subjects compared with HCs. These results are consistent with previous studies that support the important roles of these regions in chronic pain.4, 27, 28, 29 The PCL and PoG play a major role in the localisation and discrimination of pain and in brain networks that mediate and sustain chronic pain,27 and the dACC is selectively involved in pain processes.28 Many studies have shown that chronic pain patients have altered excitability in the motor cortex, including the PrG, PCL, and SMA, which may be caused by the disease itself or by patients' altered motor performance.4, 29
We also found that cLBP subjects showed increased rACC ALFF and left ACC grey matter volume compared with HCs. The rACC ALFF was also negatively and moderately correlated with pain bothersomeness in the past week in cLBP subjects. These findings may reflect the important role of the rACC in the pain modulation process,5, 30 suggesting that cLBP subjects may be associated with a more activated endogenous pain modulation system. We reported previously that RS functional connectivity between the PAG and rACC was increased in cLBP subjects compared with HCs14 and that fibromyalgia is associated with decreased cortical thickness and grey matter volume in the rACC.31 Fibromyalgia is a central and general pain condition, while LBP is a localised pain condition. The differences in brain volume changes in the same region may indicate a difference in neuropathology of the two chronic pain disorders.
Developing a machine learning classifier for pain is an emerging concept in neuroimaging research. Machine learning techniques have been used to identify specific conditions such as physical pain states and drug-induced analgesia.32 33 We found that the PoG/PrG, PCL/SMA, rACC, and ACC can discriminate cLBP subjects from HCs in a large cohort. However, the PCL/SMA was the only region that could discriminate cLBP from HC subjects in an independent cohort using a different scanner. Our results are consistent with a previous study that found grey matter density of the PCL to be a contributor of the SVM classifier for discriminating chronic pain patients from controls.32 The representation of the low back locates near the PCL,34 which may be the reason that the PCL ALFF showed the highest reliability for discriminating cLBP subjects from HCs. This result is also consistent with a previous study, which found that fMRI activity in the SMA is an important part of a machine learning classifier for pain.33
Brain regions sensitive to manoeuvre-induced low back pain intensity changes
The ALFF in the mPFC, rACC, precuneus, PCC, and TPJ decreased in cLBP subjects when LBP intensity increased after patient manoeuvres. Change in mPFC/rACC ALFF was negatively correlated with manoeuvre-induced back pain intensity change. The mPFC, rACC, precuneus, and PCC are critical hubs in the default mode network (DMN), which has been implicated in the spontaneous disengagement of attention to pain and is suppressed when attending to pain.35 Decreased DMN low-frequency oscillations after pain intensity increases may be because of greater attentional shift during intense pain. The TPJ is a main component of the ventral attention network36 and DMN. Decreased TPJ ALFF may reflect a self-regulation/distraction strategy in cLBP subjects.
ALFF in the anterior insula, hippocampal gyrus, parahippocampal gyrus, amygdala, and thalamus increased in cLBP subjects when back pain intensity increased. The thalamus, amygdala, hippocampal gyrus, and parahippocampal gyrus are all key regions of the limbic system. A previous study found that persistence of chronic pain is predetermined by corticolimbic neuroanatomical factors.37 Our results further support the important roles of the limbic regions in cLBP and suggest that regional low-frequency oscillations of these regions are sensitive to LBP intensity changes.
The anterior insula is a key node of the salience network (SN), a network responsible for detecting and filtering salient stimuli38 that is activated when subjects receive pain stimulation.39 Consistent with our findings, a previous study found a positive correlation between pain intensity and cerebral blood flow changes in the anterior insula when LBP intensity increased.40 Our findings of increased SN and decreased DMN activity in the high pain condition compared with the low pain condition provide further evidence for the hypothesis that interplay between the DMN and SN reflects interactions between mind wandering and ongoing pain.35
There are several limitations to our study. First, we applied a manoeuvre model to increase LBP. Therefore, there may be a potential order effect. Secondly, we did not collect a second set of fMRI data for HC subjects. However, in a previous study, we collected both pre- and post-manoeuvre fMRIs for cLBP and HC subjects and found that brain functional changes were significantly associated with the presence of clinical pain in cLBP subjects but found no behaviour association in HCs who performed the same maneuver.40 Thus, we believe that the ALFF differences we observed in this study between cLBP subjects with high pain and low pain conditions are related to back pain intensity differences. Finally, the physiological significance of ALFF is still under investigation. A previous study found both high cerebral blood flow and high fluctuation amplitude, as measured by ALFF, in the same brain areas in the same cohort of healthy subjects,9 and another study showed a significant correlation between ALFF and task activations from a bilateral finger tapping task.10 Using a middle cerebral artery occlusion (MCAO) stroke model in rats, investigators also found that the signal intensities of abnormal ALFF increased and migrated from the core of ischaemic lesion areas to the edges after MCAO, suggesting that ALFF may reflect neural processes.11 Nevertheless, more studies including multimodal imaging approaches (e.g., ALFF, cerebral blood flow, task-related fMRI) are needed to further elucidate the physiological significance of ALFF and the mechanism of cLBP.
In conclusion, compared with HCs, cLBP subjects showed increased ALFF in the PCL, SMA, PoG, PrG, dACC, and rACC and grey matter volume increases in the ACC. Discriminative analysis showed that PCL/SMA ALFF may hold the potential to classify cLBP. Further, ALFF in the DMN, insula, and limbic areas is sensitive to manoeuvre-induced spontaneous LBP intensity changes in cLBP, which may have potential in monitoring cLBP symptom changes. Our findings may shed light on the pathophysiology of cLBP and the applications of brain-imaging in translational medicine.
Authors' contributions
Contributed to the study design: JK, RG, STC, AW, RE, VN, TK, BR.
Contributed to the data acquisition: AO, JG, IM, JL.
Contributed to the data analysis and interpretation: BZ, MJ, YT, JK.
Wrote the manuscript: BZ, MJ, CL, JP, JK, GW, AW.
Approved the final manuscript: all authors.
Declarations of interest
JK has equity in a start-up company (Massachusetts Neuro Technology) and pending patents to develop new neuromodulation tools, but declares no conflict of interest. All other authors declare no conflict of interest.
Funding
US National Institutes of Health (NIH) P01 AT006663 to BR and RG; JK is supported by US NIH R01 AT008563, R21 AT008707, and R33 AT009310.
Handling editor: H.C. Hemmings Jr
Editorial decision: 24 February 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.02.021.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hartvigsen J., Hancock M.J., Kongsted A. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 2.Deyo R.A., Von Korff M., Duhrkoop D. Opioids for low back pain. BMJ. 2015;350 doi: 10.1136/bmj.g6380. g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X., Xu M., Jorgenson K., Kong J. Neurochemical changes in patients with chronic low back pain detected by proton magnetic resonance spectroscopy: A systematic review. NeuroImage Clin. 2017;13:33–38. doi: 10.1016/j.nicl.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kregel J., Meeus M., Malfliet A. Structural and functional brain abnormalities in chronic low back pain: A systematic review. Semin Arthritis Rheum. 2015;45:229–237. doi: 10.1016/j.semarthrit.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Tu Y., Jung M., Gollub R.L. Abnormal medial prefrontal cortex functional connectivity and its association with clinical symptoms in chronic low back pain. Pain Adv Access Published. January 29 2019 doi: 10.1097/j.pain.0000000000001507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derbyshire S.W. The use of neuroimaging to advance the understanding of chronic pain: from description to mechanism. Psychosom Med. 2014;76:402–403. doi: 10.1097/PSY.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 7.Ung H., Brown J., Johnson K., Younger J., Hush J., Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cereb Cortex. 2014;24:1037–1044. doi: 10.1093/cercor/bhs378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis K.D., Flor H., Greely H.T. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol. 2017;13:624–638. doi: 10.1038/nrneurol.2017.122. [DOI] [PubMed] [Google Scholar]
- 9.Zou Q., Wu C.W., Stein E.A., Zang Y., Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. NeuroImage. 2009;48:515–524. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan R., Di X., Kim E.H., Barik S., Rypma B., Biswal B.B. Regional homogeneity of resting-state fMRI contributes to both neurovascular and task activation variations. Magn Reson Imaging. 2013;31:1492–1500. doi: 10.1016/j.mri.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Q-l, Zhang H.-Y., Nie B-b, Fang F., Jiao Y., Teng G.-J. MRI assessment of amplitude of low-frequency fluctuation in rat brains with acute cerebral ischemic stroke. Neurosci Lett. 2012;509:22–26. doi: 10.1016/j.neulet.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Lu B., Yan C.G. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum Brain Mapp. 2018;39:300–318. doi: 10.1002/hbm.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linn K.A., Gaonkar B., Satterthwaite T.D., Doshi J., Davatzikos C., Shinohara R.T. Control-group feature normalization for multivariate pattern analysis of structural MRI data using the support vector machine. NeuroImage. 2016;132:157–166. doi: 10.1016/j.neuroimage.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu R., Gollub R.L., Spaeth R., Napadow V., Wasan A., Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. NeuroImage Clin. 2014;6:100–108. doi: 10.1016/j.nicl.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn K.M., Croft P.R. Classification of low back pain in primary care: using "bothersomeness" to identify the most severe cases. Spine. 2005;30:1887–1892. doi: 10.1097/01.brs.0000173900.46863.02. [DOI] [PubMed] [Google Scholar]
- 16.Cherkin D.C., Sherman K.J., Avins A.L. A randomized trial comparing acupuncture, simulated acupuncture, and usual care for chronic low back pain. Arch Intern Med. 2009;169:858–866. doi: 10.1001/archinternmed.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z., Zeng F., Yin T. Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. NeuroImage Clin. 2017;15:367–375. doi: 10.1016/j.nicl.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan C.G., Cheung B., Kelly C. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winkler A.M., Ridgway G.R., Douaud G., Nichols T.E., Smith S.M. Faster permutation inference in brain imaging. NeuroImage. 2016;141:502–516. doi: 10.1016/j.neuroimage.2016.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tracey I., Mantyh P.W. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Bliss T.V., Collingridge G.L., Kaang B.K., Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17:485–496. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 22.Fischl B. FreeSurfer NeuroImage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischl B., van der Kouwe A., Destrieux C. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 24.Kliuchko M., Puolivali T., Heinonen-Guzejev M. Neuroanatomical substrate of noise sensitivity. NeuroImage. 2017;167:309–315. doi: 10.1016/j.neuroimage.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 25.Chang C.-C., Lin C.-J. LIBSVM: A library for support vector machines. ACM Trans Intell Syst Technol. 2011;2:1–27. [Google Scholar]
- 26.Tu Y., Zhang Z., Tan A. Alpha and gamma oscillation amplitudes synergistically predict the perception of forthcoming nociceptive stimuli. Hum Brain Mapp. 2016;37:501–514. doi: 10.1002/hbm.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim W., Kim S.K., Nabekura J. Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J Neurochem. 2017;141:499–506. doi: 10.1111/jnc.14012. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman M.D., Eisenberger N.I. The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci U S A. 2015;112:15250–15255. doi: 10.1073/pnas.1515083112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker R.S., Lewis G.N., Rice D.A., McNair P.J. Is motor cortical excitability altered in people with chronic pain? A systematic review and meta-analysis. Brain Stimul. 2016;9:488–500. doi: 10.1016/j.brs.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 31.Jensen K.B., Srinivasan P., Spaeth R. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum. 2013;65:3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagarinao E., Johnson K.A., Martucci K.T. Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. PAIN. 2014;155:2502–2509. doi: 10.1016/j.pain.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.W., Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramachandran V.S., Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 35.Kucyi A., Salomons T.V., Davis K.D. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A. 2013;110:18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vachon-Presseau E., Tetreault P., Petre B. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139:1958–1970. doi: 10.1093/brain/aww100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 39.Downar J., Crawley A.P., Mikulis D.J., Davis K.D. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 40.Wasan A.D., Loggia M.L., Chen L.Q., Napadow V., Kong J., Gollub R.L. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115:364–374. doi: 10.1097/ALN.0b013e318220e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.