Summary
Over the past decade, the mechanisms underlying placebo effects have begun to be identified. At the same time, the placebo response appears to have increased in pharmacological trials and marked placebo effects are found in neurostimulation and surgical trials, thereby posing the question whether non-pharmacological interventions should be placebo-controlled to a greater extent. In this narrative review we discuss how the knowledge of placebo mechanisms may help to improve placebo control in pharmacological and non-pharmacological trials. We review the psychological, neurobiological, and genetic mechanisms underlying placebo analgesia and outline the current problems and potential solutions to the challenges with placebo control in trials on pharmacological, neurostimulation, and surgical interventions. We particularly focus on how patients' perception of the therapeutic intervention, and their expectations towards treatment efficacy may help develop more precise placebo controls and blinding procedures and account for the contribution of placebo factors to the efficacy of active treatments. Finally, we discuss how systematic investigations into placebo mechanisms across various pain conditions and types of treatment are needed in order to ‘personalise’ the placebo control to the specific pathophysiology and interventions, which may ultimately lead to identification of more effective treatment for pain patients. In conclusion this review shows that it is important to understand how patients' perception and expectations influence the efficacy of active and placebo treatments in order to improve the test of new treatments. Importantly, this applies not only to assessment of drug efficacy but also to non-pharmacological trials on surgeries and stimulation procedures.
Keywords: implantable neurostimulators, operative, placebo effect, randomised controlled trial, surgical procedures, treatment outcome
Editor's key points.
-
•
The placebo response is often high in pain studies. A wide range of factors (genetic, psychological, and biological) need to be considered, that may impact on the placebo response.
-
•
Patient expectation of analgesic response can have a significant impact on reported efficacy.
-
•
Improved understanding of the mechanisms may help improve clinical trial design to identify potentially effective novel interventions (pharmacological and non-pharmacological).
-
•
Future clinical analesia trials could then use a more precise approach to control for the individual components of the placebo response, according to pain type and intervention.
Placebos are typically conceptualised as inactive treatments such as sugar pills, saline injections, or sham surgeries that are used as controls for the active treatment under investigation in RCTs.1, 2, 3 During the past decade, however, an independent research field focused on understanding placebo mechanisms has emerged.3, 4, 5, 6 This line of research investigates how the treatment context and patients' perception of receiving a treatment contribute to the overall treatment efficacy and specifies psychological and neurobiological mechanisms responsible for placebo analgesia.5, 6, 7
Pharmacological treatments must show an effect over and above a placebo response in RCTs to be approved for human use. Such a proof of efficacy is not required in the case of surgical procedures or neurostimulation procedures. At the same time, substantial placebo responses have been demonstrated in neurostimulation techniques8 and surgeries,9,10 which has opened the discussion on when and how placebo procedures should be used as a control.11 Moreover, the magnitude of the placebo response in RCTs has increased during the past decade, which may potentially contribute to the growing problem of showing an advantage of the active medication over placebo.12, 13, 14, 15
In this article, we will briefly summarise the current knowledge of placebo analgesia mechanisms and outline to which extent this knowledge may help improve the test of pharmacological treatments, neurostimulation techniques, and surgical interventions.
Placebo analgesia
Definitions
The placebo phenomenon is related to patients' perception or direct experience of a treatment, that is seeing, smelling, and hearing verbal information about the treatment and actively integrating this sensory information with memories of previous experiences and current expectations.16, 17 The placebo response is measured as the change in pain observed after administration of the placebo treatment.18, 19 The placebo effect, however, is measured as the difference in pain across an untreated and a placebo-treated group/condition, thereby separating changes in the placebo group from spontaneous fluctuations in pain, regression to the mean, and other confounding factors (Fig. 1).3, 19, 20, 21 The well-controlled placebo effect is usually assessed in placebo mechanism studies, whereas the uncontrolled placebo response is typically measured in RCTs although there are several three-arm RCTs that include an active, a placebo, and a no-treatment control group.
Fig 1.
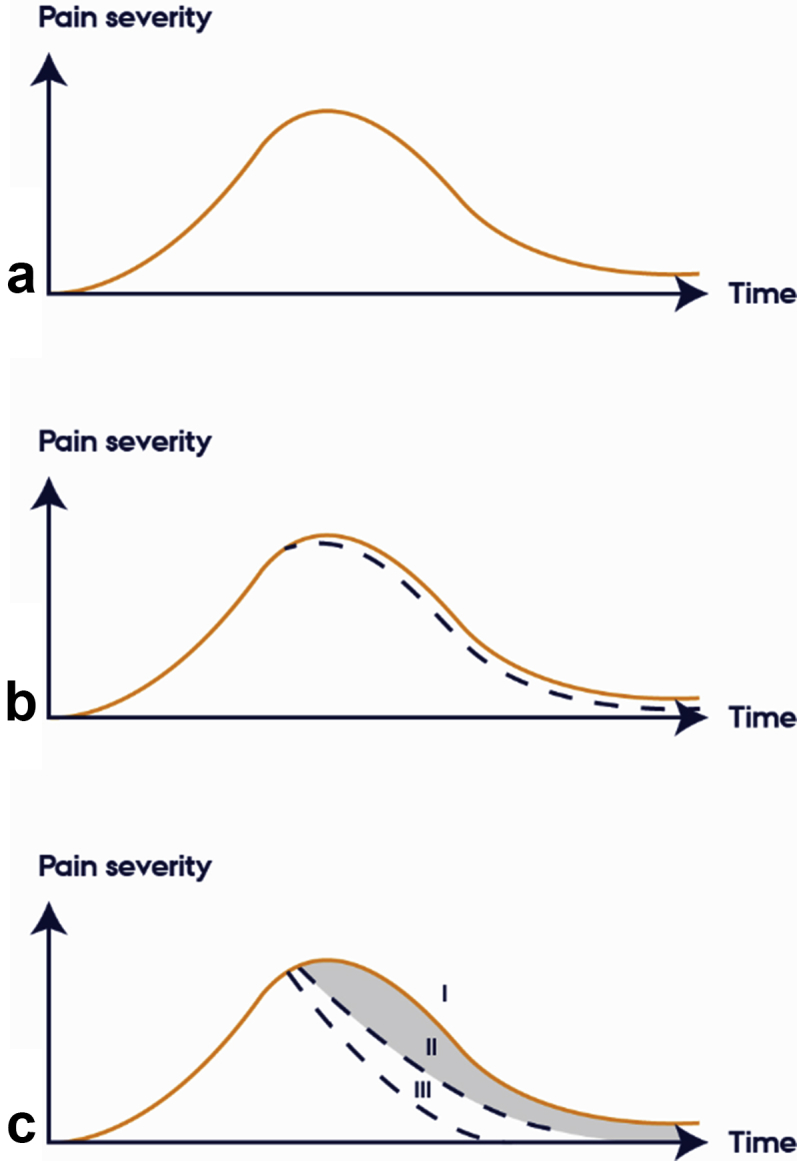
The natural course of pain and the course of pain after treatments. (a) The natural course of pain over time. (b) ‘Reduction in pain’ after a placebo treatment (dashed line). The uncontrolled placebo response. (c) Reduction in pain after no treatment (l), placebo treatment (II), and active treatment (III). The placebo effect is the difference in pain levels in a placebo treated (II) and no-treatment (I) group/condition. The figure is inspired by Fields and Levine.20
Magnitude and mechanisms
The magnitude of placebo analgesia effects is highly variable,22, 23, 24 and it appears to be influenced by prior experiences and verbal suggestions accompanying administration of the placebo agent. If patients are told ‘this agent has been shown to powerfully reduce pain in some patients’, they experience larger placebo effects than if they are told ‘this agent may either be an active painkiller or an inactive placebo agent’.25, 26 In fact, the verbal suggestions that accompany the treatment may enhance the effect of placebo and decrease the effect of anti-migraine medicine to an extent where there is no longer a significant difference between the effect of the two treatments.27 Likewise, prior conditioning with an active agent increases the magnitude of placebo analgesia.3, 28 Expected pain levels have been shown to account for up to 77% of the variance in post-treatment pain ratings after placebo administration.7, 26 Also, emotional factors such as satisfaction with the treatment, feeling of reward, and reduced levels of anxiety and stress contribute to placebo effects,29, 30, 31 whereas fear may block these effects.32
Brain imaging studies and their meta-analyses have illustrated that the anticipation/expectation of (placebo) analgesia is associated with increased activity in the left anterior cingulate, right precentral and lateral prefrontal cortex, and the left periaqueductal grey matter.33 Pain relief after placebo administration, by contrast, is associated with decreased activity in pain processing regions, including the medial thalamus, primary and secondary somatosensory cortex, the dorsal posterior insula, the mid-insula, anterior insula, and the dorsal anterior cingulate cortex (dACC) with the most consistent effects in the dACC, the thalamus, and the anterior insula.33 Placebo analgesia has also been shown to reduce nociception at the level of the spinal cord.34 A recent meta-analysis of patient-level data only found a small effect of placebo treatments on the neurological pain signature,35 whereas a new study shows that expectation, a core element in placebo effects, influences the neurological pain signature.36 These mixed findings may at least in part be attributable to the meta-analysis investigating the effect of heterogeneous placebo treatments as opposed to the placebo effect per se. Also, the understanding of how psychological modulation of pain and pain medication influence the neurological pain signature is still a topic of ongoing investigations.37
Neurotransmitter studies have shown that placebo analgesia effects may be mediated by the release of endogenous opioids.28, 38, 39 Interestingly, placebo effects that are non-opioid mediated can be blocked by the cannabinoid receptor antagonist CB1.40 Moreover, by changing the meaning of pain from negative to positive via verbal suggestions for pain relief (ischaemia is aversive vs ischaemia is beneficial for the muscles), opioid and cannabinoid systems are co-activated and, in turn, increase pain tolerance.41 Positron emission tomography (PET) studies have supported the assumption that reward and release of dopamine may be central to placebo effects,42, 43 but the dopamine antagonist haloperidol does not seem to block the placebo effect.44, 45 Genetics investigation has likewise suggested the gene for catechol-O-methyltransferase (COMT), an enzyme involved in the metabolism of dopamine and other catecholamines, to be a potential genetic locus of the placebo response46, 47, 48 although more investigations are necessary to specify the role of genetics in placebo effects. Finally, hormones such as oxytocin and vasopressin, which are involved in pro-social behaviours (e.g. bonding), have been shown to increase the placebo effect in some contexts and populations.49, 50
The majority of placebo mechanism studies are conducted in healthy volunteers exposed to experimental or acute pain. Studies of chronic pain patients include patients suffering from irritable bowel syndrome,26, 51 migraine,27 low back pain,52 and neuropathic pain,53, 54 and although they appear to find similar involvement of expectations, emotions, and brain structures, they have not been able to replicate the involvement of neurotransmitters.31, 45, 55 This points to the importance of investigating the magnitude and the mechanisms of placebo effects systematically across various pain conditions.
Placebo control in pharmacological trials
Current problems
New pharmacological treatments are tested in RCTs including an active arm and a placebo arm to minimise bias in the test of new treatments.56 Currently, however, the RCT is facing several challenges and some of the main assumptions underlying the RCT are questioned.57, 58, 59, 60, 61
First, some meta-analyses have shown that the magnitude of the placebo response in RCTs have increased during the past decade,12, 13, 14, 62 whereas other meta-analyses have not found this increase.63 Large placebo effects may make it difficult for a trial to demonstrate the superiority of active treatment over placebo and thereby hamper the development of new drugs.13, 64, 65, 66 As the RCTs in these meta-analyses only involved an active and a placebo arm, it is not possible to differentiate the placebo response from changes in the natural history of pain. Hence, it is difficult to know whether the placebo effect has increased or whether the natural history of the pain has changed, for example by including more patients who report high levels of pain at study entry and subsequently regress to lower pain levels. Still, at present, the apparent problem of an increasing placebo response is approached by identifying placebo responders through psychological tests, genetic analyses, or both, and excluding them from trials, for example via placebo run-in where placebo responders are identified early in the trial and subsequently removed from the trial.47, 65, 67 Although these approaches are designed to reduce the placebo response and enhance the drug vs placebo comparison (i.e. assay sensitivity), they do not necessarily succeed in doing so.68 Furthermore, the ecological validity of this approach can be questioned58 as a highly selected study population makes the findings less applicable to clinical practice.
Second, a core element of the RCT and its use of placebo control is that the patients and everyone who may affect the trial result by knowing the treatment allocation are blinded to the administration of active and placebo treatments.56, 69 This ensures that the perception and expectations towards the two treatments are held constant so that only the presence or the absence of the active ingredient differs across the two treatments. Yet, although most RCTs are set up to be double-blind and guidelines recommend reporting the success of blinding,70, 71 only a few trials assess to what extent blinding has been achieved,72, 73, 74 and even fewer trials directly test how un-blinding might have influenced the results of the trial.75, 76 In pharmacological studies, it is well known that patients and healthcare providers often correctly identify treatment allocation based on, for example patients' experience of adverse events.58, 77 This problem has been approached by developing so-called ‘active placebos’ that elicit adverse events similar to those of the active treatment,76, 77 but even in these studies full double-blinding is not achieved.76
Third, a basic assumption of the RCT is that the drug response and the placebo response are additive so the effect of the active treatment can be calculated by subtracting the placebo response from the total treatment response.78 However, recent studies and meta-analyses have questioned the additivity assumption27, 78, 79, 80 as they have found that the total treatment effect is less than the active response plus the placebo response, especially in high placebo responders, thereby potentially underestimating the efficacy of the drug.78 Interestingly, however, high placebo responders also tend to have the highest drug response,78 which could be related to the circumstance that placebo effects appear to take effect through similar mechanisms as active treatments.3 This notion may at least in part explain why removal of placebo responders do not necessarily improve the drug vs placebo comparison.
Potential solutions
At present, the problems with the placebo response in RCTs are primarily approached within the framework of the RCT, for example by investigating patient characteristics (e.g. ethnicity) and trial characteristics (e.g. country in which the trial is conducted) that may relate to the placebo response in RCTs.12, 13, 65, 81 Yet, the scientific evidence to support each of the factors is low.
In order to overcome some of the problems of the RCT, it may instead be helpful to turn to the placebo mechanism literature. Here it is evident that the magnitude of placebo effects is highly variable22,23 and related to patients' perception of the therapeutic intervention.16,17 Several attempts have been made to identify stable factors such as demographic variables,12 personality traits,82, 83, 84 genetic profiles,46, 47, 85 and illness characteristics86 that may predict a high placebo response, but consistent findings across studies are rare.82, 83, 84 In fact, experimental studies have shown that the same person responds differently to different placebos,87,88 thereby complicating the notion of a generic placebo responder, that is one that responds consistently to placebo across different situations.87 Thus, instead of trying to identify stable factors that can predict the placebo responder, it may be more helpful to tap into patients' perception of the specific therapeutic intervention and test how that influences the outcome.
One way to tap into patients' perceptions is by measuring their expectations towards the treatment and testing the extent to which this influences the outcome of the trial. In RCTs, patients' expectations towards treatment outcome are typically not assessed. Still, two meta-analyses have made approximations of patients' expectations in RCTs and found that these predicted the outcome.63,89 In the first meta-analyses, adverse events were compared in the placebo arm of three different types of anti-migraine treatment: NSAIDs, triptans, and anti-convulsants. Although patients only received placebo, they experienced a high frequency of adverse events and the type of adverse events followed the information they had received in the informed consent. For example, patients who received placebo as a control for anti-convulsants experienced insomnia more frequently than patients who received placebo as a control for NSAIDS; this suggests that verbal suggestion and patients' expectations influence adverse events.89 In the second meta-analysis, an approximation of expectations was based on a higher number of interactions with healthcare professionals and knowledge of an opioid rather than a non-opioid drug as the active comparator; these approximations significantly predicted the placebo response in analgesic RCTs whereas stable factors and trials characteristics in general did not.63
Patients' perception of the treatment may directly influence the outcome of the trial. For example, active acupuncture shows no effect over placebo acupuncture when data are analysed according to actual treatment allocation. Yet, when data are analysed according to perceived treatment allocation, the patients who believe they received active acupuncture experienced significantly more pain relief as compared with those who believed they received placebo acupuncture.90, 91 Furthermore, the efficacy of active topical or opioid analgesia can be either enhanced or abolished by verbal suggestions that induce positive or negative expectations and emotions, respectively.92,93
Thus, by measuring patients' perception of the intervention and their expectations towards treatment outcome early in the trial, it may be possible to test how that influences the outcome in the placebo and active arm thereby offering an alternative way of measuring the placebo component of the treatment. Such an approach may also give a better indication of whether blinding has been maintained. Although it has correctly been argued that assessment of blinding at the end of the trial makes it difficult to distinguish between un-blinding and ‘haunches’ of efficacy,94 assessment of perceived treatment allocation early in the trial has proved helpful for accurate interpretation of data57,95 and it is recommended for future trials.57
Hence, instead of trying to minimise the placebo response, it may be more advantageous to begin to understand how patients' perception of the therapeutic intervention influences the placebo response, the drug response, the adverse events, and the interactions between these outcomes. To do this most precisely, it would be helpful to have three-arm RCTs so the efficacy of placebo can be separated from confounding factors such as spontaneous remission and regression to the mean, and to directly assess perceived treatment allocation and expectations of efficacy.
Placebo control in non-pharmacological trials
Current problems
Unlike pharmacological treatments, neurostimulations and surgical interventions are not required to demonstrate their safety and efficacy in a placebo-controlled RCT.96 It seems sensible to require a solid proof of evidence that a small tablet has powerful clinical effects. The perception of surgical procedures and clinical devices is the opposite; it is difficult to believe that a procedure that involves implantation of a device or change of anatomy will not have powerful clinical effects. There is an assumption that any improvement observed after this treatment will be caused by this mechanical change. However, when non-pharmacological trials use subjective outcomes, such as pain, it may be difficult to demonstrate the true efficacy of the treatment without a placebo control.11
The placebo mechanism literature suggests that more invasive procedures have larger placebo effect, possibly because patients perceive an invasive procedure as more effective and hence develop greater expectations of relief.97 Placebo responses after implantation of a neurostimulation device or after a surgical procedure may be particularly large because of the personal involvement of the person delivering the treatment, that is the surgeon, the environment of the operating theatre, and the investment in care required from the patient.98 Meta-analyses of the placebo effect in surgical trials demonstrate a large and sustained improvement in the placebo arm even a year after the surgical procedure.99,100 As the placebo response in surgical trials is large for patient-reported outcomes,100 it is necessary to either use a placebo-controlled blinded trial design or use objective or assessor-rated outcomes. When debating placebo control of neurostimulations and surgical interventions at least three problems occur: (i) identifying the crucial therapeutic element that should be omitted in the placebo procedure, (ii) creating a successful blinding so that the placebo control is indistinguishable from the real surgical procedure, and (iii) the ethical aspect of the placebo control for interventional procedures.
First, the main problem with neurostimulation and surgical trials is that a sugar pill cannot be used as a placebo control. In trials on neurostimulation the crucial therapeutic element is the stimulation itself, and the placebo control can be created by switching the stimulation off.11 However, the two main challenges in these trials are that even the control group requires implantation of the device and that the process of implantation may change some anatomical structures and create a clinically meaningful effect.101 Surgeries and implantations of stimulators are often complex and involves several procedures, which may also have a clinical effect. Unlike neurostimulation trials, in surgical trials, it may be difficult to identify the crucial therapeutic element, that is the key procedure gives the therapeutic effect and which should be omitted during the placebo surgery.102, 103, 104 For example, in a trial on knee osteoarthritis, both tissue removal (debridement) with joint wash-out (lavage) and only lavage could have been therapeutic.105 In addition, surgical trials require operating surgeons to accept that the procedure may not be truly effective and to be willing to test it in the trial conditions by performing an imitation surgery.106 Also, not all patients may agree to take part in a surgical trial, especially if one of the arms is a sham procedure.107
Second, in order to ensure blinding it is important that the placebo control is indistinguishable from a real surgery, so an imitation procedure has to be performed. This imitation procedure has to involve an incision and analgesia both of which are associated with risks.102 Maintaining blinding during surgery is straightforward while patients are under general anaesthesia; however, for safety reasons local anaesthesia may be preferred, but then it is necessary to imitate the sounds, smells, and sensory sensations as if the real surgery were taking place. Imitating the incision wound may be easy when the active surgery is performed using minimally invasive techniques because there is no visible scar after endoscopy or bronchoscopy and only a small skin incision is required to imitate portal access used for other ‘-scopies’.103 Creating a placebo control for an open surgery is much more challenging as it would require a large skin incision and this is one of the reasons why, after the internal mammary artery ligation trials in the 1950s and 1960s, open surgery trials with placebo control have not been attempted.104 The first surgical placebo-controlled trials were performed by Cobb and colleagues (1959)108 and by Dimond and colleagues (1960)109 to test the efficacy of the internal mammary artery ligation on symptoms of coronary heart disease. In these studies, some patients had a placebo procedure, that is, a skin incision, instead of the surgical procedure changing vasculature to provide additional blood supply to the coronary vessels. Patients in both groups demonstrated an improvement in exercise tolerance and reduction in the use of nitrates, but there was no improvement in the ECG in either of the groups. As patients were not informed that they might undergo a placebo procedure, all patients expected the full surgical procedure. Therefore, as Cobb and colleagues108 concluded, the marked improvement in subjective outcomes after the skin incision was caused not by the changes in the blood supply but by the psychological effects of the surgery.
Third, the ethical aspect of placebo for non-pharmacological procedures is controversial. For example, Macklin110 argued that placebo surgery should not be performed unless it could be done for therapeutic purposes because it endangers the trust between patients and doctors and deprives patients of an effective treatment.
Potential solutions
Although placebo control for non-pharmacological interventions may be challenging, a recent review has shown108 109 that published trials used clever methods to make sure that the active and the placebo procedure were indistinguishable so they created similar perceptions of the interventions and equal expectations. For example, studies used opaque goggles or drapes to obscure the field of view, imitated the sounds of devices and the conversations between the operating staff, manipulated the operated leg and splashed saline on it as during actual arthroscopy, and even imitated smell of substances used during the active procedure. In some trials, the patients were operated by a surgical team in one hospital but the postoperative care and assessment were done at another hospital by a surgical team that was blinded to the treatment allocation. In a few trials, even surgeons were blinded as the implanting device was prepared by the manufacturer to either contain the implant or not.103,104 Also, it was possible to test more than one element of the surgery and to investigate whether it has therapeutic benefits. For example, in the trial on arthroscopic debridement and lavage in knee osteoarthritis, there were two interventional arms debridement and lavage and lavage only, and both were compared against the placebo control (a skin incision).105 Moreover, blinding and analgesia were not necessarily obstacles to perform a placebo-controlled surgical trial. Anaesthesia was not mentioned as a problem in any of the reviewed trials, and only one trial reported an adverse effect caused by anaesthesia and it was a bruise at the injection site.103
Furthermore, surgeons are generally not against surgical placebo111 but they are strongly opposed to deception.111 As for patients, they are also opposed to deception but they find placebo treatment acceptable and are willing to take part in placebo-controlled surgical trials.107,112,113 Still, many trials under-recruited or had to be terminated prematurely because they could not recruit more than one patient a month. This is because of patient preference and the fact that surgical trials have many exclusion criteria and often use additional inclusion criteria such as presence or absence of certain pathologies on MRI scans.103,107
With respect to the ethical issues, the counter-argument to Macklin's110 opinion that surgery is unethical unless it can be used for therapeutic purposes, is that many non-pharmacological therapies never had their efficacy confirmed in an RCT,114, 115 and it is unethical to consent patients into a potentially risky treatment of unproven efficacy. A placebo-controlled trial can be ethically justified when outcomes are subjective and only this type of trial can provide an unbiased proof of treatment efficacy. An ethical trial should avoid deceiving patients or exposing them to unnecessary risks,11, 102, 116, 117 which is possible as placebo surgery tends to be much safer than active surgery because the crucial therapeutic element and some additional treatments are omitted.104
Conclusions
We have shown how patients' perception of an intervention is central to the modern understanding of placebo mechanisms. We have also illustrated how the understanding of placebo mechanisms may not only improve the understanding of pain processing but also help develop better control of new treatments. In pharmacological trials, placebo control may be improved by including a no-treatment control condition to accurately assess the placebo effect and test whether that is increasing in trials over time. Also, by assessing patients' perception of the intervention and their expectations and emotions towards treatment efficacy, it may be possible to calculate how that influences the outcome of the active treatment, the placebo treatment, and the interaction between the two, which may give a more precise estimation of treatment efficacy. It is also crucial to recognise that placebo effects may be large in trials on non-pharmacological interventions and should be controlled for even if the tested procedure is a surgery or a neurostimulation. Interestingly, by taking patients' perception of an intervention into consideration, it is possible to perform adequate placebo control and blinding where patients cannot distinguish the active from the placebo intervention. Furthermore, it can be argued that it is more ethical to use placebo control to test the efficacy of treatments so patients are offered safe and effective treatment as opposed to continuing to administer treatment where the efficacy is questionable and the risks may be substantial.
Although placebo mechanism research has come a long way during the past decade, there are still several unsolved issues. Placebo effects are primarily investigated in healthy volunteers exposed to experimental pain stimuli, but in order to utilise knowledge of placebo mechanisms to improve clinical trials or treatment of patients in clinical practice it is pivotal to understand the specific mechanisms in chronic pain conditions and potentially the differences in mechanisms between different types of pain. Likewise, it is important to map the placebo effect across various pharmacological and non-pharmacological interventions including neurostimulation and surgery in order to understand how the efficacy of treatments can be tested most optimally. This would require systematic and comparable investigations of psychological, neurophysiological, and genetic placebo mechanisms across different types of pain and interventions. As a result of this, it would be possible to specify and ‘personalise’ the placebo control to each type of pain and each type of intervention, which would enable a more precise test of treatment efficacy. Such knowledge will lead to approval of more effective treatments and to better optimisation of these treatments in clinical practice, which ultimately will benefit chronic pain patients.
Authors' contributions
Study conception: both authors.
Writing paper: both authors.
Declaration of interest
The authors declare that they have no conflicts of interest.
Handling editor: L. Colvin
Editorial decision: 28 January 2019
Contributor Information
Lene Vase, Email: lenevase@psy.au.dk.
Karolina Wartolowska, Email: karolina.wartolowska@phc.ox.ac.uk.
References
- 1.Harrington A. Boston University Press; Cambridge: 1997. The placebo effect. An interdisciplinary exploration. [Google Scholar]
- 2.Finniss D. Historical aspects of placebo analgesia. In: Colloca L., Flaten M.A., Meissner K., editors. Placebo and pain. From bench to bedside. Elsevier Academic Press; London: 2013. pp. 1–8. [Google Scholar]
- 3.Benedetti F. 2nd edn. Oxford University Press; Oxford: 2014. Placebo effects. Understanding the mechanisms in health and disease. [Google Scholar]
- 4.Colloca L., Flaten M., Meissner K. Elsevier Academic Press; London: 2013. Placebo and pain. From bench to bedside. [Google Scholar]
- 5.Benedetti F., Enck P., Frisaldi E., Schedlowski M. Handbook of experimental pharmacology. Springer; Berlin: 2014. Placebo. [Google Scholar]
- 6.Wager T.D., Atlas L.Y. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16:403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price D.D., Finniss D.G., Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 8.Pollo A., Torre E., Lopiano L. Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. NeuroReport. 2002;13:1383–1386. doi: 10.1097/00001756-200208070-00006. [DOI] [PubMed] [Google Scholar]
- 9.Al-Lamee R., Thompson D., Dehbi H.M. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391:31–40. doi: 10.1016/S0140-6736(17)32714-9. [DOI] [PubMed] [Google Scholar]
- 10.Beard D.J., Rees J.L., Cook J.A. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet. 2018;391:329–338. doi: 10.1016/S0140-6736(17)32457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George A.J.T., Collett C., Car A.J. When should placebo surgery as a control in clinical trials be carried out? Bull R Coll Surg Engl. 2016;98:75–79. [Google Scholar]
- 12.Häuser W., Bartram-Wunn E., Bartram C., Reinecke H., Tölle T. Systematic review: placebo response in drug trials of fibromyalgia syndrome and painful peripheral diabetic neuropathy–magnitude and patient-related predictors. Pain. 2011;152:1709–1717. doi: 10.1016/j.pain.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle A.H., Tohyama S., Ramsay T. Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain. 2015;156:2616–2626. doi: 10.1097/j.pain.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 14.Walsh B.T., Seidman S.N., Sysko R., Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 15.Finnerup N.B., Haroutounian S., Baron R. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain. 2018;159:2339–2346. doi: 10.1097/j.pain.0000000000001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vase L., Petersen G.L., Lund K. Placebo effects in idiopathic and neuropathic pain conditions. Handb Exp Pharmacol. 2014;225:121–136. doi: 10.1007/978-3-662-44519-8_7. [DOI] [PubMed] [Google Scholar]
- 17.Shaibani A., Frisaldi E., Benedetti F. Placebo response in pain, fatigue, and performance: possible implications for neuromuscular disorders. Muscle Nerve. 2017;56:358–367. doi: 10.1002/mus.25635. [DOI] [PubMed] [Google Scholar]
- 18.Kirsch I. The placebo effect revisited: lessons learned to date. Complement Ther Med. 2013;21:102–104. doi: 10.1016/j.ctim.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Evers A.W.M., Colloca L., Blease C. Implications of placebo and nocebo effects for clinical practice: expert consensus. Psychother Psychosom. 2018;87:204–210. doi: 10.1159/000490354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields H.L., Levine J.D. Placebo analgesia — a role for endorphins? Trends Neurosci. 1984;7:271–273. [Google Scholar]
- 21.Fields H.L., Price D.D. Toward a neurobiology of placebo analgesia. In: Harrington A., editor. The placebo effect. Harvard University Press; Boston, MA: 1997. pp. 93–116. [Google Scholar]
- 22.Vase L., Riley J.L., III, Price D.D. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 23.Vase L., Petersen G.L., Riley J.L., III, Price D.D. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain. 2009;145:36–44. doi: 10.1016/j.pain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Forsberg J.T., Martinussen M., Flaten M.A. The placebo analgesic effect in healthy individuals and patients: a meta-analysis. Psychosom Med. 2017;79:388–394. doi: 10.1097/PSY.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 25.Pollo A., Amanzio M., Arslanian A., Casadio C., Maggi G., Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 26.Vase L., Robinson M.E., Verne G.N., Price D.D. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients: an empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 27.Kam-Hansen S., Jakubowski M., Kelley J.M. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6:218ra5. doi: 10.1126/scitranslmed.3006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amanzio M., Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaten M.A., Aslaksen P.M., Lyby P.S., Bjørkedal E. The relation of emotions to placebo responses. Philos Trans R Soc Lond B Biol Sci. 2011;366:1818–1827. doi: 10.1098/rstb.2010.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flaten M.A. Pain-related negative emotions and placebo analgesia. Handb Exp Pharmacol. 2014;225:81–96. doi: 10.1007/978-3-662-44519-8_5. [DOI] [PubMed] [Google Scholar]
- 31.Vase L., Robinson M.E., Verne G.N., Price D.D. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain. 2005;115:338–347. doi: 10.1016/j.pain.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Lyby P.S., Forsberg J.T., Asli O., Flaten M.A. Induced fear reduces the effectiveness of a placebo intervention on pain. Pain. 2012;153:1114–1121. doi: 10.1016/j.pain.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 33.Amanzio M., Benedetti F., Porro C.A., Palermo S., Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2013;34:738–752. doi: 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eippert F., Finsterbuch J., Bingel U. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 35.Zunhammer M., Bingel U., Wager T.D. For the Placebo Imaging Consortium. Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol. 2018;75:1321–1330. doi: 10.1001/jamaneurol.2018.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jepma M., Koban L., van Doorn J., Jones M., Wager T.D. Behavioural and neural evidence for self-reinforcing expectancy effects on pain. Nat Hum Behav. 2018;2:838–855. doi: 10.1038/s41562-018-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.W., Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine J.D., Gordon N.C., Fields H.L. The mechanism of placebo analgesia. Lancet. 1978;312:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 39.Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain. 1996;64:535–543. doi: 10.1016/0304-3959(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 40.Benedetti F., Amanzio M., Rosato R., Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nat Med. 2011;17:1228–1230. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 41.Benedetti F., Thoen W., Blanchard C., Vighetti S., Arduino C. Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain. 2013;154:361–367. doi: 10.1016/j.pain.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Scott D.J., Stohler C.S., Egnatuk C.M., Wang H., Koeppe R.A., Zubieta J.K. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Scott D.J., Stohler C.S., Egnatuk C.M., Wang H., Koeppe R.A., Zubieta J.K. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 44.Wrobel N., Wiech K., Forkmann K., Ritter C., Bingel U. Haloperidol blocks dorsal striatum activity but not analgesia in a placebo paradigm. Cortex. 2014;57:60–73. doi: 10.1016/j.cortex.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Skyt I., Moslemi K., Baastrup C. Does conditioned pain modulation predict the magnitude of placebo effects in patients with neuropathic pain? Eur J Pain. 2018;22:784–792. doi: 10.1002/ejp.1164. [DOI] [PubMed] [Google Scholar]
- 46.Hall K.T., Lembo A.J., Kirsch I. Catechol-O-methyltransferase val158met polymorphism predicts placebo effect in irritable bowel syndrome. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall K.T., Loscalzo J., Kaptchuk T.J. Genetics and the placebo effect: the placebome. Trends Mol Med. 2015;21:285–294. doi: 10.1016/j.molmed.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aslaksen P.M., Forsberg J.T., Gjerstad J. The opioid receptor mu 1 (OPRM1) rs1799971 and catechol-O-methyltransferase (COMT) rs4680 as genetic markers for placebo analgesia. Pain. 2018;159:2585–2592. doi: 10.1097/j.pain.0000000000001370. [DOI] [PubMed] [Google Scholar]
- 49.Colloca L., Pine D.S., Ernst M., Miller F.G., Grillon C. Vasopressin boosts placebo analgesic effects in women: a randomized trial. Biol Psychiatry. 2016;79:794–802. doi: 10.1016/j.biopsych.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kessner S., Sprenger C., Wrobel N., Wiech K., Bingel U. Effect of oxytocin on placebo analgesia: a randomized study. JAMA. 2013;310:1733–1735. doi: 10.1001/jama.2013.277446. [DOI] [PubMed] [Google Scholar]
- 51.Kaptchuk T.J., Kelley J.M., Conboy L.A. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carvalho C., Caetano J.M., Cunha L., Rebouta P., Kaptchuk T.J., Kirsch I. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. 2016;157:2766–2772. doi: 10.1097/j.pain.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen G.L., Finnerup N.B., Grosen K. Expectations and positive emotional feelings accompany reductions in ongoing and evoked neuropathic pain following placebo interventions. Pain. 2014;155:2687–2698. doi: 10.1016/j.pain.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 54.Petersen G.L., Finnerup N.B., Nørskov K.N. Placebo manipulations reduce hyperalgesia in neuropathic pain. Pain. 2012;153:1292–1300. doi: 10.1016/j.pain.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Kupers R., Maeyaert J., Boly M., Faymonville M.E., Laureys S. Naloxone-insensitive epidural placebo analgesia in a chronic pain patient. Anesthesiology. 2007;106:1239–1242. doi: 10.1097/01.anes.0000265418.68005.8a. [DOI] [PubMed] [Google Scholar]
- 56.Hróbjartsson A., Boutron I. Blinding in randomized clinical trials: imposed impartiality. Clin Pharmacol Ther. 2011;90:732–736. doi: 10.1038/clpt.2011.207. [DOI] [PubMed] [Google Scholar]
- 57.Benedetti F., Carlino E., Piedimonte A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurol. 2016;15:736–747. doi: 10.1016/S1474-4422(16)00066-1. [DOI] [PubMed] [Google Scholar]
- 58.Enck P., Bingel U., Schedlowski M., Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12:191–204. doi: 10.1038/nrd3923. [DOI] [PubMed] [Google Scholar]
- 59.Blease C.R., Bishop F.L., Kaptchuk T.J. Informed consent and clinical trials: where is the placebo effect? BMJ. 2017;356:j463. doi: 10.1136/bmj.j463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rief W., Bingel U., Schedlowski M., Enck P. Mechanisms involved in placebo and nocebo responses and implications for drug trials. Clin Pharmacol Ther. 2011;90:722–726. doi: 10.1038/clpt.2011.204. [DOI] [PubMed] [Google Scholar]
- 61.Vase L., Amanzio M., Price D.D. Nocebo vs. placebo: the challenges of trial design in analgesia research. Clin Pharmacol Ther. 2015;97:143–150. doi: 10.1002/cpt.31. [DOI] [PubMed] [Google Scholar]
- 62.Silberman S. Placebos are getting more effective. Drugmakers are desperate to know why. Wired Mag. 2009;17:1–8. [Google Scholar]
- 63.Vase L., Vollert J., Finnerup N.B. Predictors of the placebo analgesia response in randomized controlled trials of chronic pain: a meta-analysis of the individual data from nine industrially sponsored trials. Pain. 2015;156:1795–1802. doi: 10.1097/j.pain.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 64.Dworkin R.H., Turk D.C., Peirce-Sandner S. Meta-analysis of assay sensitivity and study features in clinical trials of pharmacologic treatments for osteoarthritis pain. Arthritis Rheumatol. 2014;66:3327–3336. doi: 10.1002/art.38869. [DOI] [PubMed] [Google Scholar]
- 65.Dworkin R.H., Turk D.C., Peirce-Sandner S. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012;153:1148–1158. doi: 10.1016/j.pain.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Dworkin R.H., Katz J., Gitlin M.J. Placebo response in clinical trials of depression and its implications for research on chronic neuropathic pain. Neurology. 2005;65:7–19. doi: 10.1212/wnl.65.12_suppl_4.s7. [DOI] [PubMed] [Google Scholar]
- 67.Dworkin R.H., Turk D.C., Peirce-Sandner S. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149:177–193. doi: 10.1016/j.pain.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Simpson D.M., Rice A.S., Emir B. A randomized, double-blind, placebo-controlled trial and open-label extension study to evaluate the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with human immunodeficiency virus neuropathy. Pain. 2014;155:1943–1954. doi: 10.1016/j.pain.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 69.Kaptchuk T.J. Powerful placebo: the dark side of the randomised controlled trial. Lancet. 1998;351:1722–1725. doi: 10.1016/S0140-6736(97)10111-8. [DOI] [PubMed] [Google Scholar]
- 70.Moher D., Hopewell S., Schulz K.F. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10:28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Schulz K.F., Altman D.G., Moher D., for the CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med. 2011:152. doi: 10.3736/jcim20100702. [DOI] [PubMed] [Google Scholar]
- 72.Hróbjartsson A., Forfang E., Haahr M.T., Als-Nielsen B., Brorson S. Blinded trials taken to the test: an analysis of randomized clinical trials that report tests for the success of blinding. Int J Epidemiol. 2007;36:654–663. doi: 10.1093/ije/dym020. [DOI] [PubMed] [Google Scholar]
- 73.Margraf J., Ehlers A., Roth W. How "blind" are double-blind studies? J Consult Clin Psychol. 1991;59:184–187. doi: 10.1037//0022-006x.59.1.184. [DOI] [PubMed] [Google Scholar]
- 74.Rabkin J.G., Markowitz J.S., Stewart J. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19:75–86. doi: 10.1016/0165-1781(86)90094-6. [DOI] [PubMed] [Google Scholar]
- 75.Hróbjartsson A., Emanuelsson F., Thomsen A.S.S., Hilden J., Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol. 2014;43:1272–1283. doi: 10.1093/ije/dyu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turner J., Jensen M., Warms C., Cardenas D. Blinding effectiveness and association of pretreatment expectations with pain improvement in a double-blind randomized controlled trial. Pain. 2002;99:91–99. doi: 10.1016/s0304-3959(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 77.Rief W., Glombiewski J.A. The hidden effects of blinded, placebo-controlled randomized trials: an experimental investigation. Pain. 2012;153:2473–2477. doi: 10.1016/j.pain.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 78.Lund K., Vase L., Petersen G.L., Jensen T.S., Finnerup N.B. Randomised controlled trials may underestimate drug effects: balanced placebo trial design. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finnerup N.B., Sindrup S.H., Jensen T.S. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 80.Katz J., Finnerup N.B., Dworkin R.H. Clinical trial outcome in neuropathic pain: relationship to study characteristics. Neurology. 2008;70:263–272. doi: 10.1212/01.WNL.0000275528.01263.6c. [DOI] [PubMed] [Google Scholar]
- 81.Macedo A., Farré M., Baños J.E. A meta-analysis of the placebo response in acute migraine and how this response may be influenced by some of the characteristics of clinical trials. Eur J Clin Pharmacol. 2006;62:161–172. doi: 10.1007/s00228-005-0088-5. [DOI] [PubMed] [Google Scholar]
- 82.Kaptchuk T.J., Kelley J.M., Deykin A. Do "placebo responders" exist? Contemp Clin Trial. 2008;29:587–595. doi: 10.1016/j.cct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Darragh M., Booth R.J., Consedine N.S. Who responds to placebos? Considering the "placebo personality" via a transactional model. Psychol Health Med. 2014;76:414–421. doi: 10.1080/13548506.2014.936885. [DOI] [PubMed] [Google Scholar]
- 84.Geers A.L., Kosbab K., Helfer S.G., Weiland P.E., Wellman J.A. Further evidence for individual differences in placebo responding: an interactionist perspective. J Psychosom Res. 2007;62:563–570. doi: 10.1016/j.jpsychores.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 85.Peciña M., Martínez-Jauand M., Hodgkinson C., Stohler C., Goldman D., Zubieta J. FAAH selectively influences placebo effects. Mol Psychiatry. 2014;19:385–391. doi: 10.1038/mp.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cepeda M.S., Berlin J.A., Gao C.Y., Wiegand F., Wada D.R. Placebo response changes depending on the neuropathic pain syndrome: results of a systematic review and meta-analysis. Pain Med. 2012;13:575–595. doi: 10.1111/j.1526-4637.2012.01340.x. [DOI] [PubMed] [Google Scholar]
- 87.Whalley B., Hyland M.E., Kirsch I. Consistency of the placebo effect. J Psychosom Res. 2008;64:537–541. doi: 10.1016/j.jpsychores.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 88.Lieberman R.P. The elusive placebo reactor. Neuro Psychopharm. 1967;5:557–566. [Google Scholar]
- 89.Amanzio M., Corazzini L.L., Vase L., Benedetti F. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain. 2009;146:261–269. doi: 10.1016/j.pain.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 90.Vase L., Baram S., Takakura N. Specifying the nonspecific components of acupuncture analgesia. Pain. 2013;154:1659–1667. doi: 10.1016/j.pain.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bausell R.B., Lao L., Bergman S., Lee W.L., Berman B.M. Is acupuncture analgesia an expectancy effect? Preliminary evidence based on participants' perceived assignments in two placebo-controlled trials. Eval Health Prof. 2005;28:9–26. doi: 10.1177/0163278704273081. [DOI] [PubMed] [Google Scholar]
- 92.Bingel U., Wanigasekera V., Wiech K. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 93.Aslaksen P.M., Zwarg M.L., Eilertsen H.I., Gorecka M.M., Bjørkedal E. Opposite effects of the same drug: reversal of topical analgesia by nocebo information. Pain. 2015;156:39–46. doi: 10.1016/j.pain.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 94.Sackett D.L. Commentary: measuring the success of blinding in RCTs: don't, must, can't or needn't? Int J Epidemiol. 2007;36:664–665. doi: 10.1093/ije/dym088. [DOI] [PubMed] [Google Scholar]
- 95.McRae C., Cherin E., Yamazaki T.G. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatry. 2004;61:412–420. doi: 10.1001/archpsyc.61.4.412. [DOI] [PubMed] [Google Scholar]
- 96.McCulloch P., Altman D.G., Campbell W.B. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 97.Kaptchuk T.J., Goldman P., Stone D.A., Stason W.B. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786–792. doi: 10.1016/s0895-4356(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 98.Johnson A.G. Surgery as a placebo. Lancet. 1994;344:1140–1142. doi: 10.1016/s0140-6736(94)90637-8. [DOI] [PubMed] [Google Scholar]
- 99.Holtedahl R., Brox J.I., Tjomsland O. Placebo effects in trials evaluating 12 selected minimally invasive interventions: a systematic review and meta-analysis. BMJ Open. 2015;5:e007331. doi: 10.1136/bmjopen-2014-007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wartolowska K.A., Gerry S., Feakins B.G. A meta-analysis of temporal changes of response in the placebo arm of surgical randomized controlled trials: an update. Trials. 2017;18:323. doi: 10.1186/s13063-017-2070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Freeman T.B., Vawter D.E., Leaverton P.E. Use of placebo surgery in controlled trials of a cellular-based therapy for Parkinson’s disease. N Engl J Med. 1999;341:988–992. doi: 10.1056/NEJM199909233411311. [DOI] [PubMed] [Google Scholar]
- 102.Tenery R., Rakatansky H., Riddick F.A.J. Surgical “placebo” controls. Ann Surg. 2002;235:303–307. doi: 10.1097/00000658-200202000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wartolowska K.A., Collins G.S., Hopewell S. Feasibility of surgical randomised controlled trials with a placebo arm: a systematic review. BMJ Open. 2016;6:e010194. doi: 10.1136/bmjopen-2015-010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wartolowska K., Judge A., Hopewell S. Use of placebo controls in the evaluation of surgery: systematic review. BMJ. 2014;348:g3253. doi: 10.1136/bmj.g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moseley J.B., O’Malley K., Petersen N.J. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 106.Wright J.G., Katz J.N., Losina E. Clinical trials in orthopaedics research: Part I. Cultural and practical barriers to randomized trials in orthopaedics. J Bone Jt Surg. 2011;93:e15. doi: 10.2106/JBJS.J.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hare K.B., Lohmander L.S., Roos E.M. The challenge of recruiting patients into a placebo-controlled surgical trial. Trials. 2014;15:167. doi: 10.1186/1745-6215-15-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cobb L.A., Thomas G.I., Dillard D.H., Merendino K.A., Bruce R.A. An evaluation of internal-mammary-artery ligation by a double-blind technic. N Engl J Med. 1959;260:1115–1118. doi: 10.1056/NEJM195905282602204. [DOI] [PubMed] [Google Scholar]
- 109.Diamond E.G., Kittle C.F., Crockett J.E. Comparison of internal mammary artery ligation and sham operation for angina pectoris. Am J Cardiol. 1960;5:483–486. doi: 10.1016/0002-9149(60)90105-3. [DOI] [PubMed] [Google Scholar]
- 110.Macklin R. The ethical problems with sham surgery in clinical research. N Engl J Med. 1999;341:992–996. doi: 10.1056/NEJM199909233411312. [DOI] [PubMed] [Google Scholar]
- 111.Wartolowska K., Beard D.J., Carr A.J. Attitudes and beliefs about placebo surgery among orthopedic shoulder surgeons in the United Kingdom. PLoS One. 2014;9:e91699. doi: 10.1371/journal.pone.0091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hull S.C., Colloca L., Avins A. Patients’ attitudes about the use of placebo treatments: telephone survey. BMJ. 2013;347:f3757. doi: 10.1136/bmj.f3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frank S.A., Wilson R., Holloway R.G. Ethics of sham surgery: perspective of patients. Mov Disord. 2008;23:63–68. doi: 10.1002/mds.21775. [DOI] [PubMed] [Google Scholar]
- 114.Gelijns A.C., Ascheim D.D., Parides M.K., Kent K.C., Moskowitz A.J. Randomized trials in surgery. Surgery. 2009;145:581–587. doi: 10.1016/j.surg.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wenner D.M., Brody B.A., Jarman A.F. Do surgical trials meet the scientific standards for clinical trials? J Am Coll Surg. 2012;215:722–730. doi: 10.1016/j.jamcollsurg.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Savulescu J., Wartolowska K., Carr A. Randomised placebo-controlled trials of surgery: ethical analysis and guidelines. J Med Ethics. 2016;42:776–783. doi: 10.1136/medethics-2015-103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horng S.B.A., Miller F.G. Ethical framework for the use of sham procedures in clinical trials. Crit Care Med. 2003;31:126–130. doi: 10.1097/01.CCM.0000054906.49187.67. [DOI] [PubMed] [Google Scholar]


