Abstract
Background
Paediatric chronic pain is a significant problem that can have devastating impacts on quality of life. Multimodal interdisciplinary interventions are the mainstay of paediatric treatment. The aim of this article is to provide a comprehensive review of the effectiveness of interdisciplinary interventions in the management of paediatric chronic pain.
Methods
Studies were identified via a search of nine databases. The search strategy included concept blocks pertaining to type of pain, study population, and type of intervention. Eligible studies reported the effects of an intervention co-ordinated by two or more healthcare professionals of different disciplines, and recruited a sample aged 22 yr or below with chronic pain. Twenty-eight studies were included, and 21 provided data for inclusion in between- and within-groups meta-analyses.
Results
Patients randomised to interdisciplinary interventions reported significantly lower pain intensity 0–1 month post-intervention compared with patients randomised to the control groups. Within-groups analysis of patients receiving interdisciplinary interventions showed significant improvements pre- to post-intervention in pain intensity, functional disability, anxiety, depression, catastrophising, school attendance, school functioning, and pain acceptance. Few differences were found between interventions delivered in inpatient vs outpatient settings. Significant heterogeneity due mainly to differing outcome variables and intervention content was found in most analyses.
Conclusions
Overall, interdisciplinary interventions show promise in providing a range of clinical benefits for children with chronic pain. Methodologically robust randomised controlled trials using standardised outcome measures are needed, however, to guide clinical care.
Keywords: meta-analysis, interdisciplinary pain clinic, multimodal analgesia, paediatrics, chronic pain, systematic review
Editor's key points.
-
•
The authors address the difficult problem of chronic pain in children, examining the effectiveness of interdisciplinary interventions using a systematic review of 28 studies with meta-analysis of data from 21 studies.
-
•
They found good evidence of the effectiveness of interdisciplinary interventions in a variety of relevant outcome measures in the paediatric chronic pain setting.
Paediatric chronic pain (PCP) is defined as persistent or recurring pain of any aetiology lasting longer than 3 months.1, 2 Although prevalence rates differ across reports and between conditions,3 PCP is a significant problem worldwide.4 Children with CP report substantially worse quality of life than their healthy peers in physical, emotional, social, and school functioning.5 Their parents report greater anxiety and depression compared with parents of healthy children, and feelings of helplessness and a lack of control over their own lives.6 A biopsychosocial approach highlighting the interplay of biological and psychosocial processes in the onset and maintenance of CP is well accepted.7 Liossi and Howard8 recently summarised the evidence that suggests a purely biological model of PCP is outdated and incorrect, and provided a roadmap for its biopsychosocial assessment and formulation, and subsequent multimodal, interdisciplinary management.9, 10
Hechler and colleagues11 reviewed ten randomised and non-randomised trials exploring the effects of intensive interdisciplinary interventions12, 13 in the treatment of PCP. Meta-analysis showed significant reductions in pain intensity, disability, and depression from pre-treatment to immediate post-treatment and short-term follow-up (2–6 months). Pooled estimates of additional outcomes were not provided because of the small number of included studies and the substantial heterogeneity in outcome measures used. The aim of the present review was to provide an up-to-date comprehensive review of the effectiveness of interdisciplinary interventions in the management of PCP. Unlike Hechler and colleagues11 which included studies reporting coordinated interventions involving at least three disciplines, the present review included studies involving at least two or more disciplines based on the premise that treatment is guided by the initial formulation and different combinations of key interventions (e.g. medication, physiotherapy, psychology) are warranted depending on the patient's clinical presentation.8, 9, 10 Furthermore, single-group pre- to post-intervention studies were included, because even though randomised controlled trials are higher in the evidence hierarchy than single-group or observational studies,14 the latter are informative for assessing change over time.
Methods
This review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15 The protocol16 was developed in line with PRISMA-P guidelines17, 18 and published on International prospective register of systematic reviews (PROSPERO)19 (CRD42018091825). To further ensure the high quality of reporting of this systematic review that includes both randomised and non-randomised studies, the AMSTAR 2 critical appraisal tool was closely followed.20
Literature search
MEDLINE, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL; title), Web of Science (title), SCOPUS (title), Cochrane Library (title, abstract, keywords), PubMed (title), and PubPsych (main search field) were searched. The grey literature was searched using OpenGrey (main search field) and the reference lists of included studies. Databases were searched from inception until March 22, 2018. The search strategy included three concept blocks pertaining to the type of pain (e.g. pain, migraine), study population (e.g. youth, teenager), and type of intervention (e.g. interdisciplinary team, physiotherapy) (see Supplementary Material 1 for the complete search terms). The search strategy was developed after an extensive reading of the relevant literature, including original articles and a recent meta-analysis,11 followed by a discussion among the research team to ensure all relevant terms had been included. The final strategy was finalised with the help of a medical librarian. Search results were transferred into Endnote X8.1 and scanned for duplication using an automated function. Titles and abstracts were then screened independently by two review authors (LJ and DS) to identify potentially eligible studies. The full text of these studies were retrieved and assessed for eligibility independently by the same two authors. Disagreements at any stage were resolved via discussion with a third author (CL).
Inclusion criteria
Studies were eligible for inclusion if they met the following criteria: (1) available in the English language; (2) reported the effects of an intervention co-ordinated by two or more healthcare professionals of different disciplines that occurred in an inpatient or outpatient setting; (3) reported pain frequency, intensity, or both as measured by self-report or as reported by caregiver or health-care professional; (4) recruited a sample aged 22 yr or below with chronic pain, including primary pain (i.e. pain in one or more anatomic regions that has lasted for longer than 3 months and cannot be better explained by any other chronic pain condition) and secondary pain (i.e. pain that is symptomatic of another health condition)21; and (5) studies using single-group pre-test to post-test, parallel-group, crossover designs and randomised or non-randomised trials.
Studies were excluded if they reported no pain frequency/intensity outcomes. We acknowledge that even though during treatment emphasis is placed on improving function rather than directly decreasing pain, pain is nevertheless a primary reason for patients to seek healthcare22, 23 and the reason patients were referred for specialist treatment. Conference abstracts, case studies, and qualitative studies were also not eligible for review.
Data extraction
Data from the eligible studies were extracted using standardised forms (Supplementary Tables S1 and S2). Data were extracted by LJ and subsequently checked by DS and CL. Where necessary information was not available within the publication study, authors were contacted.
Study quality assessment
The quality of randomised and non-randomised controlled trials was assessed via the Cochrane Risk of Bias tool24 (Supplementary Table S3). Single-group studies were assessed using the Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group25 (Supplementary Table S4). The quality of the reporting of the intervention was assessed using the Template for Intervention Description and Replication (TIDieR) checklist.26 Full details and results are provided in Supplementary Material 2. According to Grading of Recommendations Assessment, Development and Evaluation (GRADE)27 guidelines, if the total number of participants in a systemic review is less than that required for a single adequately powered study of the intervention (a threshold known as the optimal information size) the quality of evidence may be downgraded.28 A power calculation was conducted in GPower29 using conventional statistical parameters (two-tailed, power=0.80, alpha=0.05). Cohen30 proposed benchmarks for small (d=0.3), medium (d=0.5), and large (d=0.8) effect sizes. A medium effect size (d=0.50)30 was selected after a preliminary review of the relevant literature. The optimal information size for between-groups analyses was 128 participants.
Outcome assessment
A tabulation of outcome measures used in each study, along with their reported psychometric properties, is provided in Supplementary Table S5. A graphical display of heterogeneity in outcome measures is provided as an outcome matrix31, 32, 33 in Supplementary Figure S1.
Meta-analytic procedures
Meta-analyses were planned for primary outcomes of patient pain frequency and intensity, and secondary outcomes of disability, quality of life, school functioning, anxiety, depression, and sleep, and caregiver outcomes of quality of life, anxiety, depression, catastrophising, coping, and family functioning. These outcomes were selected a priori based on current clinical practice and a detailed reading of existing literature including the numerous domains impacted by PCP. Sufficient data was only available to conduct analyses for pain intensity, headache frequency, functional disability, anxiety, depression, catastrophising, school attendance, school functioning/aversion, and pain acceptance. Data were not available to conduct planned analyses on caregiver outcomes. Sub-groups analyses were planned a priori comparing interventions delivered to patients with cancer-related pain with those delivered to patients with non-cancer-related chronic pain. Only one study recruited patients with cancer-related pain, however, and therefore sub-groups analyses could not be performed. Planned between-groups analyses examined differences between patients receiving interdisciplinary interventions compared with those allocated to single disciplinary interventions, placebo conditions, or waiting-lists conditions (referred to as control groups) at any follow-up period. Planned within-groups analyses examined differences between pre- and post-test outcomes for participants receiving interdisciplinary interventions. Different forms of control were combined in the within-groups analysis as planned. Sufficient data was not available to perform subgroups analyses based on type of control as planned. Data from all possible follow-up time-points were analysed.
Between-groups analyses
Hedges' adjusted g effect sizes (standardised mean differences) for between-group comparisons were computed using group means and standard deviations in Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). Random-effects models were used which assumes the average effect size varies between studies and therefore heterogeneity is to be expected.34, 35 Cochrane's Q and the I2 statistic were used to assess study heterogeneity. With Cochrane's Q, a significant result is indicative of heterogeneity. The I2 statistic describes the percentage of variability in effect estimates because of heterogeneity as opposed to sampling error.36
Within-groups analyses
Cohen's d effect sizes were computed for pre- vs post-intervention score comparisons based on study means and the average standard deviations.37, 38 Random-effects models were used to compute average effect sizes using Exploratory Software for Confidence Intervals (ESCI).37 An unbiased estimate of the population effect size, referred to as dunb, was calculated.37 As d overestimates the population effect size, especially for smaller sample sizes, the adjustment is advocated.37, 39, 40 For all analyses, a positive effect size indicates an improvement in the outcome variable (i.e. a reduction pre- to post-intervention for pain intensity, headache frequency, functional disability, anxiety, depression, catastrophising, and an increase in school attendance, school functioning, and pain acceptance). Cochrane's Q and the I2 statistic were used to assess study heterogeneity. Small study effects were assessed via funnel plots for analyses including 10 or more studies.36
Results
Search results
The literature search and study selection process is shown in Supplementary Figure S2. From an initial identification of 6337 records, 28 studies meeting the inclusion criteria were retained for the review, of which 21 provided data for inclusion in one or more meta-analysis. Nineteen studies had a single-group pre-post design, and nine were classified as a randomised control trial or a randomised trial (i.e. comparing interdisciplinary vs an alternative intervention). A description of each study is provided in Supplementary Table S1.
Summary of identified studies
Twelve studies were conducted in the USA, three each in the UK, The Netherlands, and Germany, and the remainder in Sweden, Italy, France, Denmark, and Egypt. Twenty-four studies were published within the past 10 yr, and 14 within the past 5 yr. The majority recruited patients with mixed chronic pain diagnoses, with chronic headache, abdominal pain, and complex regional pain syndrome particularly prevalent. All but five studies recruited more female than male patients (two studies only recruited males), and the average age across studies was 13.8 yr (3–22 yr across all studies). Interdisciplinary interventions varied in their content, number of sessions, and follow-up time-points, although all were co-ordinated by two or more healthcare professionals of different disciplines (see Supplementary Table S2 for details). Fifteen interventions were conducted in an outpatient setting, and 13 studies in an inpatient setting. Outcome variables varied widely between studies as shown in Supplementary Table S5 and Figure S1. Pain intensity was the most commonly assessed outcome variable (which is to expected given the inclusion criteria for this review), which was assessed via a numeric rating scale in 18 studies and VAS in 10 studies. Sixteen studies clearly reported significant reductions in pain intensity by patients receiving interdisciplinary interventions pre-to post-intervention.41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56
Methodological quality
Risk of bias ratings for randomised controlled trials are provided in Supplementary Table S3 and methodological quality assessments for pre-post single group interventions in Supplementary Table S4. A detailed narrative summary of methodological quality results are provided in Supplementary Material 2. To briefly summarise, a total of 57 ratings were provided across six domains, 37 of which were ‘low risk of bias’ and only two of which were ‘high risk of bias’. A number of limitations were highlighted in the pre-post studies. For example, none provided a power calculation, and seven did not pre-specify and clearly describe their participation inclusion criteria.
Meta-analysis results
Between-groups analyses
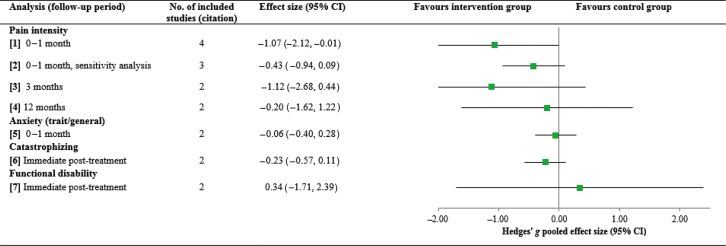
Meta-analyses compared patients receiving multidisciplinary interventions to patients recruited to a control/comparison group (i.e. placebo, waiting-list, single disciplinary intervention) on pain intensity, anxiety, catastrophising, and functional disability. Full details and results are provided in Table 1 and Figure 1 (individual forest plots are provided in Supplementary Figure S3). As noted, a power calculation revealed the optimal information size for between-groups analyses to be 128 participants.
Table 1.
Between-groups meta-analysis effect sizes for pain intensity, anxiety, catastrophising, and functional disability at all possible follow-up points. Note: The standardised mean difference and random effects model were used for all analyses. CI, confidence interval
| Analysis (follow-up period) | Notes | No. of included studies (citation) | No. participants in intervention group | No. participants in control group | Effect size (95% CI) | Test for overall effect (Z and P-value) | Cochrane's Q | I2 (%) |
|---|---|---|---|---|---|---|---|---|
| Pain intensity | ||||||||
| (1) 0–1 Month | – | 442, 45, 46, 48 | 102 | 92 | −1.07 (−2.12, −0.01) | 1.99, 0.05 | 27.02, <0.001 | 89 |
| (2) 0–1 Month, sensitivity analysis | 46Removed which had the largest effect size and could potentially be an outlier | 342, 45, 48 | 87 | 77 | −0.43 (−0.94, 0.09) | 1.61, 0.11 | 4.14, 0.13 | 52 |
| (3) 3 Months | – | 242, 45 | 35 | 25 | −1.12 (−2.68, 0.44) | 1.40, 0.16 | 7.11, 0.008 | 86 |
| (4) 12 Months | – | 242, 67 | 31 | 23 | −0.20 (−1.62, 1.22) | 0.28, 0.78 | 6.14, 0.01 | 84 |
| Anxiety (trait/general) | ||||||||
| (5) 0–1 Month | – | 242, 48 | 72 | 62 | −0.06 (−0.40, 0.28) | 0.35, 0.72 | 0.07, 0.79 | 0 |
| Catastrophising | ||||||||
| (6) Immediate post-treatment | – | 248, 67 | 66 | 67 | −0.23 (−0.57, 0.11) | 1.33, 0.18 | 0.11, 0.74 | 0 |
| Functional disability | ||||||||
| (7) Immediate post-treatment | – | 248, 67 | 66 | 67 | 0.34 (−1.71, 2.39) | 0.32, 0.75 | 20.02, <0.001 | 95 |
Fig 1.
Overall pooled effect sizes for each between-groups analysis on pain intensity, anxiety, catastrophising, and functional disability. CI, confidence interval.
Primary outcomes. Pain intensity was significantly lower 0–1 month post-intervention in patients randomised to the multidisciplinary intervention group than the control group (analysis 1: k=4, intervention n=102, control n=92; Hedges' g=−1.07, P=0.05), although it was no longer significant in a sensitivity analysis removing the study with the largest effect size (analysis 2: k=3, intervention n=87, control n=77; Hedges' g=−0.43, P=0.11). No significant differences in pain intensity were found between groups at 3 month follow-up (analysis 3: k=2, intervention n=35, control n=25; Hedges' g=−1.12, P=0.16) and 12 month follow-up (analysis 4: k=2, intervention n=31, control n=23; Hedges' g=−0.20, P=0.78).
Secondary outcomes. No significant differences were found between intervention and control groups for anxiety (trait/general anxiety) 0–1 month post-intervention (analysis 5: k=2, intervention n=72, healthy control n=62; Hedges' g=−0.06, P=0.72), catastrophising immediately post-intervention (analysis 6: k=2, intervention n=66, healthy control n=67; Hedges' g=−0.23, P=0.18), or functional disability immediately post-intervention (analysis 7: k=2, chronic pain n=66, healthy control n=67; Hedges' g=0.34, P=0.75).
Within-groups analyses
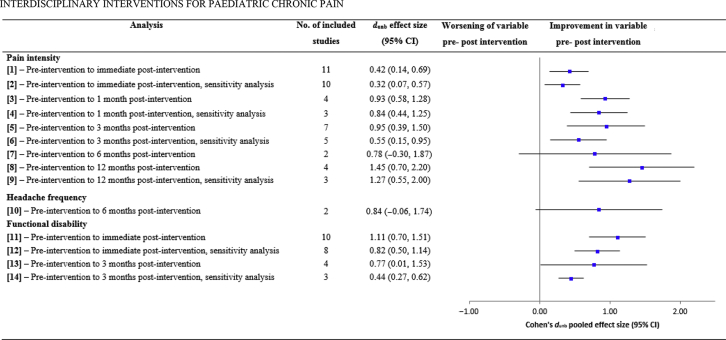
Meta-analyses compared pre- with post-intervention scores on pain intensity, headache frequency, functional disability, anxiety (trait/general), depression, catastrophising, school attendance, school functioning/aversion, and acceptance for patients receiving multidisciplinary interventions. Full details and results are provided in Table 2 and Fig 2, Fig 3, Fig 4 (individual forest plots are provided in Supplementary Figure S4). Heterogeneity was high in most analyses, and where stated, sensitivity analyses were conducted removing studies with large effect sizes that could potentially be outliers.
Table 2.
Within-groups meta-analyses of pre- to post-intervention change scores for pain intensity, headache frequency, functional disability, anxiety, depression, catastrophising, school attendance, school functioning, and acceptance. Note: A random effects model was used for all analyses. CI, confidence interval.
| Analysis | Notes | No. of included studies (citation) | No. participants | dunb effect size (95% CI) | Test for overall effect (t and P-value) | Cochrane's Q, P-value | I2 (%) |
|---|---|---|---|---|---|---|---|
| Pain intensity | |||||||
| (1) Pre-intervention to immediate post-intervention | – | 1143, 46, 48, 50, 51, 53, 55, 57, 67, 81, 82 | 698 | 0.42 (0.14, 0.69) | 2.96,0.003 | 109.55, <0.001 | 91 |
| (2) Pre-intervention to immediate post-intervention, sensitivity analysis | 46Removed which had the largest effect size and is an outlier | 1043, 48, 50, 51, 53, 55, 57, 67, 81, 82 | 683 | 0.32 (0.07, 0.57) | 2.51, 0.012 | 86.24, <0.001 | 90 |
| (3) Pre-intervention to 1 month post-intervention | – | 441, 42, 45, 53 | 299 | 0.93 (0.58, 1.28) | 5.26, <0.001 | 14.23, 0.003 | 79 |
| (4) Pre-intervention to 1 month post-intervention, sensitivity analysis | 53Removed which had the largest sample size and a large effect size | 341, 42, 45 | 154 | 0.84 (0.44, 1.25) | 4.12, <0.001 | 5.05, 0.08 | 60 |
| (5) Pre-intervention to 3 months post-intervention | – | 742, 43, 45, 49, 57, 82, 83 | 396 | 0.95 (0.39, 1.50) | 3.32, 0.001 | 118.32, <0.001 | 95 |
| (6) Pre-intervention to 3 months post-intervention, sensitivity analysis | 45, 49Removed which had the two effect sizes and could be outliers;49 also had the largest sample size | 542, 43, 57, 82, 83 | 240 | 0.55 (0.15, 0.95) | 2.69, 0.007 | 32.22, <0.001 | 88 |
| (7) Pre-intervention to 6 months post-intervention | – | 242, 83 | 67 | 0.78 (−0.30, 1.87) | 1.42, 0.15 | 10.67, 0.001 | 91 |
| (8) Pre-intervention to 12 months post-intervention | – | 442, 47, 49, 67 | 334 | 1.45 (0.70, 2.20) | 3.80, <0.001 | 52.50, <0.001 | 94 |
| (9) Pre-intervention to 12 months post-intervention, sensitivity analysis | 49Removed which had the largest effect size and could be an outlier | 342, 47, 67 | 198 | 1.27 (0.55, 2.00) | 3.44, <0.001 | 11.76, 0.002 | 83 |
| Headache frequency | |||||||
| (10) Pre-intervention to 6 months post-intervention | – | 252, 83 | 86 | 0.84 (−0.06, 1.74) | 1.83, 0.07 | 12.26, <0.001 | 92 |
| Functional disability | |||||||
| (11) Pre-intervention to immediate post-intervention | – | 1048, 50, 51, 53, 57, 67, 81, 82, 84, 85 | 869 | 1.11 (0.70, 1.51) | 5.37, <0.001 | 191.57, <0.001 | 95 |
| (12) Pre-intervention to immediate post-intervention, sensitivity analysis | 51, 53Removed which had the two largest effect sizes and could be outliers | 848, 50, 57, 67, 81, 82, 84, 85 | 668 | 0.82 (0.50, 1.14) | 5.03, <0.001 | 83.99, <0.001 | 92 |
| (13) Pre-intervention to 3 months post-intervention | – | 449, 57, 67, 82 | 271 | 0.77 (0.01, 1.53) | 1.99, 0.05 | 73.24, <0.001 | 96 |
| (14) Pre-intervention to 3 months post-intervention, sensitivity analysis | 49Removed which had the largest effect size and could be an outlier | 357, 67, 82 | 130 | 0.44 (0.27, 0.62) | 4.90, <0.001 | 0.66, 0.71 | 0 |
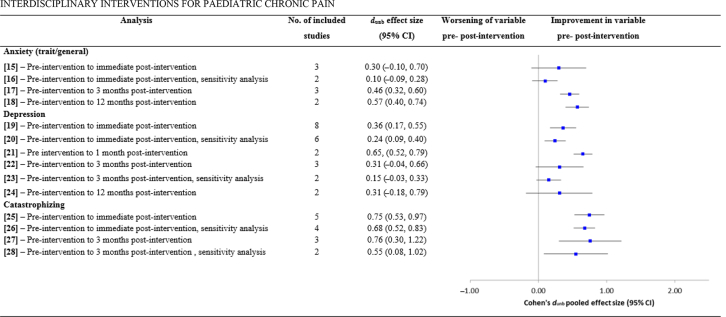
| Anxiety (trait/general) | |||||||
| (15) Pre-intervention to immediate post-intervention | – | 348, 51, 57 | 164 | 0.30 (−0.10, 0.70) | 1.48, 0.138 | 12.96, 0.002 | 85 |
| (16) Pre-intervention to immediate post-intervention, sensitivity analysis | 51Removed which had the largest effect size and could be an outlier | 248, 57 | 108 | 0.10 (−0.09, 0.28) | 1.02, 0.301 | 0.21, 0.65 | 0 |
| (17) Pre-intervention to 3 months post-intervention | – | 342, 49, 57 | 204 | 0.46 (0.32, 0.60) | 6.31, <0.001 | 1.74, 0.42 | 7 |
| (18) Pre-intervention to 12 months post-intervention | – | 242, 49 | 156 | 0.57 (0.40, 0.74) | 6.64, <0.001 | 0.13, 0.72 | 0 |
| Depression | |||||||
| (19) Pre-intervention to immediate post-intervention | – | 848, 51, 53, 55, 57, 67, 82, 84 | 564 | 0.36 (0.17, 0.55) | 3.68, <0.001 | 31.98, <0.001 | 78 |
| (20) Pre-intervention to immediate post-intervention, sensitivity analysis | 53, 84Removed which had the two largest effect sizes and sample sizes | 648, 53, 55, 57, 67, 82 | 307 | 0.24 (0.09, 0.40) | 3.03, 0.003 | 8.87, 0.11 | 44 |
| (21) Pre intervention to 1 month post-intervention | – | 241, 53 | 264 | 0.65, (0.52, 0.79) | 9.67, <0.001 | 0.56, 0.45 | 0 |
| (22) Pre-intervention to 3 months post-intervention | – | 349, 57, 82 | 230 | 0.31 (−0.04, 0.66) | 1.75, 0.08 | 12.76, 0002 | 84 |
| (23) Pre-intervention to 3 months post-intervention, sensitivity analysis | 49Removed which had the largest effect size and could be an outlier | 257, 82 | 116 | 0.15 (−0.03, 0.33) | 1.59, 0.11 | 0.31, 0.58 | 0 |
| (24) Pre-intervention to 12 months post-intervention | – | 249, 67 | 152 | 0.31 (−0.18, 0.79) | 1.24, 0.22 | 3.94, 0.05 | 75 |
| Catastrophising | |||||||
| (25) Pre-intervention to immediate post-intervention | – | 548, 57, 67, 82, 84 | 328 | 0.75 (0.53, 0.97) | 6.67, <0.001 | 11.41, 0.02 | 65 |
| (26) Pre-intervention to immediate post-intervention, sensitivity analysis | 57Removed which had the largest effect size and could be an outlier | 448, 67, 82, 84 | 272 | 0.68 (0.52, 0.83) | 8.49, <0.001 | 3.92, 0.27 | 23 |
| (27) Pre-intervention to 3 months post-intervention | – | 357, 67, 82 | 132 | 0.76 (0.30, 1.22) | 3.25, 0.001 | 9.05, 0.01 | 78 |
| (28) Pre-intervention to 3 months post-intervention, sensitivity analysis | 57Removed which had the largest effect size and could be an outlier | 267, 82 | 89 | 0.55 (0.08, 1.02) | 2.30, 0.02 | 2.91, 0.08 | 66 |
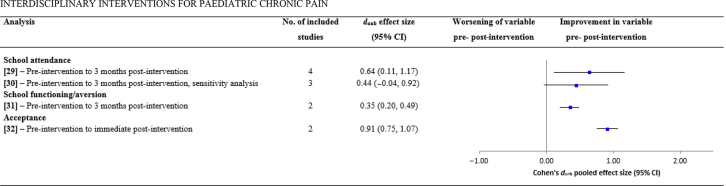
| School attendance | |||||||
| (29) Pre-intervention to 3 months post-intervention | – | 449, 57, 82, 83 | 304 | 0.64 (0.11, 1.17) | 2.35, 0.02 | 48.03, <0.001 | 94 |
| (30) Pre-intervention to 3 months post-intervention, sensitivity analysis | 49Removed which had the largest effect size and could be an outlier | 357, 82, 83 | 163 | 0.44 (−0.04, 0.92) | 1.80, 0.07 | 16.73, <0.001 | 88 |
| School functioning/aversion | |||||||
| (31) Pre-intervention to 3 months post-intervention | – | 249, 83 | 188 | 0.35 (0.20, 0.49) | 4.64, <0.001 | 0.73, 0.39 | 0 |
| Acceptance | |||||||
| (32) Pre-intervention to immediate post-intervention | Positive effect size indicates increase in acceptance | 282, 84 | 206 | 0.91 (0.75, 1.07) | 11.05, <0.001 | 0.70, 0.40 | 0 |
| Parental measure of pain intensity | |||||||
| (33) – Pre-intervention to immediate post-intervention | – | 255, 57 | 84 | 0.42 (−0.50, 1.34) | 0.90, 0.37 | 13.39, <0.001 | 93 |
Fig 2.
Overall pooled effect sizes for each pre- vs post-intervention analysis on outcomes pain intensity, headache frequency, and functional disability. CI, confidence interval.
Fig 3.
Overall pooled effect sizes for each pre- vs post-intervention analysis on outcomes anxiety, depression, and catastrophising. CI, confidence interval.
Fig 4.
Overall pooled effect sizes for each pre- vs post-intervention analysis on outcomes of school attendance, school functioning/aversion, and acceptance. CI, confidence interval.
Primary outcomes. Pain intensity reduced significantly from pre- to immediate post-intervention (analysis 1; k=11, n=698, dun=0.42, P=0.003), 1 month post-intervention (analysis 3; k=4, n=299, dun=0.93, P<0.001), 3 months (analysis 5; k=5, n=396, dun=0.95, P=0.001), and 12 months (analysis 8; k=4, n=334, dun=1.45, P<0.001) post-intervention. Significant reductions were not found pre- to 6 months post-intervention (analysis 7; k=2, n=67, dun=0.78, P=0.15), although data were only available from two studies. These effects remained significant in subsequent sensitivity analyses. Two studies reported data on headache frequency, with no significant reduction in frequency pre- to 6 months post-intervention (analysis 10; k=2, n=86, dun=0.74, P=0.07).
Secondary outcomes. Significant improvements were shown in functional disability from pre- to immediate post-intervention (analysis 11; k=10, n=869, dun=1.11, P<0.001) and 3 months post-intervention (analysis 13; k=4, n=271, dun=0.77, P=0.05). These effects remained significant when sensitivity analysis were conducted. Significant reductions were found in anxiety (trait/general) from pre- to 3 months post-intervention (analysis 17; k=3, n=204, dun=0.46, P<0.001) and 12 months post-intervention (analysis 18; k=2, n=156, dun=0.57, P<0.001), but not pre- to immediate post-intervention (analysis 15; k=3, n=164, dun=0.30, P=0.14).
Significant reductions were found in depression from pre- to immediate post-intervention (analysis 19; k=8, n=564, dun=0.36, P<0.001) which remained significant when sensitivity analysis was conducted, and also pre- to 1 month post-intervention (analysis 21; k=2, n=264, dun=0.65, P<0.001). No significant reduction was shown from pre- to 3 months post-intervention (analysis 22; k=3, n=230, dun=0.31, P=0.08) which remained non-significant in a sensitivity analysis. No significant reduction was also shown pre- to 12 months post-intervention (analysis 24; k=2, n=152, dun=0.31, P=0.22).
Significant reductions were found in catastrophising from pre- to immediate post-intervention (analysis 25; k=5, n=328, dun=0.75, P<0.001) and 3 months post-intervention (analysis 27; k=3, n=132, dun=0.76, P<0.001). Both effects remained significant in subsequent sensitivity analyses. School attendance significantly improved pre- to 3 months post-intervention (analysis 29; k=4, n=304, dun=0.64, p=0.02), although this was non-significant in a subsequent sensitivity analysis (analysis 30; k=3, n=163, dun=0.44, p=0.07). School functioning also significantly improved from pre- to 3 months post-intervention (analysis 31; k=2, n=188, dun=0.35, P<0.001), as did acceptance pre- to 3 months post-intervention (analysis 32; k=2, n=206, dun=0.90, P<0.001).
Parental measures. Two studies provided parental ratings of their child's pain intensity pre- and immediately post-intervention.55, 57 No significant reduction was observed (analysis 33; k=2, n=84, dun=0.42, P=0.37).
Inpatient vs outpatient settings. Where possible, effect sizes were computed separately for multidisciplinary interventions recruiting patients to inpatient and outpatient settings. Full details and results for each analysis are provided in Supplementary Table S6, and a summary of results is provided in Supplementary Material 3. To summarise, interventions delivered in both inpatient and outpatient treatment settings were successful in significantly reducing (1) pain intensity pre- to immediate post-intervention, 1, 3, and 12 months post-intervention, (2) functional disability pre- to immediate post-intervention, and (3) catastrophising from pre- to immediate post-intervention. Significant reductions were shown in functional disability from pre-to 3 months post-intervention for outpatient studies, but not inpatient studies. Significant reductions were shown in depression from pre- to immediate post-intervention for inpatient studies but not outpatient studies. School attendance significantly improved pre- to 3 months post-intervention for inpatient studies but not outpatient studies.
Funnel plots
There was evidence of asymmetry in the within-groups analysis of pain intensity (analysis 1 and analysis 2) and functional disability (analysis 11) pre- to immediate post-intervention (Supplementary Figure S5). Caution has been advocated in the interpretation of funnel plots,36, 37 although the asymmetry observed in these three analyses may be because of publication bias, or heterogeneity between studies because of variations in intervention design.
Discussion
The aim of this review was to examine the effectiveness of interdisciplinary interventions for the management of PCP. Between-groups analyses revealed significantly lower pain intensity 0–1 month post-intervention in patients receiving interdisciplinary interventions compared with those in the control group. This result aligns with those of Hechler and colleagues11 in a former review. Significant heterogeneity was observed, however (also reported by Hechler and colleagues11), and a subsequent sensitivity analysis failed to find a significant difference between groups. No significant differences were shown in anxiety, catastrophising, or functional disability. Between-groups analyses therefore provide little evidence for the benefits of interdisciplinary interventions compared with control conditions, although only five studies provided usable data for these analyses. Additionally, none of the between-groups analyses met the GRADE optimal information size of 128 participants.28 Further methodologically rigorous randomised controlled trials are therefore needed.
Although there are concerns about the conduct and reporting of systematic reviews of non-randomised studies, almost half of published systematic reviews now include non-randomised studies of intervention effects.20, 58, 59 Analyses of pre-post intervention change scores showed more consistent evidence for the clinical benefits of paediatric interdisciplinary interventions. Data were available from 21 studies, and it was possible to compute effect sizes for many more outcome variables. Significant reductions were found in pain intensity pre-intervention to immediate post-intervention, and at 3 and 12 month follow-up points. Significant improvements were also found in functional disability pre-intervention to immediate post-intervention and 3 month follow-up, anxiety pre-intervention to immediate post-intervention, 3 and 12 month follow-up points, depression pre-intervention to immediate post-intervention and 1 month follow-up, catastrophising pre-intervention to immediate post-intervention and 3 month follow-up, school attendance and school functioning pre-intervention to 3 month follow-up, and pain acceptance pre-intervention to immediate post-intervention. Significant heterogeneity was found in most analyses, however. Although the pattern of results remained the same in subsequent sensitivity analyses (with the exception of school attendance which was no longer significant), heterogeneity also remained high in most instances.
It is very encouraging for patients and their families that pain intensity and disability are immediately reduced after interventions and remain reduced over the long term. Interestingly, while anxiety is not immediately reduced after intervention, but is reduced at follow-ups, depression is immediately reduced, but this impact does not keep up in the long term. Without knowing the exact content and order of administration of the interventions delivered, it is difficult to explain such effects. It is possible that improved mood resulting perhaps from intense intervention and social support is superficial and transient; without having been underpinned by addressing depressogenic cognitive schemata it is difficult to consolidate, generalise, and maintain the long term. Alternatively, anxiety may be mostly related to fear-avoidance and somatic sensations and take longer to reduce, but as pain and disability are reduced and remain reduced in the long term, it is possible that anxiety also remains low in the long term.
Overall, the results of the pre-post analyses are in agreement with those of Hechler and colleagues'11 former review, which reported significant reductions in pain intensity, disability, and depression from pre-treatment to immediate post-treatment and short-term follow-up (2–6 months). We also agree with this former review in advising caution in the interpretation of results, however, because of high levels of heterogeneity observed in most analyses. Heterogeneity can stem from many sources,60 although notable variation was found in the structure and content of the interventions included in this review. This is to be expected, however, and may be because of not only the different paediatric populations recruited, but also factors such as hospital budgets, healthcare professional preferences, and different clinical guidelines across countries.61 Interestingly, interventions delivered in both in- and outpatient treatment settings were successful in reducing pain intensity, functional disability (except 3 months post-intervention), and catastrophising. Given the substantial cost associated in inpatient treatment,47 if outpatient treatment is equally effective this is an important finding with significant implications for service commissioners. We recommend further research to test this specifically, as many of the outpatient analyses in the present review were underpowered.
The results of the present review also align with other recent reviews of the PCP literature which have reported reductions in pain frequency and intensity. A review of 47 studies explored psychological therapies for the management of chronic and recurrent pain in young people.62 Psychological therapies were found to reduce pain frequency immediately after treatment in children with headaches, but effects were not maintained at follow-up. In contrast, small benefits were shown in disability at follow-up but not immediately after the interventions. For patients with mixed chronic pain conditions, psychological therapies reduced pain intensity immediately post-treatment but not at follow-up. Another review of eight remotely delivered psychological interventions (internet, CD Rom, audiotape, telephone) found significant reductions in headache severity and pain intensity immediately post-treatment for children with headache conditions and mixed pain conditions, respectively.63
We recommend caution in interpreting the results of non-randomised studies of interventions which are inevitably subject to a range of biases that are either not present, or are less prominent in randomised controlled trials. Furthermore, if precise estimates of intervention effects, which may nevertheless be inaccurate because of residual biases, are combined with those from the (generally smaller) randomised controlled trials, the meta-estimates will be weighted towards the observational study estimates, therefore biasing conclusions.20 As is often the case, however, caution should be balanced with the need to collect and utilise real-world evidence. While the definition of real-world evidence is still evolving, most proponents associate this with data derived from medical practice among heterogeneous sets of patients in real life practice settings. Such evidence is increasingly seen as a way to tailor healthcare decision making more closely to the characteristics of individual patients, and thus as a step towards making healthcare more personalised and effective.64
A detailed assessment of methodological quality was conducted. Considering randomised trials, a total of 57 ratings were provided across six domains, 37 of which were ‘low risk of bias’. Seventeen ratings of ‘unclear risk of bias’ were given, although nine of these were for the domain ‘blinding of participants and personnel’ as blinding was not possible in the majority of instances. Only two studies received a single ‘high risk of bias’ rating. Pre-post single intervention trials are lower in the evidence hierarchy than randomised trials, and possess the obvious limitation of featuring no control group. It was also notable from the quality assessment of these studies that none reported a power calculation, although it should be noted that four of the randomised trials also did not report performing a power calculation.42, 65, 66, 67
The quality of reporting of the interventions delivered was specifically addressed via the completion of the TIDieR checklist26 for each study. A number of notable omissions were apparent, including failure to sufficiently describe the informational materials used, whether the intervention was modified during the course of the study, and whether the intervention was planned to be personalised. We encourage authors to use the TIDieR checklist themselves when preparing their study reports, as it is essential that full details are provided to facilitate scientific reproducibility. Related to this, it is also essential that published reports include sufficient details of control arms and intervention arms in randomised trials. Indeed, a recent systematic review of paediatric clinical trials found that descriptions of control arms were more often incomplete than descriptions of intervention arms, and that terms such as ‘treatment as usual’, ‘usual care’, and ‘existing practice’ are often unclear.68
Another limitation of the reviewed body of literature was that outcome domains and associated measures were inconsistently measured across studies (Supplementary Table S5 and Figure S1). Aside from pain intensity, the most commonly assessed outcomes were functioning and depression. It is readily apparent from Supplementary Figure S1 that large gaps exist in the published literature for many key outcomes, including fear of pain, pain acceptance, and sleep quality among others. There was also considerable variation in the actual measures used. For example, the Children's Depression Inventory69 was the most frequently used measure of depression, although other measures included the Center for Epidemiological Studies Depression Scale for Children,70 the Depression Inventory for Children and Adolescents,71 and the Bath Adolescent Pain Questionnaire.72 It is important that researchers justify the choice of the specific measures used, and provide psychometric properties for their recruited samples. Harmonising outcome collection and reporting in future clinical studies could make a profound contribution to advancing the reach and relevance of research to inform clinical practice, enhance patient care, and improve patient outcomes.73
A number of limitations with the present review should be mentioned. First, when conducting the between-groups analyses, the control groups comprised a mixture of single disciplinary interventions, placebo interventions, and waiting-list conditions. Because of the small number of studies, we were unable to perform meaningful subgroups analyses. Second, within-groups analyses were performed separately for interventions conducted in in- and outpatient settings. We were unable to perform meta-regression examining the influence of variables such as the number of sessions provided and the length of intervention, however, because of the recommendation that data from a minimum of 10 studies are required for such analyses.60 For the same reason, we were unable to explore whether dose and type of pharmacological interventions moderated any of the effects found, nor whether baseline characteristics such as anxiety, depression, or functioning moderated pain outcomes. Third, the interdisciplinary interventions included in this review varied considerably in terms of content, structure, and duration. As a result of these limitations, our review is not able to identify key components of interdisciplinary practice. It is still the case that not all published reports provide full details on their interventions, however,74 and there remains a crucial need to identify the most effective components in such PCP interventions.62, 75 Fourth, the majority of studies included patients with mixed pain diagnoses and we were unable to explore effectiveness according to diagnosis. While diagnosis and aetiology are undeniably important,8 pain management clinics need to provide effective treatment for all PCP patients, including those with primary and secondary chronic pain, and therefore an understanding of how interdisciplinary interventions work across conditions is informative. Lastly, it is important to note that the majority of interventions were conducted in the USA and, aside from one study conducted in Egypt, the remainder were conducted in European countries. Healthcare systems, policies, and resources differ between countries. Common barriers include poor education of healthcare professionals76, 77 and limited resources,78 which are particularly problematic in the developing world.79 Although challenges exist in conducting research in developing countries,80 it is nevertheless essential that research explores the effectiveness of pain management interventions for children and adolescents in all regions of the world.
In conclusion, the results of the present review provide support for the clinical benefits of interdisciplinary interventions for PCP. Even though the evidence is promising, most evaluations are limited, however, because of small sample sizes and geographically constrained samples, thereby preventing strong conclusions and generalisability. Furthermore, support for the beneficial impact of interdisciplinary treatments stems mostly from within-group analyses. More efforts are needed to expand our between-group evidence base to make strong conclusions on the benefits of interdisciplinary treatment over and above standard care.
Authors' contributions
Conceived the initial idea and design of the review: CL.
Data acquisition, preparation, analysis, and drafting of the review: CL, DS.
Data acquisition, preparation, analysis and drafting of the first version of the review: LJ.
Provided important intellectual content and made critical revisions to the manuscript: SL, LC, GW.
Approved the final manuscript as submitted and agree to be accountable for all aspects of the work: all authors.
Declarations of interest
The authors declare that they have no conflicts of interest.
Handling editor: J.G. Hardman
Editorial decision date: 15 January 2019
Footnotes
This article is accompanied by an editorial: Trickle-down healthcare in paediatric chronic pain by McCarthy & de Leeuw., Br J Anaesth 2019:123:e188–e190, doi: 10.1016/j.bja.2019.04.043
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.01.024.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huguet M.J. The severity of chronic pediatric pain: an epidemiological study. J Pain. 2008;9:226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Merskey H. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 3.King S., Chambers C.T., Huguet A. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Miró J., McGrath P.J., Finley G.A., Walco G.A. Pediatric chronic pain programs: current and ideal practice. Pain Rep. 2017;2:e613. doi: 10.1097/PR9.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varni J.W., Limbers C.A., Burwinkle T.M. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcome. 2007;5:43. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palermo T.M., Eccleston C. Parents of children and adolescents with chronic pain. Pain. 2009;146:15–17. doi: 10.1016/j.pain.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatchel R.J., Peng Y.B., Peters M.L., Fuchs P.N., Turk D.C. The Biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 8.Liossi C., Howard R.F. Pediatric chronic pain: biopsychosocial assessment and formulation. Pediatrics. 2016;138:e20160331. doi: 10.1542/peds.2016-0331. [DOI] [PubMed] [Google Scholar]
- 9.Rajapakse D., Liossi C., Howard R.F. Presentation and management of chronic pain. Arch Dis Child. 2014;99:474–480. doi: 10.1136/archdischild-2013-304207. [DOI] [PubMed] [Google Scholar]
- 10.Williams G., Howard R.F., Liossi C. Persistent postsurgical pain in children and young people: prediction, prevention, and management. Pain Rep. 2017;2:e616. doi: 10.1097/PR9.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hechler T., Kanstrup M., Holley A.L. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. Pediatrics. 2015;136:115–127. doi: 10.1542/peds.2014-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ospina M., Harstall C. Alberta Heritage Foundation for Medical Research Edmonton; Canada: 2003. Multidisciplinary pain programs for chronic pain: evidence from systematic reviews. [Google Scholar]
- 13.Peng P., Stinson J.N., Choiniere M. Dedicated multidisciplinary pain management centres for children in Canada: the current status. Can J Anaesth. 2007;54:985–991. doi: 10.1007/BF03016632. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt G.H., Oxman A.D., Vist G. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone L., Schoth D., Lilley S., Caes L., Williams G., Liossi C. Systematic review and meta-analysis of interdisciplinary interventions for the management of paediatric chronic pain. PROSPERO. 2018 doi: 10.1016/j.bja.2019.01.024. CRD42018091825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Shamseer L., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamseer L., Moher D., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health Research . 2016. PROSPERO: international prospective register of systematic reviews.https://www.crd.york.ac.uk/prospero/ Available from: [Google Scholar]
- 20.Shea B.J., Reeves B.C., Wells G. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treede R.-D., Rief W., Barke A. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elnegaard S., Andersen R.S., Pedersen A.F. Self-reported symptoms and healthcare seeking in the general population-exploring “The Symptom Iceberg”. BMC Public Health. 2015;15:685. doi: 10.1186/s12889-015-2034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAteer A., Elliott A.M., Hannaford P.C. Ascertaining the size of the symptom iceberg in a UK-wide community-based survey. Br J Gen Pract. 2011;61:e1–e11. doi: 10.3399/bjgp11X548910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins J.P.T., Altman D.G., Gøtzsche P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Heart L., Institute B. Systematic evidence reviews and clinical practice guidelines. National Institutes of Health; Washington DC: 2014. Quality assessment tool for before-after (pre-post) studies with no control group. [Google Scholar]
- 26.Hoffmann T.C., Glasziou P.P., Boutron I. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G., Oxman A.D., Akl E.A. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt G.H., Oxman A.D., Kunz R. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Erdfelder E., Faul F., Buchner A. GPOWER: a general power analysis program. Behav Res Method Instrum Comput. 1996;28:1–11. [Google Scholar]
- 30.Cohen J. 2nd Edn. Erlbaum; Hillsdale: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 31.Kapadia M.Z., Joachim K.C., Balasingham C. A core outcome set for children with feeding tubes and neurologic impairment: a systematic review. Pediatrics. 2016;138:e20153967. doi: 10.1542/peds.2015-3967. [DOI] [PubMed] [Google Scholar]
- 32.Kirkham J.J., Dwan K.M., Altman D.G. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ. 2010;340:c365. doi: 10.1136/bmj.c365. [DOI] [PubMed] [Google Scholar]
- 33.Williamson P.R., Altman D.G., Blazeby J.M. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132. doi: 10.1186/1745-6215-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borenstein M., Hedges L.V., Higgins J., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Method. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 35.Borenstein M., Hedges L.V., Higgins J., Rothstein H.R. Wiley Online Library; Cornwall: 2009. Introduction to meta-analysis. [Google Scholar]
- 36.Higgins J.P.T., Green S. The Cochrane Collaboration; 2008. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 37.Cumming G. Routledge; New York: 2012. Understanding the new statistics: effect sizes, confidence intervals, and meta-analysis. [Google Scholar]
- 38.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:1–12. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedges L.V. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Stat. 1981;6:107–128. [Google Scholar]
- 40.Cumming G. The new statistics: why and how. Psychol Sci. 2014;25:7–29. doi: 10.1177/0956797613504966. [DOI] [PubMed] [Google Scholar]
- 41.Benore E., D'Auria A., Banez G.A., Worley S., Tang A. The influence of anxiety reduction on clinical response to pediatric chronic pain rehabilitation. Clin J Pain. 2015;31:375–383. doi: 10.1097/AJP.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 42.Bussone G., Grazzi L., D'Amico D., Leone M., Andrasik F. Biofeedback-assisted relaxation training for young adolescents with tension-type headache: a controlled study. Cephalalgia. 1998;18:463–467. doi: 10.1111/j.1468-2982.1998.1807463.x. [DOI] [PubMed] [Google Scholar]
- 43.De Blécourt A.C.E., Preuper H.R.S., Van Der Schans C.P., Groothoff J.W., Reneman M.F. Preliminary evaluation of a multidisciplinary pain management program for children and adolescents with chronic musculoskeletal pain. Disabil Rehabil. 2008;30:13–20. doi: 10.1080/09638280601178816. [DOI] [PubMed] [Google Scholar]
- 44.Delivet H., Dugue S., Ferrari A., Postone S., Dahmani S. Efficacy of self-hypnosis on quality of life for children with chronic pain syndrome. Int J Clin Exp Hypn. 2018;66:43–55. doi: 10.1080/00207144.2018.1396109. [DOI] [PubMed] [Google Scholar]
- 45.Elnaggar R.K., Elshafey M.A. Effects of combined resistive underwater exercises and interferential current therapy in patients with juvenile idiopathic arthritis: a randomized controlled trial. Am J Phys Med Rehabil. 2016;95:96–102. doi: 10.1097/PHM.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 46.El-Shamy S.M., Abdelaal A.A.M. Efficacy of pulsed high-intensity laser therapy on pain, functional capacity, and gait in children with haemophilic arthropathy. Disabil Rehabil. 2018;40:462–468. doi: 10.1080/09638288.2016.1261416. [DOI] [PubMed] [Google Scholar]
- 47.Evans J.R., Benore E., Banez G.A. The cost-effectiveness of intensive interdisciplinary pediatric chronic pain rehabilitation. J Pediatr Psychol. 2015;41:849–856. doi: 10.1093/jpepsy/jsv100. [DOI] [PubMed] [Google Scholar]
- 48.Hechler T., Ruhe A.-K., Schmidt P. Inpatient-based intensive interdisciplinary pain treatment for highly impaired children with severe chronic pain: randomized controlled trial of efficacy and economic effects. Pain. 2014;155:118–128. doi: 10.1016/j.pain.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Hirschfeld G., Hechler T., Dobe M. Maintaining lasting improvements: one-year follow-up of children with severe chronic pain undergoing multimodal inpatient treatment. J Pediatr Psychol. 2013;38:224–236. doi: 10.1093/jpepsy/jss115. [DOI] [PubMed] [Google Scholar]
- 50.Kempert H., Pearson R., Daghstani S., Benore D. Adolescents' change in functional abilities after completion of an intensive chronic pain rehabilitation program using subjective measures. J Pain. 2017;16:S94. [Google Scholar]
- 51.Logan D.E., Carpino E.A., Chiang G. A day-hospital approach to treatment of pediatric complex regional pain syndrome initial functional outcomes. Clin J Pain. 2012;28:766–774. doi: 10.1097/AJP.0b013e3182457619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Przekop P., Przekop A., Haviland M.G. Multimodal compared to pharmacologic treatments for chronic tension-type headache in adolescents. J Bodyw Mov Ther. 2016;20:715–721. doi: 10.1016/j.jbmt.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Simons L.E., Kaczynski K.J., Conroy C., Logan D.E. Fear of pain in the context of intensive pain rehabilitation among children and adolescents with neuropathic pain: associations with treatment response. J Pain. 2012;13:1151–1161. doi: 10.1016/j.jpain.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simons L.E., Sieberg C.B., Conroy C. Children with chronic pain: response trajectories after intensive pain rehabilitation treatment. J Pain. 2018;19:207–218. doi: 10.1016/j.jpain.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeltzer L.R., Tsao J.C.I., Stelling C., Powers M., Levy S., Waterhouse M. A phase I study on the feasibility and acceptability of an acupuncture/hypnosis intervention for chronic pediatric pain. J Pain Symptom Manag. 2002;24:437–446. doi: 10.1016/s0885-3924(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 56.Hechler T., Blankenburg M., Dobe M., Kosfelder J., Hubner B., Zernikow B. Effectiveness of a multimodal inpatient treatment for pediatric chronic pain: a comparison between children and adolescents. Eur J Pain. 2010;14:97–103. doi: 10.1016/j.ejpain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Eccleston C., Malleson P.N., Clinch J., Connell H., Sourbut C. Chronic pain in adolescents: evaluation of a programme of interdisciplinary cognitive behaviour therapy. Arch Dis Child. 2003;88:881–885. doi: 10.1136/adc.88.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egger M., Schneider M., Smith G.D. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 60.Higgins J.P., Green S. John Wiley & Sons; Chichester, UK: 2011. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 61.Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:46–54. doi: 10.1097/00005650-200108002-00003. [DOI] [PubMed] [Google Scholar]
- 62.Fisher E., Law E., Dudeney J., Palermo T.M., Stewart G., Eccleston C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2018;9 doi: 10.1002/14651858.CD003968.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fisher E., Law E., Palermo T.M., Eccleston C. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2015;3:CD011118. doi: 10.1002/14651858.CD011118.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan H., Ali M.S., Brouwer E.S. Real-world evidence: what it is and what it can tell us according to the international society for pharmacoepidemiology (ISPE) comparative effectiveness research (CER) special interest group (SIG) Clin Pharmacol Ther. 2018;104:239–241. doi: 10.1002/cpt.1086. [DOI] [PubMed] [Google Scholar]
- 65.Kanstrup M., Wicksell R.K., Kemani M., Wiwe Lipsker C., Lekander M., Holmström L. A clinical pilot study of individual and group treatment for adolescents with chronic pain and their parents: effects of acceptance and commitment therapy on functioning. Children (Basel) 2016;3:E30. doi: 10.3390/children3040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kroner J.W., Hershey A.D., Kashikar-Zuck S.M. Cognitive behavioral therapy plus amitriptyline for children and adolescents with chronic migraine reduces headache days to ≤4 per month. Headache. 2016;56:711–716. doi: 10.1111/head.12795. [DOI] [PubMed] [Google Scholar]
- 67.Wicksell R.K., Melin L., Lekander M., Olsson G.L. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain: a randomized controlled trial. Pain. 2009;141:248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Yu A.M., Balasubramanaiam B., Offringa M., Kelly L.E. Reporting of interventions and “standard of care” control arms in pediatric clinical trials: a quantitative analysis. Pediatr Res. 2018;84:393–398. doi: 10.1038/s41390-018-0019-7. [DOI] [PubMed] [Google Scholar]
- 69.Kovacs M. North Tonawanda, Multi-Health System; N.Y: 1992. Children's depression inventory. [Google Scholar]
- 70.Roberts R.E., Andrews J.A., Lewinsohn P.M., Hops H. Assessment of depression in adolescents using the center for epidemiologic studies depression scale. Psychol Assess. 1990;2:122–128. [Google Scholar]
- 71.Stiensmeier-Pelster J., Schürmann M., Duda K. Hogrefe; Göttingen: 2000. Depression inventory for children and adolescents. [Google Scholar]
- 72.Eccleston C., Jordan A., McCracken L.M., Sleed M., Connell H., Clinch J. The Bath Adolescent Pain Questionnaire (BAPQ): Development and preliminary psychometric evaluation of an instrument to assess the impact of chronic pain on adolescents. Pain. 2005;118:263–270. doi: 10.1016/j.pain.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 73.Liossi C., Anderson A.-K., Howard R.F., NC-CCi Pain, Care P. Development of research priorities in paediatric pain and palliative care. Br J Pain. 2017;11:9–15. doi: 10.1177/2049463716668906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coakley R., Wihak T. Evidence-based psychological interventions for the management of pediatric chronic pain: new directions in research and clinical practice. Children. 2017;4:9. doi: 10.3390/children4020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caes L., Fisher E., Clinch J., Eccleston C. Current evidence-based interdisciplinary treatment options for pediatric musculoskeletal pain. Curr Treatm Opt Rheumatol. 2018;4:223–234. doi: 10.1007/s40674-018-0101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bond M. Pain education issues in developing countries and responses to them by the International Association for the Study of Pain. Pain Res Manag. 2011;16:404–406. doi: 10.1155/2011/654746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hurley-Wallace A., Wood C., Franck L.S., Howard R.F., Liossi C. Paediatric pain education for health care professionals. Pain Rep Adv Access. 2019;4 doi: 10.1097/PR9.0000000000000701. e701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Albertyn R., Rode H., Millar A., Thomas J. Challenges associated with paediatric pain management in Sub Saharan Africa. Int J Surg. 2009;7:91–93. doi: 10.1016/j.ijsu.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Size M., Soyannwo O., Justins D. Pain management in developing countries. Anaesthesia. 2007;62:38–43. doi: 10.1111/j.1365-2044.2007.05296.x. [DOI] [PubMed] [Google Scholar]
- 80.Varmus H., Satcher D. Ethical complexities of conducting research in developing countries. N Engl J Med. 1997;337:1003–1005. doi: 10.1056/NEJM199710023371411. [DOI] [PubMed] [Google Scholar]
- 81.Kempert H., Benore E., Heines R. Easily administered patient-reported outcome measures: adolescents' perceived functional changes after completing an intensive chronic pain rehabilitation program. Arch Phys Med Rehabil. 2017;98:58–63. doi: 10.1016/j.apmr.2016.08.471. [DOI] [PubMed] [Google Scholar]
- 82.Gauntlett-Gilbert J., Connell H., Clinch J., McCracken L.M. Acceptance and values-based treatment of adolescents with chronic pain: outcomes and their relationship to acceptance. J Pediatr Psychol. 2012;38:72–81. doi: 10.1093/jpepsy/jss098. [DOI] [PubMed] [Google Scholar]
- 83.Claar R.L., Kaczynski K.J., Minster A., McDonald-Nolan L., LeBel A.A. School functioning and chronic tension headaches in adolescents: improvement only after multidisciplinary evaluation. J Child Neurol. 2013;28:719–724. doi: 10.1177/0883073812450945. [DOI] [PubMed] [Google Scholar]
- 84.Weiss K.E., Hahn A., Wallace D.P., Biggs B., Bruce B.K., Harrison T.E. Acceptance of pain: associations with depression, catastrophizing, and functional disability among children and adolescents in an interdisciplinary chronic pain rehabilitation program. J Pediatr Psychol. 2013;38:756–765. doi: 10.1093/jpepsy/jst028. [DOI] [PubMed] [Google Scholar]
- 85.Westendorp T., Verbunt J.A., de Groot I.J.M., Remerie S.C., ter Steeg A., Smeets R.J.E.M. Multidisciplinary treatment for adolescents with chronic pain and/or fatigue: who will benefit? Pain Pract. 2017;17:633–642. doi: 10.1111/papr.12495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.