Abstract
Background
Impaired cardiorespiratory reserve is an accepted risk factor for patients having major surgery. Ventilatory inefficiency, defined by an elevated ratio of minute ventilation to carbon dioxide excretion (VE/VCO2), and measured by cardiopulmonary exercise testing (CPET), is a pathophysiological characteristic of patients with cardiorespiratory disease. We set out to evaluate the prevalence of ventilatory inefficiency in a colorectal cancer surgical population, and its influence on surgical outcomes and long-term cancer survival.
Methods
In this retrospective study of 1375 patients who had undergone preoperative CPET followed by colorectal cancer surgery, we used receiver operating characteristic curve analysis to identify an optimal value of VE/VCO2 associated with 90-day mortality. Binary logistic regression was used to evaluate whether this degree of ventilatory inefficiency was independently associated with decreased survival, both after surgery and in the longer term.
Results
We identified an optimal VE/VCO2 >39 cut-off for predicting 90-day mortality; 245 patients (17.8%) had VE/VCO2 >39, of which 138 (10% of total cohort) had no known cardiorespiratory risk factors. Ventilatory inefficiency was independently associated with death at 90-days (8.2% mortality vs 1.9%; adjusted odds ratio [OR], 4.04; 95% confidence interval [CI], 2.09–7.84), with death after unplanned critical care admission (OR=4.45; 95% CI, 1.37–14.46) and with decreased survival at 2 yr (OR=2.21; 95%, 1.49–3.28) and 5 yr (OR=2.87; 95% CI, 1.54–5.37) after surgery.
Conclusions
A significant proportion of patients having colorectal cancer surgery have ventilatory inefficiency observed on CPET, the majority of whom have no history of cardiorespiratory risk factors. This group of patients has significantly decreased survival both after surgery and in the long-term, irrespective of cancer stage. Survival might be improved by formal medical evaluation and intervention in this group.
Keywords: cardiopulmonary exercise testing, colorectal surgery, mortality, perioperative risk factors, pre-operative evaluation, ventilatory inefficiency
Editor’s key points.
-
•
Traditional perioperative risk factors typically account for less than half of the variance of mortality and serious complications.
-
•
Cardiopulmonary exercise testing is the accepted standard to evaluate cardiorespiratory reserve.
-
•
Ventilatory inefficiency is defined by a relatively high minute ventilation to carbon dioxide production ratio.
-
•
This study identified that ventilatory inefficiency detected by cardiopulmonary exercise testing had added predictive value for early and longer-term mortality.
In the UK, despite the 90-day mortality rate after colorectal cancer surgery decreasing from 3.8% in 2010–1 to 2.1% in 2014–5,1 this group of patients still have significant risks associated with surgery. Mortality and morbidity after colorectal surgery arises either directly from specific cardiorespiratory complications, or indirectly by the inability of the patient to respond appropriately to surgical complications because of impaired functional reserve, mainly of the cardiorespiratory system.2, 3, 4
It is recommended to evaluate the functional cardiorespiratory status of patients about to undergo major surgery, in order to detect those with impaired functional capacity from undiagnosed cardiorespiratory disease, who are likely to be at increased risk from the surgery.5 Cardiopulmonary exercise testing (CPET) is an objective method of assessing functional capacity, providing measures of aerobic fitness, by measuring oxygen uptake at the anaerobic threshold (AT), and ventilatory efficiency as defined by the value of the slope of minute ventilation divided by carbon dioxide excretion (VE/VCO2, known as the ventilatory equivalent for CO2). Both of these parameters are used in assessing functional status and prognosis of patients with cardiac and respiratory disease,6, 7, 8, 9, 10, 11 with some evidence that VE/VCO2 may have superiority over measures of oxygen uptake.12
CPET is now regularly used in UK centres for assessing the fitness of surgical patients, traditionally involving the classification of patients with reduced AT as being unfit and therefore at increased risk from surgery.13, 14, 15 The values of AT that are associated with an increased risk of death after major surgery are similar to those that represent the pathophysiology associated with moderate to severe heart failure.6
Practical problems with using AT as the definitive marker of cardiorespiratory fitness, include difficulty in its identification, the interpretation of the effect of body weight (as AT is expressed in ml kg–1 min–1), and new evidence suggesting a marked variability in where the true value occurs.16 It is also unclear whether a reduction in AT is always attributable to a disease state, or whether it just represents deconditioning through lack of activity.
Ventilatory inefficiency, defined by an increase in the VE/VCO2 slope, has potential advantages in that it is straightforward to identify, is a simple numerical ratio without incorporating other parameters such as body weight, and a raised value usually represents an underlying pathophysiological process that interferes with the normal ventilation to perfusion relationship in the pulmonary gas exchange system. The utility of ventilatory inefficiency in predicting adverse outcomes for surgical patients remains unclear, with conflicting published results to date in a mixture of surgical groups.17, 18, 19 As a result we set out to explore the influence of CPET-measured ventilatory inefficiency on both postoperative outcomes and long-term survival, in the single subgroup of colorectal cancer surgery.
Methods
York Teaching Hospital Foundation Trust sponsored the study, which was approved by the UK Health Research Authority (IRAS 226341). As this was a retrospective analysis of prospectively gathered anonymised data that had been recorded and stored for clinical use, the requirement for individual consent was waived.
Data were analysed for patients older than 55 yr, or younger if any known cardiorespiratory risk factors were present, who were referred for a preoperative evaluation, including CPET, and subsequently underwent surgery for colorectal cancer between June 2004 and December 2016. Those patients who were either unable to exercise to AT, who did not proceed to surgery, or who were operated on for benign disease were excluded from analysis. Where patients had had more than one procedure, only the first CPET result was included in the analysis.
Preoperative evaluation consisted of recording known medical history, medications, and standard observations, followed by a CPET on a bicycle ergometer, as described previously.15 For most of the study period we practiced sub-maximal testing, stopping the test after we were confident that AT had been reached, without necessarily reaching a true peak oxygen consumption (VO2 peak). For this reason we have not included VO2 peak in this analysis. We recorded VE/VCO2 as the value recorded at AT, or the lowest value observed if AT was not reached, based on previous evidence that this value is, in practice, equivalent to using the VE vs VCO2 slope.20
Clinicians were informed of the results, to aid management and decision making. Patients were classified as either high or standard risk, based on a combination of CPET data (high risk if AT < 11 ml kg–1 min–1 and VE/VCO2 > 34, based on our previous experience), and clinical risk factors.
Intraoperatively, all patients received goal-directed fluid therapy. Where possible, high-risk patients were allocated to high-dependency care (HDU), or to a Level 1 bed on the nursing enhanced unit (NEU) on the general surgical floor; standard-risk patients all went to Level 1 care for a minimum of 24 h after surgery. Postoperative care was generally under the control of the surgical team, or intensivists if the patient was on HDU or in ICU, although for the past 18 months of the study period, perioperative physicians (anaesthetists) provided immediate postoperative care for colorectal patients on the surgical ward.
All preoperative data were recorded prospectively on a secure Trust database. Aside from basic patient characteristics and CPET variables of AT and VE/VCO2, we recorded the known presence of cardiac clinical risk factors based on Lee’s Revised Cardiac Risk Index: ischaemic heart disease, heart failure, diabetes mellitus (any), cerebrovascular disease, and renal insufficiency (creatinine >170 μmol L–1).2 In addition, we recorded the presence of chronic obstructive pulmonary disease (COPD), asthma, and other lung disease (e.g. pulmonary fibrosis, previous pulmonary embolism). We recorded whether surgery was open or laparoscopically assisted, whether there was evidence of tumour spread (irresectable local spread, positive lymph nodes, or known distal metastases).
Outcome data were gathered after hospital discharge from the Trust’s core patient database. These included postoperative and longer-term mortality, unplanned use of HDU or ICU (described collectively as Critical Care [CC] in this paper), prolonged hospital length of stay (LOS), long-term status, and survival time. Unplanned CC use was defined as any unexpected admission or re-admission to CC from the general surgical floor. Prolonged hospital LOS was defined as greater than the 75th centile.
Statistics and analysis
All data were exported into SPSS version 25.0 (IBM Corp 2017. Armonk NY, USA) for analysis. The primary outcome measure was 90-day mortality after surgery, this being the end point for surgical outcome used in the national UK bowel cancer audit.1 Secondary outcome measures were unplanned admission to CC after surgery, and longer-term survival (at 2 and 5 yr).
Statistics
Scale variables (age, AT, and VE/VCO2) were tested for distribution, then subjected to *receiver operating characteristic (ROC) curve analysis to define optimal cut-off points. If significantly predictive of the primary outcome on ROC analysis, the cut-off value defined risk groups for entering into binary logistic regression. Odds ratios (ORs) were derived for the distribution of risk factors between survivors and non-survivors for 90-day and CC outcomes. Binary logistic regression was used to determine which of these risk factors were independently associated with non-survival at each stage, provided a minimum of 10 events per cell were available.
Kaplan–Meier curves were constructed to assess which variables were significantly associated with long-term mortality, as determined by the log-rank method. Adjusted ORs for those significant variables for 2 and 5 yr mortality risk were then obtained using logistic regression, on patients for whom we had complete data for 2 and 5 yr follow-up.
Sample size
We estimated that we would have approximately 1300–1400 patients operated on for colorectal cancer surgery, of whom approximately 45% (630) would have a VE/VCO2 of >34, the cut-off value for increased postoperative risk that we had been using in practice, before this analysis.15 We calculated that this cohort size would give an estimated study power of 95% at the 0.05 alpha level of detecting a three-fold increase in 90-day mortality for patients with VE/VCO2 >34.
Results
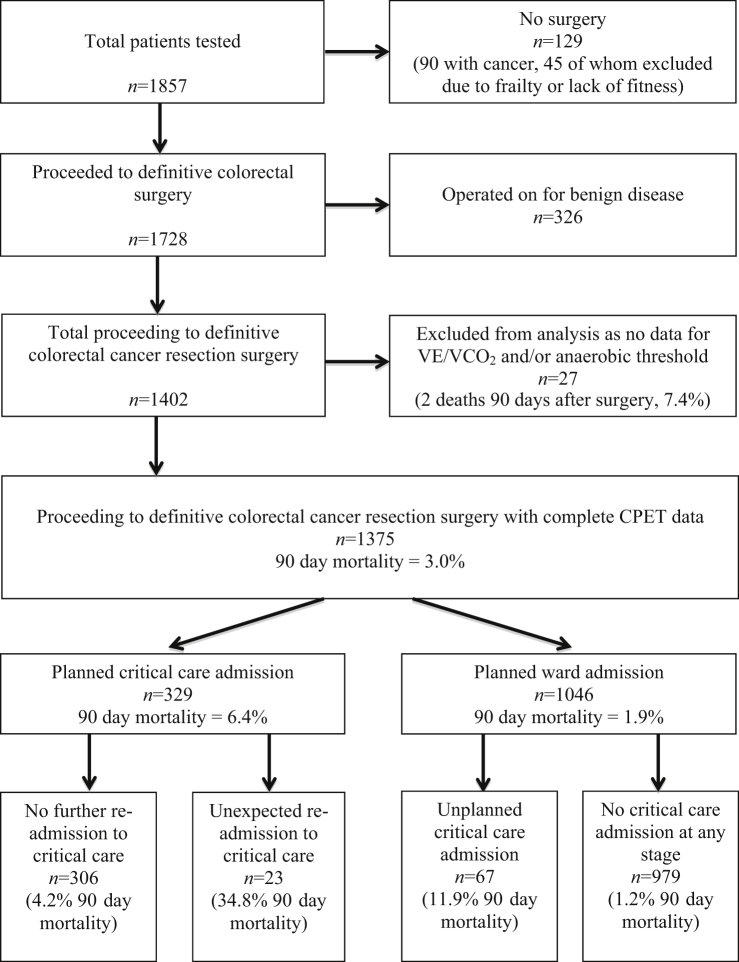
A total of 1857 patients presented for preoperative assessment and CPET for all colorectal surgery procedures between June 2004 and December 2016. Overall, 1375 met criteria for this analysis after resection surgery for colorectal cancer (Fig. 1).
Fig 1.
Flow diagram including reasons for exclusion from analysis, and the postoperative location and outcomes for the analysis cohort. CPET, cardiopulmonary exercise testing; VE/VCO2, ratio of minute ventilation to carbon dioxide excretion.
The null hypotheses that age, AT, and VE/VCO2 were normally distributed were all rejected by Kolmogorov–Smirnov testing, so non-parametric tests were used to compare differences in these parameters. ROC analyses demonstrated that age (AUC=0.63; 95% confidence interval [CI], 0.54–0.72; P=0.004), and VE/VCO2 (AUC=0.67; 95% CI, 0.0.57–0.76; P<0.001) were significantly predictive of 90-day mortality. Optimal cut-off points for predicting 90-day mortality risk were identified for age of >80 yr, and for VE/VCO2 of >39, the latter defining a cohort of 245 patients (17.8%) with ventilatory inefficiency. ROC analysis for AT demonstrated a lack of predictive ability (AUC=0.55; 95% CI, 0.47–0.64; P=0.27), so we excluded AT from inclusion in the remaining analyses.
Postoperative outcomes
Overall mortality at 90-days was 3.0%. Patients with VE/VCO2 >39 had a mortality risk of 8.2%, compared with 1.9% for those with normal VE/VCO2 (OR=4.04; 95% CI, 2.09–7.84). Age >80 yr and male gender were also independently associated with death at 90-days (Table 1). The prevalence of laparoscopic surgery significantly increased from 7.3% to 28.5% (P<0.001), but there was no variation in 90-day mortality (P=0.77) over the period of the study.
Table 1.
Patient characteristics and co-morbidities as risk factors for death at 90 days
| All patients (n=1375) | Survivors (n=1334) | Non-survivors (n=41) | P-value | |||
|---|---|---|---|---|---|---|
| Age (median, IQR) | 72 (65–79) | 72 (65–78) | 76 (70–83) | 0.004 | ||
| Anaerobic threshold (median, IQR) | 11.2 (9.6–13.0) | 11.2 (9.6–13.0) | 10.9 (9.1–12.1) | 0.27 | ||
| VE/VCO2 (median, IQR) | 33 (30–38) | 33 (30–37) | 39 (32–43) | <0.001 | ||
| Potential demographic risk factors | ||||||
| All patients (risk factor present/absent), n/N (%) | 90-day mortality (%) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | |||
| Risk factor | No risk factor | |||||
| Male sex | 813/562 (59.1) | 3.8 | 1.8 | 2.19 (1.06–4.50) | 2.44 (1.17–5.09) | |
| Cancer spread | 519/856 (37.7) | 3.1 | 2.9 | 1.06 (0.56–2.00) | N/A | |
| Open surgery | 1083/292 (78.8) | 3.1 | 2.4 | 1.32 (0.55–3.30) | N/A | |
| Age 80 yr or older (Y/N) | 243/1131 (17.7) | 6.6 | 2.2 | 3.12 (1.64–5.93) | 2.18 (1.10–4.32) | |
| VE/VCO2 >39 | 245/1130 (17.8) | 8.2 | 1.9 | 4.69 (2.50–8.80) | 4.04 (2.09–7.84) | |
| Potential clinical co-morbidity risk factors | ||||||
| All patients (risk factor present/absent), n/N (%) | 90-day mortality (%) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | |||
| Risk factor | No risk factor | |||||
| Ischaemic heart disease | 189/1186 (13.7) | 5.3 | 2.6 | 2.08 (1.00–4.31) | 1.61 (0.76–3.41) | |
| Heart failure | 24/1351 (1.7) | 4.2 | 3.0 | 1.42 (0.19–10.8) | N/A | |
| Cerebrovascular disease | 78/1297 (5.7) | 2.6 | 3.0 | 0.85 (0.20–3.58) | N/A | |
| Diabetes mellitus (any) | 164/1211 (11.9) | 3.7 | 2.9 | 1.28 (0.52–3.08) | N/A | |
| Renal insufficiency | 50/1325 (3.6) | 12.0 | 2.6 | 5.03 (2.01–12.6) | N/A∗ | |
| COPD | 86/1289 (6.3) | 8.1 | 2.6 | 3.27 (1.40–7.61) | N/A∗ | |
| Asthma | 82/1293 (6.0) | 0.0 | 3.2 | 0.97 (0.96–0.98) | N/A | |
| Other lung disease | 53/1322 (3.9) | 5.7 | 2.9 | 2.03 (0.60–0.96) | N/A | |
Excluded from binary logistic regression as less than 10 events in some cells. CI, confidence interval; COPD, chronic obstructive pulmonary disease; IQR, inter-quartile range; N/A, not available; VE/VCO2, ratio of minute ventilation (L min–1) to carbon dioxide excretion (mL kg–1 min–1)
Fig. 1 details the number of patients with planned and unplanned admission to CC, and their respective outcomes. Male gender, heart failure, and renal insufficiency were the only patient factors associated with unexpected CC admission. Only VE/VCO2 >39 was significantly associated with death after unexpected CC (OR=4.45; 95% CI, 1.37–14.5; Table 2).
Table 2.
Unexpected critical care admission after colorectal cancer surgery
| Potential risk factors for unexpected critical care admission | ||||
|---|---|---|---|---|
| Unexpected CC admission (%) |
Univariate odds ratio∗ (95% CI) | |||
| Risk factor present | Risk factor absent | |||
| Male sex | 7.7 | 4.8 | 1.66 (1.05–2.65) | |
| Age >80 yr | 7.4 | 6.4 | 1.18 (0.69–2.01) | |
| Ischaemic heart disease | 9.5 | 6.1 | 1.63 (0.95–2.80) | |
| Heart failure | 20.8 | 6.3 | 3.92 (1.43–10.8) | |
| Cerebrovascular disease | 11.5 | 6.2 | 1.96 (0.94–4.06) | |
| Diabetes mellitus (any) | 9.8 | 6.1 | 1.66 (0.94–2.93) | |
| Renal insufficiency | 18.0 | 6.1 | 3.38 (1.58–7.18) | |
| COPD | 11.6 | 6.2 | 1.99 (0.99–3.99) | |
| Asthma | 4.9 | 6.7 | 0.72 (0.26–2.01) | |
| Other lung disease | 3.8 | 6.7 | 0.55 (0.13–2.30) | |
| Open surgery | 7.1 | 4.5 | 1.64 (0.87–3.15) | |
| Evidence of cancer spread | 6.0 | 6.9 | 0.86 (0.55–1.34) | |
| VE/VCO2 > 39 | 7.3 | 6.4 | 1.16 (0.68–1.99) | |
| Potential risk factors for 90-day mortality after unexpected critical care admission | ||||
| Mortality after unexpected CC admission (%) | Univariate odds ratio∗(95% CI) | |||
| Risk factor present | Risk factor absent | |||
| Male sex | 19.0 | 14.8 | 1.35 (0.39–4.65) | |
| Age >80 yr | 27.8 | 15.3 | 2.13 (0.63–7.19) | |
| Ischaemic heart disease | 33.3 | 13.9 | 3.10 (0.95–10.2) | |
| Heart failure | 20.0 | 17.6 | 1.17 (0.12–11.2) | |
| Cerebrovascular disease | 11.1 | 18.5 | 0.55 (0.06–4.74) | |
| Diabetes mellitus (any) | 18.8 | 17.6 | 1.08 (0.27–4.35) | |
| Renal insufficiency | 33.3 | 16.0 | 2.61 (0.58–11.8) | |
| COPD | 40.0 | 15.0 | 3.78 (0.93–15.41) | |
| Asthma | 0.0 | 18.6 | 0.81 (0.74–0.90) | |
| Other lung disease | 0.0 | 18.2 | 0.81 (0.74–0.90) | |
| Open surgery | 16.9 | 23.1 | 0.68 (0.14–3.61) | |
| Evidence of cancer spread | 9.7 | 22.0 | 0.38 (0.10–1.45) | |
| VE/VCO2 > 39 | 38.9 | 12.5 | 4.45 (1.37–14.5) | |
Binary logistic regression is not possible because none of the cells have more than the required minimum 10 events. CC, critical care; CI, confidence interval; COPD, chronic obstructive pulmonary disease; VE/VCO2, ratio of minute ventilation (L min–1) to carbon dioxide excretion (mL kg–1 min–1)
Longer-term outcomes
Overall survival was 86.9% at 2 yr and 64.5% at 5 yr. The strongest predictor of long-term mortality was evidence of cancer spread at the time of surgery but VE/VCO2 > 39, age >80 yr, diabetes, renal disease, and COPD also impact significantly on long-term survival. Unexpected CC admission and prolonged LOS after surgery, markers of postoperative morbidity, were also independently predictive of decreased long-term survival (Table 3).
Table 3.
Longer-term survival after colorectal cancer surgery. COPD, chronic obstructive pulmonary disease; N/A, not available; VE/VCO2, ratio of minute ventilation (L min–1) to carbon dioxide excretion (ml kg–1 min–1)
| Risk factor | Logrank P-value |
2 yr survival (n=1175, 86.9% overall survival) Adjusted odds ratio |
5 yr survival (n=872, 64.3% overall survival) Adjusted odds ratio |
|---|---|---|---|
| Sex (male) | 0.13 | N/A | N/A |
| Age >80 yr | <0.001 | 1.43 (0.95–2.17) | 2.15 (1.47–3.20) |
| Ischaemic heart disease | 0.14 | N/A | N/A |
| Heart failure | 0.055 | N/A | N/A |
| Cerebrovascular disease | 0.15 | N/A | N/A |
| Diabetes mellitus (any) | 0.003 | 1.44 (0.89–2.33) | 1.74 (1.09–2.76) |
| Renal insufficiency | 0.001 | 1.73 (0.83–3.61) | 2.86 (1.25–6.54) |
| COPD | 0.001 | 3.63 (2.10–6.25) | 1.71 (0.96–3.06) |
| Asthma | 0.087 | N/A | N/A |
| Other lung disease | 0.76 | N/A | N/A |
| Open surgery | 0.93 | N/A | N/A |
| Evidence of cancer spread | <0.001 | 4.38 (3.09–6.19) | 4.68 (3.39–6.45) |
| AT < 11 ml–1 kg–1 min | 0.055 | N/A | N/A |
| VE/VCO2 > 39 | <0.001 | 2.21 (1.49–3.28) | 2.67 (1.83–3.89) |
| Unexpected critical care admission | <0.001 | 2.35 (1.31–4.20) | 2.87 (1.54–5.37) |
| Prolonged hospital length of stay | <0.001 | 1.81 (1.23–2.66) | 1.64 (1.14–2.36) |
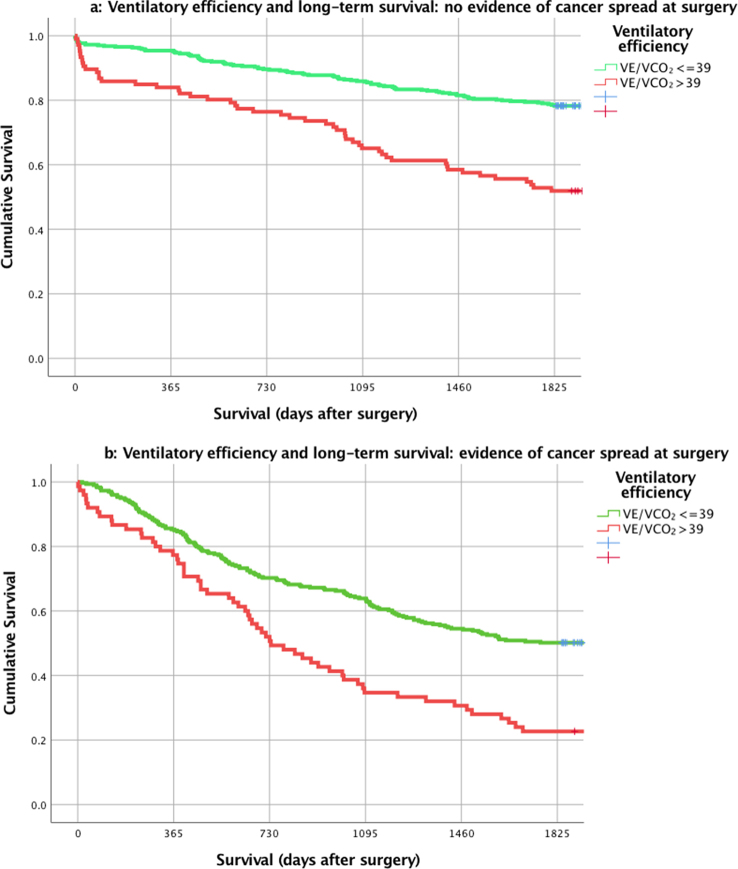
After stratification for evidence of cancer spread, VE/VCO2 > 39 is associated with significantly worse survival in both groups (Fig. 2a and b). Patients with VE/VCO2 > 39 but no evidence of cancer spread had the equivalent 5 yr survival as those patients with evidence of cancer spread but normal VE/VCO2.
Fig 2.
Effects of ventilatory inefficiency on long-term survival after surgery for patients with no evidence of metastatic spread at time of surgery (a) and evidence of metastatic spread (b).
Characteristics of patients with ventilatory inefficiency
A total of 245 patients (17.8% of the total cohort) had VE/VCO2 > 39. These patients were older, had lower ATs, and were more likely to have ischaemic heart disease and COPD (Table 4). However, 138 patients (57% of those with ventilatory inefficiency, or 10% of the total cohort) had no clinical cardiac risk factors or history of chronic respiratory disease.
Table 4.
Characteristics of patients with ventilatory inefficiency (VE/VCO2 >39). COPD, chronic obstructive pulmonary disease; IQR, inter-quartile range; NS, not significant; VE/VCO2, ratio of minute ventilation (L min–1) to carbon dioxide excretion (mL kg–1 min–1)
| No ventilatory inefficiency (n=1130) | Ventilatory inefficiency (n=245) | P-value | |
|---|---|---|---|
| Age (median, IQR) | 71 (64–77) | 78 (73–82) | 0.001 |
| Anaerobic threshold (median, IQR) | 11.4 (10.0–13.3) | 10.0 (8.3–11.5) | 0.001 |
| Male sex (%) | 60.4 | 53.5 | NS |
| Ischaemic heart disease (%) | 12.7 | 18.4 | 0.024 |
| Heart failure (%) | 1.4 | 3.3 | NS |
| Cerebrovascular disease (%) | 6.0 | 4.1 | NS |
| Diabetes mellitus (any) (%) | 11.7 | 13.1 | NS |
| COPD (%) | 4.4 | 14.7 | <0.001 |
| Asthma (%) | 6.4 | 4.1 | NS |
| Other lung disease (%) | 3.6 | 4.9 | NS |
| Renal insufficiency (%) | 3.2 | 5.7 | NS |
Discussion
Nearly 20% of colorectal cancer surgical patients presenting for CPET in our pre-assessment clinic had ventilatory inefficiency, which was associated with an increased risk of death at 90-days after surgery, an increased risk of death after unplanned CC admission, and an increased longer-term risk of death up to 5 yr, independent of cancer spread, and co-existing cardiorespiratory disease.
We have demonstrated that patients with ventilatory inefficiency but no oncological spread at the time of surgery have a similar long-term survival to those with oncological spread at the time of surgery and good ventilatory efficiency, implying that cardiac reserve or fitness has a major impact in long-term cancer survival.
Nearly 60% of our patients with ventilatory inefficiency had no known history of cardiac or respiratory co-morbidity to alert the clinician, one out of every 10 patients tested. Elevated VE/VCO2 reflects underlying abnormalities of the matching of ventilation and perfusion in the lungs (V/Q mismatch). Impaired lung perfusion is usually associated with impaired cardiac output, from conditions such as heart failure or pulmonary hypertension. In our cohort of patients with VE/VCO2 >39, only 3.3% had a diagnosis of heart failure, although this is relatively comparable with current levels of diagnosed heart failure in the UK, being 2% in the 65–74 yr age group, rising to 7% in those older than 75 yr.21 The Rotterdam Heart Failure study followed a cohort of healthy 55 yr olds from the late 1980s onwards,22 with regular cardiac evaluation, and observed an actual prevalence of 4% in the 65–74 age group, 9.7% in the 75–84 yr age group, and 17.4% in those older than 85 yr, suggesting heart failure is under-diagnosed in routine UK practice. Our data would seem to reinforce this, suggesting many patients with heart failure remain undiagnosed, maybe attributing the onset of breathlessness and fatigue to old age, and not seeking medical evaluation. In the absence of known cardiac risk factors, standard current guidelines are likely to pass these patients as fit for surgery. Guidelines acknowledge the utility of assessing functional capacity,23, 24 but only recommend further evaluation in those patients deemed to be at increased risk, based on the presence of known clinical risk factors.
A poor cardiorespiratory response to a surgical complication such as anastomotic leak can lead to multi-organ failure and death; our data show that patients with VE/VCO2 are at a high risk of death when major complications occur that require unplanned CC admission. If unrecognised as high risk, these patients are more likely to be allocated to a location with less monitoring after surgery, are more likely to be at an increased risk of ‘failure to rescue’ if complications occur, and once complications are established, will have significantly worse chances of survival.25
Respiratory causes of elevated VE/VCO2 include COPD and interstitial pulmonary disease, and CPET has utility in evaluating severity of these diseases and in differentiating them from heart failure.10, 11, 26 Elevated VE/VCO2 has been shown to be the strongest predictor of pulmonary complications and death in patients having lung resection.27 In our dataset the prevalence of known COPD was 14.7% and did indeed have an adverse effect on long-term outcomes, but as with heart failure, it is possible that a significant proportion of the surgical population has undiagnosed respiratory disease, and are at risk of being placed on a ‘standard-risk’ pathway, if not evaluated properly.
Previous work on ventilatory inefficiency and long-term outcomes for patients having major surgery has been equivocal. In a small cohort of 130 patients undergoing aneurysm surgery, a VE/VCO2 > 42 was independently associated with increased mortality at 18–24 months after open repair.17 However, Snowden and colleagues19 reported on 389 patients undergoing hepato-biliary surgery and found no difference in mean VE/VCO2 between survivors and non-survivors. In a large series of 1725 patients Colson and colleagues28 found no clear associations between distinct CPET variables and 5 yr survival, but this study looked at patients from seven distinctly different surgical groups, in which other factors affecting longer-term survival, such as malignancy, were varied and inconsistent between groups, consequently making it impossible to draw conclusions about the true influence of CPET variables on a purely cancer surgery population.
In terms of morbidity, ventilatory inefficiency was not predictive of a higher incidence of complications requiring unplanned CC admission, a finding consistent with that of Snowden and colleagues,18 who found no relationship between ventilatory inefficiency and the incidence of postoperative complications in a series of 123 patients undergoing major abdominal or vascular surgery.
The strength of our research is that we chose to study a large series of patients with a single surgical diagnosis, colorectal cancer, which allowed us to evaluate the influence of ventilatory inefficiency against a consistent background of other factors including malignancy and known cardiorespiratory disease, and thus establish a clearer signal for the influence of a raised VE/VCO2. The size of our cohort, the strength of the signal for ventilatory inefficiency, and the fact that the prevalence of cardiorespiratory disease may well be higher in other groups, would all suggest our results would be applicable to other major surgery cohorts such as aneurysm repair or thoracic surgery.
There are weaknesses to be addressed. This was a retrospective study, although all preoperative data were entered prospectively, but outcomes are representative of current UK practice.1 At 21.2%, the prevalence of laparoscopic-assisted procedures is relatively low, but this reflects the period studied, during which the incidence of laparoscopic surgery was increasing; our data suggest no impact on 90-day mortality resulted, and there is little other evidence that laparoscopic surgery confers a significant mortality and morbidity benefit that would affect the results of this study.29
We were unable to show any significant independent effect of low AT on outcomes, in contrast to our earlier series.15 There is some overlap of patients from the current study, but overall the two cohorts are different, with the previous study also containing patients undergoing radical urological surgery and patients having colorectal excision for benign conditions. The highest risk patients have a combination of low AT and elevated VE/VCO2,30 and testing for independence in this present study reveals VE/VCO2 to be the more influential CPET variable, when taking other confounders into account. A recent study looking at zones of uncertainty in evaluating CPET variables demonstrated a 60% zone of indeterminate risk for AT, compared with a 40% zone for VE/VCO2, suggesting that this wider variation may make AT a less reliable parameter when assessing fitness for surgery.16
Having identified a high-risk cohort of patients with ventilatory inefficiency, what can the perioperative physician do to reduce the risk? A dual approach consisting of medical optimisation and exercise training may be indicated. The former would involve evaluating the underlying cause for ventilatory inefficiency and starting relevant treatments, for example beta-blockade for heart failure,31 or inhaler therapy, smoking cessation, physiotherapy, and incentive spirometry for COPD.32, 33
In patients undergoing neoadjuvant cancer treatment before surgery, exercise training has been shown to reverse the adverse effects on oxygen uptake parameters caused by the treatment, although it was unclear whether ventilatory efficiency specifically was affected.34 However, as exercise training has been shown to improve ventilatory inefficiency in patients with heart failure and pulmonary hypertension, and improves quality of life in patients with COPD, it merits investigation in the context of the surgical cancer patient with ventilatory inefficiency 35, 36, 37
In summary, 10% of patients presenting for colorectal cancer resection have undiagnosed ventilatory inefficiency of sufficient severity to increase risk of death after surgery, and decreased survival in the long term, irrespective of cancer staging. Without formal testing these patients would likely remain undiagnosed. Further research is required to evaluate the effect, if any, of formal cardiorespiratory evaluation, training, and treatment, on long-term survival after cancer surgery and the postoperative period.
Authors’ contributions
Study design, data entry, analysis, manuscript review and write up: All authors
Responsibility for the manuscript: RJTW
Acknowledgements
The authors thank their colleagues Drs Stone, Redman, Biddulph, and Kannakaraj for aiding in initial testing of patients and data entry after CPET.
Handling editor: P.S. Myles
Editorial decision: 20 January 2019
Declaration of interest
The authors declare that they have no conflicts of interest.
References
- 1.Healthcare Quality Improvement Partnership Ltd . 24–7. 2016. (National Bowel Cancer Audit 2016). [Google Scholar]
- 2.Lee T.H., Marcantonio E.R., Mangione C.M. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 3.Hammill B.G., Curtis L.H., Bennett-Guerrero E. Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology. 2008;108:559–567. doi: 10.1097/ALN.0b013e31816725ef. [DOI] [PubMed] [Google Scholar]
- 4.Canet J., Gallart L., Gomar C. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee D., Eagle K.A. Perioperative cardiac assessment for noncardiac surgery — eight steps to the best possible outcome. Circulation. 2003;107:2771–2774. doi: 10.1161/01.CIR.0000072248.24921.D6. [DOI] [PubMed] [Google Scholar]
- 6.Gitt A.K., Wasserman K., Kilkowski C. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3079–3084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 7.Arena R., Myers J., Abella J. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115:2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira A.M., Tabet J., Frankenstein L. Ventilatory efficiency and the selection of patients for heart transplantation. Circ Heart Failure. 2010;3:378–386. doi: 10.1161/CIRCHEARTFAILURE.108.847392. [DOI] [PubMed] [Google Scholar]
- 9.Arena R., Sietsema K.E. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation. 2011;123:668–680. doi: 10.1161/CIRCULATIONAHA.109.914788. [DOI] [PubMed] [Google Scholar]
- 10.Bonini M., Fiorenzano G. Exertional dyspnoea in interstitial lung diseases: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev. 2017;26:160099. doi: 10.1183/16000617.0099-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell D.E., Elbehairy A.F., Faisal A. Exertional dyspnoea in COPD: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev. 2016;25:333–347. doi: 10.1183/16000617.0054-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arena R., Myers J., Hsu L. The minute ventilation/carbon dioxide production slope is prognostically superior to the oxygen uptake efficiency slope. J Cardiac Failure. 2007;13:462–469. doi: 10.1016/j.cardfail.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Older P., Smith R., Courtney P., Hone R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest. 1993;104:701–704. doi: 10.1378/chest.104.3.701. [DOI] [PubMed] [Google Scholar]
- 14.Older P., Hall A., Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116:355–362. doi: 10.1378/chest.116.2.355. [DOI] [PubMed] [Google Scholar]
- 15.Wilson R.J.T., Davies S.D., Yates D., Redman J., Stone M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth. 2010;105:297–303. doi: 10.1093/bja/aeq128. [DOI] [PubMed] [Google Scholar]
- 16.Rose G.A., Davies R.G., Davison G.W. The cardiopulmonary exercise test grey zone; optimizing fitness stratification by application of critical difference. Br J Anaesth. 2018;120:1187–1194. doi: 10.1016/j.bja.2018.02.062. [DOI] [PubMed] [Google Scholar]
- 17.Carlisle J., Swart M. Mid-term survival after abdominal aortic aneurysm surgery predicted by cardiopulmonary exercise testing. Br J Surg. 2007;94:966–969. doi: 10.1002/bjs.5734. [DOI] [PubMed] [Google Scholar]
- 18.Snowden C.P., Prentis J.M., Anderson H.L. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251:535–541. doi: 10.1097/SLA.0b013e3181cf811d. [DOI] [PubMed] [Google Scholar]
- 19.Snowden C.P., Prentis J., Jacques B. Cardiorespiratory fitness predicts mortality and hospital length of stay after major elective surgery in older people. Ann Surg. 2013;257:999–1004. doi: 10.1097/SLA.0b013e31828dbac2. [DOI] [PubMed] [Google Scholar]
- 20.Sun X.G., Hansen J.E., Garatachea N., Storer T.W., Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166:1443–1448. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 21.Bhatnagar P., Wickramasinghe K., Williams J., Rayner M., Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart. 2015;101:1182–1189. doi: 10.1136/heartjnl-2015-307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosterd A., Cost B., Hoes A.W. The prognosis of heart failure in the general population – the Rotterdam Study. Eur Heart Journal. 2001;22:1318–1327. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 23.Fleisher L.A., Fleischmann K.E., Auerbach A.D. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215–2245. doi: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 24.The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) European Society of Anaesthesiology (ESA) 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J. 2014;35:2383–2431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 25.Ghaferi A.A., Birkmeyer J.D., Dimick J.B. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 26.Teopompi E., Tzani P., Aiello M. Ventilatory response to carbon dioxide output in subjects with heart failure and in patients with COPD with comparable exercise capacity. Respir Care. 2014;59:1034–1041. doi: 10.4187/respcare.02629. [DOI] [PubMed] [Google Scholar]
- 27.Brunelli A., Belardinelli R., Pompili C. Minute ventilation-to-carbon-dioxide output (VE/VCO2) slope is the strongest predictor of respiratory complications and death after pulmonary resection. Ann Thorac Surg. 2012;93:1802–1806. doi: 10.1016/j.athoracsur.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Colson M., Baglin J., Bolsin S., Grocott M.P.W. Cardiopulmonary exercise testing predicts 5 yr survival after major surgery. Br J Anaesth. 2012;109:735–741. doi: 10.1093/bja/aes263. [DOI] [PubMed] [Google Scholar]
- 29.Vennix S., Pelzers L., Bouvy Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2014;4:CD005200. doi: 10.1002/14651858.CD005200.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson R.J.T. Shades of grey: embracing uncertainty in the exercise room. Br J Anaesth. 2018;120:1145–1146. doi: 10.1016/j.bja.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Agostini P., Apostolo A., Cattadori G. Effects of beta-blockers on ventilation efficiency in heart failure. Am Heart J. 2010;159:1067–1073. doi: 10.1016/j.ahj.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Szafranski W., Cukier A., Ramirez A. Efficacy and safety of budenoside/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 33.Licker M., Schweizer A., Ellenberger C. Perioperative medical management of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:493–515. [PMC free article] [PubMed] [Google Scholar]
- 34.West M.A., Loughney L., Lythgoe D. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. 2015;114:244–251. doi: 10.1093/bja/aeu318. [DOI] [PubMed] [Google Scholar]
- 35.Flynn K.E., Pina I.L., Whellan D.J. Effects of exercise training on health status in patients with chronic heart failure. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehani S.H.M., Abdeen H.A.A. Cardiopulmonary rehabilitation program impact on prognostic markers in selected patients with resting and exercise-induced ventilatory inefficiency: a clinical trial. J Phys Ther Sci. 2017;29:1803–1810. doi: 10.1589/jpts.29.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spruit M.A., Burtin C., DeBoever COPD and exercise: does it make a difference? Breathe. 2016;12:1–12. doi: 10.1183/20734735.003916. [DOI] [PMC free article] [PubMed] [Google Scholar]